Abstract
Matrix metalloproteinases (MMPs) are endopeptidases that contribute to growth, development and wound healing as well as to pathologies such as arthritis and cancer. Until recently, it has been thought that MMPs participate in these processes simply by degrading extracellular matrix (ECM) molecules. However, it is now clear that MMP activity is much more directed and causes the release of cryptic information from the ECM. By precisely cleaving large insoluble ECM components and ECM-associated molecules, MMPs liberate bioactive fragments and growth factors and change ECM architecture, all of which influence cellular behavior. Thus, MMPs have become a focal point for understanding matrix biology.
Introduction
The extracellular matrix (ECM) is composed of a complex mixture of insoluble molecules including collagens, laminins, fibronectin, entactin/nidogen and heparan sulfate proteoglycans. The ECM not only provides a solid-state support for cells, it also acts a reservoir for embedded cytokines and growth factors and harbors cryptic information within molecules that make up the ECM network. ECM receptors at the cell surface provide outside-in signals for cells to sense their microenvironment and react to stimuli [1–4]. Thus, the state of macromolecules within the ECM is of critical importance and proteolysis is a major factor leading to changes in the ECM. Proteolysis can affect the adherence of cells to the ECM as well as releasing bioactive fragments, sequestered growth factors and cytokines [5,6•,7].
Because matrix metalloproteinases (MMPs) degrade all the components of the ECM, they have been extensively studied in the context of modulating matrix function. MMPs belong to the metzincin superfamily of metalloproteinases, which also includes astacins, ADAMs (a protein with a disintegrin and metalloprotease domain) and ADAM-TS (an ADAM with a thrombospondin-like motif) proteases [8]. Because of the similarity in their metalloproteinase domains, there is potential for functional overlap within the MMP family as well as overlap between other metalloproteinase families. The importance of MMP in the regulation of ECM homeostasis in humans has been demonstrated by the discovery of mutations in the MMP-2 (gelatinase A) gene [9]. Individuals with these mutations manifest a disorder involving characteristic facial features, lytic bone lesions, arthritis and subcutaneous nodules. Interestingly, these features are not present in the MMP-2-null mouse; however, some of the same features are observed in the membrane type 1 MMP (MT1-MMP)-null mouse. MT1-MMP is a known activator of MMP-2, suggesting that in different species a deficiency in either of these enzymes can lead to a common pathology. The specific biochemical and physiological mechanisms of this disorder are not clear. However, it has been postulated that the balance of MMP activity has been tipped in such a way as to interfere with the release of growth factors or disrupt ECM maintenance [10]. Thus, it is important to expand our knowledge of how MMP activity regulates matrix biology and cellular behavior. This review highlights some specific instances where MMPs have been shown to participate in the regulation of matrix biology.
MMPs release cryptic fragments and neo-epitopes from ECM macromolecules
Fragments of ECM molecules have been studied for some time. In some instances this has been for practical reasons associated with the large insoluble nature of the molecules. However, ECM fragments were found to have bioactivities that the parent molecules do not. Furthermore, the presence of some of these fragments in medium conditioned by cells or in bodily fluids suggests that they have physiological and/or pathological functions and that they are released by ECM proteolysis in vivo. Because of the nature of their substrates, MMPs have been a major focus for characterizing the generation of ECM bioactive fragments.
