Summary
Mammary gland development is a complex process that is dependent on interactions between the developing mammary epithelium and the surrounding stromal tissues. We show that mice lacking the triglyceride synthesis enzyme acyl CoA:diacylglycerol transferase 1 (DGAT1) have impaired mammary gland development, characterized by decreased epithelial proliferation and alveolar development, and reduced expression of markers of functional differentiation. Transplantation studies demonstrate that the impaired development results from a deficiency of DGAT1 in both the stromal and epithelial tissues. Our findings are the first to link defects in stromal lipid metabolism to impaired mammary gland development.
Keywords: Mammary gland, Acyl CoA:diacylglycerol acyl transferase, Lipid, Triacylglycerol
Introduction
Mammary gland development is a complex process resulting in lactation. Normal development occurs through the influences of systemic hormonal factors and locally acting growth factors that cause the mammary epithelium to proliferate and differentiate upon conception. The mammary epithelium grows and branches until mid-pregnancy, and differentiates functionally during late pregnancy and the early postpartum period. This epithelial differentiation is characterized by the expression of milk protein genes, such as whey acidic protein (Wap) and β-casein, and by the production of milk droplets (Robinson et al., 1995). Milk is rich in triglycerides, which provide concentrated sources of energy and essential fatty acids for newborn offspring.
There is increasing evidence that interactions between the mammary epithelium and surrounding stroma are crucial for normal mammary gland development. The stroma is a complex tissue composed of myoepithelial cells, extracellular matrix components within the basement membrane, fibroblasts, and adipocytes. Several studies showed that extracellular matrix components (Wiseman and Werb, 2002) and stromal fibroblasts (Darcy et al., 2000; Lukashev and Werb, 1998) can influence epithelial development. However, the role of adipocytes, the most abundant cell type in the stroma, in mammary gland development is poorly understood. Mammary gland adipocytes have been hypothesized to serve as a source of growth factors and lipids that may influence mammary epithelium development and function (Hovey et al., 1999). Co-culture of mammary epithelial cells with 3T3L1-derived adipocytes (Levine and Stockdale, 1985; Wiens et al., 1987), or with isolated rat mammary adipocytes (Zangani et al., 1999), enhances differentiation and milk protein expression. In mice that lack adipose tissue, the proliferation of mammary epithelium is impaired, although differentiation proceeds normally (Couldrey et al., 2002).
Stromal adipocytes synthesize and store large amounts of triglycerides, which may serve as reservoirs of substrates for milk production by the mammary epithelium. During this process, adipocyte triglycerides must be hydrolyzed and the fatty acids transferred to the epithelial cells for re-esterification. Triglyceride synthesis is catalyzed by acyl CoA:diacylglycerol acyltransferase (DGAT) enzymes, which covalently join diacylglycerol with fatty acyl CoA (Bell and Coleman, 1980; Brindley, 1991; Lehner and Kuksis, 1996). Studies from our group have identified genes encoding two mammalian DGAT enzymes, DGAT1 and DGAT2 (Cases et al., 1998; Cases et al., 2001). The Dgat1 gene is expressed in nearly all tissues, including the mammary glands (Farese et al., 2000). Mice lacking DGAT1 (Dgat1−/−) have decreased triglyceride content in tissues, and are resistant to diet-induced obesity and diabetes mellitus (Chen et al., 2002; Smith et al., 2000). In addition, female Dgat1−/− mice are unable to lactate (Smith et al., 2000). This result was surprising because other mouse knockout models that are deficient in protein components of milk are capable of producing milk with an altered composition. For example, α-lactalbumin-deficient mice (Stacey et al., 1994; Stinnakre et al., 1994) produce highly viscous milk containing little or no lactose, and β-casein-deficient mice (Kumar et al., 1994) produce milk with a reduced total protein concentration. This suggests that the lactation defect in Dgat1−/− mice might result from impaired mammary gland development.
In the present study, we characterized mammary gland development in Dgat1−/− mice at different stages, and used tissue transplantation techniques to determine whether the expression of DGAT1 was required in extraglandular, stromal and epithelial compartments. Our results indicate that DGAT1 plays important functional roles in both the mammary epithelial and stromal compartments during pregnancy.
Materials and methods
Mice
Dgat1−/− mice (Smith et al., 2000) were backcrossed into the C57BL/6J strain for 10 generations. Mice were housed in a pathogen-free barrier facility (12-hour light/12-hour dark cycle) and fed a standard chow diet. All experiments were approved by the Committee on Animal Research of the University of California, San Francisco. For timed pregnancies, male and female mice were mated overnight, and female mice were examined for vaginal plugs the next morning. The presence of a vaginal plug was considered to represent pregnancy day 0.5.
Antibodies
Mouse monoclonal antibodies for E-cadherin, smooth muscle actin and β-catenin were from Transduction Laboratories and Pharmingen (San Diego, CA). Rabbit polyclonal antibody for STAT5A (L-20) and mouse monoclonal antibody for phosphotyrosine (PY99) were from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal antibodies for NKCC1 (sodium-potassium-chloride co-transporter characteristic for ductal epithelium), NPT2B (sodium-phosphate co-transporter isoform characteristic for lactating epithelium) and WAP have been described (Miyoshi et al., 2001; Shamay et al., 1992).
Histological and whole-mount analyses
For histology, inguinal mammary glands were removed from wild-type and Dgat1−/− mice on day 18 of pregnancy (P18) or day 1 of lactation (L1), fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) at 4°C overnight, washed for 15 minutes in PBS and embedded in paraffin. Tissue sections (6 μm) were stained with Hematoxylin and Eosin, and mounted on pre-treated glass slides (Superfrost Plus Micro Slides, VWR).
For whole-mount analyses, mammary glands (3 or 4) were removed at different times during pregnancy and immediately spread onto glass slides. The glands were then immersed in Carnoy’s fixative (3:1, ethanol:glacial acetic acid) overnight, washed in 70% ethanol for 15 minutes, washed under running tap water for 5 minutes, drained and then stained overnight with Alum Carmine stain [2 g/l carmine dye (C6152, Sigma, St Louis, MO) and 5 g/l aluminum potassium sulfate in distilled water]. The glands were then dehydrated by three 15-minute immersions in 70%, 90% and 100% ethanol, and the lipids were removed by a 15-minute immersion in xylenes. The tissues were stored in methyl salicylate (Sigma).
For neutral lipid staining, inguinal mammary glands were fixed with 2% glutaraldehyde/2% paraformaldehyde, stained in 1% osmium tetroxide in sodium phosphate buffer (0.1 M, pH 7.4), dehydrated in a series of ethanols, transitioned into propylene oxide, and embedded in Epon resin. Sections (1 μm) were cut with a glass knife and stained in warm Toluidine Blue.
