Abstract
Background
Oxidative stress, generated by excessive reactive oxygen species (ROS) promotes cardiovascular disease. Cyclophilin A (CyPA) is a 20 kD chaperone protein secreted from vascular smooth muscle cells (VSMC) in response to ROS, which stimulates VSMC proliferation and inflammatory cell migration in vitro. However, the role CyPA plays in vascular function in vivo remains unknown.
Methods and Results
We tested the hypothesis that CyPA contributes to vascular remodeling by analyzing the response to complete carotid ligation in CyPA knockout mice, wild-type mice, and mice that overexpress CyPA in VSMC (VSMC-Tg). After carotid ligation, CyPA expression in vessels of wild-type mice dramatically increased, and was significantly greater in VSMC-Tg mice. ROS-induced secretion of CyPA from mouse VSMC correlated significantly with intracellular CyPA expression. Intimal and medial hyperplasia correlated significantly with CyPA expression after 2 weeks of carotid ligation with marked decreases in CyPA knockout and increases in VSMC-Tg mice. Inflammatory cell migration into the intima was significantly reduced in CyPA knockout mice and increased in VSMC-Tg mice. Additionally, VSMC proliferation assessed by Ki67+ cells was significantly less in CyPA knockout mice and increased in VSMC-Tg mice. The importance of CyPA for intimal and medial thickening was shown by strong correlations between CyPA expression and the number of both inflammatory cells and proliferating VSMC in vivo and in vitro.
Conclusion
In response to low flow, CyPA plays a crucial role in VSMC migration and proliferation as well as inflammatory cell accumulation, thereby regulating flow-mediated vascular remodeling and intima formation.
Keywords: reactive oxygen species, vasculature, remodeling, atherosclerosis, restenosis
Introduction
The interaction between endothelial cells (EC) and vascular smooth muscle cells (VSMC) plays an important role in regulating vascular integrity. EC secrete several vasoactive substances including NO and prostacyclin, which maintain vascular integrity and limit intima formation.1 VSMC contain numerous sources of reactive oxygen species (ROS; i.e., H2O2, O2-, and .OH), including NADPH oxidases, xanthine oxidase, the mitochondrial respiratory chain, lipoxygenases and nitric oxide synthases.2 It has become clear that increases in ROS represent one of the pathogenic mechanisms for vascular disease.3,4 ROS have been implicated in the pathogenesis of intima formation in part by promoting VSMC growth,5,6 as well as stimulating pro-inflammatory events.7-9 Recently, we proposed a pathogenic role for a newly discovered class of ROS mediators that we term SOXF, for Secreted OXidative stress induced Factors.10,11 Among these factors cyclophilin A (CyPA) expression is induced by ROS, and CyPA is secreted in response to ROS.10-12 We demonstrated that CyPA stimulates pro-inflammatory signals in EC and VSMC, including expression of E-selectin and vascular cell adhesion molecule (VCAM)-1.13 Furthermore, we showed that secreted CyPA stimulates the ERK1/2 and JAK/STAT pathways in vitro, thereby increasing DNA synthesis in VSMC.10 In addition to effects on vascular cells, CyPA has been shown to be a chemoattractant for inflammatory cells14,15 and promotes activation of matrix metalloproteinases (MMPs), especially MMP-1 and MMP-9.14,16 Therefore, CyPA is a key mediator that affects EC, VSMC and inflammatory cell function during oxidative stress. Here, we tested the hypothesis that CyPA contributes to vascular remodeling by analyzing the response to complete carotid ligation in CyPA knockout (CyPA−/−) mice, wild-type (WT) mice and mice that overexpress CyPA specifically in VSMC (VSMC-Tg).
Methods
CyPA Knockout Mice
All animal experiments were conducted in accordance with the experimental protocols that were approved by the Institutional Animal Care and Use Committee at the University of Rochester (2002-135A). CyPA−/− mice were purchased from Jackson Laboratory (Bar Harbor, Maine) and were backcrossed to C57BL/6J mice for 7 generations. Wild-type littermates (CyPA+/+) were used as controls, and all mice were genotyped by PCR on tail clip samples.
Generation of CyPA Overexpressing Transgenic Mouse
We utilized a Cre/LoxP strategy to prepare CyPA transgenic mice. In brief, a LacZflox-CyPA construct was prepared using the pZ/EG vector (Supplement Figure 1). The pZ/EG double reporter construct was a kind gift from the Nagy lab.17 This vector contains LacZ floxed by two loxP sites, driven by the chicken β-actin promoter and a cytomegalovirus (CMV) enhancer with enhanced green fluorescent protein (EGFP) downstream.18 We replaced EGFP with full-length wild type mouse CyPA carrying a Flag tag to make the LacZflox-Flag-CyPA construct. ES cells transfected by electroporation with linearized LacZflox-Flag-CyPA cDNA were screened by neomycin resistance and LacZ expression. ES clones with a single copy by Southern blotting were used to generate chimeric mice by ES cell – embryo aggregation. The chimeric mice were bred to C57BL/6J mice to produce hemizygous transgenic offspring. Hemizygous offspring with germline transmission were identified by PCR of DNA harvested from tail snippets of weaned offspring. We obtained 9 germline mice from the 2A3 ES cell clone and 8 from the 3H9 ES cell clone. Transgenic mice were backcrossed to C57BL/6J mice for 7 generations to establish experimental lines.
VSMC-Specific Overexpression of CyPA
For VSMC-specific overexpression of CyPA in transgenic mice, the LacZflox-CyPA transgenic mouse and SM22α-Cre mouse (C57BL/6J background)19 were crossed. Previously we showed that expression of SM22α promoter when linked to LacZ was restricted to VSMC in the day 12.5 embryo, without expression in other smooth muscle. Breeding the LacZflox-CyPA mice to SM22α-Cre mice19 resulted in excision of LacZ and expression of CyPA in VSMC.
Complete Common Carotid Artery Ligation
Six- to 8 week old male mice underwent complete carotid artery ligation.20,21 Mice were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (5 mg/kg). The left common carotid artery was exposed through a small midline incision in the neck and was completely ligated with 6-0 silk just proximal to the carotid bifurcation (Supplement Figure 2A).20,21 In the right common carotid artery (sham), the suture was passed under the exposed carotid artery but not tightened. Four groups of operated animals were processed for morphological studies at 14 days after the operation. Survival rate of the operated mice was more than 95% and all mice showed no neurological deficits.
