Abstract
Objective
It has been suggested that both endogenous reproductive hormones and hormone therapy may play a protective role against coronary artery disease (CAD). However, recent clinical trials have failed to demonstrate the benefit of a variety of forms of hormone therapy. The observational data on the role of endogenous reproductive hormones, using surrogate measures such as number of birth, age at menarche, and age at menopause are inconsistent. In addition, the longer-term associations have not been evaluated. The aim of this study was to evaluate the relationships between detailed measurements of endogenous and exogenous estrogen exposure time with angiographic CAD and major adverse cardiovascular events.
Methods
We assessed total estrogen exposure time, quantitative CAD by a core angiography laboratory, and prospectively measured major adverse cardiovascular events in 646 postmenopausal women undergoing coronary angiography for evaluation for suspected ischemia in the Women's Ischemia Syndrome Evaluation (WISE) study.
Results
Timing of postmenopausal exogenous hormone therapy (HT) use was associated with reduced CAD. Two summarized total estrogen time scores (TET and sTET) were not related to angiographic CAD after accounting for HT use. In addition, these scores were not related to cardiovascular events over a median of 6.0 years of follow-up.
Conclusions
There was no independent relation of estrogen exposure time to angiographic CAD or major adverse cardiovascular events in a contemporary cohort of postmenopausal women evaluated for suspected ischemia. Our results suggest that the paradigm of estrogen protection from CAD in women may be more complex than estrogen exposure duration alone.
Introduction
Women have a relatively lower risk of coronary artery disease (CAD) compared to age-matched men,1 suggesting that endogenous reproductive hormones may play a protective role against CAD. Both animal models2 and human epidemiological studies3 suggest that oophorectomy is a risk factor for accelerated CAD, while exogenous hormone therapy (HT) appears to have anti-atherosclerotic effects.4–6 However, recent clinical trials have failed to show protective cardiovascular effects in postmenopausal women randomly assigned to a variety of forms of HT.7–11 The observational data on the role of endogenous reproductive hormone levels are inconsistent.12 Finally, while large numbers of premenopausal women are now exposed to relatively high levels of exogenous estrogen in the form of oral contraceptives, little is known regarding its long-term atherosclerotic impact. The clinical importance and the data discrepancies on this issue call for additional research and clinical trials.
With new methods, including quantitative coronary angiography as a measure of atherosclerosis, we can more precisely address this question within existing observational cohorts. The Women's Ischemia Syndrome Evaluation (WISE) is a prospective, multi-center NHLBI-sponsored study designed to improve diagnostic testing for ischemic heart disease and investigate female-specific ischemic heart disease pathophysiology in a large sample of women.13 We undertook a detailed study to examine the relationship between total estrogen exposure time with CAD, measured by quantitative coronary angiography and prospectively attained adverse cardiovascular events.
Methods
The WISE study population was comprised of 936 consecutive women with chest pain symptoms or suspected ischemia who were clinically referred for coronary angiography at four clinical sites. The WISE study protocol has been described previously.13 Briefly, between 1998 and 2002, the women underwent an initial evaluation that included a clinical examination as well as collection of demographic, medical history, psychosocial, and symptom data, and have been followed for a median of 6 years. Blood for reproductive hormone determinations was drawn following an overnight fast in close temporal proximity to the WISE testing. All women signed an informed consent form and the study was approved by all involved sites' Institutional Review Boards.
Reproductive Status Questionnaire
The determination of reproductive status in the WISE study has been validated to be an accurate assessment of menopausal status using both a detailed questionnaire and blood reproductive hormone levels.14 Reproductive hormone levels were assessed using validated steroid and protein assay methods for total estradiol, bioavailable estradiol, estrone, progesterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH)15 at the WISE reproductive hormone core laboratory. The WISE reproductive status questionnaire includes a detailed history of menarche, date of last menstrual period, current and prior menstrual cycling patterns, prior reproductive events (pregnancy, hysterectomy, uni- and bilateral oophorectomy), current and prior perimenopausal symptoms, and current and prior oral contraceptive or hormone therapy use.14 Documentation of type of gynecological surgery was obtained when the reproductive hormone levels in the blood were not consistent with the reported status during expert consensus review for menopausal status determination.
