Summary
Bisphenol A (BPA) is one of the most prevalent and best studied endocrine disruptors. After years of exposure to consumer products containing BPA, most individuals tested have circulating BPA at the low nanomolar levels. In addition to its well documented actions on the reproductive system, BPA exerts a wide variety of metabolic effects. This review summarizes recent findings on the ability of BPA, at environmentally relevant doses, to inhibit adiponectin and stimulate the release of inflammatory adipokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNFα) from human adipose tissue. Expression of several classical and non-classical estrogen receptors in human adipose tissue raises the possibility of their involvement as mediators of BPA actions. The implications of these observations to the obesity-related metabolic syndrome and its sequalae are discussed.
Keywords: bisphenol A, adiponectin, inflammatory cytokines, human adipose tissue, metabolic syndrome
BPA: Overview and Controversial Issues
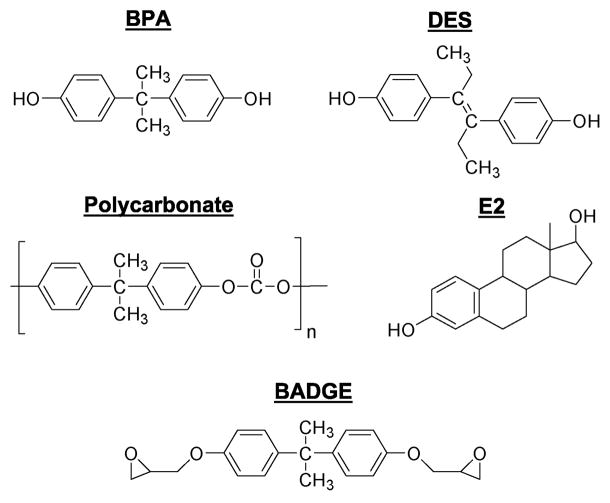
BPA is a small (228 Da) molecule which is used as a monomer in polymerization reaction to produce polycarbonate plastics (Fig 1). Polycarbonates are used in numerous consumer products, including food and water containers, baby bottles, lining of food and beverage metal cans, medical tubing, epoxy resins and dental fillings (Ben-Jonathan and Steinmetz, 1998; Welshons et al., 2006). Small amounts of BPA can migrate from the polymers to food or water especially upon heating (Le et al., 2008). Studies conducted in the USA, Europe and Japan, have documented widespread human exposure to BPA, with detected levels ranging from 0.3 to 5 ng/ml (approximately 1–20 nM) in serum and breast milk (Welshons et al., 2006)). Being lipophilic, BPA also accumulates in human fat (Fernandez et al., 2007). Bisphenol A diglycidyl ether (BADGE), is a component of epoxy resins (Fig 1). Workers who spray epoxy resins had higher urinary and plasma levels of both BADGE and BPA than controls, indicating that BPA can be endogenously generated from BADGE (Hanaoka et al., 2002).
Fig 1.
Structures of bisphenol A (BPA) and related compounds: estradiol (E2), polycarbonates, diethylstilbestrol (DES), and bisphenol A diglycidyl ether (BADGE).
In 1993, Krishnan et al discovered a substance that leached from polycarbonate flasks during autoclaving and acted like an estrogen by increasing the proliferation of breast cancer cells (Krishnan et al., 1993). Using sequential chromatography they identified this compound as BPA. Soon thereafter, BPA was detected in food cans and dental cement (Brotons et al., 1995; Olea et al., 1996). At about the same time, we were studying interactions between prolactin (PRL) and estradiol (E2). Upon noticing a striking structural similarity between BPA and the potent estrogen diethylstilbestrol (DES) (Fig 1), we set out to examine if BPA altered PRL production. Our study (Steinmetz et al., 1997) was the first to show estrogen-like properties of BPA within the neuroendocrine axis in vitro and in vivo. This was followed by reporting on the effects of BPA on the reproductive tract in female rats (Steinmetz et al., 1998), and the induction of prolonged hyperprolactinemia following treatment of neonatal rats with BPA (Khurana et al., 2000). We also found exquisite sensitivity of some rat strains (i.e., Fischer 344) to the estrogen-mimetic effects of BPA (Long et al., 2000).
A recent PubMed search revealed more than 2000 publications with a focus on BPA. Studies with rodents demonstrate that BPA elicits a wide range of activities, including neurochemical alterations, abnormalities in sperm and oocyte maturation, disruption of fertility, changes in growth rates and immune dysfunctions (Richter et al., 2007). This is supported by reports on the effects of BPA on proliferation, differentiation, ion transport, and hormone release in cultured cells (Wetherill et al., 2007). BPA also has an ecological impact on wildlife (Crain et al., 2007).