It has long been accepted that MMPs play an important role in angiogenesis, but the exact mechanisms are not well characterized. Recently, the release of cryptic fragments and neo-epitopes from basement membrane proteins has been the subject of intense evaluation in the angiogenesis field (Table 1). Both MMP-2 and MMP-9 expose a cryptic epitope within collagen IV that promotes angiogenesis [11,12]. Interestingly, MMP-2 correlates with sites of tumor angiogenesis while MMP-9 correlates with retinal angiogenesis. This suggests that different MMPs regulate ECM function in a similar fashion under specific microenvironments. What is even more confounding regarding the role of MMPs in angiogenesis is that MMPs also generate anti-angiogenic factors. Endo-statin, the NC1 domain of collagen XVIII, can be released by direct cleavage with MMP-7 or by a multistep process involving elastase and MMPs (reviewed in [7]). Fragments generated from the NC1 domain of collagen IV α1, α2 and α3 chains, named arresten, canstatin and tumstatin respectively, are also anti-angiogenic (reviewed in [7,13]). It is currently unclear whether MMPs are involved in the release of arresten and canstatin. However, MMP-9 can release tumstatin in vitro. Furthermore, in vivo experiments show that MMP-9 null mice have decreased circulating tumstatin and accelerated tumor growth [14••]. Other fragments, such as restin from collagen XV and vastatin from collagen VIII, are also involved in angiogenesis. However, the mechanisms for their release have not been clearly elucidated. It will be of importance to determine what role MMPs play in the generation of these and other fragments derived from collagen family members.
Table 1.
Cryptic basement membrane fragments involved in angiogenesis.
| ECM protein |
Fragment |
Function |
Reference |
|---|---|---|---|
| Collagen IV | Cryptic epitope | Angiogenic | [11,12] |
| Collagen IV | NC1 α1, arresten | Anti-angiogenic | [47] |
| NC1 α 2, canstatin | Anti-angiogenic | [48,49] | |
| NC1 α 3, tumstatin | Anti-angiogenic | [49,50] | |
| NC1 α 6 | Anti-angiogenic | [49] | |
| Collagen VIII | NC1 α1, vastatin | Anti-angiogenic | [51] |
| Collagen XV | NC1, restin | Anti-angiogenic | [52] |
| Collagen XVIII | NC1, endostatin | Anti-angiogenic | [53] |
| Perlecan | C-term, endorepellin | Anti-angiogenic | [15] |
Recently, a fragment of perlecan, a modular proteoglycan found in basement membranes, has also been identified as an angiogenesis inhibitor [15]. This fragment, called endorepellin, is derived from the C terminus of perlecan. Although in vivo mechanisms for its release are not well characterized, it is probable that endorepellin is released by proteolytic processing similar to the processing that releases other anti-angiogenic fragments and will probably involve MMP cleavage.
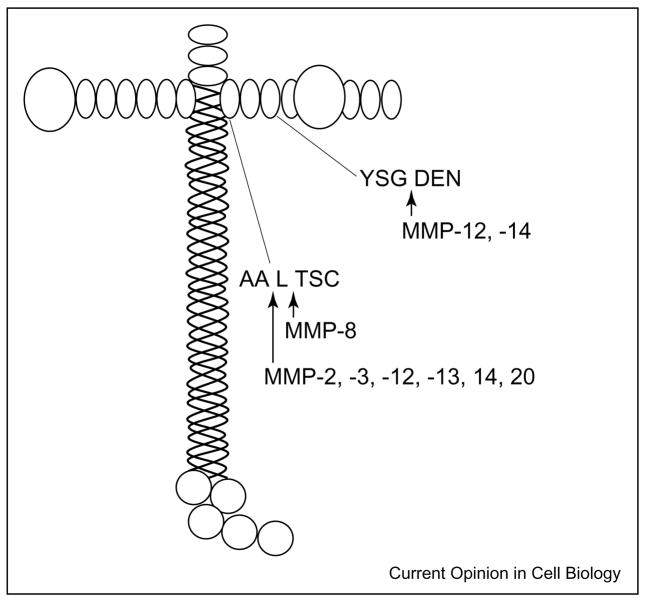
Another component of basement membranes, laminin-5, also harbors cryptic information that is released by MMP activity. Quaranta and colleagues have characterized a cryptic fragment from the γ2 chain of laminin-5 that induces epithelial cell migration [16,17]. MT1-MMP and MMP-2 release laminin-5 γ2 chain domain III, which is comprised of epidermal-growth-factor-like repeats (Figure 1) [17,18]. Recombinant domain III binds epidermal growth factor receptor, activates downstream signaling events and induces cell motility. Domain III is not detected in the quiescent stages of the mammary gland, during which little MMP dependent remodeling