Abstract
Objectives
Low-income women have high rates of smoking during pregnancy, but little is known about the costs, benefits, and cost-effectiveness of motivational interviewing (MI), focused on the medical and psychosocial needs of this population, as an intervention for smoking cessation and relapse prevention.
Methods
A sample of 302 low-income pregnant women was recruited from multiple obstetrical sites in the Boston metropolitan area into a randomized controlled trial of a motivational intervention for smoking cessation and relapse prevention versus usual care (UC). The findings of this clinical trial were used to estimate the costs, benefits, and cost-effectiveness of the intervention from a societal perspective, incorporating published quality-adjusted life-year (QALY) and life-year (LY) estimates. Outcomes included smoking cessation and relapse, maternal and infant outcomes, economic costs, LYs and QALYs saved, and incremental cost-effectiveness ratios.
Results
The cost-effectiveness of MI for relapse prevention compared to UC was estimated to be $851/LY saved and $628/QALY saved. Including savings in maternal medical costs in sensitivity analyses resulted in cost savings for MI for relapse prevention compared to UC. For smoking cessation, MI cost more but did not provide additional benefit compared to UC. In one-way sensitivity analyses, the incremental cost-effectiveness of MI versus UC would have been $117,100/LY saved and $86,300/QALY saved if 8% of smokers had quit. In two-way sensitivity analyses, MI was still relatively cost-effective for relapse prevention ($17,300/QALY saved) even if it cost as much as $2000/participant and was less effective. For smoking cessation, however, a higher level of effectiveness (9/110) and higher cost ($400/participant) resulted in higher incremental cost-effectiveness ratios ($112,000/QALY).
Conclusions
Among low-income pregnant women, MI helps prevent relapse at relatively low cost, and may be cost-saving when net medical cost savings are considered. For smoking cessation, MI cost more but provided no additional benefit compared to UC, but might offer benefits at costs comparable to other clinical preventive interventions if 8–10% of smokers are induced to quit.
Keywords: cost-effectiveness, low-income, pregnant women, relapse prevention, smoking cessation
Introduction
Smoking during and after pregnancy is associated with adverse maternal and infant health outcomes [1] and an increased risk of nicotine dependence among off-spring [2]. Nevertheless, only about one-third of female smokers quit when they become pregnant (spontaneous quitters) [3], and rates are lower among unmarried, low-income, poorly educated, non- Hispanic white, or American Indian women, and heavy smokers [1,4–6]. In 1998, 26% of women who did not complete high school smoked during pregnancy versus 2% of women with a college degree [1]. Furthermore, for women who smoke but quit at some time during pregnancy, relapse rates range from 70% to 85%, stressing the difficulty in preventing relapse during pregnancy and in the postpartum period [7].
Traditional cessation counseling—brief, low-intensity interventions—offer modest benefits to pregnant clients [8–17]. To our knowledge, no published studies have estimated clinical benefits in terms of life-years (LYs) and quality-adjusted life-years (QALYs) saved when low-income pregnant women quit smoking and continue to abstain; nor have cost-effectiveness analyses been performed for these interventions in this target population as recommended by the US Panel on Cost-Effectiveness in Health and Medicine [18].
Factors that are associated with smoking during and post pregnancy include a lack of awareness of fetal damage, heavy smoking before pregnancy, being in a relationship with a smoker, low self-efficacy, and not breast-feeding [12–14]. Traditional smoking cessation and relapse prevention programs are also difficult to implement among lower-income populations because of social and environmental factors.
We conducted a randomized, controlled trial and cost-effectiveness analysis of individually tailored motivational interviewing (MI), which public health nurses delivered to low-income women. This client-centered technique explores perceptions and concerns about smoking, clarifies conflicting motivations, focuses on the social context in which women live, and provides support and skills of training. It also aims to reduce household levels of nicotine, increase readiness to quit, and lower relapse rates.