Image analysis
For quantification of epithelial areas, images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) and converted into a binary format with Adobe Photoshop 5.0.1 (Adobe Systems, San Jose, CA) and the Image Processing Tool Kit (Reindeer Games, Gainesville, FL). The binary black and white images were compared with the original images to ensure an accurate conversion. Areas (mm2) were calculated with the command ‘Measure All’, and epithelial and lumen areas were then expressed as percentage of total area.
Immunohistochemistry and analysis of proliferation and apoptosis
NKCC1, WAP and NPT2B were detected as described (Shillingford et al., 2003; Shillingford et al., 2002). Briefly, paraffin-embedded mammary tissue sections were cleared in xylene and rehydrated. Antigen was retrieved by heat treatment using an antigen-masking solution (1:100 dilution; Vector Laboratories, Burlingame, CA), and tissue sections were blocked for 30 minutes in PBS with 0.05% Tween-20 (PBST) containing 3% goat serum.
Sections were incubated with solutions containing various combinations of diluted primary antibodies for smooth muscle actin (1:1000), NKCC1 (1:1000), β-catenin (1:100), WAP (1:600), NPT2B (1:200) and E-cadherin (1:100). After 30 minutes at 37°C, the slides were rinsed in PBST to remove nonspecific antibody binding. The primary antibodies were detected by incubation with either anti-mouse FITC-conjugated (1:400) or anti-rabbit Texas Red-conjugated (1:400) secondary antibodies (Molecular Probes, Eugene, OR) for 30 minutes at room temperature in the dark. Sections were then washed twice in PBST and mounted in Vectashield (Vector Laboratories), and fluorescence was visualized with an Olympus BX51 microscope equipped with DAPI, FITC, TRITC and FITC:TRITC filters. Images were captured with a Nikon 1200DXM digital camera (Nikon) and composed in Adobe PhotoShop. Three mice of each genotype were analyzed.
For analysis of cellular proliferation, mice at P15 were injected with bromodeoxyuridine (BrdU) (Amersham Biosciences, Piscataway, NJ) (1 mg/10 g body weight) 2 hours before dissection of mammary glands (4). After paraffin embedding and sectioning of glands, BrdU was detected with a biotinylated mouse anti-BrdU antibody (Zymed Laboratories, South San Francisco, CA) according to the manufacturer’s instructions. Images of the sections were captured, and epithelial areas were determined as described earlier in ‘Image analysis’. Positive nuclei were counted in five random fields per mammary gland and their number was expressed per unit of epithelial area. Four to five mice of each genotype were analyzed.
For analysis of apoptosis, mammary glands were dissected at P18, and sections were prepared as described above. Apoptotic cells were detected by terminal deoxynucleotide UTP nick-end labeling (TUNEL), using an in situ cell death detection kit (Roche Molecular Biochemicals, Indianapolis, IN) according to the manufacturer’s instructions. Nuclei were counterstained with DAPI. TUNEL-positive epithelial nuclei were counted in 10 random microscopic fields, and expressed as a percentage of DAPI-stained nuclei. Four mice of each genotype were analyzed.
RNA analyses
Total RNA from wild-type and Dgat1−/− mammary glands from virgin or pregnant mice was extracted with Trizol (Invitrogen, Carlsbad, CA). For northern blots, samples of total RNA (10 μg) were separated by gel electrophoresis, transferred to membranes, and hybridized with 1 ng of [γ-32P]dATP-labeled oligonucleotide probes in 0.4 M NaCl, 1% SDS, 1×Denhardt’s solution, and salmon sperm DNA. Membranes were washed twice for 15 minutes in 1×SSC and 0.1% SDS, and twice for 15 minutes each in 0.2×SSC and 0.1% SDS. Membranes were stripped and rehybridized with a 655-bp [α-32P]dCTP-labeled DNA probe for mouse keratin 18 (K18). WAP oligonucleotide was 5′-CAACGCATGGTACCGGTGTCA-3′, and β-casein oligonucleotide was 5′-GTCTCTCTTGCAAGAGCAAGGGCC-3′. The template for synthesizing the K18 probe was obtained by amplifying mouse mammary cDNA with the following primers: sense, 5′-AGACC-GAGAAAGAGACCATGCAAG-3′; antisense, 5′-GAGACCACA-CTCACGGAGCTGA-3′.
Immunoblot analysis and immunoprecipitation
Mammary glands were homogenized in lysis buffer (5 ml/g of tissue), containing 40 mM Tris-HCl (pH 7.5), 275 mM NaCl, 20% glycerol, 2% Nonidet P-40, 4 mM EDTA, and a cocktail of protease and phosphatase inhibitors (Calbiochem, San Diego, CA). Lysate (500 μl) containing 1.5 mg total protein was used for immunoprecipitation for 1 hour at 4°C with 1 μl of rabbit polyclonal anti-STAT5A antibody. Immunocomplexes were captured overnight with 50 μl of protein A-Sepharose beads (Pharmacia Biotech, Piscataway, NJ), washed three times with lysis buffer, and resuspended in 2×sample buffer (Bio-Rad). Samples were boiled for 5 minutes, centrifuged briefly, and analyzed by gel electrophoresis using 10% SDS-PAGE pre-cast gels (Bio-Rad). Separated proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Membranes were blocked with 5% milk in PBS for 1 hour at room temperature (RT), incubated with anti-STAT5A (1:500) or mouse monoclonal anti-phosphotyrosine (PY99, 1:500) antibodies for 2 hours at room temperature, and washed three times for 15 minutes each with 0.05% Tween-20 in PBS. The blots were then incubated for 1 hour at room temperature with horseradish peroxidase-conjugated rabbit IgG (1:2000), washed three times for 5 minutes each with 0.05% Tween-20 in PBS, incubated for 1 minute with enhanced chemiluminescence substrate (Pierce Biotechnology, Rockford, IL) and exposed to X-ray film.
Mammary tissue transplantations
For whole mammary gland transplantations, the inguinal mammary glands from 8- to 10-week-old mice were removed and transplanted into the subcutaneous space in the interscapular region of 8- to 10-week-old recipient female mice, in a manner similar to that described for transplantation of the reproductive fat pad (Chen et al., 2003; Gavrilova et al., 2000). Two weeks after surgery, the recipient mice were mated. Endogenous and transplanted mammary glands were removed at L1 for histological analyses.