Morphometric Analysis
For morphological analysis, 48 animals were perfused with normal saline and fixed with 10% phosphate-buffered formalin at physiological pressure for 5 minutes.22 The carotid arteries were harvested, fixed for 24 hours, embedded in paraffin, and cross-sections (5 μm) were prepared.22 Because lesion thickness varies longitudinally, the entire length of the left and right carotid arteries was sectioned and 5 sections located at 250 μm intervals from the carotid bifurcation were examined (Supplement Figure 2A). Vessel areas were measured with Image Pro Plus software (Media Cybernetics Inc.) and morphological parameters calculated as described.20,21 In brief, the intimal area was calculated as the internal elastic lamina (IEL) area minus luminal area, the medial area was the external elastic lamina (EEL) area minus IEL area, and the adventitial area was the vascular area minus EEL area (Supplement Figure 2B).
Harvest of Mouse Aortic Smooth Muscle Cells (MASM)
MASM were isolated from each strain of mice (WT, CyPA-/-, VSMC-Tg, Control mice) and maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS at 37°C in a humidified atmosphere of 5% CO2/95% air as described.23,24 Passage 4 to 6 MASM at 70-80% confluence were used for experiments.
Preparation of Conditioned Medium
MASM from each mouse strain were serum starved in DMEM for 24 hours, stimulated with 1 μM LY83583 to generate intracellular ROS,10,11 and medium was collected and centrifuged for 10 minutes at 800 × g to remove cell debris. The medium was concentrated 100-fold with a Centricon Plus-20 filter (Millipore Corporation, Bedford, MA) to yield concentrated conditioned medium (CM).10,11
Proliferation and scratch wound assays
MASM were seeded in 96-well plates in DMEM supplemented with 10% FBS. For proliferation assays, medium was changed to DMEM without FBS and starved for 24 hours and stimulated with CM for up to 5 days. CM was changed at day 3 and cells were counted at day 2 and day 5. For scratch wound, confluent cells were scratched with a pipette tip, medium replaced with CM from different MASM (Tg, WT, and KO) and allowed to migrate for 24 hr. To block protein synthesis (cell proliferation), MASM were pretreated with anisomycin (10 μM) for 2 hours. MASM were positive for smooth muscle actin (αSMA) (green).
Boyden chamber migration Assays
For the Boyden chamber assay, MASM were starved overnight, suspended, and seeded (50 μl containing 5.0 × 104 cells) in the upper chamber on collagen-precoated polyvinylpyrrolidone-free polycarbonate membrane. Control medium (DMEM/0.1% BSA) or conditioned medium (CM) 30 μl was placed in the lower chamber. After incubation at 37 °C and 95% air/5% CO2 for 8 hours, cells attached to the lower side were fixed in 4% formaldehyde/0.1% glutaraldehyde in 0.1 phosphate buffer, pH 7.2, and then stained for 30 min in 0.1% cresyl violet. The relative increases in cell number were determined by quantitative densitometry (Image Pro plus).
Statistical Analysis
Quantitative results are expressed as mean ± SD. Comparisons of parameters among 2 groups were made by the unpaired Student's t-test. Comparisons of parameters among the 3 groups were made by one-way analysis of variance (ANOVA), and comparisons of different parameters between the 2 genotypes were made by two-way analysis of variance (ANOVA), followed by a post hoc analysis using the Bonferroni test. Statistical significance was evaluated with StatView (StatView 5.0, SAS Institute Inc. Cary, NC). A value of P<0.05 was considered to be statistically significant.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written. Supplementary methods is available online.
Results
Vascular Remodeling after Carotid Ligation Was Significantly Reduced in CyPA-/- Mice
To test the hypothesis that CyPA contributes to vascular remodeling, we performed complete carotid ligation in wild-type (WT) and CyPA knockout (CyPA−/−) mice. ROS generation and proliferation of VSMC play significant roles in intima formation in this model.25,26 Immunostaining and western blotting revealed that expression of CyPA increased after carotid ligation in WT mice with highest expression in the intima (Figure 1A, arrows). In arteries that underwent a sham procedure, no increase in CyPA expression or intima formation was observed (Figure 1B). Quantification by western blot showed a significant increase in CyPA expression after ligation in WT mice (Figure 1, E and F). In CyPA−/− mice there was no CyPA expression in either sham or ligated vessels (Figure 1, C and D). These results indicate that blood flow cessation increases local CyPA expression. In sham WT and sham CyPA−/− controls, there was no intimal hyperplasia (Figure 2, A and D), no differences in the thickness of elastic lamina (Figure 2, B and E), similar α-smooth muscle actin (SMA) expression (Figure 2, C and F), and no differences in medial area (Figure 2, M–O). The ligated arteries of WT mice (14 days) exhibited lumen narrowing (Figure 2, G–I), which was primarily a result of intimal hyperplasia and medial thickening. These changes were obviously reduced in CyPA−/− mice (Figure 2, J–L). Morphometric analysis of sham controls (Figure 2, M–O) showed no differences in intima, media or intima/media (I/M) ratio in CyPA−/− mice versus WT mice. In contrast, analysis of ligated arteries on day 14 revealed that the intimal area was significantly smaller in CyPA−/− mice than WT mice (Figure 2M). Additionally, we observed significantly increased medial thickening in WT mice compared with CyPA−/− mice (Figure 2N), suggesting that CyPA plays a crucial role in the development of vascular remodeling after blood flow cessation. Note that there was an increase in intima area of CyPA−/− ligated vessels (Figure 2, K and M) compared to sham, indicating that other mechanisms also contribute to vascular remodeling. However, the I/M ratio of CyPA−/− mice was significantly less than WT mice (0.16+0.06 vs. 0.29+0.13, p <0.01, Figure 2O) indicating a crucial role of CyPA in vascular remodeling.
Figure 1.