The growing interest by scientists and the public alike has placed BPA at the center of the debate over adverse effects of endocrine disruptors on fetal development, reproductive fecundity and carcinogenesis. Yet, attribution of such actions to BPA has been controversial. Indeed, differences of opinion and disagreements over data interpretation underlie the inability of several expert panels to reach binding decisions as to potential hazards of BPA to human health. A major issue, often raised by the chemical and food industries, is the micromolar doses of BPA used in many studies. Unless BPA is shown to be active at environmentally relevant concentrations (low nanomolar range), it is difficult to make a persuasive argument that BPA is hazardous to health. Another issue is a lack of linear dose-dependency of BPA effects, often showing a ‘U’ or an inverted ‘U’ shaped curves. Thus, extrapolation from action, or lack of action, of BPA at high doses to its presumed bioactivity at low doses is unwarranted.
The mechanism by which BPA exerts its actions is enigmatic. BPA and DES share structural features, and the estrogen receptor (ER) binds many dissimilar compounds. While BPA binds both ERα and ERβ (Kuiper et al., 1998), its binding affinity is ≈10,000 fold lower than that of E2, suggesting that it should mimic or compete with estrogens only at the μM range. Yet, BPA at nanomolar concentrations often exhibits estrogen-like activities that are similar to, or stronger than, E2. Several speculations have been put forward to reconcile this disparity. One, differences in binding to serum proteins, transport and metabolism, as compared to estrogens, account for the low dose effect of BPA in animal studies, especially during fetal development (Welshons et al., 2006). Two, BPA binds differently within the ligand binding domain of ERs and recruits a different set of co-regulators (Safe et al., 2002). Three, BPA elicits rapid responses via non-genomic mechanisms by activating membrane-anchored ERs (Watson et al., 2007), unidentified non-classical membrane estrogen receptor (ncmER; (Alonso-Magdalena et al., 2005), or G-protein-coupled receptor 30 (GPR30; (Filardo and Thomas, 2005)). Four, BPA binds to estrogen related receptors (ERR), of which ERRγ has high binding affinity to BPA (Takayanagi et al., 2006).
Metabolic Actions of BPA
Although the pancreatic islets and adipocytes are not considered classical targets of estrogen, both express functional ERs (Le May et al., 2006; Pedersen et al., 2001). Nadal et al found that 1 nM doses of BPA, E2 and DES were equally potent in suppressing calcium oscillations in glucagon-producing mouse islet cells (Alonso-Magdalena et al., 2005). This rapid effect was mediated by a G-protein-coupled receptor which they named ncmER. Mice treated with low doses of E2 or BPA showed rapid increases in insulin release and reduced plasma glucose; this effect was not blocked by pretreatment with the ER antagonist ICI182,780 (Alonso-Magdalena et al., 2006). Prolonged treatment with BPA or E2 caused chronic hyperinsulinemia and induction of insulin resistance, an effect which was blocked by ICI. The authors suggested that BPA mimics the actions of E2 on blood glucose homeostasis via two pathways: a rapid pathway involving ncmER and a prolonged pathway involving ER.
Studies on the impact of BPA on adipose tissue yielded conflicting and hard to interpret data, most likely due to the wide range of administered doses. BPA accumulation in rat adipose tissue was low in one study (Shin et al., 2004) but high in another (Nunez et al., 2001). When given to rats at high doses for 15 days, BPA caused a reduction in body weight and a lower feeding efficiency (Nunez et al., 2001), while others found that feeding BPA to rats for three months did not alter body weight, fat depots or triglyceride levels (Seidlova-Wuttke et al., 2005). Using 3T3-F442A adipocytes, Sakurai et al reported that BPA stimulated insulin-dependent glucose uptake and increased Glut4 expression; E2 was ineffective and ICI did not antagonize BPA (Sakurai et al., 2004). However, only the highest dose of BPA tested (100 μM) was effective. Others (Masuno et al., 2002; Masuno et al., 2005) reported that BPA accelerated adipogenesis in 3T3-L1 adipocytes. Again, BPA was active only at doses of 80 μM and above.