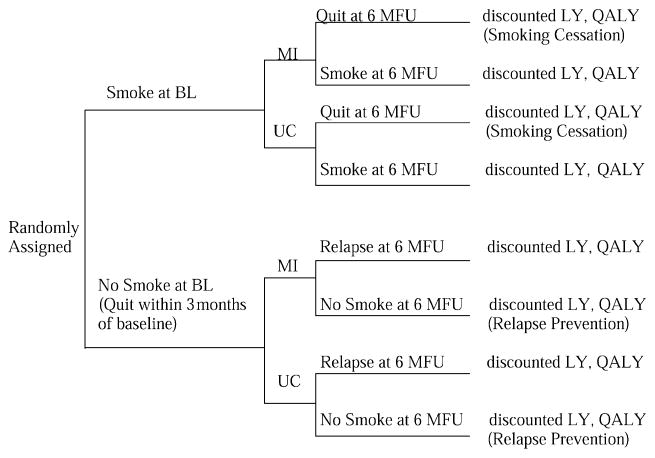
This study extends previous economic studies of smoking cessation and relapse prevention during and after pregnancy by examining a specific patient sub-group, including microcosts of the programs, estimating costs per LYs and QALYs saved, and following recommendations from the US Panel on Cost-Effectiveness in Health and Medicine [18]. We developed a model for examining the cost-effectiveness of MI as compared to usual care (UC) for low-income pregnant women. The model is represented as a decision tree in Figure 1. The effectiveness and cost-effectiveness of MI were evaluated separately for two groups of women: current smokers (smoking cessation: SC) or recent quitters (relapse prevention: RP) at baseline. We examined the cost-effectiveness analysis (CEA) of the intervention for each of these groups separately.
Figure 1.
A model of the strategies for smoking cessation and relapse prevention among pregnant women. BL, baseline; LY, life-year; 6 MFU, 6 months follow-up; MI, motivational interviewing; QALY, quality-adjusted life-year; UC, usual care.
Methods
Recruitment, Design, and Sample
Participants were recruited by several hospital and health clinics that deliver obstetrical care in the Boston metropolitan area. Eligibility criteria included: 1) being pregnant for less than 28 weeks and receiving prenatal care at a participating site; 2) being a current smoker (smoking cessation) or having been a smoker within 3 months of baseline (relapse prevention); 3) not receiving inpatient drug treatment; and 4) speaking English or Spanish. Eligible clients were introduced to the study by the site nurse. Those who were interested received additional information and, once formal informed consent was obtained, research assistants conducted baseline assessments at the clinical site or client’s home. Of 549 women referred, 65 were ineligible, 68 could not be located, 114 refused to participate, and 302 (72.6% of eligible clients) were enrolled.
We randomized participants to two treatment conditions: motivational interviewing (MI = 156) and usual care (UC = 146). Participants’ responses were assessed at baseline, 1 month after the intervention, and 6 months postpartum.
Intervention Conditions
Motivational intervention
Women randomly assigned to MI received an average of three home visits that specifically employed MI [19,20] to deliver a smoking intervention. The MI sessions: 1) educated clients about the impact of smoking on mothers, fetuses, and new-borns; 2) helped clients evaluate their smoking behavior; 3) helped increase self-efficacy for smoking cessation and abstinence; 4) provided information on reducing exposure to environmental tobacco smoke and set goals on changes in smoking; and 5) provided feedback about household nicotine levels. The MI components were tailored to each client’s stage of readiness and MI sessions lasted 1 hour on average. MI subjects also received self-help smoking cessation manuals.
Usual care
These women received standard prenatal care from their health-care provider at the clinic site. An up-to-5-minute intervention outlined the harmful effects of smoking during and after pregnancy. Self-help materials were also provided.
Primary cost data for interventions
All inputs consumed in the interventions were measured and valued alongside the clinical trial to enhance the reliability and validity of intervention costs. We developed a process-tracking form for completion at the time of intervention and used a checklist to identify the following: 1) components delivered; 2) amount of time spent with each client for intervention delivery and follow-up; 3) materials provided; and 4) travel time and distance. Costs collected were those necessary to reproduce the intervention in a non-research setting [18], including: 1) staff time related to intervention delivery; 2) costs of analyzing environmental nicotine (used in MI); 3) cost of training staff (nurses); and 4) costs of producing self-help materials. The process tracking system tracked staff time and distinguished intervention time from research and evaluation time. We did not include productivity costs [18] (work time lost because of morbidity or mortality) or the cost of setting up the program, but we included patient time as a direct cost. Overhead costs were minimal and similar in both the MI and UC groups and therefore were excluded. All costs were reported in 1997 dollars and updated, where necessary, using the medical care component of the consumer price index from the Bureau of Labor Statistics [21]. The costs of the components were summed to obtain per participant costs.
