Abstract
We demonstrate that incorporation of MnSalen into a protein scaffold enhances the chemoselectivity in sulfoxidation of thioanisole and found that both the polarity and hydrogen bonding of the protein scaffold play an important role in tuning the chemoselectivity.
Metalloenzymes have set a golden standard for carrying out reactions with high reactivity and selectivity. Understanding how proteins confer such reactivity and selectivity is important not only to providing deeper insight in biological functions, but also to its application in chemical transformations.1-11 Toward this goal, much work has focused on the study of native metalloenzymes, such as cytochrome P-450s, a metalloenzyme with high chemoselectivity in the oxidation of C-H bonds.12-14 These studies indicate that the protein scaffold is capable of creating the proper environment to modulate the reactive pathways of active intermediates so as to inhibit side reactions such as over oxidization. In contrast to the tremendous progress made in biochemical and biophysical studies of native metalloenzymes and their variants, much less has been reported regarding the application of the insight gained from such studies for designing artificial enzymes. In addition to testing our knowledge of metalloenzymes, designing artificial enzymes can provide new information that otherwise may be difficult to obtain from studying native enzymes.1-8, 12, 15 By carefully choosing protein scaffolds that are small, stable and easy to produce, such artificial enzymes may find interesting applications in chemical transformations to generate fine chemical intermediates. An emerging area in artificial enzyme design is the incorporation of non-native metal catalysts into proteins to expand the reactivity and functionality of metalloenzymes, thus transforming achiral and water-insoluble metal catalysts into asymmetric aqueous solution catalysts for reactions such as sulfoxidation, hydrogenation, and cycloaddition (Diels-Alder reaction).7, 8, 16-24
A notable bonus to such an approach is the opportunity to compare how the selectivity of the metal catalyst can be fine-tuned using biological and chemical approaches.7 Understanding how such systems control catalysis can enrich our knowledge of catalyst design, generating more selective catalysts. The majority of artificial metalloenzyme design studies have been devoted to exploring the use of the protein scaffold to tune enantioselectivity.7, 8, 16-19 However, learning to control chemoselectivity in these artificial biocatalysts, especially in catalytic oxidation, is equally important. To demonstrate the ability of the protein scaffold to tune the oxidative reactivity of metal catalysts and to discover factors involved in tuning such chemoslectivity, we report here that introducing manganese salen (salen=N,N′-bissalicylidene-1,2-ethanediamino anion, MnSalen, 1) as a non-native metal cofactor into apo sperm whale myoglobin (Mb) markedly improves chemoselectivity in catalytic sulfoxidation. For comparison, selectivity of the salen catalyst in organic solvents and of artificial metalloproteins in water showed that in both systems, hydrophobicity and hydrogen bonding play critical roles.
We previously reported the incorporation of an achiral MnSalen into apo-Mb using a dual covalent anchoring approach and the resulting artificial enzyme displayed higher enantioselectivity than those constructed using either a non-covalent or single covalent anchoring approach.26 We choose MnSalen as the metal catalyst for protein incorporation because use of MnSalen complexes for asymmetric sulfoxidation has been explored for over 20 years.27, 28 Consequently, considerable effort has been directed toward finding conditions to tune the reactivity and enantioselectivity of the catalysts by chemical means. In contrast to the remarkable success obtained in tuning the enantioselectivity of these catalysts, few studies have demonstrated the ability to prevent the overoxidation of the product sulfoxide to the undesired sulfone.29 Considering this remaining challenge, we wondered if introducing MnSalen into a protein scaffold could also control the chemoselectivity of the reaction, as observed with natural metalloenzymes, and if so, what are the important factors for controlling chemoselectivity?