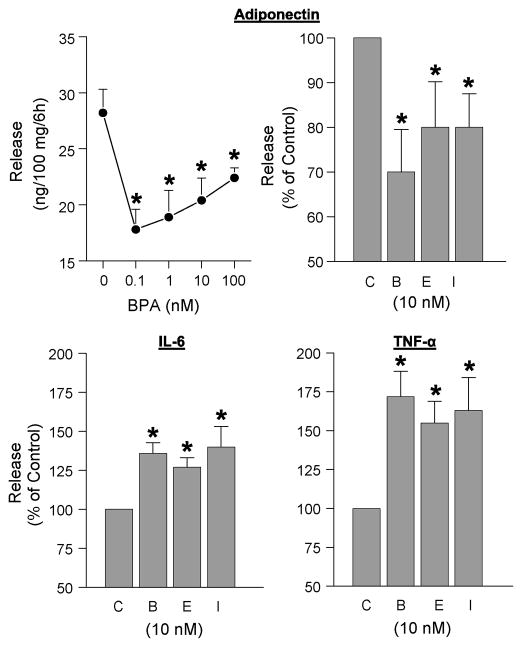
Until recently, there has been no information on BPA effects on human adipocytes. Using breast, subcutaneous and visceral adipose tissue explants as well as isolated mature adipocytes from over 20 patients, we reported that BPA at 1 and 10 nM concentrations inhibits adiponectin release (Hugo et al., 2008). Both BPA and E2 showed a clear ‘U’-shaped dose-response curve. We subsequently found that BPA stimulated the release of two inflammatory cytokines, IL-6 and TNFα. Unexpectedly, ICI mimicked, rather than antagonized, the effects of both BPA and E2 on the release of all three adipokines (Fig 2). To underscore the relevance of these findings to the metabolic syndrome and its sequelae, we discuss the key role of adiponectin as an insulin sensitizer, summarize salient features of IL-6 and TNFα as inflammatory adipocytokines, and speculate on the mechanisms by which BPA affects their release.
Fig 2.
Comparison of the effects of BPA (B), estradiol (E), and ICI (I) on adipokine release from human adipose tissue explants. Upper left panel: Dose-dependent effects of BPA on adiponectin release from a representative patient (Means±SEM of 5 replicates). All other panels show release as percent of control (C). Values are means±SEM; N = 4 patients. Subcutaneous adipose tissue explants, obtained from patients undergoing abdominoplasty, were incubated for 6 hrs with the various compounds. Conditioned media were analyzed for adiponectin, interleukin-6 (IL-6) and tumor necrosis factor α (TNFα) by their respective ELISAs. * designates significant difference from controls (P<0.05).
AdiposeTissue and the Metabolic Syndrome
Human fat is distributed in discrete visceral and subcutaneous depots which differ in morphology and function (Wajchenberg et al., 2002). Visceral fat comprises 6% of total body fat in women but 20% in men, reflecting the greater propensity of men to accumulate excess abdominal fat. Adipose tissue is composed of many cell types embedded in connective tissue matrix (Ailhaud et al., 1992). The major cell type is the very large, terminally-differentiated adipocyte, with a small number of proliferation-competent preadipocytes. The latter are fibroblast-like cells that are committed to the adipocyte lineage. The stromal fraction also contains endothelial and mast cells, fibroblasts and infiltrating macrophages (Rink et al., 1996). Obesity results from increased adipocyte size (hypertrophy) and number (hyperplasia). Mature adipocytes carry out the bulk of adipose tissue functions, given their ability to metabolize glucose, store and release free fatty acids, and secrete adipokines. Stromal macrophages contribute to the overall metabolic activity of adipose tissue by releasing adipocytokines such as TNFα and IL-6.
Increased abdominal visceral fat is a critical factor in the development and manifestation of the metabolic syndrome. This syndrome is defined by glucose intolerance, hyperinsulinemia, hypertriglyceremia, altered lipoprotein levels and hypertension (Ritchie and Connell, 2007). Visceral fat is more sensitive to β-adrenergic agonists and less responsive to insulin and α2-adrenergic agents than subcutaneous fat, making it more lipolytically active (Arner, 2001). Since the output of visceral fat drains into the hepatic portal blood, an increased influx of free fatty acids leads to inhibition of insulin clearance by the liver, thus contributing to hyperinsulinemia. Chronic elevation of free fatty acids also impairs glucose metabolism and insulin sensitivity in both the liver and muscle and reduces pancreatic β cell function (Lewis et al., 2002). There are significant differences between fat depots in the secretion of adipokines, with visceral fat secreting more IL-6 but less leptin and adiponectin than subcutaneous fat (Arner, 2001).
