Abstract
γ-Vinyl-γ-aminobutyric acid (GVG) elevates central nervous system γ-aminobutyric acid (GABA) levels by irreversibly inhibiting GABA transaminase. An open-label clinical trial in humans suggested that GVG may reduce cocaine and methamphetamine use. To test safety and to obtain preliminary data on efficacy of GVG for treating methamphetamine dependence, we conducted a double-blind, placebo-controlled, parallel groups study of GVG interaction with the cardiovascular and subjective effects produced by methamphetamine. Non-treatment seeking methamphetamine-dependent volunteers received either GVG (N=8) or placebo (N=9) by random assignment. GVG treatment was initiated at 1 g/day and increased to 5 g/day. After reaching the target dose of 5 g/day, participants received methamphetamine (15 + 30 mg, IV), and cardiovascular and subjective effects were assessed. No serious adverse events were noted, and the total number of adverse events was similar between the treatment groups. Considering the full time-course and peak effects independently, no significant differences were detected between the groups for systolic or diastolic blood pressures, or heart rate following methamphetamine exposure. Some methamphetamine-induced cardiovascular changes approached significance (p<0.10) and may warrant attention in future trials. Methamphetamine-induced subjective effects (“any drug effect”, “high”, “crave methamphetamine” were statistically similar between GVG and placebo treatment groups. Pharmacokinetic data indicate that GVG treatment did not alter methamphetamine or amphetamine plasma levels, and there was no association between methamphetamine or amphetamine plasma levels and peak cardiovascular effects. Taken together, the data indicate that GVG treatment is generally well tolerated but not efficacious in attenuating the positive subjective effects of methamphetamine in the laboratory.
Keywords: GVG, Methamphetamine, Addiction
Introduction
Pre-clinical research has shown that compounds that modulate mesolimbic dopamine (DA) neurotransmission alter the reinforcing effects of several drugs of abuse. In this regard, γ-aminobutyric acid (GABA) inhibits striatal DA release, and attenuates cocaine-induced increases in extracellular DA in the striatum and nucleus accumbens (Molina et al. 1999). Several, but not all, rodent and non-human primate studies have suggested that compounds that target GABA could be useful treatments for cocaine- and methamphetamine-dependence.
The compound of interest for the current report was γ-vinyl-γ-aminobutyric acid (GVG), an anti-epileptic medication that irreversibly inhibits GABA transaminase, a key enzyme in the metabolic disposition of GABA. GVG inhibits methamphetamine-, heroin-, and ethanol-induced increases in extracellular DA in the nucleus accumbens in rodents (Gerasimov et al. 1999) and reduces cocaine-induced striatal DA release in non-human primates (Dewey et al. 1998). In addition, GVG blocks conditioned place preference for heroin in the rat (Paul et al. 2001), decreases morphine and cocaine (Kushner et al. 1999) self-administration in rats, and reduces cocaine-seeking behavior in baboons (Weerts et al. 2005). Prefrontal cortical GABA levels are low in cocaine-dependent individuals (Streeter et al. 2005), and magnetic resonance spectroscopy have shown increases brain GABA levels 2-3-fold above baseline following treatment of human participants with GVG (3 g/day)(Verhoeff et al, 1999).
Collectively, these findings suggest that GVG may be a useful treatment for stimulant dependence. Moreover, results from two open-label trials and one placebo-controlled trial suggests that GVG may reduce cocaine and methamphetamine use. In the first study, involving 20 cocaine-dependent volunteers (most also abused methamphetamine, marijuana, and other drugs), 12 participants dropped out before completion, and most of the 8 who remained had a considerable number of days (~50) without cocaine or methamphetamine use (Brodie et al. 2003). A follow-up study confirmed this finding (Brodie et al. 2005) and included participants who met criteria for methamphetamine dependence (N=10), methamphetamine and cocaine dependence (N=17), or cocaine dependence (N=3). Eleven participants dropped out, and among completers, 15 were methamphetamine- and cocaine-free for >4 consecutive weeks. Preliminary results from a subsequent, double-blind, placebo-controlled trial support the view that GVG has efficacy as a treatment for cocaine dependence (Catalyst 2008).
As an initial step in the clinical development of GVG for methamphetamine dependence, it is important to assess the safety, tolerability, and to obtain preliminary data on efficacy of the compound in methamphetamine-dependent participants. We therefore conducted a double-blind, placebo-controlled, parallel groups study to determine the cardiovascular, subjective, and reinforcing effects of methamphetamine in volunteers treated with GVG or placebo.
Research Design and Methods
Participants
A total of 17 participants were randomized to receive GVG (n=8) or placebo (n=9). Participants were recruited using advertisements and paid for their participation. All participants met DSM-IV-TR criteria for methamphetamine dependence and did not meet criteria for dependence on other drugs other than nicotine or marijuana. Additional inclusion criteria included being between 18-45 years of age, having a history of using methamphetamine by the smoked or IV route of administration, and being otherwise healthy, as confirmed by a physical examination and safety laboratories. Exclusion criteria included having a history of seizure disorder or head trauma, having a history of prior adverse event associated with methamphetamine abuse, or the presence of any axis I psychiatric disorder other than those noted above. Serious medical conditions, such as symptomatic HIV disease, heart disease, or neurologic disease, were also exclusionary.
Study Design
This double-blind, placebo-controlled, between-subjects study was conducted in the general clinical research center at UCLA. The institutional review board at UCLA approved the study. All participants give informed consent after having the potential risks fully explained to them.