Mammary epithelial transplantations were performed essentially as described (Young, 2000). The nipple region of a mammary gland (4) containing epithelial structures was removed under anesthesia from 21-day-old mice, and the cleared fat pad was immediately implanted with a small piece (0.5–1.0 mm diameter) of donor mammary gland containing epithelium from 8- to 10-week-old virgin mice. To assess complete clearing of the recipient mammary fat pad, the surgically removed mammary tissue was used for whole-mount staining. The contralateral mammary fat pad served as an unoperated control. Transplanted mice were mated 5–7 weeks after surgery. Recipient unoperated control and transplanted mammary glands were removed at L1 for histological analyses.
Results
Lactation is defective in female Dgat1−/− mice
As reported previously (Smith et al., 2000), Dgat1−/− female mice failed to lactate. Milk production from Dgat1−/− female mice was totally absent, even after stimulation by oxytocin injection or by a second pregnancy. Dgat1+/− female mice lactated normally. Pups born to Dgat1−/− female mice died shortly after birth, apparently from starvation as no milk stripes were seen in their stomachs. By contrast, pups fostered to lactating wild-type mothers suckled and developed normally.
Impaired mammary gland development during pregnancy in Dgat1−/− mice
Defective milk production can result from various developmental and functional abnormalities in the mammary gland. To investigate the cause of the lactation defect in Dgat1−/− mice, we first examined the morphology of the mammary ductal tree in virgin mice. Whole-mount analyses (not shown) and immunostaining for the sodium-potassium-chloride co-transporter NKCC1 (Fig. 1A), a marker for ductal epithelial cells, revealed no differences in mammary structures in wild-type and Dgat1−/− virgin mice. No gross abnormalities were observed in the mammary fat pads, and Dgat1−/− mammary adipocytes appeared morphologically normal as assessed by histological techniques. The weights of Dgat1−/− mammary fat pads were similar to those of wild-type mice during pregnancy, but were reduced at L1 (Fig. 1B), probably reflecting their lack of milk production. These data suggest that the impairment in development occurred during pregnancy.
Fig. 1.
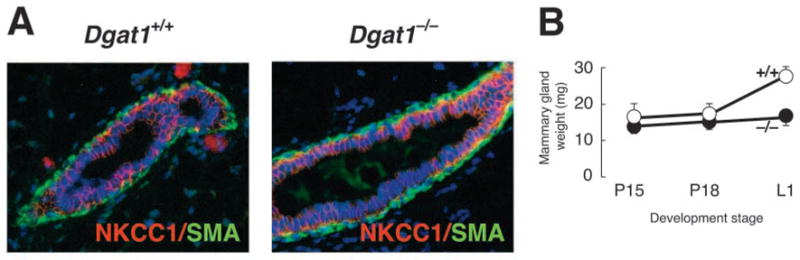
Characterization of mammary glands of virgin and pregnant Dgat1−/− mice. (A) Immunohistochemistry of mammary glands from 8-week-old virgin female Dgat1+/+ and Dgat1−/− mice. After fixation, mammary tissue was embedded in paraffin, sectioned, and stained with antibodies specific for the ductal cell marker NKCC1 (red) and smooth muscle actin (SMA, green). (B) Weights of inguinal mammary glands from wild-type and Dgat1−/− mice during pregnancy.
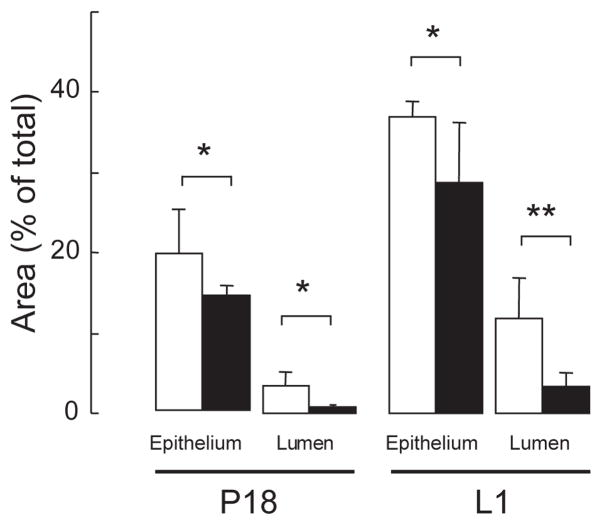
To determine the stage at which mammary gland development goes awry in Dgat1−/− mice, we analyzed whole mounts and histological sections of wild-type and Dgat1−/− mammary glands at different times during pregnancy. At P8, ductal side branching and formation of the initial alveolar buds appeared normal in Dgat1−/− mammary glands (data not shown). However, at P15, Dgat1−/− mammary glands exhibited a slight decrease in ductal structures, suggesting a developmental delay (Fig. 2A,B). By P18, lobuloalveolar structures were clearly less developed in Dgat1−/− mammary glands than in wild-type glands, as assessed in whole mounts (Fig. 2C,D) and histological sections (Fig. 2E,F). This difference was also observed in glands on L1 (Fig. 2G,H). Quantification of areas in histological sections of P18 and L1 Dgat1−/− mammary glands revealed a ~25% reduction in epithelial structures and a ~60% reduction in luminal expansion (Fig. 3).
Fig. 2.
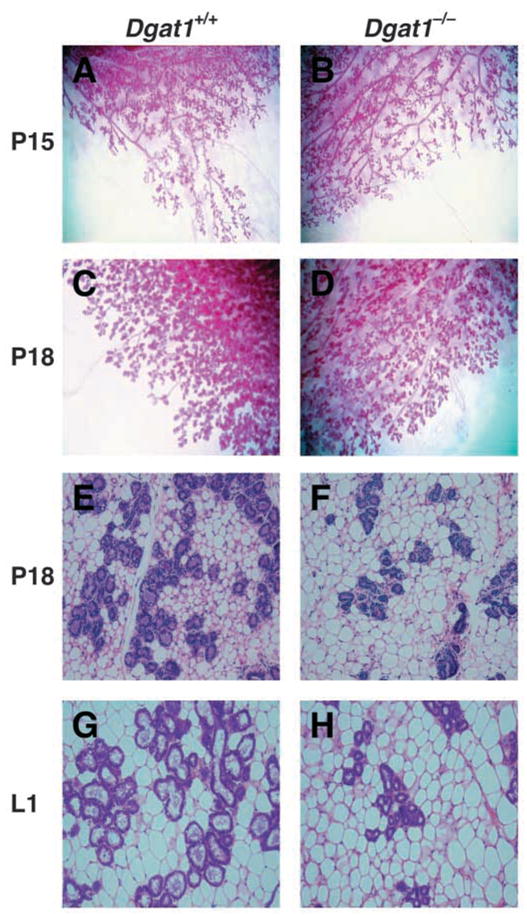
Reduced alveolar development in mammary glands of Dgat1−/− mice. Whole mounts (A–D) and histological sections (E–H) of mammary glands of wild-type and Dgat1−/− mice at P15, P18 and L1. At least five mice of each genotype were analyzed. Representative sections are shown.