Adiponectin
Adiponectin is a 30 kDa adipocyte-specific hormone which forms several multimers: high molecular weight, low molecular weight and trimeric complexes (Trujillo and Scherer, 2005). These circulate at ≈10 μg/ml, comprising as much as 0.01% of total serum proteins in humans. The biological effects of adiponectin depend upon distinct properties of the multimers and tissue-specific expression of two receptors: skeletal AdipoR1 and hepatic AdipoR2 (Kadowaki and Yamauchi, 2005). Adiponectin increases fatty acid oxidation and glucose metabolism in muscle, and reduces glucose output and enhances insulin sensitivity in liver. It also has anti-inflammatory and anti-atherogenic actions (Kadowaki et al., 2006). Serum adiponectin levels are reduced before the development of type 2 diabetes, are paradoxically lower in obese than in lean subjects, and increase after weight loss (Trujillo and Scherer, 2005). Thus, adiponectin is considered a key regulator of insulin sensitivity and tissue inflammation. Consequently, any factor which suppresses adiponectin release could promote insulin resistance and increase the risk of developing the metabolic syndrome.
Adiponectin availability is controlled at three levels: biosynthesis, assembly and release (Trujillo and Scherer, 2005). Synthesis is stimulated by PPARγ agonists, insulin and IGF-1 and inhibited by TNFagr; and catecholamines (Lihn et al., 2005). Increased adiponectin expression by PPARγ is of special interest since one mechanism by which BPA could suppress adiponectin is by antagonizing PPARγ. Indeed, BADGE, the ether conjugated BPA (Fig 1), is a potent antagonist of PPARγ actions in adipocytes (Knouff and Auwerx, 2004; Wright et al., 2000). Correct formation of disulfide bonds is critical for the assembly and retention of adiponectin (Trujillo and Scherer, 2005). Disulfide bond formation within the endoplasmic reticulum is catalised by oxyreductases, of which protein disulfide isomerase (PDI) is a critical player (Tsao et al., 2003; Wang et al., 2007). Since BPA was reported to bind PDI and inhibit its enzymatic activity (Hiroi et al., 2006), this could represent another mechanism by which BPA suppresses adiponectin.
The control of adiponectin release is poorly understood. Unlike most neuroendocrine cells, adipocytes do not have high storage capacity secretory granules and lack calcium-dependent exocytosis (Mora and Pessin, 2002). Thus, the secretory pathway of adipokines is often referred to as ‘constitutive’. Although adipocytes have Glut4-containing vesicles which rapidly translocate to the cell membrane in response to insulin, these vesicles do not contain adiponectin (Bogan and Lodish, 1999). This raises the question of whether adipocytes can regulate the release of adipokines once they are synthesized. Scherer and co-workers (Wang et al., 2007) reported that both assembly and release of adiponectin depend upon thiol-mediated retention within the endoplasmic reticulum. This process involves reciprocal interactions between PDI and several chaperones. This concept stipulates that a large proportion of de novo synthesized adiponectin is not immediately secreted but is retained within the endoplasmic reticulum. Such a pool is regulatable by compounds that modify disulfide bond formation or alter chaperone functions.
IL-6 and TNFα
IL-6 is a pleiotropic cytokine made of a single chain of 185 amino acids which configures as two pairs of anti-parallel α-helices (Kamimura et al., 2003). Binding of IL-6 to the IL-6Rα receptor recruits gp130, a non-ligand binding protein. The activated receptor complex induces phosphorylation of Jak kinases, followed by activation of STAT3 and MAP kinase. In addition to most immune cells, IL-6 is produced by preadipocytes, adipocytes, and macrophages residing within adipose tissue. IL-6 is intimately involved with inflammatory states, hematopoiesis, immune responses and host defense mechanism (Heinrich et al., 2003).
Within adipose tissue, IL-6 stimulates lipolysis, inhibits lipoprotein lipase activity, antagonizes insulin-stimulated glucose uptake and suppresses adiponectin release (Kamimura et al., 2003). Elevated serum IL-6 levels are associated with increased cardiovascular risk in obese and diabetic patients and contribute to the low grade inflammation that accompanies the metabolic syndrome (Spranger et al., 2003). IL-6 expression in adipocytes is stimulated by insulin, TNFα, and β adrenergic agonists, and is inhibited by glucocorticoids (Hoene and Weigert, 2008). We recently reported that IL-6 gene expression and release in human preadipocytes are differentially regulated (LaPensee et al., 2008). Whereas insulin-stimulated IL-6 expression is mediated by the cGMP/PKG/CREB pathway, insulin-induced IL-6 release also requires MAPK signaling. The pathway by which BPA increases IL-6 release remains to be determined.