The cost analysis was extended to the societal perspective by including net resource costs: 1) the intervention costs described above; 2) cost savings for neonatal intensive care, chronic medical conditions, and acute conditions during the first year of life; and 3) cost savings for maternal health care (cardiovascular and lung diseases). To be consistent with previous studies [18] and because estimates of projected cost savings for infants and mothers (maternal lifetime medical expenditures) were obtained from the literature, these estimates were included in the sensitivity analysis but conservatively assumed to be $0 (“no savings”) in the base case since there were no statistically significant differences in infant health outcomes or Neonatal Intensive Care Unit (NICU) admissions between the groups. Published estimates of net lifetime medical costs are $6239 (discounted 1990 dollars) more for smokers than for nonsmokers [22–24]. Published estimates that include net smoking-attributable medical costs for neonatal intensive care, chronic medical conditions, and acute conditions during the first year of life range from $1024 to $1228 [25] (in discounted 1996 dollars). The base-case estimate of savings in infant medical costs was $0, but we used costs ranging from $1000 to $5000 in sensitivity analyses.
Outcome Measures
Smoking status
The primary outcome measures were smoking cessation and relapse prevention. At each assessment, the participant was asked if she had smoked a cigarette, even a puff, within the previous 30 days. A “quitter” smoked at baseline but not at follow-up. A “relapse prevented” had quit smoking within 3 months of baseline and was abstinent at follow-up. Smoking status was verified biochemically by collecting saliva samples for saliva cotinine analysis.
Infant health outcomes
Birth weight and postdelivery status were assessed from medical charts.
Life-years and quality of life
Effectiveness measures were extended to the societal perspective by using published data and estimates [18,22,24,26–28] to convert quit and relapse prevention rates into LYs saved and QALYs saved. Separate estimates of life expectancy and quality-adjusted life expectancy for female smokers and former smokers by age group and duration of quitting were obtained from the literature [22,23]. These estimates were based on differences in life expectancy between ex-smokers and smokers for each age group of women using a 20-year phase-in period based on mortality ratios of quitters to never smokers derived from the American Cancer Society’s Cancer Prevention Study (CPS II) [26]. These estimates of quality-of-life-year adjustments for women by age [23], had been calculated using a Markov model and the Years of Healthy Life [29] measure constructed from questions on the annual National Health Interview Survey [28]. Specific modeling assumptions used in calculating discounted LY and QALY estimates are reported elsewhere [23], but it should be noted that the estimates did allow for a 35% lifetime probability of relapse after 1 year of abstinence as recommended in a 1990 Surgeon General’s Report [26] and future benefits were discounted at a 3% annual rate as recommended by the Panel on Cost-Effectiveness in Health and Medicine [18]. Published estimates indicated that female quitters and abstainers aged 25–29 years saved 1.43 LYs and 1.94 QALYs, discounted at a rate of 3% [22,23]. We used these estimates in our analyses.
Statistical Analysis
Univariate and bivariate analyses
Univariate analysis assessed overall sample characteristics. Bivariate analysis examined unadjusted relationships between the intervention group and various factors. Chi-square tests compared groups. Bivariate correlations between continuous variables were analyzed with Student’s t-test. Analysis of variance among multiple groups evaluated differences in unadjusted mean values between each pair of means for each group. P-values were evaluated for each group-wise comparison. Bonferroni corrections to significance levels permitted multiple comparisons.
Cost-effectiveness analysis
We estimated the incremental cost-effectiveness of MI compared to UC by calculating the ratio of the difference in intervention costs to the resulting incremental benefit among MI participants compared to those receiving UC. The analysis was performed separately for the smokers at baseline (SC) and former smokers who had quit within 3 months of baseline (RP). The cost-effectiveness analysis was extended to the societal perspective by incorporating published estimates of net economic costs and overall health consequences of smoking cessation and relapse prevention (described previously). We estimated a set of cost-effectiveness ratios, expressing them as net resource cost per LY gained or QALY gained.
Sensitivity analyses
We examined the robustness of our cost-effectiveness ratio estimates in sensitivity analyses that varied important parameters singly, and in combination, through clinically meaningful ranges. Sensitivity analyses were performed on MI’s effectiveness for smoking cessation and relapse prevention, LY gains and quality-of-life-year weights, intervention cost, inclusion of maternal medical cost savings, and inclusion of cost savings for infant health care during the first year of life.
Results
Baseline Characteristics
Table 1 presents baseline characteristics for the 302 participants. Study groups were comparable at base-line in terms of age, race/ethnicity (except for race other), education, marital status, smoking status, health insurance, and age of first smoke. Average age was 26 years in both groups.
Table 1.
