Abstract
Objective
Motivated by the central roles that VEGF and TGF-β play in the assembly and maintenance of the vasculature, we examined the impact of systemic VEGF or TGF-β signal inhibition on endothelial activation as detected by leukocyte-endothelial interactions.
Methods and Results
VEGF or TGF-β inhibition, accomplished using adenovirus expression of soluble Flt1 (Ad-sFlt1) or soluble endoglin (Ad-sEng), resulted in a significant increase in the number of leukocytes rolling along the mesenteric venous endothelium and a significant decrease in rolling velocity in Ad-sEng mice. Neutralization of VEGF or TGF-β resulted in endothelial surface expression of P-selectin and impaired peripheral vasodilatation. Neither inhibition of VEGF nor TGF-β was associated with platelet or leukocyte activation, as detected by the activation markers platelet P-selectin and the active integrin - αIIbβIII, or by leukocyte expression of L-selectin. Soluble VCAM-1 and E-selectin were increased in sEng-expressing mice, indicating higher levels of these adhesion receptors.
Conclusions
VEGF or TGF-β neutralization results in impaired endothelium-mediated vasodilatation and elevated expression of surface adhesion molecules, resulting in increased leukocyte adhesion. These results indicate an essential role for both VEGF and TGF-β in maintaining the endothelium in a non-activated state and have implications for therapeutic approaches that neutralize VEGF or TGF-β.
Keywords: VEGF, TGF-β, P-selectin, nitric oxide, inflammation
INTRODUCTION
A key property of the endothelium, which lines the entire vasculature, is maintenance of the non-thrombogenic and non-inflammatory vascular surface. During localized infection or injury the endothelium becomes activated, resulting in expression of adhesion molecules that prevent bleeding and mediate the inflammation that is associated with rolling of immune cells as well as their attachment and extravasation at the site of injury.1 These events are regulated by interplay between cytokines and adhesion receptors on both the endothelial cells (EC) and the circulating blood cells. Under non-pathological conditions the endothelium is equipped with several mechanisms to prevent thrombus formation and immune cell adhesion, including production of nitric oxide (NO),2 which acts as an anti-thrombotic factor, vasodilator, and modulator of leukocyte adhesion. Acute endothelial dysfunction is often characterized by impaired vascular autoregulation whereas chronic endothelial dysfunction leads to inflammation and altered expression of cell adhesion molecules.3
Emerging clinical and experimental observations suggest that VEGF is required for the maintenance of specialized EC stability and function.4, 5 Selective inhibition of VEGF in the kidney leads to thrombotic glomerular injury.6 Preeclampsia, a disease mediated in part by high levels of the circulating soluble Flt1 (sFlt1), which binds both VEGF and placental growth factor (PlGF), is associated with increased thrombosis7, 8 and leukocyte adhesion to endothelium.9 Interestingly, an increased incidence of thrombotic events has been reported in patients treated with the VEGF neutralizing drugs, bevacizumab (Avastin®)6, 10 and sunitinib.6, 11
TGF-β is also implicated in the pathogenesis of preeclampsia, and its associated systemic inflammation12. Circulating levels of soluble endoglin (sEng), a TGF-β1 inhibitor, are increased in preeclampsia. sEng also binds TGF-β3, which is expressed primarily by mesenchymal cells, macrophages and endothelial cells.13 Genetic knockout of TGF-β1 leads to a variety of abnormalities, the most prominent of which is multi-organ inflammation.14 Some of the clinical features of preeclampsia can be recapitulated by experimental VEGF and TGF-β1 inhibition in rodents, which results in the formation of microthrombi in the kidney,15 pancreas,16 and choroid plexus.4 While observations of endothelial dysfunction associated with experimental and clinical VEGF neutralization support a requirement for VEGF in vascular maintenance, the mechanism(s) by which VEGF maintains EC homeostasis in vivo is unknown. Similarly, though observations suggest a role for TGF-β1 signaling during inflammation,14, 17 the effect of systemic neutralization of TGF-β1 in healthy, non-pregnant mice has not been investigated.