TNFα is the most potent and best studied inflammatory cytokine (Qi and Pekala, 2000; Ryden and Arner, 2007). It is initially produced as a membrane-associated monomer which is proteolytically cleaved and circulates as a homotrimer. TNFα produced by human adipose tissue does not appear to be cleaved, but remains near the producing cells and acts as an autocrine/paracrine factor (Mohamed-Ali et al., 1997). TNFα binds to two receptors, TNFR1 and TNFR2, which can form multimeric complexes upon ligand binding. TNFR1 is an important member of the death receptor family characterize by an intracellular death domain and the induction of apoptosis upon activation. Recruitment of adaptor proteins that bind to the death domain activates a signaling cascade. A recent model suggests that occupation of TNFR1 activates two opposing pathways: one leads to apoptosis and the other, via the NF-κB pathway, protects against it. The fate of a cell depends on which pathway predominates (Qi and Pekala, 2000).
There is a strong correlation between increased TNFα production, adiposity and insulin resistance (Ryden and Arner, 2007). TNFα affects insulin resistance by downregulating the glucose transporter, interfering with insulin receptor phosphorylation and signaling, and by inhibiting transcription factors that affect insulin sensitivity. It also stimulates lipolysis, leading to increased free fatty acids and a further suppression of insulin sensitivity. Although increased TNFα production in obesity, primarily by infiltrating macrophages (Fain et al., 2004), has been extensively studied, less is known about the hormonal regulation of its release.
Estrogen Receptors in Human Adipose Tissue
The unmistakable difference in the anatomical distribution of fat between men and women raises the prospect that gonadal steroids affect the development or metabolism of adipose tissue. Both ERα and ERβ are expressed in human fat in a depot-specific manner (Dieudonne et al., 2004; Pedersen et al., 2001). Although serum adiponectin levels are moderately higher in women than men, hormone replacement therapy does not affect adiponectin release in either pre- or post-menopausal women (Sieminska et al., 2005). Thus, the gender difference in adiponectin appears to be due to a suppressive effect by androgens in men rather than to a direct stimulation by estrogens (Andersen et al., 2007). There is no evidence for gender differences in circulating IL-6 or TNFα levels in humans (Asai et al., 2001).
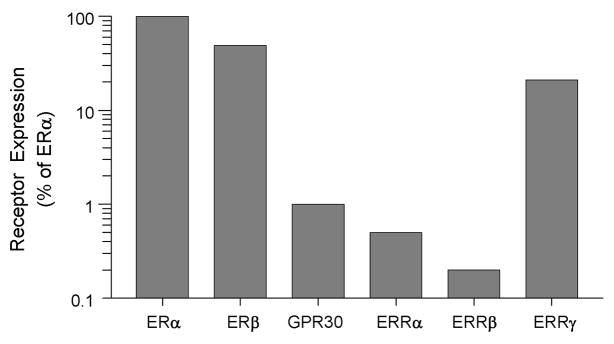
The equipotency of BPA and E2 in suppressing adiponectin and stimulating IL-6 and TNFα release (Fig 2) suggests involvement of receptors other than classical ERs. This is supported by the mimicking, rather than antagonizing, actions of ICI. Although BPA has a higher binding affinity to ERβ than ERα (Kuiper et al., 1998), it is still at a μM range, compared to a low nM for E2. The binding affinity of BPA to GPR30 is 630 nM (Thomas and Dong, 2006), and 9 nM for ERRγ (Okada et al., 2008). To compare expression of these receptors in human visceral adipose tissue, we used real-time PCR. Fig 3 shows that relative to ERα, expression of ERβ is quite significant. Of the ERR family members, ERRγ has the highest expression, approaching 25% of ERα mRNA levels. In contrast, expression of GPR30 was very low.
Fig 3.
Relative expression of classical and non-classical estrogen receptors in human visceral adipose tissue, as determined by real-time PCR. Values are presented as percent of ERα expression on a logarithmic scale to highlight low abundance receptors. ERα/β-estrogen receptor α or β; GPR30 - G-protein coupled receptor 30; ERRα/β/γ - estrogen related receptor α, β, or γ. (Redrawn and modified from Hugo et al. 2008).
Most research to-date on the biological actions of estrogens has focused on ERα. Studies with knockout mice revealed that deletion of ERα causes a much more severe phenotype than deletion of ERβ (Couse and Korach, 1999). With the exception of a few tissues such as the ovary, prostate and certain areas of the brain, ERα is more highly expressed than ERβ. Therefore, it was unexpected to find similar mRNA levels of both receptors in human visceral fat (Fig 3). This is in contrast to a previous report on the predominance of ERα over ERβ in isolated mature adipocytes (Dieudonne et al., 2004). Given adipose tissue heterogeneity, it is difficult to compare receptor expression in whole tissue, as was done in our studies, with that in isolated adipocytes. In addition, at least four different ERβ subtypes are expressed in human adipose tissue (Pedersen et al., 2001), with our primers detecting only the common isoform. As reported by us recently (Hugo et al., 2008), and depicted in Fig 3, this is the first demonstration of GPR30 and ERRγ expression in human fat.