occurs, but it is detected in the involuting mammary gland, a stage associated with extensive MMP-mediated tissue remodeling. Interestingly, the domain III fragment is found in both the quiescent and the remodeling stages of the mammary gland of tissue inhibitor of metalloproteinases-3 (TIMP-3)-null mice. This suggests that increased MMP activity due to the absence of an inhibitor leads to an increase in the release of Domain III [18]. Further in vivo evidence supporting the importance of MMP-mediated release of the laminin-5 γ2 bioactive fragment is demonstrated in the MT1-MMP null mouse, which exhibits a number of developmental abnormalities [19]. The amount of laminin-5 γ2 fragment is reduced in tissues taken from the MT1-MMP null mouse [20]. This suggests that MT1-MMP is critical for the release of the laminin-5 γ2 fragment in vivo and that this is an important developmental phenomenon providing cues for appropriate cellular behavior. MMP-3, -8, -12, -13 and -20 also cleave the laminin-5 γ2 chain in vitro, although their in vivo relevance has not been shown. Interestingly, the cleavage site targeted by MMP-8 is different from the site targeted by other MMPs tested, and MMP-8 cleavage does not induce epithelial cell migration [21•]. This observation further supports the hypothesis that the biological function is imparted by specific MMP cleavage and not simple random proteolysis of ECM molecules.
Figure 1.
MMP cleavage sites on laminin 5. A diagram of the laminin 5 structure is shown with MMP cleavage sites indicated [18,21•]. MMP-2, -3, -12, -13 and -14 cleave the γ2 chain between alanine 586 and leucine 587. MMP-12 and MMP-14 also cleave the γ2 chain between glycine 413 and aspartic acid 414. Cleavage by these MMPs can induce cell migration. MMP-8 cleaves between leucine 587 and threonine 588. This cleavage site differs only by one amino acid residue from the site of MMP-2, -3, -12, -13 or -14 cleavage. However, MMP-8 does not induce cell migration.
Cryptic biological activities are also generated by proteolysis of fibronectin, which is a known substrate of MMPs. In addition to inhibiting cell proliferation, inducing pro-teinase gene expression and promoting adipocyte differentiation, fibronectin fragments also induce cellular migration ([22] and references therein). Migration-stimulating factor (MSF), which has been identified as the gelatin-binding domain of fibronectin, promotes cell migration and is present in medium conditioned by fetal and cancer patient fibroblasts as well as in the serum of breast cancer patients. Full-length fibronectin does not produce MSF bioactivity; MSF activity is manifested by proteolytically processing of the full-length molecule. Interestingly, a novel mode of MSF generation has recently been identified: MSF can be produced by alternative splicing as a truncated isoform from the primary fibronectin gene transcript [22]. It will be of interest to characterize mechanisms driving either the genetic or the proteolytic mode of MSF production.
MMPs release growth factors
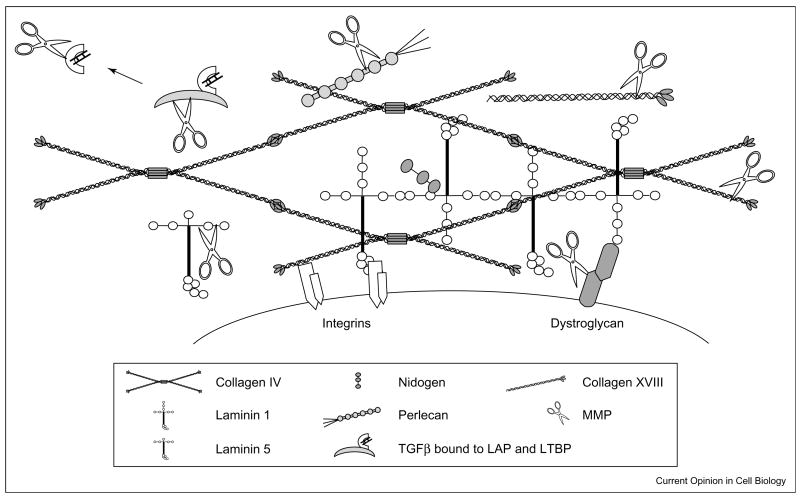
Not only does the ECM contain cryptic information released by proteolysis, it also acts as a reservoir for latent growth factors and cytokines (Figure 2). Release and activation of the embedded growth factors depends on proteolysis. Vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGFβ) represent two examples of such factors that are stored within the ECM and can be released by MMP proteolysis.