Only postmenopausal women with completed data to calculate total estrogen time were included in the current analysis (n = 646). Among the WISE women without a history of hysterectomy or oophorectomy, the mean age of the last menstrual cycle was 49 years in smokers compared to 51 years in non-smokers (p < 0.0001); thus, we imputed these menopausal ages for cessation of ovarian cycling in women with a hysterectomy prior to menopause (i.e., when her last menstrual period occurred within a year or less prior to a hysterectomy with or without unilateral oophorectomy).
Because premenopausal oral contraceptive exposure time and pregnancy time occur during premenopausal cycling time and represent times of lower ovarian estrogen production, we calculated total estrogen time (TET) as follows: (menstrual cycling time [age at last cycle − age at menarche]) −(premenopausal oral contraceptive time) − (pregnancy time) +(postmenopausal HT time). However, because oral contraceptive use and pregnancy represent relatively higher sustained blood estrogen levels compared to menstrual cycling, we also calculated a supra-total estrogen time (sTET) using the following formula: (menstrual cycling time [age at last cycle −age at menarche]) + (premenopausal oral contraceptive use time) + (pregnancy time) + (postmenopausal HT time).
Measurement of obstructive coronary artery disease
All coronary angiograms were assessed by a core laboratory used in previous NHLBI-sponsored multi-center trials.16 Measurements included quantitative assessment of the presence and severity of epicardial CAD as a measure of atherosclerosis, using previously published methods.16 For the purposes of these analyses, CAD was defined as ≥70% luminal diameter stenosis in more than one major epicardial coronary artery. We also evaluated a threshold of ≥50% stenosis as an alternative definition of obstructive CAD. An overall CAD severity score was assigned at the core lab based on severity of stenosis, location of stenosis, and presence of partial or complete collaterals.16
Adverse cardiovascular events
Major adverse cardiovascular events included cardiovascular-related mortality, myocardial infarction (MI), congestive heart failure (CHF), or stroke. Patients were contacted by telephone 6 weeks following their baseline evaluation and annually thereafter with experienced study coordinators completing a detailed scripted interview about adverse cardiovascular events or hospitalizations for up to 9 years (median, 6.0 [inter-quartile range, 3.8–7.1]). If a patient was no longer living, a primary relative was queried about the cause of death or any related cardiovascular hospitalizations during the preceding follow-up period; the site investigator was contacted for confirmation of the dates and documentation of the occurrence, including death certificates, when available. The cause of death was reviewed by two investigators blinded to the clinical and angiographic data; a third reviewer was used in case of discrepant death classification.
Statistical analysis
All data are presented as means ± standard deviation (SD) for continuous variables or as percentages for binary variables. Because of the strong association between age and CAD, and between age and reproductive variables, all p-values were age-adjusted, using logistic or linear regression where appropriate. Age-adjusted differences in prevalence and severity score of CAD by tertiles were assessed by one-way analysis of variance (ANOVA). We used Kaplan-Meier curves and the log-rank statistic to compare cardiovascular event-free survival time among TET and sTET tertiles and quartiles. Cox proportional hazards models were applied for multivariate analysis to adjust for age and presence of CAD. Logarithmic transformations and interaction terms were evaluated. The validity of the proportional hazards assumption of invariant relative risk was tested and found to be satisfactory for all models constructed. All p-values of <0.05 were considered statistically significant. All analyses were performed using the SAS 9.1 software (SAS, Cary, NC).
Results
The demographics and clinical profiles for the 646 postmenopausal women with complete reproductive hormone variables and coronary angiography results are shown in Table 1. The women represent a broad age range (36–86 years), and the majority had at least one cardiovascular risk factor. A total of 164 women (25%) had obstructive CAD defined as a ≥70% stenosis in one or more major epicardial coronary ateriers. Compared to women without obstructive CAD, those with CAD were older (p < 0.0001), more likely to have diabetes mellitus (p = 0.0009), and more likely on lipid-lowering therapy (p < 0.0001). They also had lower overall educational levels (p = 0.01) and were less likely to be currently using HT (p = 0.01).
Table 1.