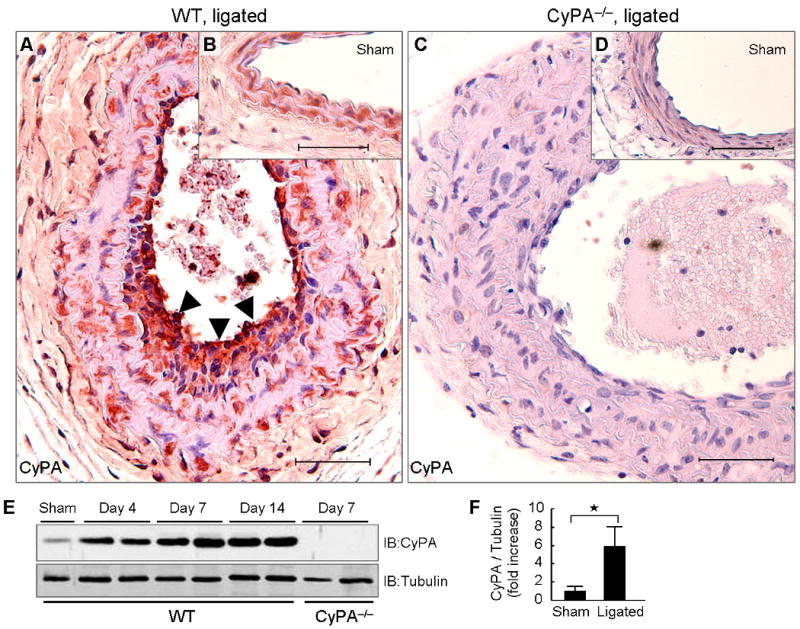
Carotid ligation enhances local CyPA expression. Representative immunostaining of CyPA in ligated (A and C) and sham (B and D) carotids from wild-type (WT) and CyPA−/− mice. Arrowheads show strong expression of CyPA in the intima. Scale bars, 50 μm. (E and F) Western blot analysis of CyPA in carotid homogenates of WT (n = 8, black bars) and CyPA−/− mice (n = 6, white bars) after ligation. Equal protein loading was confirmed with tubulin. Results are mean ± SD. ⋆P < 0.01.
Figure 2.
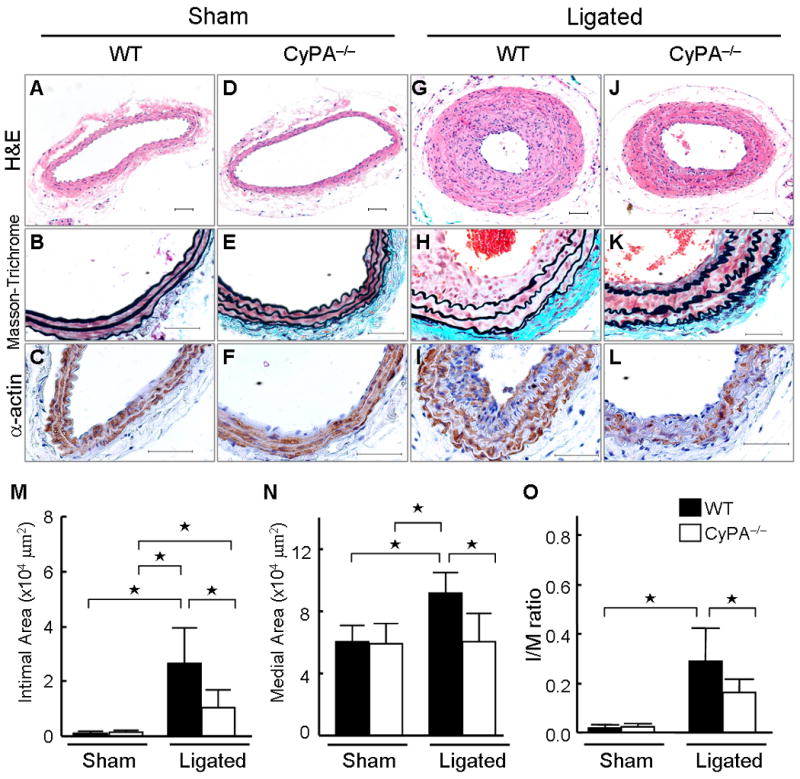
CyPA expression correlates with vascular remodeling. (A–F) Photomicrographs showing representative cross-sectional areas of sham carotids of wild-type (WT) and CyPA−/− mice. The predominant cellular component in the intima was VSMC as revealed by immunostaining for α-smooth muscle actin (SMA). Scale bars, 50 μm. (G–L) Photomicrographs showing representative cross-sectional areas of ligated carotids of WT and CyPA−/− mice. CyPA deficiency significantly reduced intimal area (M), medial area (N), and intima/media (I/M) ratio (O) in CyPA−/− mice (n = 8, white bars) compared with WT mice (n = 9, black bars). Results are mean ± SD. ⋆P < 0.01.
CyPA Induces VCAM-1 Expression and Promotes Inflammatory Cell Migration
An important initial step for the development of vascular lesions is inflammatory cell recruitment to the vascular wall.7-9 Because CyPA induces VCAM-1 in HUVEC13 and is chemotactic for inflammatory cells,14,15 we anticipated differences in inflammation based on CyPA expression. Expression of VCAM-1 was greatly increased in ligated WT carotids compared to sham (Figure 3, A, B and E). There was no difference in VCAM-1 expression in the sham carotids of WT versus CyPA−/− (Figure 3E). In contrast, expression of VCAM-1 was much less in ligated carotids of CyPA−/− mice compared to WT mice (Figure 3, C, D and E). The number of CD45+ inflammatory cells did not differ in sham arteries from WT versus CyPA−/− mice (Figure 3, G and I). There was a significant increase in CD45+ cells in ligated arteries of WT mice (Figure 3, F and G), whereas there was only a small change in CyPA−/− mice (Figure 3, H and I). Quantification of CD45+ cells per intimal or medial area showed a 2-fold greater number in WT versus CyPA−/− mice (Figure 3J). These data suggest a role for CyPA in expression of VCAM-1 and inflammatory cell migration after carotid ligation.
Figure 3.
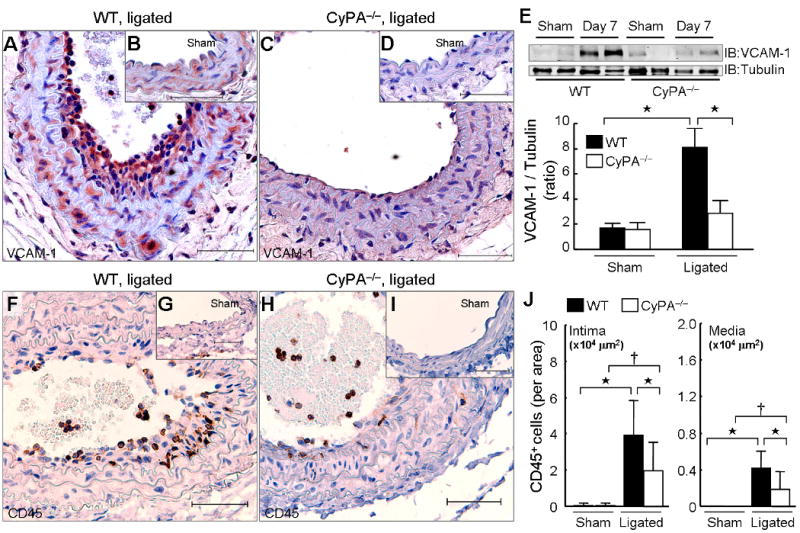
CyPA expression correlated with VCAM-1 expression and inflammatory cell accumulation. Representative immunostaining of VCAM-1 (A–D) and CD45 (F–I) in sham and ligated carotids from wild-type (WT) and CyPA−/− mice. Scale bars, 50 μm. (E) Western blot comparison of VCAM-1 in carotid homogenates of WT (n = 8, black bars) and CyPA−/− mice (n = 6, white bars) at 7 days after ligation. Equal protein loading was confirmed with tubulin. (J) The number of CD45+ cells in intima and media is increased by ligation in WT mice (n = 9, black bars) and was significantly less in CyPA−/− mice (n = 8, white bars). Results are mean ± SD. †P < 0.05; ⋆P < 0.01.