Fig. 3.
Reduced alveolar development in mammary glands of Dgat1−/− mice. Epithelial and luminal areas were quantified in Dgat1+/+ (white bars) and Dgat1−/− (black bars) mammary glands using digitalized images of the histology sections at P18 and L1. Area was quantified in five random fields. n=4 Dgat1+/+ mice; n=5 Dgat1−/− mice. *P<0.05; **P<0.01
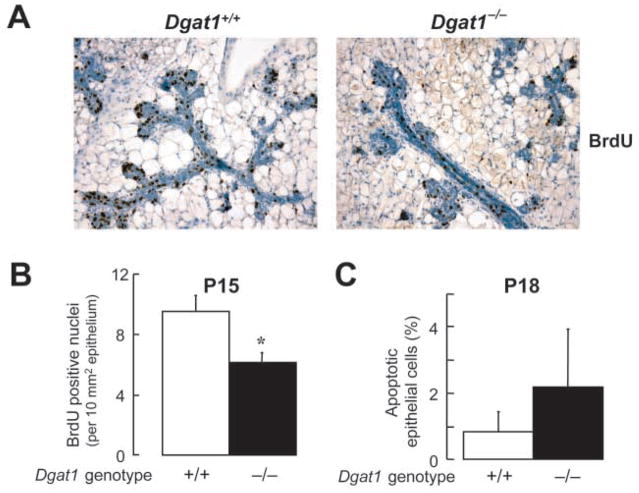
The decrease in alveolar development could arise from decreased proliferation, increased apoptosis, or both. BrdU labeling revealed a 40% reduction in proliferating nuclei in Dgat1−/− mammary epithelium at P15 (Fig. 4A,B). By contrast, staining for TUNEL-positive cells was similar in wild-type and Dgat1−/− mammary glands at P18 (Fig. 4C), indicating that increased apoptosis was not a major cause of the impaired lobuloalveolar development. A low percentage of epithelial cells were apoptotic, similar to that previously described in late pregnancy in mice (Alexander et al., 1996; Dupont et al., 2002).
Fig. 4. Reduced epithelial proliferation in Dgat1−/− mammary glands during pregnancy.
(A) Bromodeoxyuridine (BrdU) immunostaining. Pregnant (P15) mice were injected with BrdU 2 hours before mammary glands were removed. Tissues were fixed, embedded and sectioned, and proliferating nuclei were detected by BrdU immunostaining (dark brown staining).
(B) Quantification of BrdU-positive nuclei. Epithelial areas were calculated using images of histological sections (see Materials and methods), and positive nuclei from five random fields were counted and expressed per unit of area (*P<0.05, n=4 mice per genotype). The expression of BrdU-positive nuclei as a percentage of total epithelial nuclei showed a similar reduction in proliferating nuclei (9.76±1.02% for Dgat1−/− versus 17.82±0.3% for wild type, P=0.002, n=3).
(C) Similar levels of apoptosis were seen in mammary glands of wild-type and Dgat1−/− mice at P18. Apoptotic epithelial cells were detected by TUNEL staining and were counted in 10 random fields. n=5 mice per genotype.
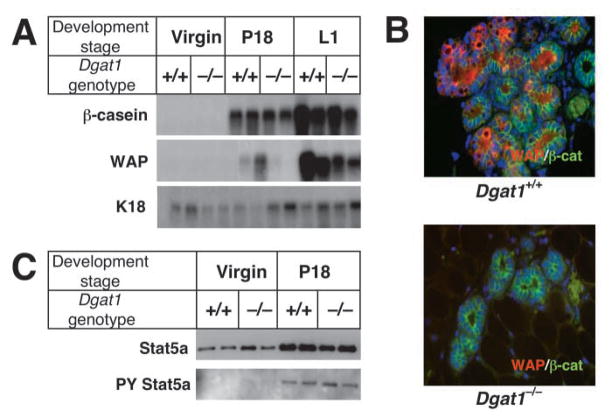
We next assessed the differentiation status of Dgat1−/− mammary glands in late pregnancy by analyzing the expression of the milk protein genes β-casein and Wap. In northern blots, the expression of β-casein, an early differentiation marker in Dgat1−/− mammary gland at P18 and L1, was similar to that in wild-type glands (Fig. 5A). Wap, a late differentiation marker, was not expressed in Dgat1−/− mammary glands at P18, and was reduced at L1 (Fig. 5A). This reduction in WAP protein in Dgat1−/− mammary glands was confirmed by immunostaining of sections from P18 mice (Fig. 5B).
Fig. 5.
Impaired differentiation of mammary epithelium in Dgat1−/− mice. (A) Reduced expression of markers of mammary epithelial differentiation in Dgat1−/− mice. Total mammary gland RNA was extracted from wild-type and Dgat1−/− virgin mice, and at P18 and L1. Expression of the milk protein genes β-casein and Wap was analyzed by northern blotting. The expression of keratin 18 (K18) was used as a control for the content of RNA from mammary epithelium. (B) Reduced immunostaining of WAP in Dgat1−/− mammary glands. Immunostaining of β-catenin (β-cat) was used as control. (C) Similar STAT5A expression and phosphorylation were observed in wild-type and Dgat1−/− mammary glands. Glands from virgin mice and from mice at P18 were dissected, and homogenates were prepared. STAT5A was immunoprecipitated from these samples and analyzed by immunoblotting with antibodies that detect STAT5A or phosphorylated tyrosine residues (PY).
Because the STAT5A transcription factor plays a central role in mammary epithelial differentiation during pregnancy (Liu et al., 1997; Miyoshi et al., 2001), we analyzed STAT5A expression and phosphorylation in Dgat1−/− mammary glands (Fig. 5C). The expression and phosphorylation of STAT5A increased to similar levels by P18 in mice of both genotypes, suggesting that decreased STAT5A activity was not responsible for the impaired mammary epithelium differentiation in Dgat1−/− mice.