Demographic and Clinical Variables
| All(n = 646) | No CAD(n = 482) | CADa(n = 164) | p | |
|---|---|---|---|---|
| Age (years ± SD) | 62 ± 10 | 61 ± 9 | 66 ± 9 | <0.0001 |
| Race (% white) | 82 | 83 | 79 | 0.11 |
| Hypertension (%) | 62 | 60 | 68 | 0.14 |
| Diabetes mellitus (%) | 26 | 23 | 37 | 0.0009 |
| Current smoking (%) | 18 | 19 | 18 | 0.34 |
| Lipid lowering Rx (%) | 33 | 26 | 51 | <0.0001 |
| Current postmenopausal HT use (%) | 44 | 50 | 28 | 0.01 |
| Education (%) | ||||
| Less than high school | 22 | 20 | 30 | |
| High school graduate | 40 | 39 | 42 | |
| College | 38 | 41 | 28 | 0.01 |
| Coronary Severity Score (±SD) | 15.8 ± 15.1 | 9.2 ± 5.8 | 35.6 ± 17.2 | <0.0001 |
Coronary disease was defined as ≥70% luminal diameter stenosis in more than one epicardial coronary artery.
All p-values are age-adjusted except age.
HT, hormone therapy; Rx, therapy; SD, standard deviation; “college” includes associate degree or vocational or training school after high school; CAD, coronary artery disease.
Menarche, menopause, and coronary disease
When adjusting for age, there were no significant differences between the two groups in age of menarche or menopause or years since final menstrual period (Table 2). Women with obstructive CAD were less likely to have a history of menopausal symptoms (p = 0.002) and had a lower prevalence of prior gynecological surgery (p < 0.0001), specifically bilateral oophorectomy (p = 0.002; Table 2). Evaluation of CAD severity scores by menopausal symptoms and the different types of gynecologic surgeries demonstrated similar differences. Repeat analyses excluding the women with imputed menopausal age did not alter these results.
Table 2.
Menarche, Menopause, and Angiographic Coronary Disease
| No CAD(n = 482) | CAD(n = 164) | pa | |
|---|---|---|---|
| Menarche age (years ± SD) | 12.6 ± 1.8 | 12.9 ± 1.8 | 0.38 |
| Regular periods (%) | 80 | 84 | 0.53 |
| Number of pregnancies (±SD) | 3.6 ± 2.3 | 3.8 ± 2.9 | 0.50 |
| Prior OC use (%) | 44 | 26 | 0.08 |
| Years of OC use (years ± SD) | 2.0 ± 4.2 | 1.2 ± 3.4 | 0.34 |
| Menopausal age (years ± SD) | 44 ± 8 | 46 ± 8 | 0.36 |
| Years since final menstrual period (years ± SD) | 16.0 ± 9.8 | 19.5 ± 10.2 | 0.36 |
| Menopausal symptoms (%) | 72 | 54 | 0.002 |
| Any gynecological surgery (%) | 70 | 46 | <0.0001 |
| BO (%) | 41 | 23 | 0.002 |
| BO before age 55 years (%) | 38 | 22 | 0.01 |
| Hysterectomy only (%) | 19 | 17 | 0.27 |
| Hysterectomy only before 55 years (%) | 18 | 15 | 0.35 |
| Hysterectomy with or without UO/BO prior to age 55 years (%) | 64 | 42 | 0.0004 |
All p-values are age-adjusted, except age.
OC, oral contraceptive; BO, bilateral oophorectomy; CAD, coronary artery disease; SD, standard deviation; UO, unilateral oophorectomy.
Components of total estrogen time and coronary disease
We compared three measures of obstructive CAD across tertiles of the components of total estrogen time (menstrual cycling time, pregnancy time, oral contraceptive time, and postmenopausal HT time; Table 3). There were significant differences across HT groups, with those never using HT having the highest prevalence and severity of CAD, followed by women with up to 5½ years of HT use, with the lowest prevalence and severity found among women using HT for more than 5½ years (p < 0.0001, p < 0.0001, p = 0.002, respectively, when comparing across all tertiles). These results remained significant when adjusted for diabetes and lipid-lowering medication use (p < 0.0001, p = 0.0007, p = 0.014, respectively). No significant differences in CAD were observed across tertiles of the other components of TET or sTET.
Table 3.