Generation of VSMC-Specific CyPA Transgenic Mice
To test the hypothesis that VSMC-derived CyPA plays a major role in vascular remodeling, we prepared VSMC-specific CyPA transgenic mice (VSMC-Tg) that overexpress a FLAG-tagged CyPA only in VSMC using a Cre/LoxP system. In brief, a LacZflox-CyPA construct (Supplement Figure 1) was prepared using the pZ/EG vector and LacZflox-CyPA mice were crossed with SM22α-Cre mice to achieve VSMC specific expression. The vessels in Figure 4A-C are from control mice (LacZflox-CyPA+/SM22α-Cre−) in which the transgene expressed is LacZ as shown by the blue β-gal staining and the low level of CyPA expression. After breeding with SM22α-Cre to express FLAG-CyPA (SM22α-Cre × LacZflox-CyPA), Figure 4E shows the efficiency of Cre recombinase mediated excision in vivo. Specifically, there is no LacZ expressed as shown by β-gal staining, while anti-FLAG reveals significant FLAG-CyPA expression. The increase in CyPA was confirmed by anti-CyPA antibody (Figure 4F). To quantify FLAG-CyPA expression we performed western blots with anti-FLAG and anti-CyPA antibody. As shown in Figure 4G, the relative expression of exogenous CyPA (FLAG tagged) is 2.0-fold greater (in aorta) compared to endogenous CyPA. These experiments show that excision by SM22α-Cre is highly efficient (>90% of cells express FLAG and <10% express LacZ), and the expression of exogenous FLAG-CyPA is 2-fold greater than endogenous CyPA. To show that FLAG-CyPA was secreted similarly to endogenous CyPA, we stimulated VSMC harvested from aorta of WT, CyPA−/− and VSMC-Tg mice with LY83583. LY83583 is a naphthoquinoleinedione that undergoes futile redox cycling generating intracellular ROS.10,11 As expected both FLAG-CyPA and endogenous CyPA were secreted in response to ROS in MASM from VSMC-Tg aorta (Figure 4H). The magnitude of secreted FLAG-CyPA was equivalent to endogenous CyPA, similar to the expression of CyPA in lysates of intact aorta (Figure 4G).
Figure 4.
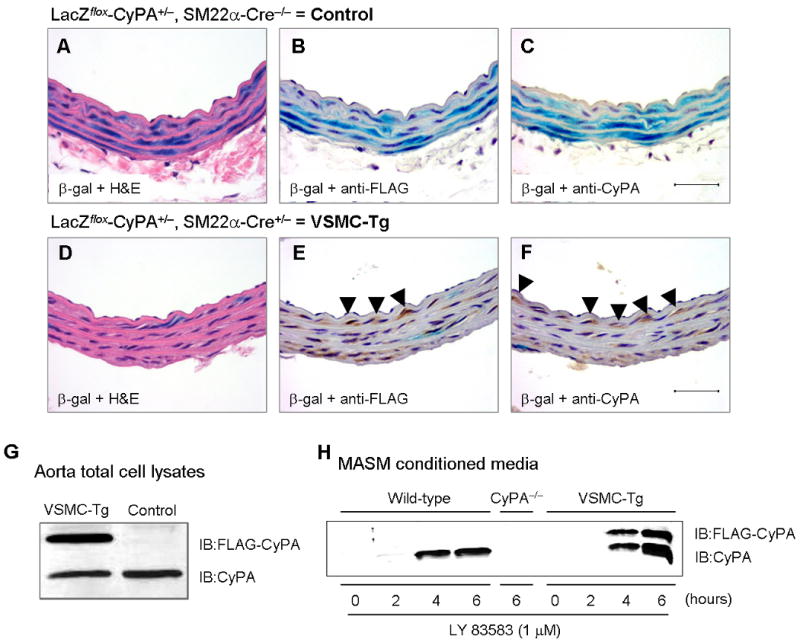
Characterization of CyPA expression in VSMC-Tg mice. (A–F) Representative immunostaining and β-gal staining of aorta from VSMC-Tg mice (LacZflox-CyPA+/SM22α-Cre+) and control mice (LacZflox-CyPA+/SM22α-Cre−). β-gal staining shows >90% expression only in VSMC (B) and complete excision with SM22α-Cre (E). FLAG-CyPA was expressed only in this VSMC-Tg mice but not in control mice. There was no change in endogenous CyPA expression. Scale bars, 50 μm. (G) Western blot demonstrated that FLAG-CyPA was expressed only in the VSMC-Tg mice aorta, but not in control mice. (H) Mouse aortic VSMC were stimulated with 1 μM LY83583 for the indicated times, conditioned media prepared, and secreted CyPA and FLAG-CyPA were detected by western blotting.
VSMC-Specific CyPA Transgenic Mice Exhibit Dramatic Intimal and Medial Thickening
To prove further that VSMC-derived CyPA promotes vascular remodeling, we performed complete carotid ligation in VSMC-Tg (LacZflox-CyPA+/SM22α-Cre+) and control (LacZflox-CyPA+/SM22α-Cre−) mice. In sham arteries, intimal thickening was not observed in VSMC-Tg and control mice (Figure 5, A–F), and the medial area did not significantly differ (Figure 5, M and N). Two weeks after carotid ligation, intimal thickness was significantly increased in VSMC-Tg mice to a much greater extent than control mice (Figure 5, G–M). Additionally, we observed significantly increased medial thickening in VSMC-Tg mice (Figure 5N). The increased intima formation was due to VSMC as revealed by immunostaining for αSMA (Figure 5, H and I). The I/M ratio was increased by 2.5-fold in the VSMC-Tg suggesting a pathogenic role for VSMC-derived CyPA in accumulation of VSMC during vascular remodeling (Figure 5O). Inflammatory cell accumulation in the remodeled carotid wall was also significantly increased in VSMC-Tg mice (Figure 6), suggesting that VSMC-derived CyPA recruits inflammatory cells.