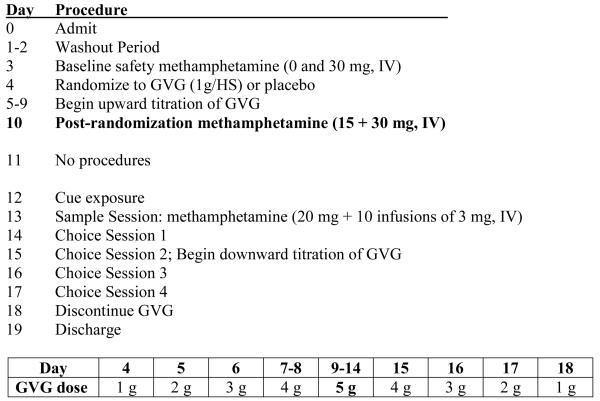
The study schema is provided in Figure 1. Days 1-2 served as a washout and stabilization period. On Day 3, participants received baseline (“pre-randomization”) infusions of methamphetamine (0 and 30 mg, IV) separated by 180 min. Outcomes were used to verify that volunteers safely tolerated the methamphetamine test doses in the laboratory. Participants were then randomized to GVG (1 g, p.o.) or placebo on Day 4 in the evening. The dose was increased to 1 g BID on Day 5, 1 g/am 2 g/hs on Day 6, 2 g twice daily on Days 7-8, followed by 2 g qam and 3 g qhs on Days 9-14.
Figure 1.
The Study Schema shows the entire 19-day inpatient procedure including titration schedule for GVG. The current report includes data obtained on Days 1-10, with emphasis on outcomes obtained during Day 10 methamphetamine infusion session. Data obtained on Days 12-17 include cue reactivity and self-administration and are being submitted for publication elsewhere.
After reaching the target dose of study medication (5 g/day), on Day 10, participants received “post-randomization” methamphetamine (15 mg plus 30 mg, IV) in two doses given 90 min apart. Cardiovascular and subjective effects data were obtained before and after methamphetamine dosing (as detailed below).
Additional tests were performed on Days 12-17, including cue reactivity and self-administration sessions and these data are being submitted for publication elsewhere. A physician was present during all methamphetamine infusion sessions and carefully monitored participant's heart rate, blood pressure, and ECG wave form. Stopping rules were in place to halt dosing if cardiovascular indices exceeded preset values. Specifically, in this study, methamphetamine administration was not initiated if there were clinically significant arrhythmias or if vital signs were outside of acceptable ranges: resting pulse < 130 bpm and blood pressure below 165mm Hg systolic and 100mm Hg diastolic. Repeated doses of methamphetamine were not administered (and the study physician halted continued methamphetamine delivery) if any of the following occurred: HR >130 bpm; Diastolic BP >100 mmHg; Systolic BP >165 mmHg; or Behavioral manifestation of methamphetamine toxicity (agitation, psychosis, inability to cooperate with study procedures).
GVG dosage tapering began on Day 15 and drug was discontinued on Day 18. Participants were discharged on Day 19 and asked to return for follow-up 2 weeks after discharge.
Subjective and Cardiovascular Measures
Subjective effects data were collected using computerized visual analog scales (VAS). VAS data were collected at 15 min before, and at several time points following the first (15 mg, IV: 5, 10, 15, 30, 45, 60 and 75 min), and second (30 mg, IV: 5, 10, 15, 30, 45, 60, 90 and 120 min) methamphetamine infusions, which were given in fixed order (and should be considered a limitation in the study design). The infusions were administered 90 min apart on the basis of our own data showing that peak effects and return to baseline occur within this time frame (De La Garza et al., 2008; Newton et al., 2005, 2006, 2008). Cardiovascular data were collected at the same time points. For VAS scales, participants reported the degree to which they feel ‘any drug effect’, ‘high’, ‘good effects’, ‘bad effects’, ‘like methamphetamine’, ‘crave methamphetamine’, ‘depressed’, ‘anxious’, ‘stimulated’, and ‘likely to use’ on a continuous scale digitized between 0 and 100. In addition, they were asked to answer the question: ‘How much would you pay for this drug’?
Concerns about Visual Field Changes
GVG has been known to produce visual field loss when used for the treatment of epilepsy. The potential for this untoward effect has slowed the approval of GVG for epilepsy in the US, and suggests that ophthalmologic evaluation and monitoring is needed. No participants receiving GVG for the treatment of cocaine or methamphetamine dependence have developed ocular or visual field adverse effects (Fechtner et al. 2006). Despite this finding, all potential study participants for the current study were required to have normal visual field examinations and electroretinogram (ERG) evaluations prior to enrollment. The rationale for requiring these prior to entry was that participants with marginal visual field functioning would likely be at higher risk for developing visual field loss following GVG treatment. The standard ERG protocol established by the International Society for Clinical Electrophysiology of Vision was used. This protocol included the 30 Hz a-b flicker amplitude, the photopic a-b amplitude, and the OP1 amplitude, which are highly correlated with visual field loss . The short duration of GVG treatment was not believed to warrant follow-up ophthalmologic evaluation.
Pharmacokinetics
Plasma samples were analyzed for concentrations of methamphetamine and amphetamine using liquid chromatographic/mass spectrometric methods at the University of Utah under the direction of David Moody, Ph.D.
Drugs
GVG and accompanying placebo were administered orally as an aqueous solution and provided by Ovation Pharmaceuticals (North Deerfield, IL). Sterile methamphetamine solution for human use was provided by a NIDA contractor (RTI International). Methamphetamine was administered intravenously by slow push over 2-min. An equal volume of sterile saline solution was used as the control and was administered at the same rate. An IND was obtained from the FDA for the use of methamphetamine and GVG in this study.
