Abstract
A temperature independent pH buffer has been develeloped from combination of buffers of opposite-sign temperature coefficients, and utility in low temperature spectroscopy and storage of pH sensitive compounds is demonstrated.
Storage and analysis of samples at low and cryogenic temperatures has become a routine practice in modern research, as these temperatures can preserve integrity of precious samples, and allow modern biophysical and bioanalytical techniques to provide information on biomolecules at an unprecedented level.1-7 Buffers are invariably used in the sample storage and analysis process. It is widely known that the pH of a buffer solution can change at low temperatures, and this has been ascribed to enthalpic effects on the proton equilibrium as well as selective precipitation of buffer components upon cooling.8-11 If left unaccounted for, these pH changes could lead to demage to the samples and erroneous conclusions about biomolecular structures and dynamics at physiological temperature.
About 75 years ago, Finn and coworkers reported the denaturation of proteins contained in muscle juice due to the variation in hydrogen ion and salt concentrations upon freezing.12 Thereafter, activity loss of aldolase, phosphofructokinase and several dehydrogenases in sodium and potassium phosphate buffer at lower temperatures has been observed and ascribed to pH effects.13, 14 Several strategies have been applied to measure the temperature-dependent pH characteristics, such as the measurement of electromotive force (emf) to determine temperature dependence of pKa values, or the use of pH-sensitive dyes to probe protonic activity at low temperatures.15-18 It was reported that EPR (electron paramagnetic resonance) based estimates of apparent pH change from the observation of several pH-sensitive systems (such as the flavin adenine dinucleotide semiquinone radical (FADH•) in xanthine oxidase) coincide well with estimates based on indicator dye optical changes.19
Despite broad awareness, and extensive research into the relevance and merits of cryotemperature studies on biological systems,20, 21 no reports of strategies to obtain buffers that resist temperature-dependent pH changes have appeared in the literature. Here we address this long standing problem through combination of buffers exhibiting an increase in pH upon freezing with those exhibiting a decrease. The utility of these temperature-independent-pH (TIP) buffers in preserving the sample integrity upon freezing of pH-sensitive pharmaceutical drugs, and in spectroscopic studies of human methemoglobin (met-Hb) is also demonstrated.
To obtain the TIP buffer, the apparent pH changes of the two commonly used buffers, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and potassium phosphate) and mixtures thereof were first measured at low temperature via ratiometric absorption spectroscopy. A 1:1 mixture of two pH-indicator dyes (bromcresol green (BCG) and bromthymol blue (BTB) was used as a probe having two band maxima in the visible absorption spectrum, allowing for ratiometric calibration of pH values at different temperatures that is not vulnerable to baseline shifts often affiliated with low temperature absorption spectral characterization (Fig. 1a).
Fig. 1.
(a) Absorption spectral changes of a 0.1 v/v% solution of BCG:BTB indicator in 50 mM mixed buffer (16.6 mM in potassium phosphate/BTP/Hepes) and 50% v/v glycerol from pH 6.0 to 8.6. Arrows indicate direction of increasing pH. (b) Apparent pH vs. temperature for selected buffers as probed by indicator dyes. Error bars indicate standard deviation of three replicates. Images show color change upon cooling from 25°C to −140°C in presence of each buffer. (BTP buffer not shown)
It has been established that the low temperature effect on the protonation state of the indicator dyes chosen is this work is governed by the buffer composition, and is not, in this case, a result of a significant pK shift of the dye itself upon cooling.18 Consideration must always be taken to separate low temperature effects on the probe itself from those inferred as pH changes.
Quantification of apparent pH change was accomplished via development of a room temperature calibration curve correlating absorption peak maxima ratios to pH, and subsequent correlation of low temperature absorption spectra to apparent pH. As shown in Fig. 1b, the apparent pH of HEPES buffer measuring pH 7.0 at room temperature increases when lowering the temperature, while the apparent pH of the phosphate buffer decreases. The apparent changes of pH for HEPES and phosphate buffers from 25 °C to −180 °C are 1.52 ± 0.20 and -0.55 ± 0.22 units, respectively, and such pH changes are so drastic as to heavily influence the quantitative cryogenic investigations of pH-sensitive biomolecules.