Coronary Artery Disease: Age-Adjusted Frequencies and Means ± SD by Tertiles of Components of TET and sTET
| CAD Tertiles | 1st tertile | 2nd tertile | 3rd tertile | pa | pb |
|---|---|---|---|---|---|
| Menstrual cycling time (years) | 4–31 (n = 225) | 31.1–37 (n = 215) | 37.1–46 (n = 206) | ||
| 70% stenosis (%) | 24 | 23 | 29 | 0.64 | 0.15 |
| 50% stenosis (%) | 41 | 37 | 45 | 0.45 | 0.10 |
| Severity Score (±SD) | 15.8 ± 14.3 | 14.6 ± 14.6 | 16.9 ± 16.0 | 0.77 | 0.16 |
| Postmenopausal HT use time (years)c | 0 (n = 282) | 0.1–5.5 (n = 180) | 5.6–41 (n = 184) | ||
| 70% stenosis (%) | 34 | 22 | 14 | <0.0001 | 0.065 |
| 50% stenosis (%) | 50 | 41 | 28 | <0.0001 | 0.017 |
| Severity Score (±SD) | 18.1 ± 17.5 | 14.7 ± 13.0 | 13.2 ± 11.5 | 0.002 | 0.31 |
| Premenopausal OC use time (years)c | 0 (n = 397) | 0.1–2 (n = 133) | 2.1–29 (n = 116) | ||
| 70% stenosis (%) | 28 | 22 | 21 | 0.28 | 0.28 |
| 50% stenosis (%) | 42 | 38 | 40 | 0.54 | 0.84 |
| Severity Score (±SD) | 16.9 ± 16.9 | 13.7 ± 10.2 | 14.0 ± 11.0 | 0.06 | 0.23 |
| Pregnancy time (years) | 0–1.5 (n = 230) | 1.6–2.9 (n = 191) | 3.0–10.8 (n = 225) | ||
| 70% stenosis (%) | 27 | 21 | 28 | 0.26 | 0.34 |
| 50% stenosis (%) | 44 | 36 | 42 | 0.16 | 0.58 |
| Severity Score (±SD) | 16.7 ± 15.1 | 14.2 ± 13.9 | 16.1 ± 16.0 | 0.19 | 0.61 |
Age-adjusted p-values.
Age-adjusted p-values comparing the highest tertile with all others.
More than one third of women had not used HT or OC. For the remaining women, we performed a median split.
CAD, coronary artery disease; SD, standard deviation; HT, hormone therapy; OC, oral contraceptive; TET, total estrogen time; sTET, supra-total estrogen time.
Summarized total estrogen time and coronary disease
When comparing the three measures of CAD across tertiles of the summary scores of lifetime estrogen exposure (TET and sTET; Table 4), we found that women in the third tertile (those with the highest number of years of estrogen exposure [sTET]), had the lowest age-adjusted prevalence and severity of CAD (p = 0.006, p = 0.014, and p = 0.016, for CAD defined as ≥70% stenosis, ≥50% stenosis, or as a severity score, respectively). No consistent difference was found among TET tertiles. To partial out the effect of HT use from TET and sTET, we additionally adjusted for history of HT use (Table 4). When further adjusting the sTET data by years of HT use, all differences became non-significant (p = 0.33, 0.55, 0.19 for 70% stenosis, 50% stenosis, and the CAD severity score, respectively). This suggests that HT was the major driving variable in the relationship of TET and sTET with CAD. Furthermore, TET and sTET did not differ when evaluating only women never on HT. These findings did not change when women with imputed menopausal age were excluded.
Table 4.
Coronary Artery Disease: Age-Adjusted Frequencies and Means ± SD by Tertiles of TET and sTET
| CAD Tertiles | 1st tertile | 2nd tertile | 3rd tertile | pa | pb |
|---|---|---|---|---|---|
| For all women | |||||
| TET (years) | 0–30.3 (n = 214) | 30.4–36.9 (n = 209) | 37–63.8 (n = 223) | ||
| 70% stenosis (%) | 30 | 25 | 21 | 0.12 | 0.10 |
| 50% stenosis (%) | 48 | 38 | 37 | 0.038 | 0.12 |
| Severity Score (±SD) | 16.9 ± 14.8 | 14.6 ± 15.2 | 15.7 ± 15.2 | 0.30 | 0.96 |
| sTET (years) | 4–38 (n = 217) | 38.1–45 (n = 210) | 45.1–71.5 (n = 219) | ||
| 70% stenosis (%) | 27 | 30 | 19 | 0.018 | 0.006 |
| 50% stenosis (%) | 43 | 46 | 35 | 0.041 | 0.014 |
| Severity Score (±SD) | 16.3 ± 15.9 | 17.2 ± 16.4 | 13.8 ± 12.5 | 0.044 | 0.016 |
| For women never on HT | |||||
| TET (years) | 0–28.9 (n = 94) | 29–34.4 (n = 90) | 34.5–43.1 (n = 98) | ||
| 70% stenosis (%) | 41 | 34 | 36 | 0.61 | 0.79 |
| 50% stenosis (%) | 58 | 49 | 54 | 0.47 | 0.94 |
| Severity Score (±SD) | 20.3 ± 17.6 | 17.7 ± 17.2 | 19.5 ± 17.7 | 0.57 | 0.83 |
| sTET (years) | 4–35.4 (n = 93) | 35.5–41 (n = 93) | 41.1–67.2 (n = 96) | ||
| 70% stenosis (%) | 34 | 32 | 44 | 0.21 | 0.08 |
| 50% stenosis (%) | 52 | 51 | 57 | 0.62 | 0.33 |
| Severity Score (±SD) | 19.8 ± 18.8 | 17.9 ± 16.9 | 19.8 ± 17.0 | 0.68 | 0.66 |
CAD, coronary artery disease; SD, standard deviation.