The elimination half-life of GVG in humans is approximately 7 hours (investigators' brochure). The drug acts as a suicide inhibitor of GABA transaminase. After inhibition therefore, new enzyme must be synthesized de novo. Thus, its duration of action is much longer than its elimination half-life. We chose twice daily dosing for ease of titration and to limit the maximum dose administered at any one time. Prior outpatient studies in cocaine and methamphetamine users used 3 g/day or 4 g/day. We selected 5 g/day as a maximum dose to be more sensitive to potential side effects. Because the participants were hospitalized we were able to provide very close monitoring, thus ensuring safety.
Data Analyses
Data were analyzed using StatView 5.0 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics were compiled for demographic variables and analyzed using appropriate non-parametric tests. For all measures, statistical significance was set at p<0.05. All data are presented as mean ± standard error.
Across-study measures
The total number of AEs was summed from days 1 to 19 and analyzed using ANOVA as a function of GVG dose (0 or 5 g). Other aspects of AE data reporting (type, severity and duration) were not analyzed since the overall number of AEs was low and not different between treatment groups. BDI scores were analyzed using repeated measures ANOVA as a function of GVG dose (0 or 5 g) and days (days 4–18). Day 4 represented the day immediately prior to study drug randomization and day 18 was the last day of drug exposure, so this time course encompassed the full treatment period. For across-study measures, all data were analyzed as between-subjects factors. Time (in days) was analyzed as a within-subjects factor.
Post-randomization measures
Post-randomization data were derived from outcomes obtained on Day 10 (methamphetamine 15 + 30 mg). Heart rate, systolic blood pressure, diastolic blood pressure, and VAS data were analyzed using repeated measures ANOVA as a function of GVG dose (0 or 5 g) and Time (in min). Time-courses reflect within-session change from baseline (value at a given time-point minus the value at T=−15 min). These data were also analyzed with respect to peak effects for each individual using one-way ANOVA.
Note: On Day 10, one participant in the placebo group experienced an adverse event that was not drug-related (infusion needle broke off into vein and had to be surgically removed). The data from this participant were incomplete and therefore not included in the final analyses. As such, heart rate, systolic blood pressure, diastolic blood pressure, and VAS data reflect N=8 for each of the treatment groups.
Pharmacokinetic data were analyzed using repeated measures ANOVA as a function of GVG dose and Time (in min). In addition, simple linear regression analyses were conducted in order to determine whether methamphetamine or amphetamine plasma levels were associated with peak changes in cardiovascular effects in GVG and placebo treatment groups.
For post-randomization measures, all data except Time were analyzed as between-subjects factors. Time (in min) was analyzed as a within-subjects factor. Methamphetamine dosages 15 and 30 mg were not analyzed separately since the infusions were given consecutively and the known half-life of methamphetamine is ~11-12h; so we did not expect the statistical outcomes of these dosages to be dissociable.
Results
Demographics and Drug Use
Detailed demographic information and drug-use data are provided in Table 1. Participants in the GVG (N=8) and placebo (N=9) treatment groups were statistically similar along all demographic and drug-use variables.
Table 1.
Demographics and Drug Use
|
GVG (N=8) |
Placebo (N=9) |
|
|---|---|---|
| Gender (N) | ||
| Male | 7 | 8 |
| Female | 1 | 1 |
| Ethnicity (N) | ||
| Caucasian | 4 | 4 |
| Hispanic | 0 | 2 |
| African American | 2 | 0 |
| Other | 2 | 3 |
| Age (yrs) | 42.3±2.6 | 37.7±2.7 |
| Education (yrs) | 11.9±0.4 | 13.2±0.6 |
| Methamphetamine Use | ||
| Years of use | 7.5±1.9 | 11.6±3.0 |
| *Last 30 days use | 15.1±3.5 | 13.7±2.3 |
| Meth Route of Admin (N) | ||
| Smoke | 6 | 4 |
| IV | 2 | 2 |
| Other | 0 | 3 |
| Nicotine Use (N) | 6/8 | 6/9 |
| Years of use | 19.2±3.3 | 8.8±2.1 |
| Last 30 days use | 25.2±4.8 | 21.3±4.7 |
| Alcohol Use (N) | 5/8 | 9/9 |
| Years of use | 10.6±4.8 | 13.6±4.0 |
| Last 30 days use | 4.6±2.8 | 5.9±3.1 |
| Marijuana Use (N) | 6/8 | 8/9 |
| Years of use | 9.8±3.9 | 12.9±1.9 |
| Last 30 days use | 5.5±2.5 | 3.5±1.8 |
Last 30 days use indicates number of days of use of that drug in the 30 days preceding entry into this study.
Adverse events
There were no serious adverse events recorded during this trial. The type, severity and duration of all other adverse events were comparable between the placebo and GVG groups (Table 2).
Table 2.
Summary of Adverse Events
| Adverse Event | GVG | Placebo |
|---|---|---|
| Insomnia | 5 | 9 |
| Headache | 2 | 5 |
| Constipation | 1 | 2 |
| Nausea | 0 | 2 |
| Toothache | 1 | 1 |
| Abdominal Cramping | 1 | 1 |
| High blood pressure | 1 | 1 |
| Arm pain | 0 | 2 |
| Yeast Infection | 1 | 0 |
| Sedation | 0 | 1 |
| Indigestion | 1 | 0 |
| Anxiety | 1 | 1 |
| Groin Pain | 1 | 0 |
| Pruritis | 1 | 0 |
| Itching Eye | 1 | 0 |
| Tinea Pedis | 1 | 0 |
| Visual Blurring | 0 | 1 |
| Rash to side of nose | 0 | 1 |
| Vessel in eye popped | 0 | 1 |
| Dyspepsia | 0 | 1 |
| Shoulder Pain | 0 | 1 |
| Difficulty breathing | 0 | 1 |
| Fatigue | 0 | 1 |
Of particular interest in a trial that includes GVG treatment are potential ophthalmological changes. All participants were pre-screened with an ERG prior to enrollment. Three adverse events fell into this category, with two (mild visual blurriness, vessel in eye popped) reported in the placebo condition, and one (itching eye) reported in the GVG condition. All were considered mild in nature and resolved within 24h.