In light of the known roles of VEGF and TGF-β in vessel integrity,18, 19 we sought to investigate the effect of their neutralization on the endothelial-blood interface in vivo. Mesenteric venules in mice are very accessible to visualization using intravital microscopy, and previous studies demonstrating both TGF-β1 and VEGF expression in mesenteric vessels indicate that this vascular bed is appropriate for these investigations. Expression of Ad-sFlt1 or Ad-sEng led to increased numbers of rolling leukocytes, as well as elevated expression of P-selectin at the EC surface. Peripheral blood flow studies revealed impaired endothelial-dependent vascular autoregulation, suggesting that VEGF or TGF-β1 neutralization leads to systemic vascular endothelial dysfunction.
MATERIALS AND METHODS
An expanded methods section is provided in the supplemental Methods.
Animals
Animal experiments were carried out at the Immune Disease Institute and at the Schepens Eye Research Institute in accordance with IACUC guidelines. For intravital microscopy, three to four week old Balb/c mice were injected intravenously on day 0 with 1×1010 viral particles (V.P.) Ad-CMV-null control (Ad-null), 2.5×109 V.P. Ad-CMV-sFlt1 (Ad-sFlt1) or 2.5×109 VP Ad-CMV-sEng (Ad-sEng) (Q·Biogene). For vascular autoregulation studies, six to eight week old CD-1 mice were used.
Analysis of platelet and leukocyte concentration and activation
Leukocyte number was determined using a Z1 particle counter (Coulter). Platelets in whole blood were analyzed by fluorescent activated cell sorting (FACS) for the levels of P-selectin and αIIbβIII in its active conformation using anti-P-selectin-FITC (BD-Pharmingen) and JONA-PE (Emfret Analytics) antibodies, respectively. To determine leukocyte activation, L-selectin (BD-Pharmingen) levels on Mac-1 (anti-Mac-1; BD-Pharmingen) positive leukocytes were detected by FACS following RBC lysis.
Analysis of circulating adhesion molecules
Plasma levels of circulating soluble P-selectin (sP-selectin), soluble E-selectin (sE-selectin) and soluble VCAM-1 (sVCAM-1) were assayed by enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions (R&D Systems) and circulating von Willebrand factor (VWF) was determined as described.20
Intravital microscopy
Phase contrast and fluorescence intravital microscopy were utilized to measure the velocity of leukocyte and expression of surface P-selectin as described.20, 21
Vascular autoregulation
After seven days of adenoviral expression, blood flow rates were recorded non-invasively in the tail vein using a CODA6 BP device (Kent Scientific), according to manufacturer’s instructions. This device measures tail vein blood flow by recording the volume (ml) that enters the tail vein between the systolic and diastolic measurements. Each cycle measurement corresponds to 20 seconds. After baseline values were determined, mice were injected retroorbitally with saline as a control or with 10 µg/kg acetylcholine (ACh) (Calbiochem), an endothelial-dependent relaxant, and recordings were taken until values returned to baseline levels. Values were averaged in 5-cycle intervals pre- and post-injection and normalized to baseline flow values per animal.
FACs
Bovine aortic endothelial cells (BAEC) were grown to confluence in DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 1x insulin-transferrin-sodium selenium (ITS), maintained in a humidified atmosphere of 5% CO2 / 95% air, and routinely used between passages 4 to 8.
For analysis of surface P-selectin, BAEC were grown in DMEM containing 1% FBS for 24 hr before addition of VEGF (10 ng/ml) or TGF-β1 (1 ng/ml) for 48 hr. Inhibitors of signaling pathways that were used include the p38 inhibitor PD169316 (Sigma P9248), the JNK inhibitor SP600125 (Sigma S5567) and an ERK inhibitor (Sigma A6355). BAEC collected by trysinization and centrifugation at 100 × g for 4 min. BAEC from each well of a 12-well plate were resuspended in 350 ul of FBS buffer (10% FBS in PBS) containing 7 ul of FITC-labeled P-selectin antibody (BD Biosciences cat. No 553744). The cells were gently rotated in the dark for 20 min, washed and resuspended in FBS buffer, then analyzed by FACS using a FACSCAN flow cytometer.
Statistical analysis
The values are presented as mean ± SEM. Statistical significance was calculated using a student unpaired T-test. P values <0.05 were considered statistically significant.