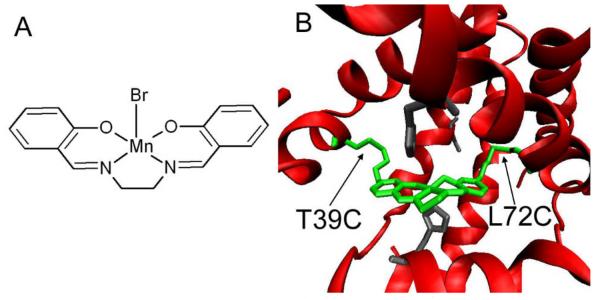
To investigate whether the protein scaffold helps control chemoselectivity, we chose Thr39Cys/Leu72Cys (T39C/L72C) as anchoring sites for MnSalen attachment (Fig. 1). As described previously,26 we used a methane thiosulfonate group modified MnSalen as the non-native metal catalyst for incorporation into apo-myoglobin (T39C/L72C). UV-vis spectroscopy and ESI mass spectrometry confirm the full incorporation of MnSalen into apo-myoglobin with covalent bonds to form MnSalen-Mb(T39C/L72C), called 1·Mb (see supplemental information)).
Fig. 1.
MnSalen 1 (A). Computer model of 1.Mb(T39C/L72C) (B), the polar residues H64, S108 and H93 are shown in grey, MnSalen and anchoring T39C and L72C are in green (PDBID 1MBO rendered using VMD).25
To compare the different reactivity and chemoselectivity of MnSalen with or without the protein scaffold, we used MnSalen catalyst 1 as a control. Catalytic sulfoxidations were carried out with H2O2 at 4°C in different buffers (pH= 7.0) for 10 min. As summarized in Table 1, catalyst 1 without protein yields 18.8% to 39.2% sulfoxide depending on the buffer used. Over oxidation resulting in sulfone was also observed under all conditions, similar to that reported in other MnSalen systems.29 In contrast, when catalyst 1 is incorporated into Mb protein, the product yields for 1·Mb are much less dependent on the type of buffer used in the reaction (<8% variation) than those of 1 (as high as 20% variation). This is not surprising as it is an indication that the metal catalyst is inside the protein and shielded from most interactions with buffer anions. More interestingly, 1·Mb exhibits higher reactivity (with yields between 44.7-52.2%, entries 5-8, Table 1) than 1 (with yields between 18.8-39.2%, entries 1-4, Table 1). Most importantly, no sulfone formation was detected in the presence of 1·Mb (entries 5-8, Table 1) while sulfone was formed under all conditions with 1 (entries 1-4, Table 1). These results strongly suggest that the protein scaffold can enhance both the reactivity and chemoselectivity of metal catalysts.
Table 1.
Reactivity and selectivity of 1 and 1·Mb in the sulfoxidation of thioanisole at pH= 7.a
| Entry | Cat. | Bufferb | Yieldc (%) |
Sulfoxide: Sulfone |
|
|---|---|---|---|---|---|
| Sulfoxide | Sulfone | ||||
| 1 | 1 | Mes | 21.0±1.3 | 1.6±0.1 | 13.1: 1 |
| 2 | 1 | Hepes | 18.8±2.0 | 1.2±0.1 | 15.6: 1 |
| 3 | 1 | His | 32.4±1.1 | 2.8±0.5 | 11.6: 1 |
| 4 | 1 | KPi | 39.2±1.9 | 4.3±0.7 | 9.1: 1 |
| 5 | 1·Mb | Mes | 52.4±2.6 | 0d | 100: 0 |
| 6 | 1·Mb | Hepes | 49.4±1.8 | 0d | 100: 0 |
| 7 | 1·Mb | His | 44.7±2.9 | 0d | 100: 0 |
| 8 | 1·Mb | KPi | 48.6±3.2 | 0d | 100: 0 |
Reactions were performed with 5 mM substrate, 10 mM H2O2, and 0.5 mM catalyst in 200 μL buffer (50 mM, pH= 7, 5% MeOH) at 4°C for 10 min.
Buffers: Mes= 2-morpholinoethanesulfonic acid; Hepes= N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid; Tris= tris(hydroxymethyl)aminomethane; His= histidine; KPi= potassium phosphate.
Yields were determined by GC with a chiral G-TA column with acetophenone as an internal standard and standard deviation as reported in parentheses.
Sulfone was not detected.