GPR30 is a seven-transmembrane receptor which activates second messengers such as adenylate cyclase and MAP kinase in response to E2 (Filardo and Thomas, 2005). Notably, ICI acts as a GPR30 agonist, similar to its action on adipokine release (Fig 2). In spite of the low expression of GPR30 in visceral fat (Fig 3), mediation of BPA actions by this receptor should not be ruled out. An especially attractive candidate is ERRγ. The ERRs are orphan nuclear receptors which are constitutively active and do not bind estrogens (Ariazi and Jordan, 2006). ERRγ is expressed in a tissue-specific manner (Heard et al., 2000), but little is known about its biological functions. Future studies should first confirm expression of all the above receptors in human fat at the protein level, and then use siRNA to determine the consequences of receptor knockdown on the ability of E2 or BPA to alter adipokine release. It would also be of interest to determine whether BPA at low doses affects other functions of human adipocytes such as adipogenesis, glucose transport and lipid metabolism.
Conclusions and Perspectives
To firmly establish the metabolic effects of BPA, it is critically important to recognize the full spectrum of its actions on human fat. Although the value of rodents and murine adipocytes as experimental models is undisputed, there are sufficient differences in adipocyte biology between rodents and humans to warrant prudence (Ben Jonathan et al., 2008). For example, the regional distribution of fat depots, their cellular composition (e.g., brown vs white fat, infiltration by macrophages), and the regulation of resistin, agouti protein, adipsin, and adrenergic receptors are dissimilar in rodents and humans. Interspecies differences in the cellular milieu are highlighted by the dependence on serum for adipogenesis in 3T3-L1 cells, while human preadipocytes undergo differentiation without serum (Hauner et al., 2000). Intrinsic differences between the species are also exemplified by the suppression of adiponectin expression in 3T3-L1 cells by insulin but its induction by insulin in human adipose tissue (Whitehead et al., 2006).
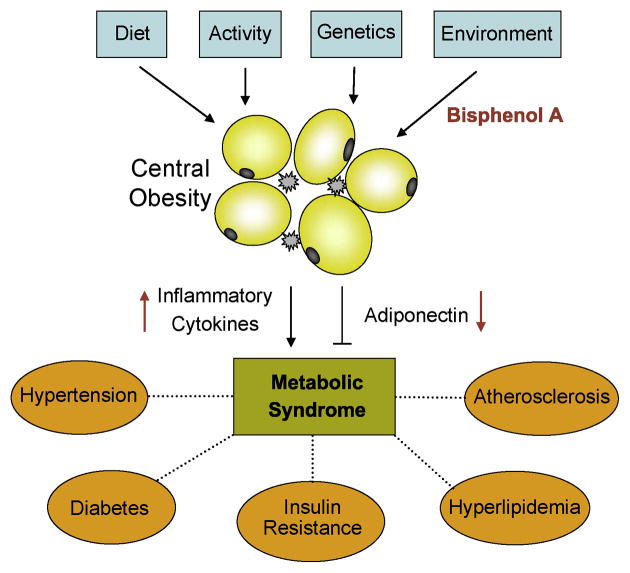
Most attention to date has focused on high caloric food intake and sedentary life style as the root causes of the obesity epidemic. However, the roles of genetic predisposition and environmental factors should not be ignored. Lower circulating adiponectin levels and elevated inflammatory cytokines are strongly associated with increased risks of obesity-related diseases. Given its prevalence in the environment, presence in serum from humans worldwide, suppression of adiponectin and increased IL-6 and TNFα release at nanomolar concentrations, BPA may be the bona fide endocrine disruptor that adversely affects metabolic homeostasis. Fig 4 presents a model which incorporates input by environmental factors to the combined effects of diet, exercise and genetics on the development and manifestation of the metabolic syndrome.
Fig 4.
A model depicting an integrated view of the various factors that affect the obesity-related metabolic syndrome. BPA suppresses adiponectin and stimulates inflammatory cytokines by acting on adipocytes and infiltrating macrophages. Adiponectin is an insulin sensitizer whereas cytokines such as IL-6 and TNFα promote insulin resistance. The opposing actions of BPA on these adipokines contribute to the development and manifestation of the metabolic syndrome.