Baseline characteristics of participants in the MI and UC groups*
| Women, n (%) or mean [95% CI or SE]† |
||
|---|---|---|
| Characteristic | Motivational intervention (MI) (N = 156) | Usual care (UC) (N = 146) |
| Age (year), mean [range] | 25.6 [24.5–26.5] | 25.7 [24.6–26.8] |
| Race/ethnicity | ||
| White | 109 (70.3) | 94 (64.4) |
| Asian/Pacific Islander | 1 (0.65) | 0 (0.0) |
| Black | 30 (19.4) | 22 (15.1) |
| Hispanic | 13 (8.3) | 16 (11.0) |
| American Indian, Aleut or Eskimo | 2 (1.3) | 1 (0.70) |
| Other | 12 (7.7) | 29 (19.9)‡ |
| Education | ||
| <High school | 54 (34.6) | 44 (30.1) |
| Completed high school | 57 (36.5) | 67 (45.9) |
| Postsecondary | 45 (28.9) | 34 (23.3) |
| Married | 34 (21.8) | 27 (18.5) |
| Smoking status | ||
| Baseline smoker | 132 (84.6) | 113 (77.4) |
| Baseline nonsmoker | 24 (15.4) | 33 (22.6) |
| Smoked during previous pregnancy | 55 (72.4) | 63 (80.8) |
| Health insurance | ||
| Major medical | 39 (25.3) | 41 (28.3) |
| Medicaid | 10 (6.5) | 7 (4.8) |
| Mass health | 110 (71.4) | 103 (71.0) |
| Other | 1 (0.65) | 2 (1.4) |
| Age of first smoke | ||
| ≤13 years | 48 (30.8) | 50 (34.3) |
| 14–17 years | 67 (43.0) | 75 (51.4) |
| ≥18 years | 39 (25.0) | 20 (13.7)* |
Percentages might not sum to 100 because of rounding.
May not sum to group total and 100% because of rounding, missing data, or multiple recording.
Statistically significant at the P < 0.05 level compared to MI.
CI, confidence interval; SE, standard error.
Costs
The mean intervention cost per participant was $309.2 for MI versus $4.85 for UC, a difference of $304.4 (CI $289.2–320.2). The main cost components of MI were intervention delivery, travel time, and training, which occurred by design. All direct program costs were in 1997 dollars.
Effectiveness
At 6 months postpartum, the two groups had similar cessation rates (7/110 [MI] vs. 8/100 [UC]), although the MI group had twice the relapse prevention rate as the UC group (9/21 [MI] vs. 5/28 [UC]; P = 0.055) (Table 2).
Table 2.
Outcome measures for motivational intervention and usual care
| Women or infants, n (%) or mean [SD] |
||
|---|---|---|
| Motivational intervention (MI) | Usual care (UC) | |
| Smoking cessation | ||
| Smokers at baseline | 110 | 100 |
| Nonsmokers at 6MPP | 7 | 8 |
| Smokers at 6MPP | 103 | 92 |
| Relapse prevention | ||
| Nonsmokers at baseline | 21 | 28 |
| Nonsmokers at 6MPP | 9 | 5* |
| Smokers at 6MPP | 12 | 23 |
| Infant health outcomes (nsd)† | ||
| Birth weight (g) | 3241.2 [586.0] | 3321.3 [612.1] |
| Low birth weight (<2500 g) | 16 (59.3) | 11 (40.7) |
| NICU/special care unit | 14 (10.1) | 23 (17.6) |
| Respiratory problems at birth | 21 (15.1) | 23 (17.8) |
Borderline statistical significance (P = 0.055) compared to MI.
Subanalyses by smoking status at baseline and 6MPP revealed no statistically significant differences.
Statistically significant at the P < 0.05 level compared to MI.
6MPP, 6 months postpartum; NICU, neonatal intensive care unit; SD, standard deviation.
Cost-Effectiveness
For smoking cessation, MI cost more but provided no additional benefit compared to UC. Therefore, the incremental cost per LY and per QALY saved from smoking cessation was not estimated in the base case, and MI was dominated by UC. The MI intervention did, however, prevent relapse more effectively than UC. The incremental cost per LY saved by relapse prevention among MI ex-smokers compared to UC ex-smokers was an estimated $851/LY (Table 3). The incremental cost per QALY of preventing relapse among MI ex-smokers compared to UC ex-smokers was estimated at $628/QALY (Table 3).
Table 3.