Figure 2.
A schematic diagram of some of the proteins that comprise the ECM and the potential for MMP cleavage. The ECM is composed of both laminin and collagen networks. Within ECM network many laminins and collagens are present as well as proteins such as nidogen, perlecan (i.e. heparan sulfate proteoglycans) and fibronectin (not shown). Cell surface receptors such as integrins and dystroglycan interact with proteins of the basement membrane network. Cleavage of ECM molecules releases bioactive fragments that have been referred to as matricryptins [6•,54] or matrikines [55]. Furthermore, growth factors, such as latent TGFβ, are embedded with in ECM, and proteolysis of binding proteins that keep the growth factors in a latent state is a major activation pathway. In addition, some of the cell surface receptors, such as dystroglycan [34], are targets for proteolysis. Cleavage of these types of molecules breaks cell–ECM contact.
VEGF is an important factor in the process of angiogenesis because it enhances vascular permeability and promotes new vessel growth. There are several isoforms of VEGF that differ in their affinity for heparin. This characteristic allows some isoforms to tightly bind heparan sulfate proteoglycans within the ECM [23]. Proteolysis of the ECM releases bound VEGF, increasing its bioavailability. Two different tumor models implicate MMP-9 in the release of VEGF from the ECM. In the RIP1-Tag2 insulinoma model, release of VEGF by MMP-9 correlates with an angiogenic switch, which promotes tumor progression [24]. In an ovarian carcinoma model, activated MMP-9 and, to a lesser extent, MMP-2 are associated with increased bioavailability of VEGF in culture. Furthermore, in vivo evidence suggests MMP-9 plays an important role in ascites formation in this model by increasing the bioavailability of VEGF [25]. These studies suggest that MMP-9, and possibly other MMPs, are important for mobilizing VEGF from the ECM. Even though these are tumor models, it is highly likely that similar mechanisms for the release of VEGF, leading to increased angiogenesis, occur in non-pathological conditions.
MMPs can also mobilize and activate TGFβ, which is a multifunctional cytokine important for maintaining tissue homeostasis. TGFβ is secreted and maintained in a latent complex where the cytokine non-covalently interacts with a latency-associated peptide (LAP). The latent TGFβ complex is sequestered within the ECM by covalent attachment between LAP and a latent TGFβ binding protein (LTBP), a member of the fibrillin protein family. Control of TGFβ activity is regulated by proteolytic release of the large latent complex from the ECM and by the disassociation of LAP from TGFβ [26]. MMPs-2, -9, -13 and MMP-14 directly activate TGFβ via cleavage of LAP [27–29]. Moreover, proteolysis is thought to release the large latent TGFβ complex from the ECM. A soluble form of LTBP is cleaved by both MMP-2 and -9, but only MMP-2 cleaves the ECM-bound form [30•]. MMP-3 has also been implicated in the cleavage of LTBP [31]. Taken together these studies suggest that MMPs are involved in the regulation of TGFβ activity either by direct cleavage of LAP or by release of latent TGFβ from the matrix. Release of the large latent TGFβ complex may occur by proteolysis of LTBP or by proteolysis of ECM molecules attached to LTBP. Dysregulation of TGFβ activity can have severe consequences, as recently described in a mouse model of Marfan syndrome [32••]. These mice, which are deficient in fibrillin-1, display early postnatal lung abnormalities and developmental impairment of distal alveolar septation. As they age, the mice develop destructive emphysema. The underlying mechanism of these abnormalities is an increase in TGFβ activity due to the inability of these mice to sequester TGFβ in the matrix. Because of their activity against ECM and ECM-associated molecules, MMPs will probably play a central role in the release of matrix-embedded cytokines and growth factors.