BDI Scores
BDI scores were low at baseline for both placebo (2.0±0.76; Mean ±S.E.M.) and GVG (2.0±0.54) treatment groups and remained consistently low throughout this study. Repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=0.61, p=0.45) or Time (F1,14=0.91, p=0.55), and no significant interaction of GVG × Time (F14,196=0.86, p=0.60).
Cardiovascular Effects
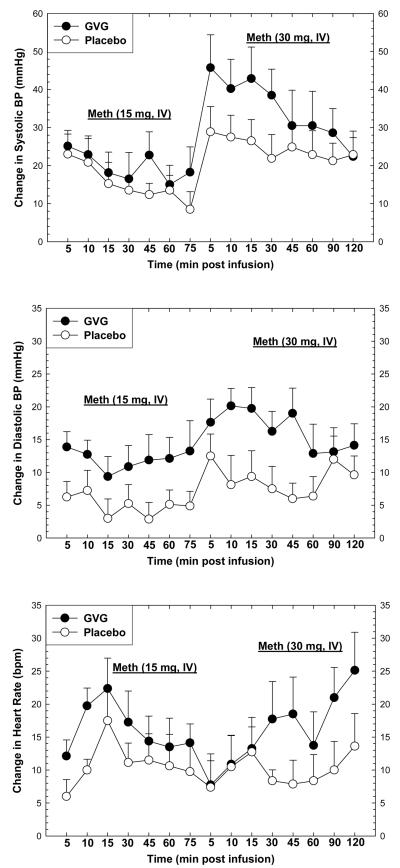
Heart rate and blood pressure were measured prior to and for several minutes following each methamphetamine infusion on Day 10 (15 + 30 mg). As expected, acute methamphetamine exposure increased heart rate and blood pressure (Figure 2). For systolic blood pressure, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=1.06, p=0.32), a significant effect of Time (F1,14=10.38, p<0.0001), and no significant interaction of GVG × Time (F14,196=1.53, p=0.10). For diastolic blood pressure, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=3.97, p=0.07), a significant effect of Time (F1,14=13.74, p<0.0001), and no significant interaction of GVG × Time (F14,196=1.17, p=0.30). For heart rate, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=1.57, p=0.23), a significant effect of Time (F1,14=2.78, p<0.0001), and no significant interaction of GVG × Time (F14,196=0.97, p=0.48).
Figure 2.
Change in systolic blood pressure (upper panel), diastolic blood pressure (middle panel), or heart rate (lower panel) following two consecutive infusions of methamphetamine (15 mg + 30 mg, i.v.) as a function of drug (GVG or placebo) and time. Data represent the mean ± S.E.M. from 16 methamphetamine-dependent participants on day 10. Values represent change from baseline (given time-point minus t=−15 min).
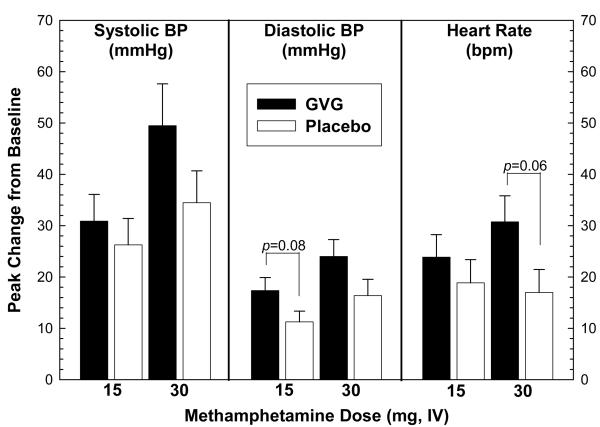
Analysis of peak effects was also performed on cardiovascular data obtained on Day 10 (Figure 3). For systolic blood pressure, ANOVA revealed no significant difference in peak effect between GVG and placebo with methamphetamine at 15 mg (F1,14=0.40, p=0.54) or 30 mg (F1,14=2.15, p=0.16). For diastolic blood pressure, ANOVA revealed no significant difference in peak effect between GVG and placebo for methamphetamine at 15 mg (F1,14=3.49, p=0.08) or 30 mg (F1,14=2.77, p=0.11). For heart rate, ANOVA revealed no significant difference in peak effect between GVG and placebo for methamphetamine at 15 mg (F1,14=0.64, p=0.44) or 30 mg (F1,14=4.16, p=0.06).
Figure 3.
Analysis of peak effects in systolic blood pressure (left), diastolic blood pressure (middle), or heart rate (right) following two consecutive infusions of methamphetamine (15 mg + 30 mg, i.v.) as a function of GVG dose (or placebo). Data represent the mean ± S.E.M. from 16 methamphetamine-dependent participants on day 10. Peak effect values represent change from baseline for a given time-point minus t=−15 min.