DGAT1 is required in both the mammary epithelial and extraepithelial tissues for normal development
The defect in lobuloalveolar development in pregnant Dgat1−/− mice could be due to the lack of DGAT1 function in the mammary gland or to changes in systemic factors. To differentiate between these possibilities, we transplanted whole mammary glands into the interscapular region of recipient mice and analyzed the endogenous control mammary glands and the transplants at L1 (Fig. 6). Twenty mice received transplants during the study, and 18 had viable grafts, as judged by visual inspection at sacrifice and by histology. In control experiments, wild-type endogenous glands (Fig. 6A) were indistinguishable from wild-type transplants (Fig. 6B). The endogenous Dgat1−/− mammary glands were underdeveloped and lacked milk secretion (Fig. 6C), whereas wild-type mammary glands transplanted into Dgat1−/− recipient mice exhibited fully developed lobuloalveolar structures and visible milk droplets (Fig. 6D). Conversely, Dgat1−/− mammary glands transplanted into Dgat1+/+ recipient mice failed to develop normally (Fig. 6F). These results suggest that local DGAT1 expression in the mammary gland is sufficient for normal mammary gland development.
Fig. 6.
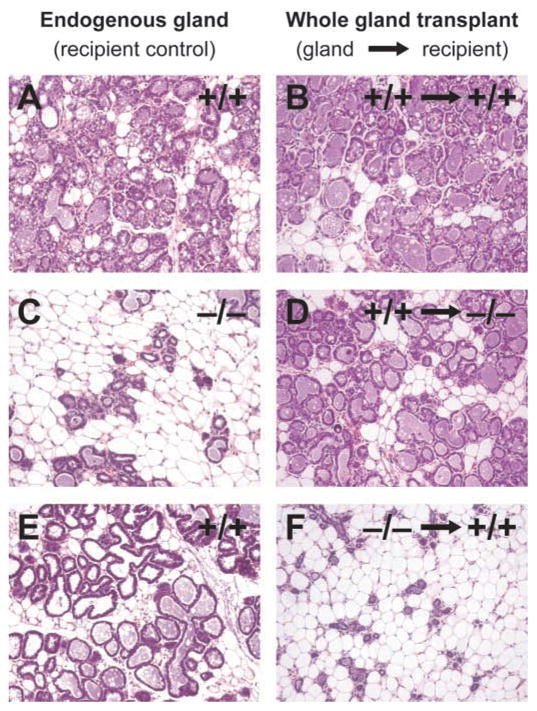
Whole mammary gland transplantations. Whole mammary glands were transplanted into the intrascapular region of recipient mice, and sections were analyzed at L1. In control experiments, whole wild-type mammary gland transplants (B) developed to the same extent as wild-type endogenous glands (A). Although control recipient Dgat1−/− glands failed to develop (C), wild-type whole mammary glands transplanted into the same Dgat1−/− recipients developed normally (D). In the converse experiment, control endogenous Dgat1+/+ glands developed normally (E), whereas Dgat1−/− mammary glands transplanted into the Dgat1+/+ recipients failed to develop (F).
To assess the requirement for DGAT1 expression in the mammary epithelium or stroma, we transplanted mammary epithelium into the cleared mammary fat pads of recipient mice and analyzed epithelial development by whole mounts and histology at P18 or L1. In 24 out of 25 mice, the grafted epithelium colonized the recipient mammary fat pad. In control experiments, wild-type mammary epithelium transplanted into cleared Dgat1+/− fat pads exhibited normal lobuloalveolar development during pregnancy, similar to that of unoperated Dgat1+/− endogenous control glands at P18 (Fig. 7A,B). By contrast, wild-type mammary epithelium transplanted into cleared fat pads of Dgat1−/− littermates failed to develop normally. The lobuloalveolar structures were underdeveloped (Fig. 7C–F) and poorly differentiated (Fig. 7G,H), the lumina were reduced or absent, and the epithelium lacked visible milk droplets. These results indicated that DGAT1 expression in the mammary stroma is crucial for normal epithelial development during pregnancy.
Fig. 7.
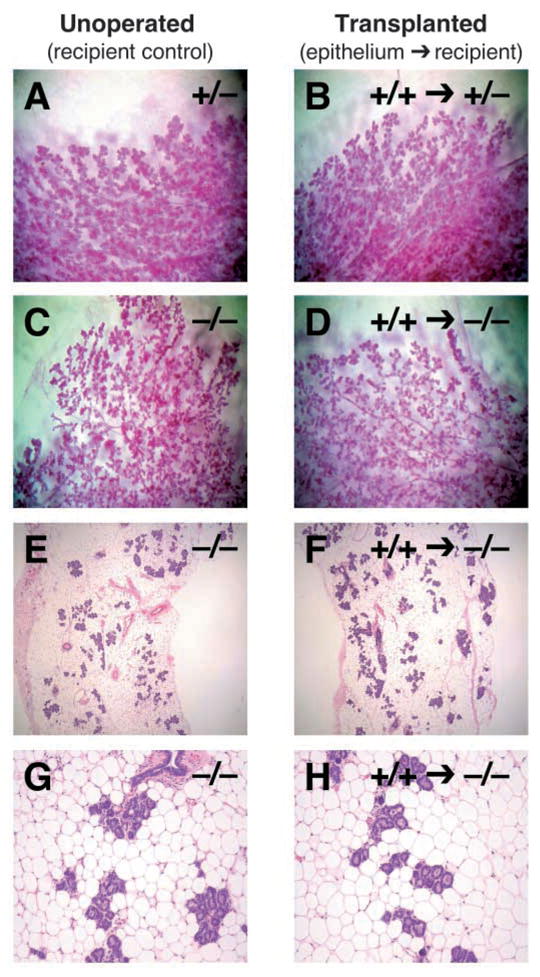
Transplantation of Dgat1+/+ mammary epithelium into Dgat1−/− recipient mice. Recipient mammary glands were cleared of endogenous epithelium and then implanted with donor epithelium. Recipient mice were mated, and epithelial morphology was analyzed at P18 by wholemounts (A-D) or histology (E-H). Shown are recipient (unoperated) control glands (A,C,E,G) and recipient glands that received transplanted epithelium (B,D,F,H). Control transplantations are shown for comparison (A,B).
To determine whether DGAT1 plays a role in epithelium during mammary gland development, we transplanted Dgat1−/− mammary epithelium into cleared mammary fat pads of wild-type mice and analyzed them histologically at L1. The extent of lobuloalveolar development and alveolar expansion was similar in the unoperated wild-type or Dgat1−/− recipient glands, in recipient glands transplanted with control Dgat1+/+ mammary epithelium, and in those transplanted with Dgat1−/− mammary epithelium (Fig. 8A–F), indicating that DGAT1 expression in extra-epithelial tissues was sufficient for these aspects of development. However, milk droplets were not detected in the transplanted Dgat1−/− epithelium by histology (Fig. 8F,H) or by osmium tetroxide staining for neutral lipids (Fig. 9A), suggesting that DGAT1 expression in the mammary epithelium is required for milk production. Additionally, WAP expression was reduced, and expression of the sodium-phosphate co-transporter isoform NPT2B, a marker of secretory function (Miyoshi et al., 2001), was absent in the transplanted Dgat1−/− epithelium (Fig. 9B), indicating a defect in functional differentiation.