The model of TGFβ activation may apply to other members of the TGFβ superfamily. For example, chordin is a substrate for the metalloproteinase Tolloid Xolloid (an astacin) and cleavage releases bone morphogenetic protein (BMP) from the inhibitory complex of chordin/BMP [33]. This cleavage releases the cysteine-rich domain (CR), which may have bioactive properties of its own. There are several molecules, including type II collagen, within the ECM that contain chordin-like CR domains, and it is interesting to speculate that their activity may be modulated by metalloproteinase proteolysis.
MMPs modify the cell–ECM interface
Even though ADAMs are considered the major shed-dases, MMPs also cleave molecules present at the cell–ECM interface that can alter cellular attachment to the ECM. Dystroglycan is the core protein of the dystrophin–glycoprotein complex and links the intracellular cytoskeleton to the ECM. β-Dystroglycan is the transmembrane component that interacts with cytoskeletal proteins and anchors α-dystroglycan to the cell surface. α-Dystroglycan binds the extracellular region of β-dystroglycan and ECM proteins such as laminin, agrin and perlecan [2]. MMP activity has been reported to cleave β-dystroglycan within its extracellular region. This disrupts the interaction between α- and β-dystroglycan, resulting in uncoupling of the intracellular cytoskeleton from the ECM [34]. This cleavage has been observed in cell culture systems and has yet not been demonstrated in vivo. However, due to the functional consequences of MMP cleavage of β-dystroglycan, it will be of interest to investigate this phenomenon in pathologies such as muscular dystrophies and cancer.
Syndecans are a family of transmembrane heparan sulfate proteoglycans that bind a variety of ECM molecules via their glycosaminoglycans. Thus, syndecans are positioned to serve as a link between the cell surface and the ECM and are likely to be involved in regulation of cell shape and tissue morphogenesis [3]. Syndecans are shed from the cell surface in metalloproteinase-dependent manner [35]. Although other metalloproteinases may participate in this process, MT-MMPs have been reported to cleave syndecan-1 and this shedding may affect cell motility [36]. Recent reports indicate that syndecan-1 plays an important role in mammary gland development and tumor progression via its influence on the Wnt signaling pathway [37,38]. Syndecan-1 may act as a co-receptor for important cell surface molecules or ECM components and thus play a role in regulating intracellular signaling pathways. In consequence, altering the conformation of syndecan-1, for example via MMP cleavage, may have important implications in tissue architecture, development and tumor progression.
New tools of the trade
For a complete understanding of MMP regulation of the ECM, new reagents will be required. Mouse models that exploit the deletion or over-expression of MMPs or their inhibitors have been indispensable for the characterization of MMP function in specific tissues and during pathological challenges. However, novel tools will be invaluable for understanding mechanisms of MMP regulation of ECM biology. For example a transgenic mouse carrying a mutated collagen α1 gene coding for resistance to collagenase digestion has been useful in characterizing MMPs in development and wound healing [39–41]. Additionally, antibodies have been developed to identify the neo-epitopes of MMP cleaved substrates. These will useful to distinguish MMP cleavage sites from those of other proteinases [42–45]. Improvement in techniques such as in situ zymography will also be useful, as will further development in the live imaging of proteolysis. These types of tools and reagents will be important resources for the investigation of MMP regulation of ECM.
Conclusions
The ECM is no longer thought of as just a passive physical support for cells. Rather, it is now realized that the ECM contains cryptic information that influences cellular behavior. Furthermore, MMPs specifically cleave both ECM and non-ECM molecules in order to release this hidden information from the ECM in a functional manner. MMPs also assist in maintaining ECM molecules in their appropriate condition. A recently published study illustrates this concept. Although levels of the mRNA for decorin are increased by stimulation of endothelial cells with interleukin-6 or -10, the decorin core protein and the post-translational processing it undergoes to become a proteoglycan are only observed if the cells are in contact with fibrillar collagen I. This suggests that if the fibrillar nature of the collagen I is disrupted, for example by proteolysis, then the production of mature decorin is altered. Thus, decorin production can be regulated by the composition and condition of the ECM [46]. We are just beginning to understand the importance of MMP activity for appropriate regulation of the ECM. Further studies to identify and characterize MMP substrates and to understand the physiological consequences of MMP proteolysis will be important in order to complete our understanding of matrix biology.