We subsequently monitored the effect of mixing the two buffers in different ratios and found that a combination of 60% HEPES and 40% potassium phosphate (TIP7) exhibits less than 0.07 ± 0.10 pH upon cooling from 25 °C to −180 °C (Fig. 1b). The spectrum in bis-tris propane (BTP) buffer is shown as a positive control, as a large pH change was expected for this buffer upon freezing.
To explore the utility of such a TIP buffer in preserving integrity of samples stored at low temperature, we employed the pH-sensitive pharmaceutical drug, oxacillin, a penicillin analog. Inactivation of β-lactam antibiotics in acidic or basic solution has been correlated with the initial opening of the β-lactam ring, irreversibly deactivating the drug towards antibiotic activity.22-24
Solutions of oxacillin were prepared at 4 °C in the presence of 50 mM HEPES, potassium phosphate, BTP, and TIP7 buffers at pH 7.0. Aliquots of each solution were then subject to storage in a freezer at −20 °C, and the degradation of oxacillin was monitored quantitatively over several days via thawing and subsequent HPLC analysis. Control samples (light colors) were thawed only at the end of the test period, and indicate that the freeze/thaw process itself was not largely responsible for the observed degradation. All buffered solutions measured pH 7.00 ± 0.05 at 25 °C after storage.
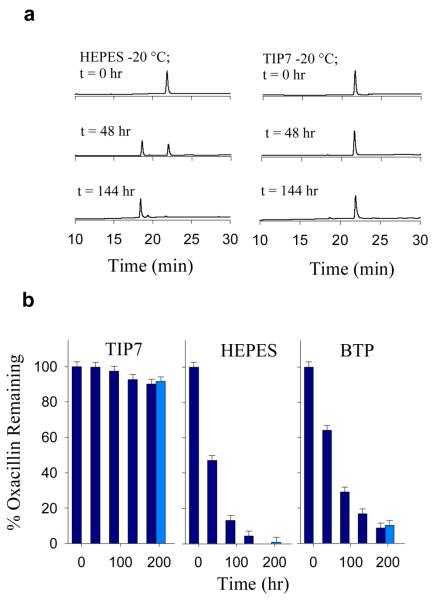
Shown in Fig. 2a are HPLC chromatograms exhibiting the conversion of oxacillin (Tr 22.5 min) to its hydrolysis product (Tr 17.8 min) over time upon storage at −20 °C the presence of HEPES and TIP7 buffers. A sample of the major hydrolysis product at Tr = 17.8 min was collected at 4 °C for each buffer and in all cases was identified as the β-lactam hydrolysis species via electrospray mass spectrometry (ESI− (m/z) obs. 208.2 ± 1.0 calc. 208.7 (M-2H+)/2). No buffer-oxacillin adducts could be observed under the investigated conditions. Consistent with indicator dye experiments, increased rates of hydrolysis were observed for bis-tris propane and HEPES buffers (Fig. 2b), and were attributed to an increase in pH of the solution upon storage at −20 °C. Remarkably, even after 144 hours, the extent of hydrolysis observed in TIP7 buffer (60:40 HEPES:potassium phosphate) is only 7.6% of that observed in HEPES alone; with 95.6% of the oxacillin remaining intact, compared with 4.4% remaining in HEPES solution. No hydrolysis was observable in potassium phosphate buffer over the reported test period, consistent with an expected decrease in pH from 7.0 into the optimal stability range for this particular antibiotic.
Fig. 2.
(a) Degradation of oxacillin in different buffers at low temperatures. (b) HPLC chromatograms showing base catalyzed conversion of 100 μM oxacillin (Tr 22.5 min) to its hydrolysis product (Tr 17.8 min) over time at −20 °C in HEPES and TIP7 buffers. Samples in dark blue were thawed every 48 hrs and refrozen after analysis. Control solutions in light blue remained frozen until end of trial. All HPLC separations were performed at 25 °C while monitoring A220. All samples measured pH 7.00 ± 0.05 at 25 °C before and after storage.