TET (Total Estrogen Time) = (menstrual cycling time [age at last cycle − age at menarche]) − (oral contraceptive use time) − (pregnancy time) + (postmenopausal hormone therapy time).
sTET (supra-Total Estrogen Time) = (menstrual cycling time [age at last cycle − age at menarche]) + (premenopausal oral contraceptive use time) + (pregnancy time) + (postmenopausal hormone therapy time).
Age-adjusted p-values.
Age-adjusted p-values comparing the highest tertile with all others.
Summarized total estrogen time and major adverse cardiovascular events
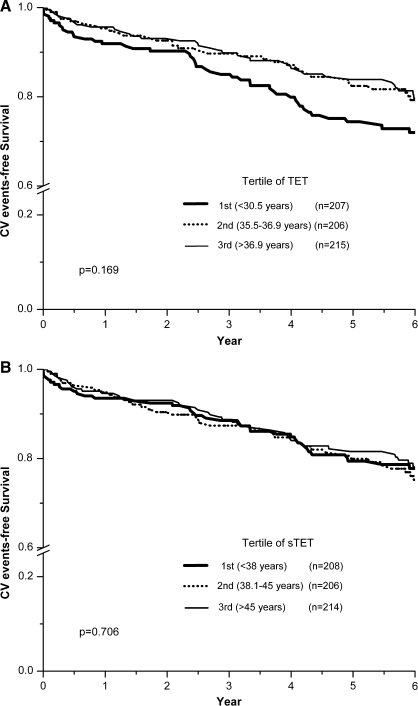
Analysis of the association of summarized total estrogen time (TET and sTET) with major adverse cardiovascular events demonstrated no relationship (p = 0.42 and p = 0.19, respectively). This lack of association was maintained when TET and sTET were log transformed (p = 0.63 and p = 0.08, respectively) or when adjusting for other covariables. Similarly, no differences in cardiovascular event rates were found among tertiles of TET or sTET (log-rank p = 0.17 and p = 0.71, respectively; Fig. 1). Assessment by quartiles did not alter this finding. Given that the two upper tertiles of TET virtually overlap, we also compared the 1st tertile with the 2nd and 3rd tertiles combined. However, only a borderline association was found (log-rank p = 0.075). A similar lack of association was found for sTET (p = 0.47).
FIG. 1.
Kaplan-Meier curves for major adverse cardiovascular events according to tertile of summarized total estrogen time in 654 postmenopausal women. (A) TET (1st tertile, <37 years; 3rd tertile, >44.4 years). (B) sTET (1st tertile, <38 years; 3rd tertile, >45 years).
Discussion
Our results show that women with the highest number of years of sustained estrogen exposure (sTET but not TET) had a lower prevalence and severity of obstructive CAD. However, this relationship disappeared after adjusting for menopausal HT use. Similarly, summarized estrogen exposure time was not related to major adverse cardiovascular events. Our analyses suggest that HT was the major driving variable in the relationship of TET and sTET with CAD. These results in a relatively large sample of women with carefully analyzed coronary angiograms hint that the paradigm of estrogen protection from CAD in women may be more complex than simple estrogen time exposure. Our findings are consistent with a prior study using a composite measure of endogenous estrogen exposure time which found that such an index failed to add to the predictive value of age at menopause for cardiovascular mortality.18
Our findings are consistent with prior epidemiological studies that have also failed to demonstrate a role of endogenous estrogen-related reproductive factors in CAD.12 While prior clinical trials have failed to provide benefit in older postmenopausal women,7–11 more recent analyses suggest that younger women may benefit from postmenopausal exogenous hormone therapy.19–21 Our results also demonstrate that the use of HRT for longer period of 5 years was associated with reduced CVD risk. Randomized trials using intermediate outcomes or subgroup analyses of larger trials are also suggestive of a benefit of exogenous hormone therapy.22–24 Prior studies had insufficient time periods to examine past oral contraceptive use and CAD, due to the relatively prolonged onset of atherosclerosis over decades combined with a relatively short recent population exposure time to oral contraceptives. No randomized clinical oral contraceptive trials using CAD outcomes exist.