Acknowledgments
This work was supported by NIH grants ES012212, ES016803, and CA096613, DOD grant BC05725, Susan G. Komen Foundation grant BCRT87406 and NIH Center for Environmental Genetics P30 ES06096.
References
- Ailhaud G, Grimaldi P, Negrel R. Cellular and molecular aspects of adipose tissue development. Annu Rev Nutr. 1992;12:207–233. doi: 10.1146/annurev.nu.12.070192.001231. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Laribi O, Ropero AB, Fuentes E, Ripoll C, Soria B, Nadal A. Low doses of bisphenol A and diethylstilbestrol impair Ca2+ signals in pancreatic alpha-cells through a nonclassical membrane estrogen receptor within intact islets of Langerhans. Environ Health Perspect. 2005;113:969–977. doi: 10.1289/ehp.8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect. 2006;114:106–112. doi: 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KK, Frystyk J, Wolthers OD, Heuck C, Flyvbjerg A. Gender differences of oligomers and total adiponectin during puberty: a cross-sectional study of 859 Danish school children. J Clin Endocrinol Metab. 2007;92:1857–1862. doi: 10.1210/jc.2006-2310. [DOI] [PubMed] [Google Scholar]
- Ariazi EA, Jordan VC. Estrogen-related receptors as emerging targets in cancer and metabolic disorders. Curr Top Med Chem. 2006;6:203–215. doi: 10.2174/1568026610606030203. [DOI] [PubMed] [Google Scholar]
- Arner P. Regional differences in protein production by human adipose tissue. Biochem Soc Trans. 2001;29:72–75. doi: 10.1042/bst0290072. [DOI] [PubMed] [Google Scholar]
- Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16:340–343. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- Ben Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Steinmetz R. Xenoestrogens: The emerging story of bisphenol A. TEM. 1998;9:124–128. doi: 10.1016/s1043-2760(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Bogan JS, Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coatings in food cans. Environ Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor null mice: What have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, Leblanc GA, Guillette LJ., Jr An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R. Evidence for functional estrogen receptors alpha and beta in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol. 2004;286:C655–C661. doi: 10.1152/ajpcell.00321.2003. [DOI] [PubMed] [Google Scholar]
- Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Arrebola JP, Taoufiki J, Navalon A, Ballesteros O, Pulgar R, Vilchez JL, Olea N. Bisphenol-A and chlorinated derivatives in adipose tissue of women. Reprod Toxicol. 2007 doi: 10.1016/j.reprotox.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Hanaoka T, Kawamura N, Hara K, Tsugane S. Urinary bisphenol A and plasma hormone concentrations in male workers exposed to bisphenol A diglycidyl ether and mixed organic solvents. Occup Environ Med. 2002;59:625–628. doi: 10.1136/oem.59.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H, Skurk T, Wabitsch M. Culture of human adipose precursor cells. In: Ailhaud G, editor. Adipose Tissue Protocols. Humana Press; Totowa, NJ: 2000. pp. 239–247. [Google Scholar]
- Heard DJ, Norby PL, Holloway J, Vissing H. Human ERRgamma, a third member of the estrogen receptor-related receptor (ERR) subfamily of orphan nuclear receptors: tissue-specific isoforms are expressed during development and in the adult. Mol Endocrinol. 2000;14:382–392. doi: 10.1210/mend.14.3.0431. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi T, Okada K, Imaoka S, Osada M, Funae Y. Bisphenol A binds to protein disulfide isomerase and inhibits its enzymatic and hormone-binding activities. Endocrinology. 2006;147:2773–2780. doi: 10.1210/en.2005-1235. [DOI] [PubMed] [Google Scholar]
- Hoene M, Weigert C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes Rev. 2008;9:20–29. doi: 10.1111/j.1467-789X.2007.00410.x. [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin reease from human adipose explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Khurana S, Ranmal S, Ben Jonathan N. Exposure of newborn male and female rats to environmental estrogens: delayed and sustained hyperprolactinemia and alterations in estrogen receptor expression. Endocrinology. 2000;141:4512–4517. doi: 10.1210/endo.141.12.7823. [DOI] [PubMed] [Google Scholar]
- Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der BB, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- LaPensee CR, Hugo ER, Ben-Jonathan N. Insulin stimulates interleukin-6 expression and release in LS14 human adipocytes through multiple signaling pathways. Endocrinology. 2008;149:5415–5422. doi: 10.1210/en.2008-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103:9232–9237. doi: 10.1073/pnas.0602956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- Long X, Steinmetz R, Ben Jonathan N, Caperell-Grant A, Young PC, Nephew KP, Bigsby RM. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect. 2000;108:243–247. doi: 10.1289/ehp.00108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuno H, Iwanami J, Kidani T, Sakayama K, Honda K. Bisphenol a accelerates terminal differentiation of 3T3-L1 cells into adipocytes through the phosphatidylinositol 3-kinase pathway. Toxicol Sci. 2005;84:319–327. doi: 10.1093/toxsci/kfi088. [DOI] [PubMed] [Google Scholar]