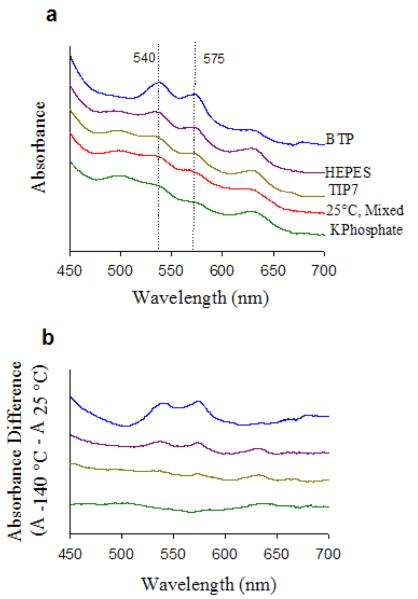
To investigate the utility of the TIP buffer for conducting accurate spectroscopic studies of biomolecules at cryotemperatures, we measured the UV-vis absorption spectrum of human methemoglobin (met-Hb) at 25 °C and upon cooling over 30 seconds to −140 °C in potassium phosphate, HEPES, bis-tris propane (BTP) and TIP buffers; all starting at pH 7.0 at 25 °C (Fig. 3a). The ferric state of human met-Hb exhibits a pH dependent equilibrium between high-spin normal state at low pH (Fe-OH2, with absorptions at 500 and 630 nm) and low-spin alkaline state at high pH (Fe-OH, with absorptions at 540 and 575 nm), with a pKa of 8.05 at 25 °C.25, 26 The difference spectra shown in Fig. 3b are arranged, from bottom to top, in order of increasing concentrations of the low-spin alkaline species generated in each buffer solution upon freezing. The met-Hb spectrum in TIP7 buffer at −140 °C showed most similarity to that at room temperature. Clearly, more alkaline signal is observable in HEPES buffer than in TIP7 buffer due to a pH increase upon freezing, as is a decrease in alkaline signal from the room temperature spectrum in the presence of phosphate buffer due to a pH decrease upon freezing.
Fig. 3.
(a) Visible spectra of adult human met-Hb (0.1 mM) in 50 mM mixed buffer (16.6 mM potassium phosphate/HEPES,/BTP) at 25 °C and upon cooling to −140 °C in presence of 50 mM potassium phosphate, TIP7, HEPES, and BTP buffers. (b) pow temperature spectra after subtraction of room temperature spectrum. All solutions measured pH 7.00 ± 0.05 at 25 °C before and after spectra were taken.
In summary, we have carried out a systematic quantification of pH changes of two commonly used buffers under low temperatures and, based on the study, we have developed a temperature independent pH buffer. We further demonstrate such a buffer can help to maintain integrity of pH-sensitive pharmaceutical compounds such as oxacillin when storing the sample under low temperature. We also show utility in pH-dependent spectroscopic analysis at cryotemperature, as in the visible absorbance spectroscopy of human methemoglobin. The ratiometric method to quantify pH changes and the strategy of mixing different buffers of opposite pH changes with temperatures to result in temperature independent pH buffers can be generally applied in other buffer systems at a different pH. Such buffers should find wide utility in cryostorage and biophysical studies of pH sensitive molecules at low temperature.
Supplementary Material
Acknowledgments
We wish to thank Cody J. Vild for help in data collection, and Dr. Mark Nilges for helpful discussions. This material is based upon work supported by the National Science Foundation (CHE- 0552008) and the National Institute of Health (GM062211).