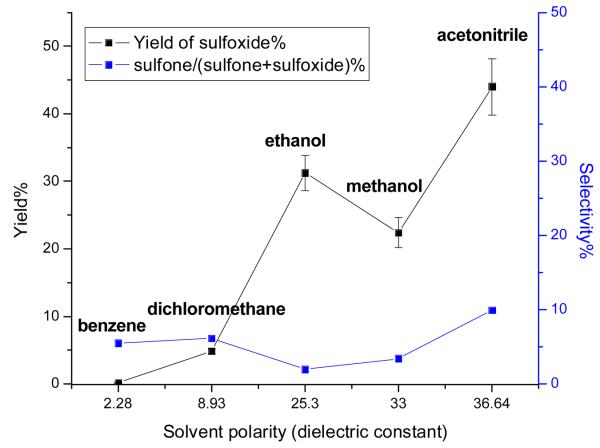
To investigate the factors responsible for the high reactivity and chemoselectivity of 1·Mb, we first considered the polarity of the protein medium surrounding the MnSalen catalyst. Such an environment inside the protein may promote MnSalen catalyst behavior similar to that observed in an organic solvent despite the aqueous solution outside the protein. To shed light on this possibility, we examined the reactivity and chemoselectivity of 1 in different organic solvents (i.e., benzene, dichloromethane, acetonitrile, methanol and ethanol) with a variety of polarities and proton donating abilities (Fig. 2). The yield of sulfoxide (Fig. 2) increased as the polarity of the solvent increased, with the highest yield obtained in acetonitrile (43.9±4.2%), being comparable to the catalyst inside the protein, 1·Mb. Therefore, we conclude that increased polarity surrounding the catalyst is responsible for the increased yield. Indeed, the environment surrounding the MnSalen in Mb contains some polar amino acids, such as H64 and S108, as well as backbone carbonyl and amide groups (see Fig. 1B).
Fig. 2.
Effect of the solvent polarity (εr= dielectric constant values30) on the reactivity and chemoselectivity of MnSalen 2 at 4°C. The following solvents have been used: benzene (εr= 2.3), acetonitrile (εr= 36.6), methanol (εr= 33.0), ethanol (εr= 25.3), and methylene chloride (εr= 8.9).
In contrast to the trend observed for reactivity, the percentage of the overoxidation product sulfone in the final combined products, and the chemoselectivity, is not dependent on the polarity of the organic solvent. Much higher chemoselectivity was observed in methanol and ethanol than in acetonitrile, benzene and dichloromethane (Fig. 2). Instead of polarity, the chemoselectivity is enhanced in good proton donating solvents such as methanol and ethanol. This enhancement may be accounted for by the proton of the alcohol group modulating the reactivity of the active intermediate(s) to favor generating sulfoxide during the catalytic process. Indeed, the same polar amino acids in Mb (H64 and S108) may also act as proton donors or acceptors to affect the generation and reactivity of active intermediate(s). Therefore, the enhanced reactivity and chemoselectivity of 1·Mb might be ascribed to a “solvent cage” effect similar to a polar proton donating organic solvent.
Since sulfone formation was still observed in both methanol and ethanol even though they are polar proton donating solvents, in constrast to that observed with the MnSalen catalyst was inside the protein (i.e., 1·Mb), there must be other effects responsible for such high chemoselectivity. We turned our attention to the protein environment around the substrate-binding site. Enzymes may be envisioned to bind the substrate and repel the product from the active site in a selective fashion; for instance, due to the large difference in polarity between sulfide (εr= 4.88) and sulfoxide (similar to sulfone, εr= 37.8) or reactant and product. Previous studies and X-ray crystal structure of sperm whale myoglobin suggest that substrates may enter the protein pocket from the right side (L72C side shown in Fig.1B), which is less bulky, but fairly hydrophobic.18, 31 Such an environment would favor entrance of the less polar sulfide compared to the corresponding sulfoxide. Therefore, increasing the polarity of the protein residues near the cavity entrance should allow observance of the formation of sulfone.