Costs, LYs, QALYs, and cost-effectiveness*
| ICER |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total costs† |
Total quitter/RPs† |
Total LYs† |
Total QALYs† |
Δ Costs |
Δ Quit/RP |
Δ LYs |
Δ QALYs |
$/quitter or $/RP |
$/LY saved |
$/QALY saved |
|
| Current smokers | |||||||||||
| UC (n = 100) | $5 | 0.08 | 0.11 | 0.16 | — | — | — | — | — | — | — |
| MI (n = 110) | $309 | 0.06 | 0.09 | 0.12 | $304 | -0.02 | -0.02 | -0.04 | D‡ | D‡ | D‡ |
| Recent quitters | |||||||||||
| UC (n = 28) | $5 | 0.18 | 0.26 | 0.35 | — | — | — | — | — | — | — |
| MI (n = 21) | $309 | 0.43 | 0.61 | 0.83 | $304 | 0.25 | 0.36 | 0.49 | $1217 | $851 | $628 |
Δ indicates change in.
Costs and effects are per participant in the target population; LYs and QALYs per participant were calculated as the quotient of the number of quitters or RPs multiplied by the LYs (1.43) and QALYs (1.94) saved per quitter/RP, respectively, and the number of participants in the target group (e.g., for UC [SC] (8 × 1.43)/100 = 0.11 LYs per participant in that group).
D, dominated (program is more costly, but less effective than comparator).
ICER, incremental cost-effectiveness ratio; LY, life-year; MI, motivational intervention; QALY, quality-adjusted life-year; RP, relapse prevented; SC, smoking cessation; UC, usual care.
Sensitivity Analyses
The program’s effectiveness for smoking cessation varied from one quitter per 110 smokers to 10 quitters per 110 smokers (baseline: 7/110). Effectiveness measured by the number of relapses prevented varied from 3 to 12 per 21 ex-smokers (baseline: 9/21). Discounted QALYs gained varied from 0.025 to 2 (baseline assumption: 1.94). Cost varied from $250 to $2000 per participant (baseline: $309). Maternal lifetime discounted medical costs saved varied from $6000 to $12,000 (baseline assumption: $0), and infant medical costs saved varied from $1000 to $5000 (baseline assumption: $0), both ranges include recent estimates of incurred maternal and infant medical costs (noted below) [22–25]. In two-way sensitivity analyses, MI’s effectiveness was varied with program costs. For cessation, because MI was dominated by UC in the base case, we explored the implications of improving the cessation effectiveness of MI on the incremental cost-effectiveness ratios. Increasing the quit rate by 2% eliminated UC domination of MI, with an incremental cost per LY saved of $117,100 and cost per QALY saved of $86,300. A 3% increase led to an incremental cost per LY saved of $19,500 and cost per QALY saved of $14,400 (Table 4). Increasing MI’s effectiveness for relapse prevention by around 15% resulted in an approximately 36% decrease in the incremental cost per QALY ratio. Thus, cost-effectiveness ratios were not exactly proportional to effectiveness.
Table 4.
One-way sensitivity analyses
| Incremental cost-effectiveness ($/LY saved)* |
Incremental cost-effectiveness ($/QALY saved)* |
|||
|---|---|---|---|---|
| Parameter varied | SC | RP | SC | RP |
| Baseline | D† | 851 | D | 628 |
| Effectiveness of MI for SC (baseline 7/110) | ||||
| 10/110 | 19,500 | ‡ | 14,400 | ‡ |
| 9/110 | 117,100 | ‡ | 86,300 | ‡ |
| 8/110 | D | ‡ | D | ‡ |
| 5/110 | D | ‡ | D | ‡ |
| 1/110 | D | ‡ | D | ‡ |
| Effectiveness of MI for RP (baseline 9/21) | ||||
| 12/21 | D | 540 | D | 400 |
| 10/21 | D | 720 | D | 530 |
| 8/21 | D | 1,050 | D | 780 |
| 6/21 | D | 2,000 | D | 1,500 |
| 5/21 | D | 3,600 | D | 2,600 |
| 3/21 | D | D | D | D |
| Discounted LYs and QALYs saved (baseline 1.43 and 1.94, respectively) | ||||
| 2 | D | 610 | D | 610 |
| 1 | D | 1,200 | D | 1,200 |
| 0.5 | D | 2,400 | D | 2,400 |
| 0.1 | D | 12,200 | D | 12,200 |
| 0.05 | D | 24,400 | D | 24,400 |
| 0.025 | D | 48,700 | D | 48,700 |
| Cost of the MI program (baseline $309) | ||||
| $250 | D | 690 | D | 510 |
| $500 | D | 1,400 | D | 1,020 |
| $1,000 | D | 2,800 | D | 2,100 |
| $2,000 | D | 5,600 | D | 4,100 |
| Maternal medical care cost savings (baseline $0) | ||||
| $6,000 | D | CS | D | CS |
| $12,000 | D | CS | D | CS |
| Cost savings for health care of newborn at birth and during first year of life (baseline $0)‡ | ||||
| $1,000 | D | CS | D | CS |
| $5,000 | D | CS | D | CS |
As compared to UC, assuming UC effectiveness for SC of 8/100 and for RP of 5/28 and direct UC program costs of $4.85 per participant.