Footnotes
Electronic Supplementary Information (ESI) available: [Experimental details including buffer preparation, low-temperature spectroscopy, and ESI-MS spectrum of oxacillin hydrolysis product.]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Williams PA, Blackburn NJ, Sanders D, Bellamy H, Stura EA, Fee JA, McRee DE. Nature. 1999;6:509–516. doi: 10.1038/9274. [DOI] [PubMed] [Google Scholar]
- 2.Blackburn NJ, de Vries S, Barr ME, Houser RP, Tolman WB, Sanders D, Fee JA. J. Am. Chem. Soc. 1997;119:6135–6143. [Google Scholar]
- 3.Sage JT, Schomacker KT, Champion PM. J. Phys. Chem. 1995;99:3394–3405. [Google Scholar]
- 4.Ondrias MR, Rousseau DL, Simon SR. Science. 1981;213:657–659. doi: 10.1126/science.7256263. [DOI] [PubMed] [Google Scholar]
- 5.van Thor JJ, Sage JT. Photochem. Photobiol. Sci. 2006;5:597–602. doi: 10.1039/b516525c. [DOI] [PubMed] [Google Scholar]
- 6.Liviu MM, Vance M, Rudd DJ, Hedman B, Hodgson KO, Solomon EI, Stack TDP. Science. 2007;308:1890–1892. doi: 10.1126/science.1112081. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Solomon EI. Proc. Natl. Acad. Sci. 2004;101:13105–13110. doi: 10.1073/pnas.0402114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazur P. Science. 1970;168:939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 9.Olien CR. Annu. Rev. Plant. Physiol. 1967;18:387–408. [Google Scholar]
- 10.Walters C, Wheeler L, Stanwood PC. Cryobiology. 2004;48:229–244. doi: 10.1016/j.cryobiol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Harding K. Cryo Lett. 2004;25:3. [PubMed] [Google Scholar]
- 12.Finn DB. Proc. Roy. Soc. 1932;B:396–411. [Google Scholar]
- 13.Chilson OP, Costello LA, Kaplan NO. Fed. Proc. 1965;24:55–65. [PubMed] [Google Scholar]
- 14.Bock P, Frieden C. Biochemistry. 1974;13:4191–4196. doi: 10.1021/bi00717a020. [DOI] [PubMed] [Google Scholar]
- 15.Bates RG, Bennetto HP, Sankar M. Anal. Chem. 1980;52:1598–1601. [Google Scholar]
- 16.Roy RN, Gibbons JJ, McGinnis T, Woodmansee R. Cryobiology. 1985;22:578–588. doi: 10.1016/0011-2240(85)90035-5. [DOI] [PubMed] [Google Scholar]
- 17.Williams-Smith DL, Bray RC, Barber MJ, Tsopanakis AD, Vincent SP. Biochem. J. 1977;167:593–600. doi: 10.1042/bj1670593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orii Y, Morita M. J. Biochem. (Tokyo) 1977;81:163–168. doi: 10.1093/oxfordjournals.jbchem.a131431. [DOI] [PubMed] [Google Scholar]
- 19.Schulze H, Ristau O, Jung C. Biochim. Biophys. Acta. 1994;1183:491–498. doi: 10.1016/0005-2728(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 20.Douzou P, Balny C, Franks F. Biochimie. 1978;60:151–158. doi: 10.1016/s0300-9084(78)80748-2. [DOI] [PubMed] [Google Scholar]
- 21.Douzou P. Proc. Roy. Soc. Lond. B. 1982;217:1–28. doi: 10.1098/rspb.1982.0091. [DOI] [PubMed] [Google Scholar]
- 22.Fleming A. Br. J. Exp. Path. 1929;10:226–236. [Google Scholar]
- 23.Deshpande AD, Baheti KG, Chatterjee NR. Curr. Sci. 2004;87:1684–1695. [Google Scholar]
- 24.Robinson-Fuentes VA, Jefferies TM, Branch SK. J. Pharm. Pharmacol. 1997;49:843–845. doi: 10.1111/j.2042-7158.1997.tb06124.x. [DOI] [PubMed] [Google Scholar]
- 25.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Reactions with pigands. North-Holland Publishing Company; Amsterdam: 1971. [Google Scholar]
- 26.Svistunenko DA, Sharpe MA, Nicholls P, Blenkinsop C, Davies NA, Dunne J, Wilson MT, Cooper CE. Biochem. J. 2000;351:595–605. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.