Figure 5.
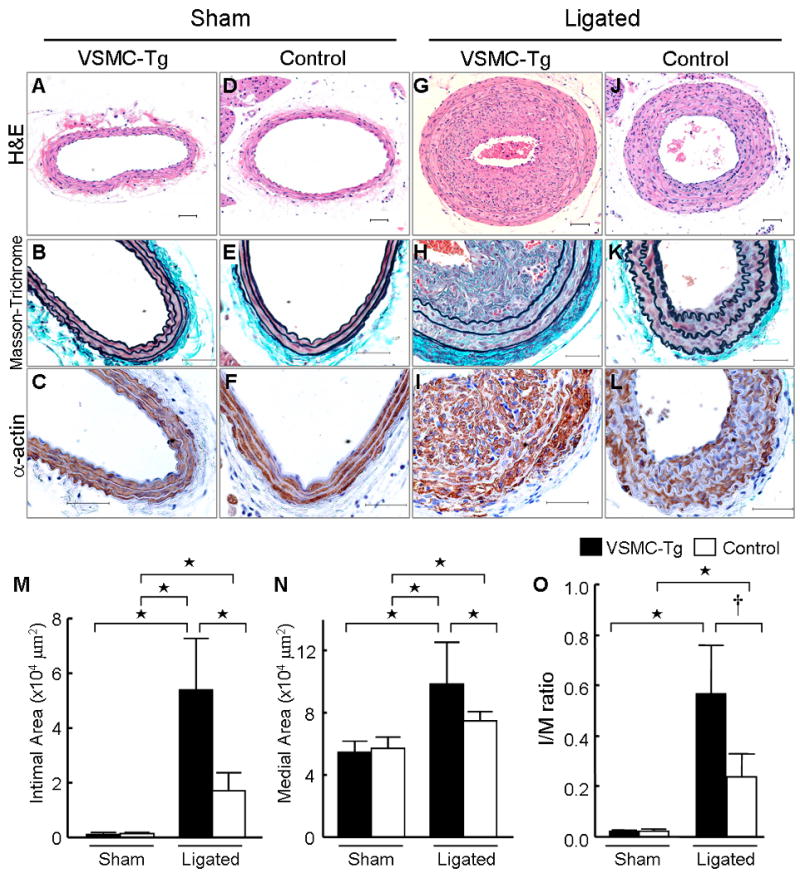
VSMC-specific overexpressed CyPA increases vascular remodeling after carotid ligation. Photomicrographs from sham (A–F) and ligated (G–L) mice carotids of VSMC-Tg and control. The predominant cellular component in the intima was VSMC as revealed by immunostaining for αSMA (α-actin). Hematoxylin and eosin staining, H&E. Scale bars, 50 μm. Intima (M), media (N), and adventitia area (O) in VSMC-Tg mice (n = 7, black bars) were greater than control mice (n = 6, white bars). Results are mean ± SD. †P < 0.05; ⋆P < 0.01.
Figure 6.
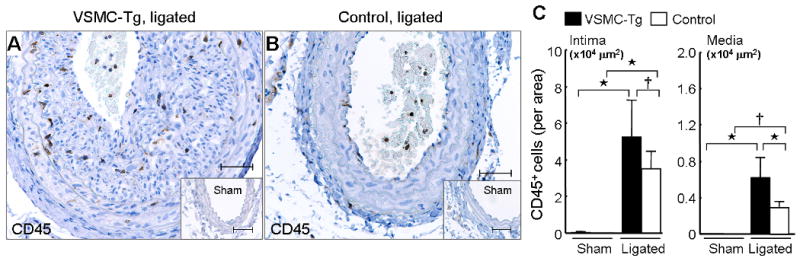
Overexpression of CyPA promotes recruitment of inflammatory cells in ligated carotids. Representative immunostaining of CD45 in ligated arteries from VSMC-Tg (A) and control mice (B). (C) The number of CD45+ cells in VSMC-Tg (n = 7, black bars) and control mice (n = 6, white bars). VSMC-specific overexpression of CyPA enhanced the recruitment of CD45+ cells to the intima and media. Results are mean ± SD. †P < 0.05; ⋆P < 0.01.
CyPA Plays a Crucial Role in VSMC Proliferation in Vivo
To strengthen the link between CyPA expression and VSMC growth, we carefully evaluated proliferation of VSMC by immunostaining for αSMA, Ki67 and ERK1/2 phosphorylation (pERK1/2) on serial sections. We previously reported that ERK1/2 phosphorylation is important for VSMC migration and growth.23 There was no difference in pERK1/2 expression (Supplement Figure 4, B and D) or Ki67+ VSMC in sham carotids (Figure 7, B and D). However, immunostaining and western blotting revealed that pERK1/2 increased after carotid ligation in WT mice with highest expression in the intima compared with CyPA−/− carotids (Supplement Figure 4, A, C, E, F). Consistently, Ki67+ VSMC were significantly increased in ligated wild-type carotids compared with CyPA−/− carotids (Figure 7, A, C and E). VSMC proliferation was even further enhanced in VSMC-Tg carotids compared to control carotids (Figure 7, F–J), suggesting that the CyPA promotes VSMC proliferation in vivo.
Figure 7.