Other evidence has shown that surgically induced early menopause with bilateral oophorectomy is a risk factor for CAD.25 However, in the present study, we found that women with bilateral oophorectomy were less likely to have CAD, overall or before age 55, compared to women without bilateral oophorectomy. Since the majority (77%) of the WISE women who underwent bilateral oophorectomy were also treated with HT, it is possible that such treatment may have counteracted the potentially detrimental effect of oophorectomy.
The current WISE methods which include a detailed reproductive medical history in a relatively large contemporary cohort of women with quantitative coronary angiography and follow-up for major adverse cardiovascular events provides an opportunity to evaluate the effect of endogenous and exogenous estrogen exposure. The current results failing to find an anti-atherosclerotic association with total estrogen time contrast with the current and prior reports from the WISE study, which suggested a beneficial anti-atherosclerotic impact of prior exogenous oral contraceptive use.26 This discrepancy raises questions whether the putative benefits of estrogen may be related to exogenous treatment as opposed to endogenous levels, treatment bias, or alternative explanations.
Among the WISE postmenopausal women without a history of hysterectomy or oophorectomy, women who smoked became menopausal at a younger age compared to non-smokers suggesting that a harmful cardiovascular risk profile may accelerate menopause. These results further support the hypothesis proposed by Kok et al., that heart disease risk factors, including serum lipids, smoking, and blood pressure, contribute to determine the age of menopause,27 although the reverse may alternatively be possible. Further work is needed.
Limitations
Some limitations of this study deserve consideration. The current study results are limited by the observational design that precludes inference regarding causality between total estrogen exposure or HT use and angiographic CAD. Also there is a possibility of residual confounding by other risk factors such as diabetes and lipid-lowering medication or potential interaction between hormonal factors and risk factors. However, our results across tertiltes of HT use remained consistent when we adjusted for these potential confounders in the logistic regression model. Third, the study design does not allow detection of early adverse cardiovascular events due to HT, as observed in prospective, randomized trials.8,9 Fourth, due to the strong correlation between OC use and HT use (72% with a history of OC use also used HT at some time), our results are likely confounded with regard to OC and HT use, therefore it is difficult to provide clear associations. Fifth, our study's total estrogen exposure time results may be limited by historical reporting inaccuracies or the fact that these measures are relatively crude estimate of estrogen exposure. These summarized estimates also do not take into account factors such as elements of menstrual cycling quality, non-ovulatory (low estrogen) cycling, variations in endogenous postmenopausal blood estrogen levels due to body mass (converted in peripheral tissues), the types of estrogen and progestin used in the oral contraceptives, or the type and dose of exogenous HT used. However, according to an analysis of our limited data regarding type of exogenous HT use and also considering the age of our cohort, the majority of exogenous estrogen exposure was oral conjugated equine estrogen and ethinyl estradiol. While additional detail regarding estrogen exposure could be valuable, to our knowledge, no other studies have been performed using this or a comparable algorithm; thus, this is exploratory work. Our algorithm has not yet been validated in other studies; however, future study is needed to improve it by collecting more precise measurements of estrogen exposure. The algorithm should also be validated for other estrogen-related diseases such as breast cancer or osteoporosis. Finally, based on the characteristics of the WISE study population, our conclusions are not representative of the general population of women but are limited to postmenopausal women with chest pain symptoms and undergoing coronary angiography for evaluation of suspected ischemia.
Conclusions
There was no independent relation of estrogen exposure time to angiographic CAD or major adverse cardiovascular events in a contemporary cohort of postmenopausal women evaluated for suspected ischemia. Our results suggest that the paradigm of estrogen protection from CAD in women may be more complex than estrogen exposure duration. The current analysis sheds light on prior data discrepancies, and may be useful for prospective HT clinical trial planning.