Fig. 8.
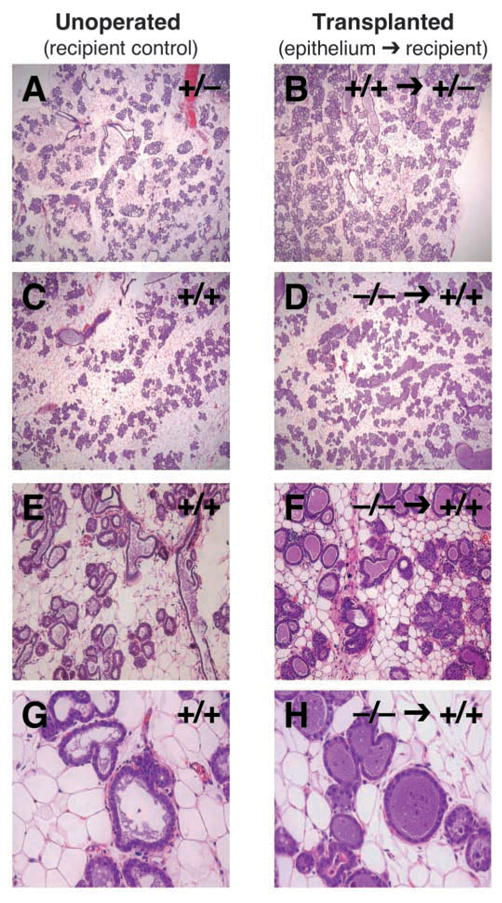
Transplantation of Dgat1−/− epithelium into Dgat1+/+ recipient mice. (A–H) After mammary epithelial transplantation, recipient mice were mated and epithelial morphology was analyzed by histology at L1. Shown are recipient (unoperated) control glands (A,C,E,G) and recipient glands that received transplanted epithelium (B,D,F,H). Control transplantations are shown for comparison (A,B).
Fig. 9.
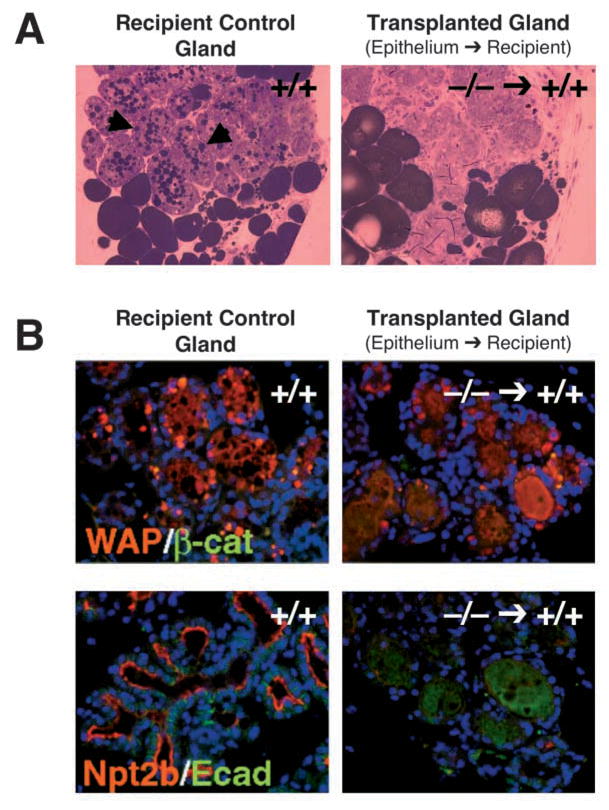
Impaired lactation and differentiation in Dgat1−/− mammary epithelium transplanted into wild-type recipient mammary glands. (A) Absence of lipid droplets in Dgat1−/− mammary epithelium transplanted into wild-type recipient mammary glands. Sections were stained with osmium tetroxide, which stains neutral lipids. Note the lipid droplets in the recipient control glands (arrowheads). (B) Reduced immunostaining of markers of epithelial differentiation in Dgat1−/− mammary epithelium transplanted into wild-type recipient mammary glands. Red fluorescence indicates the differentiation markers WAP and NPT2B. Green fluorescence indicates the control markers of epithelial cells, β-catenin (β-cat) and E-cadherin (Ecad).
Discussion
This study shows that DGAT1 function is essential for the normal development of the mammary gland during pregnancy. Impaired mammary gland development in Dgat1−/− mice was characterized by decreased epithelial proliferation and alveolar development, and by reduced expression of markers of functional differentiation in the epithelium. Transplantation studies showed that the impaired development reflects the lack of DGAT1 in both extraepithelial and epithelial compartments. The lack of DGAT1 in the extraepithelial compartment of the mammary gland appears to be responsible for the decreased alveolar growth and development of the mammary epithelium. DGAT1 deficiency in the epithelial compartment is associated with impaired functional differentiation. Thus, DGAT1 function is required both within and outside the mammary epithelium for normal development during pregnancy.
Our whole-gland transplantation studies suggested that the requirement for DGAT1 in mammary gland development is restricted to the mammary gland. Wild-type mammary glands transplanted into Dgat1−/− mice developed normally, whereas Dgat1−/− mammary glands transplanted into Dgat1+/+ mice did not. These findings indicate that systemic or hormonal factors in the recipient mice did not appreciably affect the phenotype of the transplanted gland. Thus, it is unlikely that deficiencies in the plasma hormones prolactin, estrogen or progesterone contribute to the impaired mammary gland development in Dgat1−/− mice. In support of this conclusion, Dgat1−/− female mice have normal reproduction, which is also dependent on these hormones.
The epithelial transplantation experiments suggested that DGAT1 is required in the extraepithelial compartment for normal alveolar growth and development. The transplantation of Dgat1−/− epithelium to wild-type mammary fat pads resulted in apparently normal epithelial growth, whereas the transplantation of wild-type epithelium to Dgat1−/− fat pads resulted in an underdeveloped and poorly differentiated epithelium. Thus, the presence or absence of DGAT1 in the extraepithelial compartment had a profound effect on epithelial development.