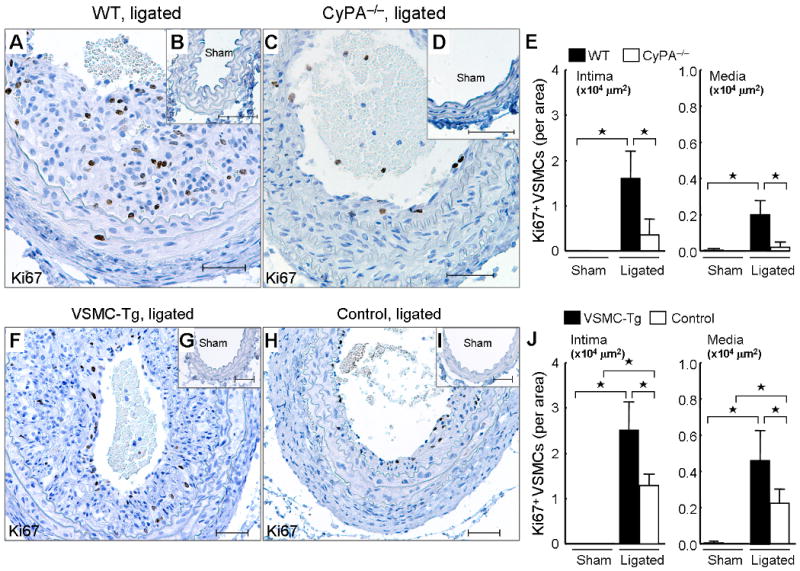
CyPA increases VSMC proliferation in ligated carotids. Representative immunostaining of Ki67 and counterstaining with hematoxylin in sham carotids and ligated carotids from WT, CyPA−/−, VSMC-Tg, and Control mice. (A–E) There is a significantly increased number of Ki67+ cells in ligated WT carotids (n = 9, black bars) compared with CyPA−/− carotids (n = 8, white bars). Scale bars, 50 μm. (E) Analysis of numbers of αSMA+ Ki67+ cells by staining of serial section (3 sections/mouse) was performed. (F–J) There is a significant increase in Ki67+ VSMC in ligated VSMC-Tg carotids (n = 7, black bars) compared with control carotids (n = 6, white bars). (J) Analysis of numbers of αSMA+ Ki67+ cells by staining of serial section (3 sections/mouse) was performed. Results are mean ± SD. ⋆P < 0.01.
CyPA Plays a Crucial Role in Migration, Chemotaxis, and Proliferation of VSMC in Vitro
To further confirm the role of CyPA in VSMC proliferation and migration, we harvested mouse aortic smooth muscle cells (MASM) from the three mice strains and evaluated their proliferation and migration. To evaluate the effect of CyPA on VSMC migration and chemotaxis we performed scratch wound and Boyden chamber assays. The scratch wound was performed using WT-MASM as the “responder” cells and conditioned medium (CM) from the three strains. Tg-CM stimulated migration more than Control-CM, and WT-CM stimulated migration more than KO-CM suggesting that CyPA secreted into CM increased VSMC migration (Supplement Figure 5). To measure the effect of CyPA on VSMC chemotaxis we studied migration in response to serum and CM (Figure 8A-B). As anticipated, chemotaxis of KO-MASM was significantly reduced compared to WT- and Tg-MASM in response to 10% serum suggesting a role for intracellular CyPA in chemotaxis (Figure 8A). Next we compared the chemotactic activity of CM from the three strains using WT-MASM as reporter cells. Migration of WT-MASM in response to Tg-CM was significantly increased compared with WT-CM (Figure 8B), and dramatically greater than migration induced by KO-CM. These results indicate that secreted CyPA strongly enhances VSMC chemotaxis.
Figure 8.
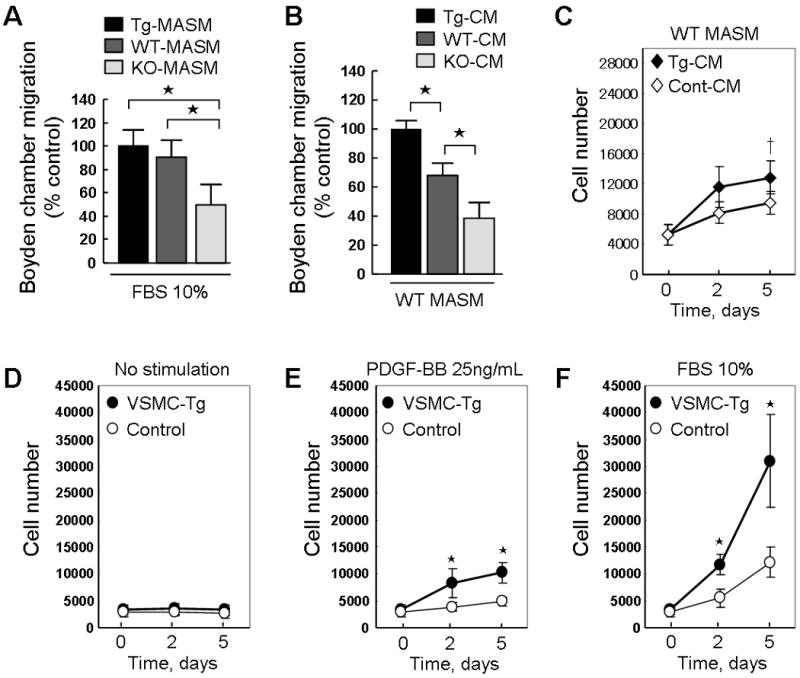
CyPA promotes migration and proliferation of mouse aortic smooth muscle cells (MASM). (A) Migration of MASM in response to 10% fetal bovine serum (FBS) in Boyden chamber assay. Tg-MASM, WT-MASM and KO-MASM were starved overnight and then seeded in the upper Boyden chamber on collagen-precoated PVP-free polycarbonate membranes. FBS (10%) was added to the lower chamber. Migration was determined and the maximum increase was normalized to 100%. (B) WT-MASM were starved overnight and then seeded in the upper Boyden chamber. Tg-CM, WT-CM or KO-CM was added to the lower chamber. Cells were incubated for 8 hours at 37 °C in a 95% air/5% CO2 humidified incubator. The membranes were removed, and cells were stained. The relative increases in cell number were determined by quantitative densitometry. ⋆P<0.01. Data are mean ± SD. n = 6 in each group. (C) Conditioned medium (CM) from VSMC-Tg (Tg-CM) or Control (Cont-CM) promotes cell proliferation. WT-MASM were seeded in 96-well plates in DMEM supplemented with 10% FBS, serum starved for 24 hours, and stimulated with Tg-CM or Cont-CM for 5 days. CM was changed at day 3 and cells were counted at day 2 and day 5. Data are mean ± SD. †P<0.05. (D-F), Effect of PDGF-BB and FBS on proliferation of Tg-MASM and Control-MASM. After starvation for 24 hours, MASM were incubated with DMEM (D) or stimulated with 25 ng/mL PDGF-BB (E) or 10% FBS (F) for 5 days. Medium was changed at day 3 and cells were counted at day 2 and day 5. Data are mean ± SD. ⋆P<0.01. n = 8 in each group.