Acknowledgments
This work was supported by grants from the NIH (AR46238, DE13058 and CA88858).
Abbreviations
- ADAM
protein with a disintegrin and metalloprotease domain
- ADAM-TS
an ADAM with a thrombospondin-like motif
- BMP
bone morphogenetic protein
- CR
cysteine-rich
- ECM
extracellular matrix
- LAP
latency-associated peptide
- LTBP
latent TGFβ binding protein
- MMP
matrix metalloproteinase
- MSF
migration-stimulating factor
- MT1-MMP
membrane type 1 MMP
- TGFβ
transforming growth factor β
- VEGF
vascular endothelial growth factor
Footnotes
This review comes from a themed issue on Cell-to-cell contact and extracellular matrix Edited by Kathleen Green and Fiona Watt
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 2.Michele DE, Campbell K. Dystrophin–glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278:15457–15460. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- 3.Yoneda A, Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003;22:25–33. doi: 10.1016/s0945-053x(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 6•.Schenk S, Quaranta V. Tales from the cryptic sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. This is a comprehensive review describing cryptic bioactive fragments from ECM proteins. [DOI] [PubMed] [Google Scholar]
- 7.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–4214. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martignetti JA, Al Aqeel A, Al Sewairi W, Boumah CE, Kambouris M, Al Mayouf S, Sheth KV, Al Eid W, Dowling O, Harris J, et al. Mutation of the matrix metalloproteinase 2 gene (MMP-2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261–264. doi: 10.1038/90100. [DOI] [PubMed] [Google Scholar]
- 10.Vu TH. Don’t mess with the matrix. Nat Genet. 2001;28:202–203. doi: 10.1038/90023. [DOI] [PubMed] [Google Scholar]
- 11.Hangai M, Kitaya N, Xu J, Chan CK, Kim JJ, Werb Z, Ryan SJ, Brooks PC. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am J Pathol. 2002;161:1429–1437. doi: 10.1016/S0002-9440(10)64418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Rodriguez D, Peticlerc E, Kim JJ, Hangai M, Yuen SM, Davis GE, Brooks PC. Proteolytic exposure of a cryptic site within collagen Type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol. 2001;154:1069–1079. doi: 10.1083/jcb.200103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalluri R. Basement membranes: structure, assembly and role in tumor angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 14••.Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R. Physiological levels of tumstatin, a fragment of collagen IV α3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via αVβ3 integrin. Cancer Cell. 2003;3:589–601. doi: 10.1016/s1535-6108(03)00133-8. This is an in vivo study showing that tumstatin is a physiologically functional angiogenesis inhibitor that is released from collagen IV by MMP-9 cleavage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of Perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 16.Gilles C, Polette M, Coraux C, Tournier J-M, Meneguzzi G, Munaut C, Volders L, Rouselle P, Birembaut P, Foidart J-M. Contribution of MT1-MMP and of human laminin-5 γ2 chain degradation to mammary epithelial cell migration. J Cell Sci. 2001;114:2967–2976. doi: 10.1242/jcs.114.16.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koshikawa N, Giannelli G, Cirulli V, Migyazaki K, Quaranta V. Role of cell surface metalloproteinase MT1-MMP in epithelial cell migration over Laminin-5. J Cell Biol. 2000;148:615–625. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a Laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole RA, Pidoux I, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 20.Koshikawa N, Schenk S, Moeckel G, Sharabi A, Miyazaki K, Gardner H, Zent R, Quaranta V. Proteolytic processing of taminin-5 by MT1-MMP in tissues and its effects on epithelial cell morphology. FASEB J. 2004;18:364–366. doi: 10.1096/fj.03-0584fje. [DOI] [PubMed] [Google Scholar]