D, MI is dominated by UC (MI is more costly and less effective than UC).
For infants of women who quit or remained abstinent—estimates are per participant in the target population.
LY, life-year; MI, motivational intervention; QALY, quality-adjusted life-year; RP, relapse prevention; SC, smoking cessation; UC, usual care; CS, cost saving.
When the discounted years of life or QALYs gained from smoking cessation or relapse prevention were assumed to be as low as 0.025, MI’s incremental cost-effectiveness for relapse prevention reached $48,700 per LY or QALY saved. Given the baseline assumptions of effectiveness and the QALY/LY estimates, if the program cost $2000 per participant, the cost-effectiveness ratios would remain favorable, at $5600/LY saved and $4100/QALY saved compared to UC when relapse prevention is considered. Including discounted expected maternal medical costs for the remaining lifetime rendered the MI “cost saving” as compared to UC for relapse prevention.
In two-way sensitivity analysis, MI was still relatively cost-effective in comparison with UC for relapse prevention ($23,400/LY saved and $17,300/QALY saved) even if it cost $2000 per participant and was less effective than the base case (5/21 vs. 9/21). For smoking cessation, a higher level of effectiveness (9/110 vs. 7/110) and a higher cost ($400/participant) resulted in an incremental cost-effectiveness ratio of $112,000/QALY.
Discussion
We examined the clinical and economic implications of two smoking cessation and relapse prevention strategies, MI and UC. The cost-effectiveness of MI for relapse prevention compared to UC was estimated to be $851/LY saved and $628/QALY saved. When cessation was considered, MI cost more than UC but provided no additional benefit. One-way sensitivity analysis revealed that the incremental cost-effectiveness of MI compared to UC was $86,300/QALY saved if 8% of smokers had quit. Including maternal medical costs in sensitivity analysis resulted in incremental “cost savings” for MI versus UC for relapse prevention, for smoking cessation MI was dominated by UC. In two-way sensitivity analysis, MI was still cost-effective compared to UC for relapse prevention ($17,300/QALY saved), if it cost $2000 per participant and was less effective (5/21). For smoking cessation, a higher level of effectiveness (9/110) and higher cost ($400/participant) resulted in a higher incremental cost-effectiveness ratio ($112,000/QALY).
In general, our analysis supports previous findings about the economic implications of smoking cessation programs among pregnant women, although the results of this study are difficult to compare to other work. When Ershoff et al. [11] evaluated an intervention consisting of an initial interview, smoking counseling by a health educator, mailed self-help books, and reinforcement at prenatal care visits, they found that the cost savings for a 100,000-member Health Maintenance Organization (HMO) was $13,432, with a net benefit of $9202 and a benefit–cost ratio of 3:1. They did not extend their analysis to the societal perspective. Assessing three cessation protocols for women in public health maternity clinics, Windsor et al. [9] found that 2%, 6%, and 14% of the participants in their respective groups stopped smoking, with costs per percentage who quit of $104, $118, and $50, respectively. Our study produced quit rates of 6% for MI and 8% for UC, although differences were not statistically significant. Marks et al. [16] modeled the benefits that would accrue from shifting low-birth-weight infants into the normal-birth-weight category, averting deaths attributable to prematurity, and avoiding the long-term costs of caring for premature infants, concluding that the ratio of savings to costs could be as high as 6:1. They did not, however, separate smoking from nonsmoking attributable infant costs. When Shipp et al. [17] modeled the break-even cost of a smoking cessation program during pregnancy, they obtained an estimate of around $32 per pregnant woman. Their sensitivity analysis revealed that this cost varied from $10 to $237, depending on the probability of adverse outcomes in various populations. Our costs exceed their estimates but fall within their range in real terms. To our knowledge, studies assessing the clinical and economic implications of relapse prevention for pregnant women are limited.