Acknowledgments
This work was supported by the National Heart, Lung and Blood Institutes (contracts N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164), the National Center for Research Resources (GCRC grant MO1-RR00425), and the Gustavus and Louis Pfeiffer Research Foundation (Denville, NJ), The Women's Guild of Cedars-Sinai Medical Center (Los Angeles, CA), and The Ladies Hospital Aid Society of Western Pennsylvania (Pittsburgh, PA), and the Edythe Broad Women's Heart Disease Research Endowment, Cedars-Sinai Medical Center (Los Angeles, CA).
Disclosure Statement
No competing financial interests exist.
References
- 1.DeStefano F. Merritt RK. Anda RF, et al. Trends in nonfatal coronary heart disease in the United States, 1980 through 1989. Arch Intern Med. 1993;153:2489. [PubMed] [Google Scholar]
- 2.Adams MR. Kaplan JR. Manuck SB, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys. Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 3.Colditz GA. Willett WC. Stampfer MJ, et al. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 4.Grodstein F. Manson JE. Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 5.Grodstein F. Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Prog Cardiovasc Dis. 1995;38:199. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 6.Wagner JD. Clarkson TB. St. Clair RW et al. Estrogen and progesterone replacement therapy reduces low density lipoprotein accumulation in the coronary arteries of surgically postmenopausal cynomolgus monkeys. J Clin Invest. 1991;88:1995. doi: 10.1172/JCI115526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrington DM. Reboussin DM. Brosnihan KB, et al. Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med. 2000;343:522. doi: 10.1056/NEJM200008243430801. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S. Grady D. Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 9.Rossouw JE. Anderson GL. Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 10.Viscoli CM. Brass LM. Kernan WN, et al. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 11.Waters DD. Alderman EL. Hsia J, et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 12.Barrett-Connor E. Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265:1861. [PubMed] [Google Scholar]
- 13.Merz CN. Kelsey SF. Pepine CJ, et al. The Women's Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnson BD. Merz CN. Braunstein GD, et al. Determination of menopausal status in women: the NHLBI-sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2004;13:872. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 15.Anderson DC. Hopper BR. Lasley BL, et al. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28:179. doi: 10.1016/0039-128x(76)90108-2. [DOI] [PubMed] [Google Scholar]
- 16.Sharaf BL. Pepine CJ. Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001;87:937. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan JM. Vander Zwaag R. Lemp GF, et al. Postmenopausal estrogen use and coronary atherosclerosis. Ann Intern Med. 1988;108:358. doi: 10.7326/0003-4819-108-3-358. [DOI] [PubMed] [Google Scholar]
- 18.de Kleijn MJ. van der Schouw YT. Verbeek AL, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 19.Grodstein F. Manson JE. Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses' health study. a prospective, observational study. Ann Intern Med. 2001;135:1. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- 20.Rossouw JE. Prentice RL. Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 21.Salpeter SR. Walsh JM. Greyber E, et al. Brief report: Coronary heart disease events associated with hormone therapy in younger and older women. A meta-analysis. J Gen Intern Med. 2006;21:363. doi: 10.1111/j.1525-1497.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brownley KA. Hinderliter AL. West SG, et al. Cardiovascular effects of 6 months of hormone replacement therapy versus placebo: differences associated with years since menopause. Am J Obstet Gynecol. 2004;190:1052. doi: 10.1016/j.ajog.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Hodis HN. Mack WJ. Azen SP, et al. Hormone therapy and the progression of coronary-artery atherosclerosis in postmenopausal women. N Engl J Med. 2003;349:535. doi: 10.1056/NEJMoa030830. [DOI] [PubMed] [Google Scholar]
- 24.Hsia J. Langer RD. Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women's Health Initiative. Arch Intern Med. 2006;166:357. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 25.Gohlke-Barwolf C. Coronary artery disease–is menopause a risk factor? Basic Res Cardiol. 2000;95(Suppl 1):I77. doi: 10.1007/s003950070014. [DOI] [PubMed] [Google Scholar]
- 26.Merz CN. Johnson BD. Berga S, et al. Past oral contraceptive use and angiographic coronary artery disease in postmenopausal women: data from the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation. Fertil Steril. 2006;85:1425. doi: 10.1016/j.fertnstert.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Kok HS. van Asselt KM. van der Schouw YT, et al. Heart disease risk determines menopausal age rather than the reverse. J Am Coll Cardiol. 2006;47:1976. doi: 10.1016/j.jacc.2005.12.066. [DOI] [PubMed] [Google Scholar]