RESULTS
Effect of VEGF and TGF-β neutralization on leukocyte number and rolling
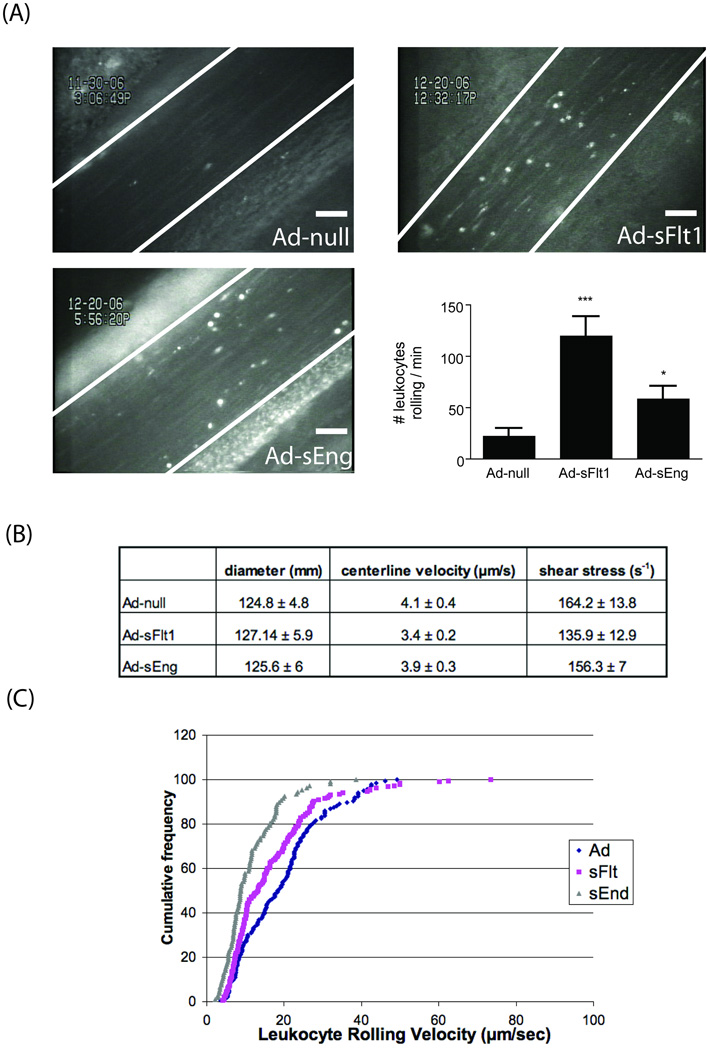
To determine the activation state of the endothelium during early stages of VEGF or TGF-β neutralization, intravital microscopy was used to observe the behavior of leukocytes in mesenteric venules. Following seven days of Ad-sFlt1, Ad-sEng or Ad-null (control) expression, there was a statistically significant increase in the number of rolling leukocytes on the endothelium of Ad-sFlt1 (120 +/−19 leukocytes/min, n=10) and Ad-sEng expressing mice (58 +/−13 leukocytes/min, n=10) when compared to Ad-null mice (22 +/−8 leukocytes/min, n=7) (Figure 1A).
Figure 1.
(A) TGF-β or VEGF neutralization increased the number of rolling leukocytes on mesenteric endothelium. Scale = 50 µm. *p<0.05, ***p<0.01 (B) Doppler measurement of blood velocity (µm/s) and shear (s−) showed similar values in each group. (C) Quantification of leukocyte rolling velocity revealed decreased velocity in sEng-expressing mice.
Since blood velocity and shear stress may influence leukocyte rolling on the endothelium, we measured mesenteric shear rates on the endothelial surface using a Doppler flow meter; veins with average shear rate of 150s- were analyzed. There was no significant difference in shear rates among Ad-sFlt1, Ad-sEng and control mice (Ad-null) (Figure 1B). The velocity of rolling leukocytes is critical to inflammation since rolling slows before stable adhesion to the endothelium and extravasation through the vessel wall.22 Quantification of leukocyte rolling velocity revealed no change in the Ad-sFlt1 mice, however, median rolling velocity decreased in Ad-sEng expressing mice (8.14 µm/sec) when compared to control Ad-null injected mice (11.72 µm/s).