Our study has limitations. First, we studied low-income women in Boston; therefore, our findings cannot be generalized to other economic (high-income) and geographic groups. Second, we analyzed savings in maternal and infant medical costs but did not have long-term morbidity and mortality data for children related to smoking-related illnesses. Because published estimates might be overestimates or underestimates, we performed sensitivity analyses to determine their impact on cost-effectiveness ratios. Third, it is difficult to know how income and pregnancy might affect health-related quality of life and life-expectancy measures. Fourth, we did not measure some nonsmoking-related costs and benefits of MI (e.g., results from instruction on general health and social services), since the intervention was broader than smoking, but the CEA did not assess those outcomes [30]. Fifth, because of the small sample size, we may not have had enough power to detect differences between groups on a number of study variables. Sixth, our study may under-estimate the importance of relapse prevention during pregnancy because it does not consider the impact of reducing maternal smoking during pregnancy on the risk of nicotine dependence among offspring.
From a policy perspective, the choice of whether to implement UC or MI will depend on available resources, alternative uses of resources, and other constraints. Comparing our results with cost-effectiveness ratios of other accepted preventive interventions demonstrates that resources devoted to smoking cessation [31] and relapse prevention during and after pregnancy might be worthwhile [32]. For example, cervical cancer screening costs have been estimated to range from $7100 (every 5 years compared to no screening) to $175,000 (every 2 years compared to every 3 years) per LY saved [33].
In conclusion, our analysis suggests that, among low-income pregnant women, MI can prevent relapse at relatively low cost whereas MI was more costly and no more effective than UC in promoting smoking cessation. Inclusion in sensitivity analyses of net medical cost savings for infants and mothers as a result of sustained cessation and abstinence results in more favorable incremental cost-effectiveness ratios.
Supplementary Material
Supplementary material for this article can be found at: http://www.ispor.org/publications/value/ViHsupplementary.asp
Acknowledgments
We thank David Paltiel, Todd Olmstead, Mark Schlesinger, Jody Sindelar, and participants of the Joint Colloquium in Health Policy and Health Services Research and Mental Health Services Research at Yale University School of Medicine for helpful comments and Ruth Lederman, Rita Butterfield, and Jackie Nolan for their participation in this study. We also thank Marina Cordeau and Linda Sage for administrative and editing assistance, respectively.
Source of financial support: This work was supported by a grant from the National Cancer Institute (grant 5R01CA07324x4). Dr. Ruger is supported by a Career Development Award from the National Institute on Drug Abuse (grant 1K01DA01635810).
References
- 1.West R. Smoking cessation and pregnancy. Fetal Matern Med Rev. 2002;3:181–94. [Google Scholar]
- 2.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mother smoked during pregnancy: a 30-year prospective study. Am J Psychiatry. 2003;160:1978–84. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- 3.Zapka JG, Pbert L, Stoddard A, et al. Smoking cessation counseling with pregnant and postpartum women: a survey of community health providers. Am J Public Health. 2000;90:78–84. doi: 10.2105/ajph.90.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews TJ. National Vital Statistics Reports. Hyattsville, MD: National Center for Health Statistics; 2001. Smoking During Pregnancy in the 1990s. [PubMed] [Google Scholar]
- 5.Walsh RA, Redman S, Brindsmead MW, et al. Predictors of smoking in pregnancy and attitudes and knowledge of risks of pregnant smokers. Drug Alcohol Rev. 1997;16:41–67. doi: 10.1080/09595239700186321. [DOI] [PubMed] [Google Scholar]
- 6.Paarlberg KM, Vingerhoets JJM, Passchier J, et al. Smoking status in pregnancy is associated with daily stressors and low well-being. Psychol Health. 1999;14:87–96. [Google Scholar]
- 7.Fang WL, Goldstein AO, Butzen AY, et al. Smoking cessation in pregnancy: a review of postpartum relapse prevention strategies. J Am Board Fam Pract. 2004;17:264–75. doi: 10.3122/jabfm.17.4.264. [DOI] [PubMed] [Google Scholar]