Subjective Effects
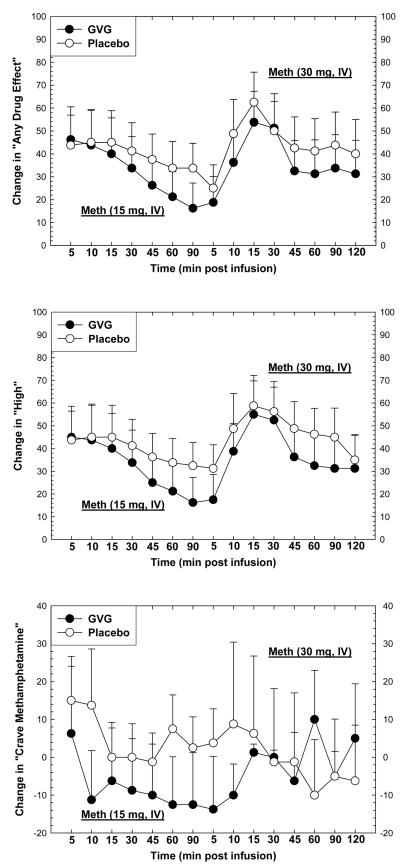
Subjective effects were measured prior to and for several minutes following each methamphetamine infusion on Day 10 (15 + 30 mg). As expected, acute methamphetamine exposure increased several positive subjective effects (Figure 4). For “Any Drug Effect”, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=0.23, p=0.64), a significant effect of Time (F1,14=3.93, p<0.0001), and no significant interaction of GVG × Time (F14,196=0.31, p=0.99). For “High”, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=0.27, p=0.61), a significant effect of Time (F1,14=5.45, p<0.0001), and no significant interaction of GVG × Time (F14,196=0.42, p=0.97). For “Crave Methamphetamine”, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,14=0.22, p=0.65) or Time (F1,14=0.55, p=0.90), and no significant interaction of GVG × Time (F14,196=1.08, p=0.38).
Figure 4.
Change in “Any Drug Effect” (upper panel), “High” (middle panel), or “Crave Methamphetamine” (lower panel) following two consecutive infusions of methamphetamine (15 mg + 30 mg, i.v.) as a function of drug (GVG or placebo) and time. Data represent the mean ± S.E.M. from 16 methamphetamine-dependent participants on day 10. Values represent change from baseline (given time-point minus t=−15 min).
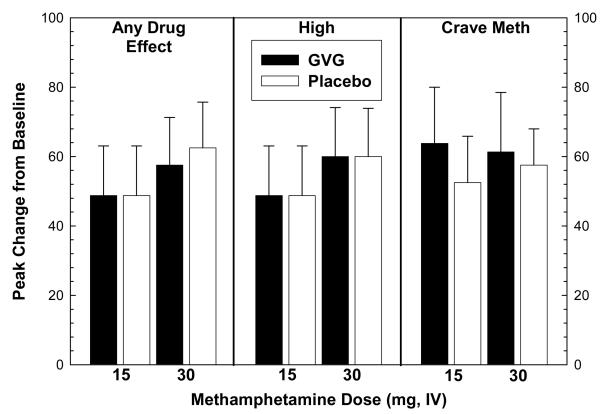
Analysis of peak effects was also performed on subjective effects data obtained on Day 10 (Figure 5). For “Any Drug Effect”, ANOVA revealed no significant effects for methamphetamine at 15 mg (means identical so statistical test could not be performed) or 30 mg (F1,14=0.07, p=0.80). For “High”, ANOVA revealed no significant effects for methamphetamine at 15 mg or 30 mg (means identical so statistical test could not be performed). For “Crave Methamphetamine”, ANOVA revealed no significant effects for methamphetamine at 15 mg (F1,14=0.29, p=0.60) or 30 mg (F1,14=0.04, p=0.85).
Figure 5.
Analysis of peak effects in “Any Drug Effect” (left), “High” (middle), or “Crave Methamphetamine” (right) following two consecutive infusions of methamphetamine (15 mg + 30 mg, i.v.) as a function of GVG dose (or placebo). Data represent the mean ± S.E.M. from 16 methamphetamine-dependent participants on day 10. Peak effect values represent change from baseline for a given time-point minus t=−15 min.
Time course and peak effects analyses were performed on all other positive and negative subjective effects recorded by the VAS instrument. These data (not shown) were similarly non-significant as those reported above.
Pharmacokinetic Data
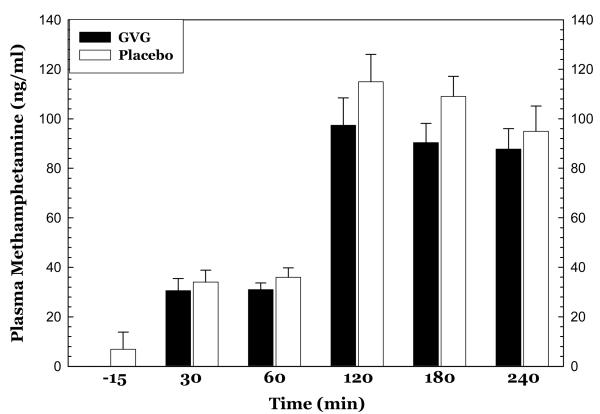
Blood samples for PK data were not available for all completers so the data presented reflect those from a subsample size of the GVG (N=3) and placebo (N=4) treatment groups. The data do not indicate any effects of GVG treatment on methamphetamine or amphetamine plasma levels. For methamphetamine (Figure 6), repeated-measures ANOVA revealed no significant effect for GVG dose (F1,5=1.39, p=0.29), a significant effect of Time (F1,5=113.8, p<0.0001), and no significant interaction of GVG × Time (F5,25=0.67, p=0.65). For amphetamine, repeated-measures ANOVA revealed no significant effect for GVG dose (F1,5=1.03, p=0.36), a significant effect of Time (F1,5=19.1, p<0.0001), and no significant interaction of GVG × Time (F5,25=1.15, p=5.75).
Figure 6.
Plasma levels of methamphetamine before and following two consecutive infusions of methamphetamine (15 mg + 30 mg, i.v.) as a function of drug (GVG or placebo) and time. Data represent the mean ± S.E.M. from only a subset (N=3 for GVG and N=4 for placebo) of methamphetamine-dependent participants on day 10.