- 21•.Pirila E, Sharabi A, Salo T, Quaranta V, Tu H, Heljasvaara R, Koshikawa N, Sorsa T, Maisi P. Matrix metalloproteinases process the laminin-5 γ2-chain and regulate epithelial cell migration. Biochem Biophys Res Commun. 2003;303:1012–1017. doi: 10.1016/s0006-291x(03)00452-2. This study identifies the sites in laminin-5 that are cleaved by MMPs and shows that the specificity of the cleavage site is important for bioactivity of the cryptic laminin-5 fragment. [DOI] [PubMed] [Google Scholar]
- 22.Schor SL, Ellis IR, Jones SJ, Baillie R, Seneviratne K, Clausen J, Motegi K, Vojtesek B, Kankova K, Furrie E, et al. Migration-stimulating factor: a genetically truncated oncofetal fibronectin isoform expressed by carcinoma and tumor-associated stromal cells. Cancer Res. 2003;63:8827–8836. [PubMed] [Google Scholar]
- 23.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4:1317–1326. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, et al. Matrix metalloproteinase 9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP-9 and MMP-2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 26.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 27.Yu Q, Stamenkovic I. Cell-surface-localized matrix metalloproteinase 9 proteolytically activates TGFβ and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 28.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGFβ1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangelo M, Sarment DP, Billings PC, Pacifici M. Activation of transforming growth factor β in chondrocytes undergoing endochondral ossification. J Bone Miner Res. 2001;16:2339–2347. doi: 10.1359/jbmr.2001.16.12.2339. [DOI] [PubMed] [Google Scholar]
- 30•.Dallas SL, Rosser JL, Mundy GR, Bonewald LF. Proteolysis of latent transforming growth factor-β (TGF-β)-binding protein-1 by osteoclasts. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. This work identifies LTBP as a novel substrate for MMPs and show that proteolysis may be the first step in the activation of growth factors sequestered in the ECM. [DOI] [PubMed] [Google Scholar]
- 31.Maeda S, Dean DD, Gomez R, Schwartz Z, Boyan BD. The first stage of transforming growth factor β 1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle Stromelysin-1 (MMP-3) Calcif Tissue Int. 2002;70:54–65. doi: 10.1007/s002230010032. [DOI] [PubMed] [Google Scholar]
- 32••.Neptune E, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. This work shows that matrix sequestration of cytokines is a critical point for the regulation of their activity. [DOI] [PubMed] [Google Scholar]
- 33.Abreu JG, Coffinier C, Larrain J, Oelgeschlager M, DeRobertis EM. Chordin-like CR domains and the regulation of evolutionarily conserved extracellular signaling systems. Gene. 2002;287:39–47. doi: 10.1016/s0378-1119(01)00827-7. [DOI] [PubMed] [Google Scholar]
- 34.Yamada H, Saito F, Fukuta-Ohi H, Zhong D, Hase A, Arai K, Okuyama A, Maekawa R, Shimizu T, Matsumura K. Processing of β-dystroglycan by matrix metalloproteinases disrupts the link between the extracellular matrix and cell membrane via the dystroglycan complex. Hum Mol Genet. 2001;10:1563–1569. doi: 10.1093/hmg/10.15.1563. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a timp-3 sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, Furukawa M, Sato H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J Biol Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 37.Liu BY, Kim YC, Leatherberry V, Cowin P, Alexander CM. Mammary gland development requires syndecan-1 to create a β-catenin/TCF-responsive mammary epithelial subpopulation. Oncogene. 2003;22:9243–9253. doi: 10.1038/sj.onc.1207217. [DOI] [PubMed] [Google Scholar]
- 38.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci USA. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beare AH, O’Kane S, Krane SM, Ferguson MW. Severely impaired wound healing in the collagenase-resistant mouse. J Invest Dermatol. 2003;120:153–163. doi: 10.1046/j.1523-1747.2003.12019.x. [DOI] [PubMed] [Google Scholar]