The extraepithelial stroma consists of many different cell types. In which type of cell is DGAT1 function required? Because Dgat1 is highly expressed in adipocytes (Cases et al., 1998), the most abundant cell type in the mammary stroma, it is likely that DGAT1 deficiency in adipocytes contributes to the defect in epithelial development. An attractive hypothesis is that DGAT1 deficiency alters the secretion of factors from mammary adipose tissue that are essential for epithelial development. In support of this hypothesis, previous studies from our laboratory indicated that DGAT1 deficiency alters the endocrine function of white adipose tissue (WAT). In these studies, the transplantation of 500 mg of Dgat1−/− WAT into wild-type recipients conveyed partial protection from diet-induced obesity and diabetes (Chen et al., 2003). Although the secreted factors responsible for these effects have not been identified, a candidate factor is adiponectin, which is secreted at twofold higher levels from Dgat1−/− WAT in obesity models (Chen et al., 2003). Adiponectin, also known as Acrp30 (Scherer et al., 1995) and Acdc (Mouse Genome Informatics), is abundant in the plasma, and enhances fatty acid oxidation and insulin sensitivity in tissues (Hu et al., 1996; Maeda et al., 1996; Scherer et al., 1995). It is unknown whether increased secretion of adiponectin from adjacent WAT could impair mammary development. However, adiponectin inhibits the proliferation of smooth muscle cells (Matsuda et al., 2002), and adiponectin levels normally fall during pregnancy (Combs et al., 2003).
Another possibility is that DGAT1 deficiency in the mammary stroma impairs signaling of hormones or stromal factors required for epithelial development (Hovey et al., 1999). For example, some effects of hormones that stimulate the mammary gland, such as prolactin (Brisken et al., 1999) and growth hormone (Gallego et al., 2001; Walden et al., 1998), are mediated by actions in the surrounding fat pad instead of targeting epithelial cells directly. In addition, several factors that regulate growth of the mammary epithelium are synthesized by the local fat pad, including epidermal growth factor receptor (Wiesen et al., 1999), inhibins (Robinson and Hennighausen, 1997), and insulin-like growth factors 1 and 2 (reviewed by Hovey et al., 1999; Plath-Gabler et al., 2001; Walden et al., 1998). We speculate that DGAT1 deficiency in stromal cells alters levels of lipids, such as DGAT1 substrates (fatty acyl CoAs and diacylglycerol), or their related metabolites in the epithelial environment, which in turn results in altered signaling for cellular proliferation and differentiation. In support of this hypothesis, several in vitro studies have demonstrated that specific fatty acids can modulate mammary epithelial cell growth and functional differentiation. For instance, oleate and linoleate can modulate functional differentiation of rat mammary epithelium in vitro, as assessed by casein accumulation (Sigurdson and Ip, 1993). In addition, the trace fatty acid conjugated linoleic acid has been shown to inhibit mammary epithelial proliferation both in vitro (Ip et al., 1999) and in vivo (Ip et al., 2001).
Our studies also show that DGAT1 function is required in the mammary epithelium for it to undergo functional differentiation. Although Dgat1−/− mammary glands initiated lactogenic differentiation, as shown by the induction of β-casein expression, they were unable to develop a secretory phenotype at the end of pregnancy. The expression of the markers of functional differentiation, WAP and NPT2B, was reduced, and milk droplets were not present. In addition, the epithelial transplantation experiments demonstrated a role for DGAT1 within the mammary epithelium. Although Dgat1−/− epithelium transplanted into wild-type fat pads grew normally, terminal events of differentiation did not occur. These findings suggest that DGAT1 deficiency in the mammary epithelial cells directly impairs intracellular processes that are required for functional differentiation.
The results indicate that DGAT2, a second DGAT that is also expressed in the WAT and mammary gland (Cases et al., 2001), is unable to compensate for the lack of DGAT1. Similarly, DGAT1 is unable to compensate for the lack of DGAT2 (Stone et al., 2004). Taken together, these results suggest that although the two DGAT enzymes catalyze the same biochemical reaction, they have distinct functions in vivo.
Lactation failure and impaired mammary gland development during pregnancy have been observed in several knockout mouse models, and these models have enhanced our understanding of the roles of hormones and growth factors on mammary epithelium growth and differentiation. Our study adds DGAT1, a lipid-synthesis enzyme, to the list of factors that are essential for normal mammary gland development. In addition, our study is the first to show that defects in lipid metabolism in the stroma can impair normal development of the mammary epithelium.
Acknowledgments
We thank Bethany Taylor for manuscript preparation, Stephen Ordway and Gary Howard for editorial assistance, and John Carroll for assistance with figures. This work was supported by NIH grants DK56084 (to R.V.F.), CA057621 and ES012801 (to Z.W.), the US Department of Defense Breast Cancer program DAMD17-99-1-9113, DAMD17-99-1-9103 (to B.W.), and the J. David Gladstone Institutes.