Simple linear regression analyses were conducted in order to determine whether methamphetamine or amphetamine plasma levels were associated with peak changes in cardiovascular effects in GVG and placebo treatment groups. The data indicate that peak systolic and diastolic blood pressure and heart rate after 15mg and 30 mg doses of methamphetamine were not significantly correlated with peak plasma levels of methamphetamine or amphetamine (data not shown).
Discussion
We conducted a double-blind, placebo-controlled, parallel groups study to determine the cardiovascular, subjective, and reinforcing effects of methamphetamine in volunteers treated with GVG or placebo. The number, type, severity and duration of all adverse events were comparable between placebo and GVG conditions, and therefore indicate that GVG was well-tolerated in this stimulant-addicted population.
In the current report, and as demonstrated previously (De La Garza et al. 2008; Newton et al. 2005; Newton et al. 2008; Newton et al. 2006), acute methamphetamine exposure increased heart rate and blood pressure. Of importance, GVG treatment tended to increase the cardiovascular effects produced by methamphetamine. Although these effects were not statistically significant in the sample tested, their magnitude may have clinical import, especially for those at risk for heart disease or stroke. The highest dose of methamphetamine delivered in the study (45 mg total) is generally toward the lower range of that used by methamphetamine-dependent individuals in their natural setting. We are not able to comment on whether individuals treated with GVG and who take doses of methamphetamine higher than tested in the laboratory would experience correspondingly greater increases in blood pressure and heart rate. Our cardiovascular findings are similar to those reported previously (Haney et al. 2005), in which gabapentin (1200 mg/day) increased heart rate following either placebo or cocaine (12 mg) administration. While the mechanism underlying these enhancements in stimulant-induced cardiovascular changes is unclear, injection of GVG into the nucleus tractus solitarius (NTS), a brainstem nucleus that receives baroreceptor afferents and is involved in autonomic control of blood pressure, produced a pressor effect (Tsukamoto and Sved 1993a; b) suggesting that GVG may augment methamphetamine-induced increases in blood pressure via inhibition of the NTS and loss of baroreceptor-mediated control of blood pressure.
In the current report, and as demonstrated previously (De La Garza et al. 2008; Newton et al. 2005; Newton et al. 2008; Newton et al. 2006), acute methamphetamine exposure increased self-reports of positive subjective effects, including Any Drug Effect, High, and Crave Methamphetamine. GVG treatment had no significant effects on subjective effects ratings as evaluated across the full time course or peak effects. In earlier research we found that a treatment (bupropion) that reduced the positive subjective effects of methamphetamine (Newton et al. 2006) also reduced methamphetamine use in subsequent clinical trials (Elkashef et al. 2008; Shoptaw et al. 2008) in a subgroup of participants who had lower level methamphetamine use at baseline. This suggests that treatment with GVG, which was not associated with alterations in the subjective effects of methamphetamine, might not be effective for the treatment of methamphetamine dependence. Although treatment-associated alterations in subjective effects have failed to predict clinical efficacy in studies of drug abuse in the past, it has been because they provided false-positive rather than false-negative predictions.
Human trials of medications that affect GABA systems have been performed with baclofen, gabapentin, topiramate, and tiagabine. An overview of these findings is provided here in order to present the status of developing agents that modulate GABAergic function for treatment of stimulant dependence.
Baclofen is a GABA-B receptor agonist that is used to treat spasticity. In a study of non-treatment-seeking volunteers who had recently used cocaine, baclofen had no significant effect on the reinforcing, subject-rated and cardiovascular effects of intranasal cocaine, nor did it have any effect on its own (Lile et al. 2004a). In an inpatient study of non-treatment-seeking, cocaine-dependent volunteers, baclofen did not alter cocaine's robust subjective effects (e.g., ‘High,’ ‘Stimulated’); but decreased self-administration of a low dose of smoked cocaine in participants who were not opioid-dependent, and decreased cocaine craving in methadone-maintained participants (Haney et al. 2006). Baclofen did not alter cocaine's robust subjective effects (e.g., ‘High,’ ‘Stimulated’) in either group. Consistent with these findings, baclofen treatment reduced cocaine use in an outpatient clinical trial, but showing a positive effect only in participants who used cocaine heavily before randomization (Shoptaw et al. 2003). More recently, the subjective and cardiovascular effects of cocaine were evaluated during treatment with the combination of amantadine and baclofen in cocaine-dependent, non-treatment-seeking individuals (Rotheram-Fuller et al. 2007). The data indicated no difference in the intensity of cocaine-induced euphoria, or reduction in the likelihood to use cocaine if given access during treatment. Only one trial has investigated the effects of baclofen as a treatment for methamphetamine dependence. In this 16-week, randomized, placebo-controlled, double-blind trial, there were no statistically significant main effects of baclofen to reduce methamphetamine use, but post-hoc analyses indicated a significant effect of baclofen (vs. placebo) in participants who reported taking a higher percentage of study medication (Heinzerling et al. 2006).
Gabapentin, originally synthesized to be a GABA receptor agonist, acts through mechanisms that are not completely known, but its administration results in increased brain GABA levels. In laboratory studies involving non-treatment-seeking, cocaine-dependent volunteers, gabapentin did not reduce cocaine choice or cardiovascular measures, but it did decrease some subjective effects and discriminative stimulus effects of smoked cocaine (Haney et al. 2005; Hart et al. 2007a; Hart et al. 2007b; Hart et al. 2004). These findings were corroborated in an outpatient clinical trial of cocaine-dependent, methadone-treated, volunteers; but gabapentin did not improve treatment retention and did not reduce cocaine use (Gonzalez et al. 2007). Further, in a 10-week outpatient study conducted using the Cocaine Rapid Efficacy and Safety Trial (CREST) study design of cocaine-dependent participants, gabapentin provided no positive data with primary outcome measures of efficacy that included urine benzoylecognine level (Berger et al. 2005), Cocaine Clinical Global Impression scale, and self-report of cocaine use. A subsequent study on the combination of gabapentin with relapse-prevention therapy for treatment of cocaine-dependence showed no greater efficacy of gabapentin than placebo, but a significant difference in the odds of cocaine use between high- and low-use groups indicated suggested that further study was warranted (Bisaga et al. 2006). The only study of gabapentin as a potential treatment for methamphetamine dependence was a 16-week, randomized, placebo-controlled, double-blind trial, which yielded no statistically significant main effects of gabapentin to reduce methamphetamine use (Heinzerling et al. 2006).