To determine the effect of varying CyPA secretion on VSMC growth, we measured the effects of CM on cell growth. Proliferation of WT-MASM in response to CM from Tg-MASM was significantly greater than CM from Control-MASM (Figure 8C) suggesting that extracellular CyPA promotes VSMC growth. To assess the effect of CyPA expression on cell growth we studied the ability of MASM from different strains to respond to both PDGF and serum (Figure 8D-F). In the absence of exogenous growth stimuli, there was no difference in growth of cells isolated from VSMC-Tg compared to Control (Figure 8D). However, growth in response to PDGF-BB and 10% serum was significantly increased in VSMC-Tg compared to Control (Figure 8E-F). These data suggest that the level of CyPA expression has powerful effects on VSMC proliferation.
Discussion
The major findings of the present study are that carotid ligation increases CyPA expression in the vascular wall, and promotes vascular remodeling due to proliferation and migration of VSMC and accumulation of inflammatory cells. These results are the first direct demonstration that CyPA contributes to vascular remodeling in vivo. The present study revealed three important pathologic consequences of CyPA activity (Supplement Figure 6). First, VSMC-derived secreted CyPA increases intimal VSMC by virtue of its ability to promote VSMC proliferation and migration. Second, secreted extracellular CyPA is pro-inflammatory because it stimulates vascular expression of VCAM-1 and recruits inflammatory cells. Third, we showed a direct role for intracellular ROS to stimulate CyPA secretion that was proportionate to intracellular CyPA expression. These data show a role for CyPA as one of the key mediators of the pathologic effects of ROS on vascular remodeling.
To strengthen the link between flow cessation, CyPA expression, and cell growth, we observed the time course and distribution of CyPA expression in carotids after ligation. There was minimal staining of CyPA in sham carotids, but a dramatic increase in the intima and media after ligation. In parallel with CyPA expression, carotid ligation induced phosphorylation of ERK1/2 in WT carotids, which was significantly less in CyPA−/− carotids, consistent with the reduced number of Ki67+ VSMC in ligated CyPA−/− carotids. The distribution of Ki67+ cells closely overlapped with areas of highest CyPA expression, especially in the rapidly proliferating intimal cells in WT mice (Supplement Figure 3). Co-localization of CyPA and αSMA staining revealed that CyPA expression was particularly high in VSMC (Supplement Figure 3). To prove further the contribution of VSMC-derived CyPA to vascular remodeling, we prepared VSMC-specific CyPA transgenic mice (VSMC-Tg). VSMC-Tg mice exhibited no significant change in sham carotids, while ligated carotids showed increases of 217% in intimal area, 32% in medial area, and 140% in I/M ratio compared with control mice expressing normal levels of CyPA. The observation that VSMC-specific CyPA overexpression not only increased the medial area but also intimal area suggests that VSMC-derived extracellular CyPA promotes the proliferation and migration of VSMC via a secreted, paracrine pathway. VSMC proliferation measured by Ki67 correlated significantly with CyPA expression (VSMC-Tg > Control ∼ WT > CyPA−/−). VSMC migration and chemotaxis similarly correlated with the magnitude of CyPA expression. The increased VSMC proliferation and migration resulting from VSMC-specific overexpression of CyPA suggests a major contribution for VSMC-derived CyPA in vascular remodeling.
Turbulent blood flow and reduced shear stress generate ROS and play a crucial role in the development of atherosclerosis due to local inflammation.7-9 In VSMC, ROS activate a pathway that induces secretion of CyPA,12 which stimulates at least 3 signaling pathways (ERK1/2, Akt and JAK).10 Extracellular CyPA activates proinflammatory pathways in EC including increased expression of VCAM-1.13 Additionally, CyPA itself is a chemoattractant and promotes migration of several cell types in vitro.13-15 Consistently, carotid ligation increased VCAM-1 expression in ligated WT carotids. VCAM-1 expression was significantly less in ligated CyPA−/− carotids, corresponding to reduced accumulation of CD45+ cells in the intima. This implies that the decreased accumulation of inflammatory cells in CyPA−/− carotids likely results from reduced expression of VCAM-1 and decreased chemotactic signaling in the absence of CyPA. Because decreased myelopoesis could also explain diminished inflammatory cell recruitment in CyPA−/− mice, we analyzed white blood cell counts from mice with WT or CyPA−/− bone marrow. The number of circulating monocytes was not altered by CyPA deficiency, nor by carotid ligation. Thus, lack of CyPA in bone marrow cells does not alter inflammatory cell production or ability to enter the peripheral circulation. In contrast to the situation in CyPA−/− mice, VSMC-specific overexpression of CyPA (VSMC-Tg) further enhanced the accumulation of inflammatory cells in ligated carotids, supporting the important role for CyPA in mediating the recruitment of inflammatory cells.
CyPA is expressed by all cell types participating in vascular pathology.25 Additionally, extracellular CyPA has recently been found to induce IL-6 release in inflammatory cells.27 We observed significant accumulation of inflammatory cells in the intima. We propose that ROS generated locally by inflammatory cells causes VSMC to release CyPA, which would promote a pro-inflammatory cycle for vascular remodeling. Therefore, in addition to the VSMC-derived CyPA, the contribution of inflammatory cells is important for intima formation in this model. Recent observations support the contribution of inflammatory cells to intimal thickening.28,29 CyPA could regulate the proteolytic activity necessary for the migration of inflammatory cells through activating MMPs, especially MMP-1 and MMP-9.14,16 Considering the importance of migrating inflammatory cells in vascular remodeling,30,31 local cytokine production by inflammatory cells could promote intima formation in this model.
Limitations of the Study
CyPA is a chaperone protein that is widely expressed including inflammatory cells and EC. Therefore, CyPA in macrophages and EC may play an important role for vascular remodeling. Future studies will be required to define the role of CyPA in the response to vascular injury of cells such as macrophages, T and B cells, mast cells, platelets, and vascular progenitor cells.
Clinical Implications and Conclusions
In summary, our data using genetically engineered mice to modulate vascular CyPA expression prove that decreasing CyPA levels has beneficial effects on the inflammatory response and vascular intima formation, as shown by significantly increased lumen diameter and decreased I/M ratio. This is consistent with our findings that the plasma level of CyPA is increased in patients with acute coronary syndromes.13 Additionally Billich et al reported increased concentrations of CyPA in synovial fluids of patients with rheumatoid arthritis.32 These results suggest that extracellular CyPA is a novel mediator for vascular disease associated with ROS and inflammation.