References
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RM, Coleman RA. Enzymes of glycerolipid synthesis in eukaryotes. Annu Rev Biochem. 1980;49:459–487. doi: 10.1146/annurev.bi.49.070180.002331. [DOI] [PubMed] [Google Scholar]
- Brindley DN. Metabolism of triacylglycerols. In: Vance DE, Vance JE, editors. Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier; 1991. pp. 171–203. [Google Scholar]
- Brisken C, Kaur S, Chavarria TE, Binart N, Sutherland RL, Weinberg RA, Kelly PA, Ormandy CJ. Prolactin controls mammary gland development via direct and indirect mechanisms. Dev Biol. 1999;210:96–106. doi: 10.1006/dbio.1999.9271. [DOI] [PubMed] [Google Scholar]
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases S, Stone SJ, Zhou P, Yen E, Tow B, Lardizabal KD, Voelker T, Farese RV., Jr Cloning of DGAT2, a second mammalian diacylglycerol acyltransferase, and related family members. J Biol Chem. 2001;276:38870–38876. doi: 10.1074/jbc.M106219200. [DOI] [PubMed] [Google Scholar]
- Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2002;109:1049–1055. doi: 10.1172/JCI14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Jensen DR, Myers HM, Eckel RH, Farese RV., Jr Obesity resistance and enhanced glucose metabolismin mice transplanted with white adipose tissue lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest. 2003;111:1715–1722. doi: 10.1172/JCI15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs TP, Berg AH, Rajala MW, Klebanov S, Iyengar P, Jimenez-Chillaron JC, Patti ME, Klein SL, Weinstein RS, Scherer PE. Sexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectin. Diabetes. 2003;52:268–276. doi: 10.2337/diabetes.52.2.268. [DOI] [PubMed] [Google Scholar]
- Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223:459–468. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]
- Darcy KM, Zangani D, Shea-Eaton W, Shoemaker SF, Lee PP, Mead LH, Mudipalli A, Megan R, Ip MM. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. In Vitro Cell Dev Biol Anim. 2000;36:578–592. doi: 10.1007/BF02577526. [DOI] [PubMed] [Google Scholar]
- Dupont J, Renou JP, Shani M, Hennighausen L, LeRoith D. PTEN overexpression suppresses proliferation and differentiation and enhances apoptosis of the mouse mammary epithelium. J Clin Invest. 2002;110:815–825. doi: 10.1172/JCI13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese RV, Jr, Cases S, Smith SJ. Triglyceride synthesis: insights from the cloning of diacylglycerol acyltransferase. Curr Opin Lipidol. 2000;11:229–234. doi: 10.1097/00041433-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Gallego MI, Binart N, Robinson GW, Okagaki R, Coschigano KT, Perry J, Kopchick JJ, Oka T, Kelly PA, Hennighausen L. Prolactin, growth hormone, and epidermal growth factor activate Stat5 in different compartments of mammary tissue and exert different and overlapping developmental effects. Dev Biol Stand. 2001;229:163–175. doi: 10.1006/dbio.2000.9961. [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey RC, McFadden TB, Akers RM. Regulation of mammary gland growth and morphogenesis by the mammary fat pad: a species comparison. J Mammary Gland Biol Neoplasia. 1999;4:53–68. doi: 10.1023/a:1018704603426. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Ip C, Dong Y, Thompson HJ, Bauman DE, Ip MM. Control of rat mammary epithelium proliferation by conjugated linoleic acid. Nutr Cancer. 2001;39:233–238. doi: 10.1207/S15327914nc392_12. [DOI] [PubMed] [Google Scholar]
- Ip MM, Masso-Welch PA, Shoemaker SF, Shea-Eaton WK, Ip C. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture. Exp Cell Res. 1999;250:22–34. doi: 10.1006/excr.1999.4499. [DOI] [PubMed] [Google Scholar]
- Kumar S, Clarke AR, Hooper ML, Horne DS, Law AJR, Leaver J, Springbett A, Stevenson E, Simons JP. Milk composition and lactation of β-casein-deficient mice. Proc Natl Acad Sci USA. 1994;91:6138–6142. doi: 10.1073/pnas.91.13.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R, Kuksis A. Biosynthesis of triacylglycerols. Prog Lipid Res. 1996;35:169–201. doi: 10.1016/0163-7827(96)00005-7. [DOI] [PubMed] [Google Scholar]
- Levine JF, Stockdale FE. Cell-cell interactions promote mammary epithelial cell differentiation. J Cell Biol. 1985;100:1415–1422. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–542. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath-Gabler A, Gabler C, Sinowatz F, Berisha B, Schams D. The expression of the IGF family and GH receptor in the bovine mammary gland. J Endocrinol. 2001;168:39–48. doi: 10.1677/joe.0.1680039. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Hennighausen L. Inhibins and activins regulate mammary epithelial cell differentiation through mesenchymal-epithelial interactions. Development. 1997;124:2701–2708. doi: 10.1242/dev.124.14.2701. [DOI] [PubMed] [Google Scholar]
- Robinson GW, McKnight RA, Smith GH, Hennighausen L. Mammary epithelial cells undergo secretory differentiation in cycling virgins but require pregnancy for the establishment of terminal differentiation. Development. 1995;121:2079–2090. doi: 10.1242/dev.121.7.2079. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Shamay A, Pursel VG, Wilkinson E, Wall RJ, Hennighausen L. Expression of the whey acidic protein in transgenic pigs impairs mammary development. Transgenic Res. 1992;1:124–132. doi: 10.1007/BF02528777. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Grimm SL, Rosen JM, Neubauer H, Pfeffer K, Hennighausen L. Jak2 is an essential tyrosine kinase involved in pregnancy-mediated development of mammary secretory epithelium. Mol Endocrinol. 2002;16:563–570. doi: 10.1210/mend.16.3.0805. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Robinson GW, Bierie B, Cao Y, Karin M, Hennighausen L. Proteotyping of mammary tissue from transgenic and gene knockout mice with immunohistochemical markers: a tool to define developmental lesions. J Histochem Cytochem. 2003;51:555–565. doi: 10.1177/002215540305100501. [DOI] [PubMed] [Google Scholar]
- Sigurdson SL, Ip MM. Casein accumulation by rat mammary epithelial cells grown within a reconstituted basement membrane is modulated by fatty acids in a hormone- and time-dependent manner. Exp Cell Res. 1993;208:333–343. doi: 10.1006/excr.1993.1254. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking DGAT. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- Stacey A, Schnieke A, McWhir J, Cooper J, Colman A, Melton DW. Use of double-replacement gene targeting to replace the murine alpha-lactalbumin gene with its human counterpart in embryonic stem cells and mice. Mol Cell Biol. 1994;14:1009–1016. doi: 10.1128/mcb.14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre MG, Vilotte JL, Soulier S, Mercier JC. Creation and phenotypic analysis of α-lactalbumin-deficient mice. Proc Natl Acad Sci USA. 1994;91:6544–6548. doi: 10.1073/pnas.91.14.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Myers H, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004 doi: 10.1074/jbc.M311000200. (in press) [DOI] [PubMed] [Google Scholar]
- Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology. 1998;139:659–662. doi: 10.1210/endo.139.2.5718. [DOI] [PubMed] [Google Scholar]
- Wiens D, Park CS, Stockdale FE. Milk protein expression and ductal morphogenesis in the mammary gland in vitro: hormone-dependent and -independent phases of adipocyte-mammary epithelial cell interaction. Dev Biol Stand. 1987;120:245–258. doi: 10.1016/0012-1606(87)90122-9. [DOI] [PubMed] [Google Scholar]
- Wiesen JF, Young P, Werb Z, Cunha GR. Signaling through the stromal epidermal growth factor receptor is necessary for mammary ductal development. Development. 1999;126:335–344. doi: 10.1242/dev.126.2.335. [DOI] [PubMed] [Google Scholar]
- Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science. 2002;296:1046–1049. doi: 10.1126/science.1067431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJT. The Cleared Mammary Fat Pad and the Transplantation of Mammary Gland Morphological Structures and Cells. In: Asch IA, editor. Methods in Mammary Gland Biology and Breast Cancer Research. New York: Kluwer Academic/Plenum Publishers; 2000. [Google Scholar]
- Zangani D, Darcy KM, Shoemaker S, Ip MM. Adipocyte–epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Exp Cell Res. 1999;247:399–409. doi: 10.1006/excr.1998.4373. [DOI] [PubMed] [Google Scholar]