Tiagabine, a GABA reuptake inhibitor that increases synaptic levels of GABA was tested for acute effects on the discriminative-stimulus, reinforcing, subject-rated, performance and cardiovascular effects of oral cocaine in non-treatment seeking cocaine users; and the findings were negative (Lile et al. 2004b). However, when cocaine was given intravenously in the laboratory, tiagabine attenuated the subjective ratings of “stimulated” and “crave cocaine” in response to cocaine administration (Sofuoglu et al. 2005). Also, in a 10-week outpatient study conducted on cocaine-dependent participants, with the CREST study design, tiagabine-treated participants showed a trend toward a significant decrease in urine benzoylecognine level from baseline to weeks 5-8 (Winhusen et al. 2005). These outcomes were not replicated, however, in a 12-week trial clinical trial of tiagabine, when both tiagabine and placebo groups improved significantly on cocaine craving and global functioning, with no significant differences between the groups (Winhusen et al. 2007). Moreover, there were no significant changes in cocaine use as measured by self-report confirmed by urine benzoylecognine or by quantitative urine toxicology results. These outcomes differ from those of a 10-week double-blind placebo-controlled trial of cocaine-dependent, methadone-treated participants. In this study, participants randomized to tiagabine had significantly reduced cocaine taking behavior as compared to those treated with placebo (Gonzalez et al. 2007). To date, there are no published studies investigating tiagabine as a treatment for methamphetamine dependence.
Topiramate is an anticonvulsant that raises cerebral GABA levels and facilitates GABAergic neurotransmission. In a 13-week, double-blind, placebo-controlled pilot trial of topiramate for treatment of cocaine dependence, the results indicated that topiramate-treated participants were more likely to be abstinent from cocaine compared to those treated with placebo (Kampman et al. 2004). Later, in a study involving methamphetamine-dependent participants, topiramate significantly increased methamphetamine-induced stimulation and euphoria (Johnson et al. 2007).
In conclusion, the data in this report reveal that GVG treatment was generally well tolerated, but do not indicate efficacy for GVG in attenuating the positive subjective effects produced by methamphetamine in the laboratory. The potential for GVG to elevate cardiovascular parameters, especially during relapse to methamphetamine use, raises concerns regarding future outpatient clinical trials of GVG for methamphetamine dependence. At the least, these observations require any Phase II design to include careful monitoring of cardiovascular functioning, particularly during periods of relapse.
Acknowledgements
This work was supported by the National Institutes of Health (DA 18185; DA 022539; RR 00865). The authors wish to thank Rachel Fintzy, Elizabeth O'Laco and Rujvi Kamat for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Interest
None
References
- Berger SP, Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Leiderman DB, Montgomery MA, Goldsmith RJ, Bloch DA, Singal BM, Elkashef A. A medication screening trial evaluation of reserpine, gabapentin and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100(Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug and Alcohol Dependence. 2006;81:267–74. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Dewey SL. Treating cocaine addiction: from preclinical to clinical trial experience with gamma-vinyl GABA. Synapse. 2003;50:261–5. doi: 10.1002/syn.10278. [DOI] [PubMed] [Google Scholar]
- Brodie JD, Figueroa E, Laska EM, Dewey SL. Safety and efficacy of gamma-vinyl GABA (GVG) for the treatment of methamphetamine and/or cocaine addiction. Synapse. 2005;55:122–5. doi: 10.1002/syn.20097. [DOI] [PubMed] [Google Scholar]
- De La Garza R, Shoptaw S, Newton TF. Evaluation of the cardiovascular and subjective effects of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. International Journal of Neuropsychopharmacology. 2008;11:729–41. doi: 10.1017/S1461145708008456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey SL, Morgan AE, Ashby CR, Jr., Horan B, Kushner SA, Logan J, Volkow ND, Fowler JS, Gardner EL, Brodie JD. A novel strategy for the treatment of cocaine addiction. Synapse. 1998;30:119–29. doi: 10.1002/(SICI)1098-2396(199810)30:2<119::AID-SYN1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, Chiang N, Kahn R, Vocci F, Ling W, Pearce VJ, McCann M, Campbell J, Gorodetzky C, Haning W, Carlton B, Mawhinney J, Weis D. Bupropion for the treatment of methamphetamine dependence. Neuropsychopharmacology. 2008;33:1162–70. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fechtner RD, Khouri AS, Figueroa E, Ramirez M, Federico M, Dewey SL, Brodie JD. Short-term treatment of cocaine and/or methamphetamine abuse with vigabatrin: ocular safety pilot results. Archives of Ophthalmology. 2006;124:1257–62. doi: 10.1001/archopht.124.9.1257. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Ashby CR, Jr., Gardner EL, Mills MJ, Brodie JD, Dewey SL. Gamma-vinyl GABA inhibits methamphetamine, heroin, or ethanol-induced increases in nucleus accumbens dopamine. Synapse. 1999;34:11–9. doi: 10.1002/(SICI)1098-2396(199910)34:1<11::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez G, Desai R, Sofuoglu M, Poling J, Oliveto A, Gonsai K, Kosten TR. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine dependent methadone-treated patients. Drug and Alcohol Dependence. 2007;87:1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart C, Collins ED, Foltin RW. Smoked cocaine discrimination in humans: effects of gabapentin. Drug and Alcohol Dependence. 