Clinical Perspective
Decreased blood flow distal to a stenosis is associated with accelerated atherosclerosis and occlusion, but the mechanisms are not fully elucidated. Accumulating evidence indicates that inflammation and vascular smooth muscle cell (VSMC) proliferation contributes to vessel narrowing. It has become clear that increased reactive oxygen species (ROS) is a key pathogenic mechanism for vascular disease. Cyclophilin A (CyPA) is a 20 kD chaperone protein secreted from VSMC in response to ROS, which stimulates VSMC proliferation and inflammatory cell migration in vitro. Here, using genetically engineered mice to modulate vascular CyPA expression we show that decreasing CyPA has beneficial effects on the inflammatory response and vascular intima formation in low flow vessels, as shown by significantly increased lumen diameter and decreased I/M ratio. Our present study may have important clinical implications, since it appears that secreted CyPA mediates the growth and inflammatory effects. This suggests that a receptor for CyPA may represent an attractive therapeutic target for vascular diseases associated with oxidative stress and inflammation.
Supplementary Material
Acknowledgments
We are grateful to the Aab Cardiovascular Research Institute members for useful suggestions and Chris Ashley, Robert Winterkorn, Mary A. Georger, Tamlyn Thomas, and Sarah A. Mack for technical assistance.
Funding Sources: This work was supported by NIH grant HL49192 (to B.C. Berk) and Japan Heart Foundation−/−Bayer Yakuhin Research Grant Abroad (to K. Satoh).
Footnotes
Conflict of interest: None.
References
- 1.Shimokawa H. Primary endothelial dysfunction: atherosclerosis. J Mol Cell Cardiol. 1999;31:23–37. doi: 10.1006/jmcc.1998.0841. [DOI] [PubMed] [Google Scholar]
- 2.Clempus RE, Griendling KK. Reactive oxygen species signaling in vascular smooth muscle cells. Cardiovasc Res. 2006;71:216–225. doi: 10.1016/j.cardiores.2006.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 4.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 5.Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells. Circ Res. 1995;77:29–36. doi: 10.1161/01.res.77.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Festa A, D'Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 2000;58:1703–1710. doi: 10.1046/j.1523-1755.2000.00331.x. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM. Novel inflammatory markers of coronary risk: theory versus practice. Circulation. 1999;100:1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 10.Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 11.Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, Berk BC. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000;275:189–196. doi: 10.1074/jbc.275.1.189. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 13.Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 14.Kim H, Kim WJ, Jeon ST, Koh EM, Cha HS, Ahn KS, Lee WH. Cyclophilin A may contribute to the inflammatory processes in rheumatoid arthritis through induction of matrix degrading enzymes and inflammatory cytokines from macrophages. Clin Immunol. 2005;116:217–224. doi: 10.1016/j.clim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007;82:613–618. doi: 10.1189/jlb.0506317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther. 2005;7:R1023–1033. doi: 10.1186/ar1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 18.Sakai K, Mitani K, Miyazaki J. Efficient regulation of gene expression by adenovirus vector-mediated delivery of the CRE recombinase. Biochem Biophys Res Commun. 1995;217:393–401. doi: 10.1006/bbrc.1995.2789. [DOI] [PubMed] [Google Scholar]
- 19.Miano JM, Ramanan N, Georger MA, de Mesy Bentley KL, Emerson RL, Balza RO, Jr, Xiao Q, Weiler H, Ginty DD, Misra RP. Restricted inactivation of serum response factor to the cardiovascular system. Proc Natl Acad Sci U S A. 2004;101:17132–17137. doi: 10.1073/pnas.0406041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Hoover JL, Simmons CA, Lindner V, Shebuski RJ. Remodeling and neointimal formation in the carotid artery of normal and P-selectin-deficient mice. Circulation. 1997;96:4333–4342. doi: 10.1161/01.cir.96.12.4333. [DOI] [PubMed] [Google Scholar]
- 21.Mukai Y, Rikitake Y, Shiojima I, Wolfrum S, Satoh M, Takeshita K, Hiroi Y, Salomone S, Kim HH, Benjamin LE, Walsh K, Liao JK. Decreased vascular lesion formation in mice with inducible endothelial-specific expression of protein kinase Akt. J Clin Invest. 2006;116:334–343. doi: 10.1172/JCI26223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003;23:2185–2191. doi: 10.1161/01.ATV.0000103120.06092.14. [DOI] [PubMed] [Google Scholar]
- 23.Ishida M, Ishida T, Thomas S, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ Res. 1998;82:7–12. doi: 10.1161/01.res.82.1.7. [DOI] [PubMed] [Google Scholar]
- 24.Ishida T, Ishida M, Suero J, Takahashi M, Berk BC. Agonist-stimulated cytoskeletal reorganization and signal transduction at focal adhesions in vascular smooth muscle cells require c-Src. J Clin Invest. 1999;103:789–797. doi: 10.1172/JCI4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin ZG, Berk BC. SOXF: redox mediators of vascular smooth muscle cell growth. Heart. 2004;90:488–490. doi: 10.1136/hrt.2003.029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tolbert T, Thompson J, Bouchard P, Oparil S. Estrogen-induced vasoprotection is independent of inducible nitric oxide synthase expression: evidence from the mouse carotid artery ligation model. Circulation. 2001;104:2740–2745. doi: 10.1161/hc4701.099581. [DOI] [PubMed] [Google Scholar]
- 27.Payeli SK, Schiene-Fischer C, Steffel J, Camici GG, Rozenberg I, Luscher TF, Tanner FC. Cyclophilin A differentially activates monocytes and endothelial cells Role of purity, activity, and endotoxin contamination in commercial preparations. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Schafer K, Schroeter MR, Dellas C, Puls M, Nitsche M, Weiss E, Hasenfuss G, Konstantinides SV. Plasminogen activator inhibitor-1 from bone marrow-derived cells suppresses neointimal formation after vascular injury in mice. Arterioscler Thromb Vasc Biol. 2006;26:1254–1259. doi: 10.1161/01.ATV.0000215982.14003.b7. [DOI] [PubMed] [Google Scholar]
- 29.Wang CH, Anderson N, Li SH, Szmitko PE, Cherng WJ, Fedak PW, Fazel S, Li RK, Yau TM, Weisel RD, Stanford WL, Verma S. Stem cell factor deficiency is vasculoprotective: unraveling a new therapeutic potential of imatinib mesylate. Circ Res. 2006;99:617–625. doi: 10.1161/01.RES.0000243210.79654.fd. [DOI] [PubMed] [Google Scholar]
- 30.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Sata M, Hirata Y, Nagai R. Diverse contribution of bone marrow cells to neointimal hyperplasia after mechanical vascular injuries. Circ Res. 2003;93:783–790. doi: 10.1161/01.RES.0000096651.13001.B4. [DOI] [PubMed] [Google Scholar]
- 32.Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997;185:975–980. doi: 10.1084/jem.185.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