To test this hypothesis, we chose to mutate alanine 71 (A71) which is an important hydrophobic residue located near the entrance to the right side of the protein pocket.31 Computational modeling showed that mutation of A71 to S results in increased hydrophilicity on the protein surface near the substrate entrance (Fig. 3, white and blue colors represent hydrophobic and hydrophilic residues, respectively). This new biocatalyst MnSalen-Mb (T39C/L72C/A71S) called 1·Mb(A71S) was then expressed and purified as described previously26 and characterized by UV-vis spectroscopy and ESI mass spectrometry (see supplemental information). As shown in Table 2, 1·Mb(A71S) exhibits similar reactivity and enantioselectivity (entry 2, Table 2) to 1·Mb (entry 1, Table 2), with the exception that the formation of sulfone was now observed (2.1±0.8%). A comparison of the GC traces of sulfoxidation catalyzed by 1·Mb(A71S) and 1·Mb (Fig. S1) indicates formation of sulfone only in the presence of 1·Mb(A71S). Therefore we conclude that the increased polarity of the protein surface in 1·Mb(A71S) allows the more polar sulfoxide to enter the pocket for oxidation to the sulfone, while the more hydrophobic surface presented by 1·Mb inhibited this side reaction. These results demonstrate that tuning the hydrophobicity of the substrate entrance channel could affect the chemoselectivity of an oxidation reaction.
Fig.3.
Protein surfaces of the 1·Mb and 1·Mb(A71S) showing the local hydrophobicity round the right side of MnSalen cofactor. White and blue colors represent hydrophobic and hydrophilic regions, respectively. Rendered using VMD).25
Table 2.
Reactivity and selectivity of 1·Mb and 1·Mb(A71S) in the sulfoxidation of thioanisole at pH= 7.a
| Entry | Catalyst | e.e. of sulfoxideb |
Yield% of sulfoxideb |
Yield% of sulfone |
|---|---|---|---|---|
| 1 | 1·Mb | 60±1.2 | 49.4±1.8 | 0c |
| 2 | 1·Mb (A71S) | 58±0.9 | 50.0±1.2 | 2.1±0.8 |
Reactions were performed with 5 mM substrate, 10 mM H2O2, and 0.5 mM catalyst in 200 μL Hepes buffer (50 mM, pH= 7, 5% MeOH) at 4°C for 10 min.
Yield and e.e. were determined by GC with a chiral G-TA column using acetophenone as an internal standard.
Sulfone was not detected.
In conclusion, we have demonstrated the effectiveness of a protein scaffold for enhancing the reactivity and chemoselectivity of MnSalen in the sulfoxidation of thioanisole in an artificial metalloenzyme. A comparison of MnSalen outside the protein in aqueous media with that in organic solvents reveals that the polar and proton-donating environment surrounding the MnSalen inside the protein is critical for enhancing reactivity and chemoselectivity. Examination of the hydrophobicity of the residues near the substrate entrance channel demonstrates that chemoselectivity is at least partially controlled by modulating the polarity at the A71 position, and occurs with minimal change in structure and reactivity. These novel insights are valuable for the furture rational design of artificial biocatalysts.
Supplementary Material
Acknowledgments
We are grateful for the support of the National Science Foundation (CHE-05-52008) and the National Institute of Health (GM062211).
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures for sulfoxidations and characterization of 1·Mb and 1·Mb (A71S). See http://dx.doi.org/10.1039/b000000x/
Notes and references
- 1.Miller VP, DePillis GD, Ferrer JC, Mauk AG, de Montellano P. R. Ortiz. J. Biol.Chem. 1992;267:8936–8942. [PubMed] [Google Scholar]
- 2.Lu Y, Berry SM, Pfister TD. Chem. Rev. Vol. 101. Washington, DC, U. S.: 2001. pp. 3047–3080. [DOI] [PubMed] [Google Scholar]