2005;80:53–61. doi: 10.1016/j.drugalcdep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–21. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Collins ED, Rubin E, Foltin RW. Smoked cocaine self-administration by humans is not reduced by large gabapentin maintenance doses. Drug and Alcohol Dependence. 2007a;86:274–7. doi: 10.1016/j.drugalcdep.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behavioral Pharmacology. 2007b;18:71–5. doi: 10.1097/FBP.0b013e328014139d. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Collins ED, Haney M, Foltin RW. Gabapentin maintenance decreases smoked cocaine-related subjective effects, but not self-administration by humans. Drug and Alcohol Dependence. 2004;73:279–87. doi: 10.1016/j.drugalcdep.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Shoptaw S, Peck JA, Yang X, Liu J, Roll J, Ling W. Randomized, placebo-controlled trial of baclofen and gabapentin for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2006;85:177–84. doi: 10.1016/j.drugalcdep.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Wells LT, Wallace CL, Dawes MA, Liu L, Wang XQ. Effects of acute topiramate dosing on methamphetamine-induced subjective mood. International Journal of Neuropsychopharmacology. 2007;10:85–98. doi: 10.1017/S1461145705006401. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O'Brien CP. A pilot trial of topiramate for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2004;75:233–40. doi: 10.1016/j.drugalcdep.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kushner SA, Dewey SL, Kornetsky C. The irreversible gamma-aminobutyric acid (GABA) transaminase inhibitor gamma-vinyl-GABA blocks cocaine self-administration in rats. Journal of Pharmacology and Experimental Therapeutics. 1999;290:797–802. [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PE, Hays LR, Rush CR. Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacology. 2004a;171:441–9. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Glaser PE, Hays LR, Rush CR. Acute administration of the GABA reuptake inhibitor tiagabine does not alter the effects of oral cocaine in humans. Drug Alcohol Depend. 2004b;76:81–91. doi: 10.1016/j.drugalcdep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Molina PE, Ahmed N, Ajmal M, Dewey S, Volkow N, Fowler J, Abumrad N. Co-administration of gamma-vinyl GABA and cocaine: preclinical assessment of safety. Life Sciences. 1999;65:1175–82. doi: 10.1016/s0024-3205(99)00351-3. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacology, Biochemistry and Behavior. 2005;82:90–7. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, Reid MS, De La Garza R, 2nd, Mahoney JJ, Abad A, Condos R, Palamar J, Halkitis PN, Mojisak J, Anderson A, Li SH, Elkashef A. Evaluation of subjective effects of aripiprazole and methamphetamine in methamphetamine-dependent volunteers. International Journal of Neuropsychopharmacology. 2008;11:1037–1045. doi: 10.1017/S1461145708009097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Paul M, Dewey SL, Gardner EL, Brodie JD, Ashby CR., Jr. Gamma-vinyl GABA (GVG) blocks expression of the conditioned place preference response to heroin in rats. Synapse. 2001;41:219–20. doi: 10.1002/syn.1078. [DOI] [PubMed] [Google Scholar]
- Rotheram-Fuller E, De La Garza R, 2nd, Mahoney JJ, 3rd, Shoptaw S, Newton TF. Subjective and cardiovascular effects of cocaine during treatment with amantadine and baclofen in combination. Psychiatry Research. 2007;152:205–10. doi: 10.1016/j.psychres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, De La Garza R, 2nd, Newton T, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2008;96:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Yang X, Rotheram-Fuller EJ, Hsieh YC, Kintaudi PC, Charuvastra VC, Ling W. Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. Journal of Clinical Psychiatry. 2003;64:1440–8. doi: 10.4088/jcp.v64n1207. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Poling J, Mitchell E, Kosten TR. Tiagabine affects the subjective responses to cocaine in humans. Pharmacology, Biochemistry and Behavior. 2005;82:569–73. doi: 10.1016/j.pbb.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Streeter CC, Hennen J, Ke Y, Jensen JE, Sarid-Segal O, Nassar LE, Knapp C, Meyer AA, Kwak T, Renshaw PF, Ciraulo DA. Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology. 2005;182:516–26. doi: 10.1007/s00213-005-0121-5. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Sved AF. Contrasting effects of the GABA transaminase inhibitors gamma-vinylGABA and aminooxyacetic acid on arterial pressure following injection into nucleus tractus solitarius. Neuropharmacology. 1993a;32:575–9. doi: 10.1016/0028-3908(93)90053-6. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993b;22:819–25. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP, Petroff OA, Hyder F, Zoghbi SS, Fujita M, Rajeevan N, Rothman DL, Seibyl JP, Mattson RH, Innis RB. Effects of vigabatrin on the GABAergic system as determined by [123I]iomazenil SPECT and GABA MRS. Epilepsia. 1999;40(10):1433–8. doi: 10.1111/j.1528-1157.1999.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Froestl W, Griffiths RR. Effects of GABAergic modulators on food and cocaine self-administration in baboons. Drug and Alcohol Dependence. 2005;80:369–76. doi: 10.1016/j.drugalcdep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Ciraulo DA, Harrer JM, Goldsmith RJ, Grabowski J, Coleman FS, Mindrum G, Kahn R, Osman S, Mezinskis J, Li SH, Lewis D, Horn P, Montgomery MA, Elkashef A. A double-blind, placebo-controlled trial of tiagabine for the treatment of cocaine dependence. Drug and Alcohol Dependence. 2007;91:141–8. doi: 10.1016/j.drugalcdep.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, Berger P, Elkashef A. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]