- Masuno H, Kidani T, Sekiya K, Sakayama K, Shiosaka T, Yamamoto H, Honda K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J Lipid Res. 2002;43:676–684. [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Mora S, Pessin JE. An adipocentric view of signaling and intracellular trafficking. Diabetes Metab Res Rev. 2002;18:345–356. doi: 10.1002/dmrr.321. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Kannan K, Giesy JP, Fang J, Clemens LG. Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere. 2001;42:917–922. doi: 10.1016/s0045-6535(00)00196-x. [DOI] [PubMed] [Google Scholar]
- Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-gamma. Environ Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olea N, Pulgar R, Perez P, Olea-Serrano F, Rivas A, Novillo-Fertrell A, Pedraza V, Soto AM, Sonnenschein C. Estrogencity of resin-based composites and sealants used in dentistry. Environ Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SB, Bruun JM, Hube F, Kristensen K, Hauner H, Richelsen B. Demonstration of estrogen receptor subtypes alpha and beta in human adipose tissue: influences of adipose cell differentiation and fat depot localization. Mol Cell Endocrinol. 2001;182:27–37. doi: 10.1016/s0303-7207(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Qi C, Pekala PH. Tumor necrosis factor-alpha-induced insulin resistance in adipocytes. Proc Soc Exp Biol Med. 2000;223:128–135. doi: 10.1046/j.1525-1373.2000.22318.x. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, Vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink JD, Simpson ER, Barnard JJ, Bulun SE. Cellular characterization of adipose tissue from various body sites of women. J Clin Endocrinol Metab. 1996;81:2443–2447. doi: 10.1210/jcem.81.7.8675558. [DOI] [PubMed] [Google Scholar]
- Ritchie SA, Connell JM. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Ryden M, Arner P. Tumour necrosis factor-alpha in human adipose tissue -- from signalling mechanisms to clinical implications. J Intern Med. 2007;262:431–438. doi: 10.1111/j.1365-2796.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- Safe SH, Pallaroni L, Yoon K, Gaido K, Ross S, McDonnell D. Problems for risk assessment of endocrine-active estrogenic compounds. Environ Health Perspect. 2002;110(Suppl 6):925–929. doi: 10.1289/ehp.02110s6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai K, Kawazuma M, Adachi T, Harigaya T, Saito Y, Hashimoto N, Mori C. Bisphenol A affects glucose transport in mouse 3T3-F442A adipocytes. Br J Pharmacol. 2004;141:209–214. doi: 10.1038/sj.bjp.0705520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidlova-Wuttke D, Jarry H, Christoffel J, Rimoldi G, Wuttke W. Effects of bisphenol-A (BPA), dibutylphtalate (DBP), benzophenone-2 (BP2), procymidone (Proc), and linurone (Lin) on fat tissue, a variety of hormones and metabolic parameters: a 3 months comparison with effects of estradiol (E2) in ovariectomized (ovx) rats. Toxicology. 2005;213:13–24. doi: 10.1016/j.tox.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Shin BS, Kim CH, Jun YS, Kim DH, Lee BM, Yoon CH, Park EH, Lee KC, Han SY, Park KL, Kim HS, Yoo SD. Physiologically based pharmacokinetics of bisphenol A. J Toxicol Environ Health A. 2004;67:1971–1985. doi: 10.1080/15287390490514615. [DOI] [PubMed] [Google Scholar]
- Sieminska L, Wojciechowska C, Niedziolka D, Marek B, Kos-Kudla B, Kajdaniuk D, Nowak M. Effect of postmenopause and hormone replacement therapy on serum adiponectin levels. Metabolism. 2005;54:1610–1614. doi: 10.1016/j.metabol.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–1786. doi: 10.1210/endo.138.5.5132. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139:2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor gamma (ERRgamma) with high constitutive activity. Toxicol Lett. 2006;167:95–105. doi: 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL, Giannella-Neto D, da Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Alyea RA. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids. 2007;72:124–134. doi: 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, Vom Saal FS. Large effects from small exposures. III Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–280. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- Wright HM, Clish CB, Mikami T, Hauser S, Yanagi K, Hiramatsu R, Serhan CN, Spiegelman BM. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J Biol Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]