- 8.Windsor RA, Cutter G, Morris J, et al. The effectiveness of smoking cessation methods for smokers in public health maternity clinics: a randomized trial. Am J Public Health. 1985;75:1389–92. doi: 10.2105/ajph.75.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Windsor RA, Warner KE, Cutter GR. A cost-effectiveness analysis of self-help smoking cessation methods for pregnant women. Public Health Rep. 1988;103:83–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Windsor RA, Lowe JB, Perkins LL, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Public Health. 1993;83:201–6. doi: 10.2105/ajph.83.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ershoff DH, Mullen PD, Quinn VP. A randomized trial of a serialized self-help smoking cessation program for pregnant women in an HMO. Am J Public Health. 1989;79:182–7. doi: 10.2105/ajph.79.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh RA, Redman S, Brinsmead MW, et al. A smoking cessation program at a public antenatal clinic. Am J Public Health. 1997;87:1201–4. doi: 10.2105/ajph.87.7.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards N, Sims-Jones N. Smoking and smoking relapse during pregnancy and postpartum: results of a qualitative study. Birth. 1998;25:94–100. doi: 10.1046/j.1523-536x.1998.00094.x. [DOI] [PubMed] [Google Scholar]
- 14.Ko M, Schulken ED. Factors related to smoking cessation and relapse among pregnant smokers. Am J Health Behav. 1998;22:83–9. [Google Scholar]
- 15.Ershoff DH, Quinn VP, Mullen PD, et al. Pregnancy and medical cost outcomes of a self-help prenatal smoking cessation program in an HMO. Public Health Rep. 1990;105:340–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Marks JS, Koplan JP, Hogue CJ, et al. A cost–benefit/cost-effectiveness analysis of smoking cessation for pregnant women. Am J Prev Med. 1990;6:282–9. [PubMed] [Google Scholar]
- 17.Shipp M, Croughan-Minihane MS, Petitti DB, et al. Estimation of the break-even point for smoking cessation programs in pregnancy. Am J Public Health. 1992;82:383–90. doi: 10.2105/ajph.82.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gold MR, Siegel JE, Russell LB, et al. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 19.Miller W, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. New York: Guilford Press; 1991. [Google Scholar]
- 20.Emmons KM, Rollnick S. Motivational interviewing in health care settings: opportunities and limitations. Am J Prev Med. 2001;20:68–74. doi: 10.1016/s0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Bureau of the Census. Statistical Abstract of the United States: 1997. 117. Washington, DC: U.S. Gov Printing Office; 1997. [Google Scholar]
- 22.Cromwell J, Bartosch WJ, Fiore MC, et al. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research. JAMA. 1997;278:1759–66. [PubMed] [Google Scholar]
- 23.Fiscella K, Franks P. Cost-effectiveness of the transdermal nicotine patch as an adjunct to physicians’ smoking cessation counseling. JAMA. 1996;275:1247–51. [PubMed] [Google Scholar]
- 24.Rogers RG, Powell-Griner E. Life expectancies of cigarette smokers and non-smokers in the United States. Soc Sci Med. 1991;32:1151–9. doi: 10.1016/0277-9536(91)90092-q. [DOI] [PubMed] [Google Scholar]
- 25.Miller DP, Villa KF, Hogue SL, et al. Birth and first-year costs of mothers and infants attributable to maternal smoking. Nicotine Tob Res. 2001;3:25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services. The Health Benefits of Smoking Cessation: A Report from the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 1990. pp. 90–8416. DHHS publication no. (CDC) [Google Scholar]
- 27.Centers for Disease Control and Prevention. Physician and other health care professional counseling of smokers to quit: United States, 1991. MMWR Morb Mortal Wkly Rep. 1993;43:925–30. [PubMed] [Google Scholar]
- 28.Adams PF, Benson V. Current estimates from the National Health Interview Survey, 1991. Vital Health Stat. 1992;10:1–232. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Years of healthy life. Stat Notes. 1995;7:1–14. [Google Scholar]
- 30.Lando HA, Valanis BG, Lichtenstein E, et al. Promoting smoking abstinence in pregnant and postpartum patients: a comparison of 2 approaches. Am J Manag Care. 2001;7:685–93. [PubMed] [Google Scholar]
- 31.Feenstra TL, Hamberg-van Reenen HH, Hoogenveen RT, et al. Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value Health. 2005;8:178–90. doi: 10.1111/j.1524-4733.2005.04008.x. [DOI] [PubMed] [Google Scholar]
- 32.West R, McNeill A, Raw M. Smoking cessation guide-lines for health professionals: an update. Thorax. 2002;55:987–99. doi: 10.1136/thorax.55.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287:2382–90. doi: 10.1001/jama.287.18.2382. [DOI] [PubMed] [Google Scholar]