- 40.Chiusaroli R, Maier A, Knight MC, Byrne M, Calvi LM, Baron R, Krane SM, Schipani E. Collagenase cleavage of type I collagen is essential for both basal and parathyroid hormone (PTH) PTH-related peptide receptor-induced osteoclast activation and has differential effects on discrete bone compartments. Endocrinology. 2003;144:4106–4116. doi: 10.1210/en.2003-0254. [DOI] [PubMed] [Google Scholar]
- 41.Lindsey ML, Yoshioka J, MacGillivray C, Muangman S, Gannon J, Verghese A, Aikawa M, Libby P, Krane SM, Lee RT. Effect of a cleavage-resistant collagen mutation on left ventricular remodeling. Circ Res. 2003;93:238–245. doi: 10.1161/01.RES.0000085580.45279.60. [DOI] [PubMed] [Google Scholar]
- 42.Stanton H, Fosang AJ. Matrix metalloproteinases are active following guanidine hydrochloride extraction of cartilage: generation of DIPEN neoepitope during dialysis. Matrix Biol. 2002;21:425–428. doi: 10.1016/s0945-053x(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 43.Chu Q, Lopez M, Hayashi K, Ionescu M, Billinghurst RC, Johnson KA, Poole RA, Markel MD. Elevation of collagenase generated type II collagen neoepitope and proteoglycan epitopes in synovial fluid following induction of joint instability in the dog. Osteoarthritis Cartilage. 2002;10:662–669. doi: 10.1053/joca.2002.0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishiguro N, Ito T, Oguchi T, Kojima T, Iwata H, Ionescu M, Poole RA. Relationships of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover and inflammation as revealed by analyses of synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 2001;44:2503–2511. doi: 10.1002/1529-0131(200111)44:11<2503::aid-art430>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Fosang AJ, Last K, Jackson DC, Brown L. Antibodies to MMP-cleaved aggrecan. Methods Mol Biol. 2001;151:425–449. doi: 10.1385/1-59259-046-2:425. [DOI] [PubMed] [Google Scholar]
- 46.Strazynski M, Eble JA, Kresse H, Schonherr E. Interleukin (IL)-6 and IL-10 induce DEcoRIn mRNA in endothelial cells, but interaction with fibrillar collagen is essential for its translation. J Biol Chem. 2004;279:21266–21270. doi: 10.1074/jbc.M309782200. [DOI] [PubMed] [Google Scholar]
- 47.Coloradao PC, Torre A, Kamphaus G, Maeshima Y, Hopfter H, Takahashi K, Volk R, Amborsky ED, Herman S, Sarkar PK, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60:2520–2526. [PubMed] [Google Scholar]
- 48.Kamphaus GD, Coloradao PC, Panka DJ, Hopfter H, Ramchandran R, Torre A, Maeshima Y, Mier JW, Sukhatme VP, Kalluri R. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275:1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 49.Peticlerc E, Boutaud A, Prestayko A, Xu J, Yoshikazu S, Ninomiya Y, Maichael P, Sarras J, Hudson BG, Brooks PC. New functions for non-collagenous domains of human collagen type IV. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 50.Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent αvβ3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 51.Xu R, Yao ZY, Xin L, Zhang Q, Li TP, Gan RB. NC1 domain of human type VIII collagen (α 1) inhibits bovine aortic endothelial cell proliferation and causes cell apoptosis. Biochem Biophys Res Commun. 2001;289:264–268. doi: 10.1006/bbrc.2001.5970. [DOI] [PubMed] [Google Scholar]
- 52.Ramchandran R, Dhanabal M, Volk R, Waterman MJ, Segal M, Lu H, Knebelmann B, Sukhatme VP. Antiangiogenic activity of restin, NC10 domain of human collagen XV: comparison to endostatin. Biochem Biophys Res Commun. 1999;255:735–739. doi: 10.1006/bbrc.1999.0248. [DOI] [PubMed] [Google Scholar]
- 53.O’Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 54.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schor SL, Schor AM. Tumour-stromal interactions: phenotypic and genetic alterations in mammary stroma — implications for tumour progression. Breast Cancer Res. 2001;3:373–379. doi: 10.1186/bcr325. [DOI] [PMC free article] [PubMed] [Google Scholar]