- 3.Qi D, Tann C-M, Haring D, Distefano MD. Chem. Rev. Vol. 101. Washington, DC, U. S.: 2001. pp. 3081–3111. [DOI] [PubMed] [Google Scholar]
- 4.Reetz MT. Tetrahedron. 2002;58:6595–6602. [Google Scholar]
- 5.Hayashi T, Hisaeda Y. Acc. Chem. Res. 2002;35:35–43. doi: 10.1021/ar000087t. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D, Pecoraro VL. Curr. Opin. Chem. Biol. 2005;9:97–103. doi: 10.1016/j.cbpa.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y. Angew. Chem., Int. Ed. 2006;45:5588–5601. doi: 10.1002/anie.200600168. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y. Inorg. Chem. 2006;45:9930–9940. doi: 10.1021/ic052007t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman CM, Choi S, Shandler S, DeGrado WF. Nat. Chem. Biol. 2007;3:252–262. doi: 10.1038/nchembio876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reedy CJ, Gibney BR. Chem. Rev. Vol. 104. Washington, DC, U. S.: 2004. pp. 617–649. [DOI] [PubMed] [Google Scholar]
- 11.Benson DE, Wisz MS, Hellinga HW. Curr. Opin. Biotechnol. 1998;9:370–376. doi: 10.1016/s0958-1669(98)80010-4. [DOI] [PubMed] [Google Scholar]
- 12.Makris TM, Davydov R, Denisov IG, Hoffman BM, Sligar SG. Drug. Metab. Rev. 2002;34:691–708. doi: 10.1081/dmr-120015691. [DOI] [PubMed] [Google Scholar]
- 13.Denisov IG, Makris TM, Sligar SG, Schlichting I. Chem. Rev. Vol. 105. Washington, DC, U. S.: 2005. pp. 2253–2277. [DOI] [PubMed] [Google Scholar]
- 14.Mueller EJ, Loida PJ, Sligar SG. In: Cytochrome P-450: Structure, Mechanism, and Biochemistry. de Montellano P. R. Ortiz., editor. Plenum Press; New York, NY: 1995. pp. 83–124. Editon edn. [Google Scholar]
- 15.Gibney BR, Dutton PL. Adv.Inorg.Chem. 2001;51:409–455. [Google Scholar]
- 16.Letondor C, Ward TR. ChemBioChem. 2006;7:1845–1852. doi: 10.1002/cbic.200600264. [DOI] [PubMed] [Google Scholar]
- 17.Reetz MT, Jiao N. Angew. Chem., Int. Ed. 2006;45:2416–2419. doi: 10.1002/anie.200504561. [DOI] [PubMed] [Google Scholar]
- 18.Ueno T, Abe S, Yokoi N, Watanabe Y. Coord. Chem. Rev. 2007;251:2717–2731. [Google Scholar]
- 19.Reetz MT, Peyralans JJP, Maichele A, Fu Y, Maywald M. Chem. Commun. Cambridge, U. K.: 2006. pp. 4318–4320. [DOI] [PubMed] [Google Scholar]
- 20.Ward TR. Chem.--Eur. J. 2005;11:3798–3804. doi: 10.1002/chem.200401232. [DOI] [PubMed] [Google Scholar]
- 21.Thomas CM, Ward TR. Appl. Organomet. Chem. 2005;19:35–39. [Google Scholar]
- 22.Letondor C, Humbert N, Ward TR. Proc. Natl. Acad. Sci. U.S.A. 2005;102:4683–4687. doi: 10.1073/pnas.0409684102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueno T, Koshiyama T, Abe S, Yokoi N, Ohashi M, Nakajima H, Watanabe Y. J. Organomet. Chem. 2007;692:142–147. [Google Scholar]
- 24.Ohashi M, Koshiyama T, Ueno T, Yanase M, Fujii H, Watanabe Y. Angew. Chem., Int. Ed. 2003;42:1005–1008. doi: 10.1002/anie.200390256. [DOI] [PubMed] [Google Scholar]
- 25.Humphrey W, Dalke A, Schulten K. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 26.Carey JR, Ma SK, Pfister TD, Garner DK, Kim HK, Abramite JA, Wang Z, Guo Z, Lu Y. J. Am. Chem. Soc. 2004;126:10812–10813. doi: 10.1021/ja046908x. [DOI] [PubMed] [Google Scholar]
- 27.Kokubo C, Katsuki T. Tetrahedron. 1996;52:13895–13900. [Google Scholar]
- 28.Palucki M, Hanson P, Jacobsen EN. Tetrahedron lett. 1992;33:7111–7114. [Google Scholar]
- 29.Katsuki T. Coord. Chem. Rev. 1995;140:189–214. [Google Scholar]
- 30.Handbook of Chemistry and Physics. 73rd Edition edn. CRC Press; Boca Raton: 1992. [Google Scholar]
- 31.Ueno T, Koshiyama T, Ohashi M, Kondo K, Kono M, Suzuki A, Yamane T, Watanabe Y. J. Am. Chem. Soc. 2005;127:6556–6562. doi: 10.1021/ja045995q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.