Effects of VEGF and TGF-β neutralization on activation of leukocytes and platelets
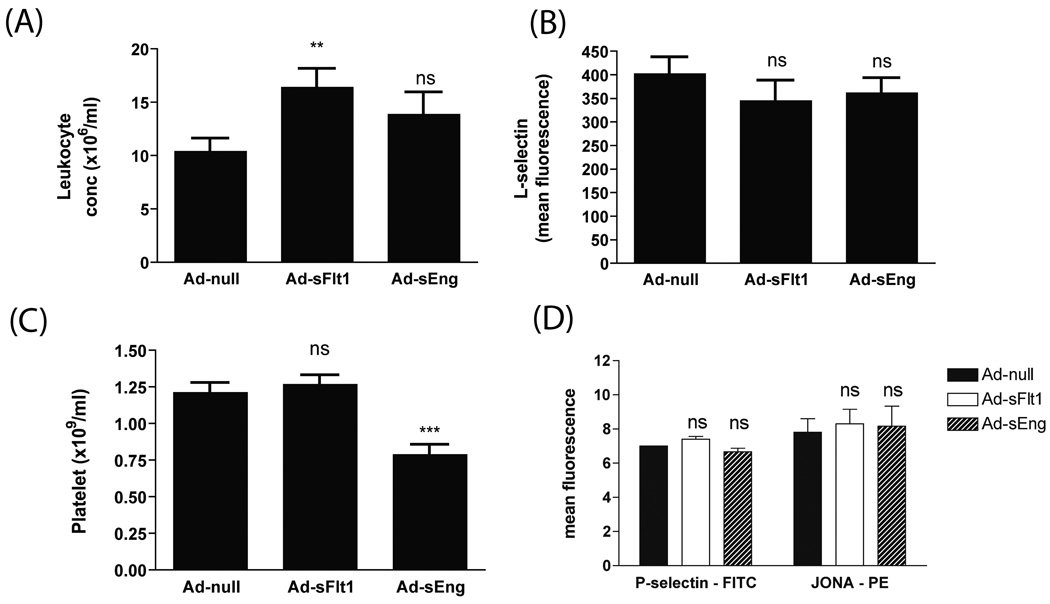
Since increased leukocyte rolling number may also result from an elevated number of circulating leukocytes, the concentration of leukocytes in the blood was examined. VEGF neutralization led to an increase in the number of peripheral leukocytes (Ad-sFlt1: 15.9 +/−1.5 × 106 /ml, n=24) compared to control (Ad-null: 11 +/−0.8/ × 106 /ml, n=23); leukocyte concentration was not significantly altered in sEng-expressing mice (Ad-sEng: 14.5 +/−1.9 × 106 /ml, n=20) (Figure 2A). Although there was a 40% increase in leukocyte concentration in Ad-sFlt1 mice, the number of rolling leukocytes was increased by 500% so that the increased rolling cannot be due solely to increased concentration.
Figure 2.
(A) Neutralization of VEGF, but not TGF-β, increased circulating leukocyte numbers. **p<0.01 (B) Activation status of leukocytes, as detected by L-selectin surface expression, revealed no change in sFlt1- or sEng-expressing mice. (C, D) Platelet counts decreased in sEng expressing mice, whereas FACS analysis revealed no change in surface platelet P-selectin or αIIbβIII in its active conformation. ***p<0.0001
Examination of the surface expression of L-selectin on Mac-1-positive leukocytes, as a reflection of their activation state, revealed no change in the levels of L-selectin, indicating that the leukocytes were not activated (Figure 2B). In light of the ability of activated platelets to induce Weibel-Palade body secretion and leukocyte rolling via endothelial P-selectin expression,20 we examined the concentration and activation state of platelets. Overexpression of Ad-sFlt1 did not alter platelet concentration (Ad-sFlt: 1.26 +/− 0.06×109/ml; n=24, versus Ad-null: 1.21 +/− 0.07 ×109/ml; n=23), whereas Ad-sEng-expressing mice exhibited decreased platelet numbers when compared to Ad-null mice (Ad-sEng: 0.78 +/−0.08 ×109/ml; n=20) (Figure 2C). FACS analysis revealed no change in the surface levels of P-selectin (FITC) (Ad-sFlt: 7.4 +/−0.2, n=10; Ad-sEng: 6.7 +/−0.2, n=6; Ad-null: 7 +/−0; n=5) or activated αIIbβ3 (JONA-PE) (Ad-sFlt: 8.3 +/−0.9, n=10; Ad-sEng: 8.2 +/−1.2, n=6; Ad-null: 7.8 +/−0.8; n=5) on circulating platelets in the sFlt1 and the sEng-overexpressing mice compared to control mice (P>0.05) (Figure 2D), indicating that platelets were not activated.
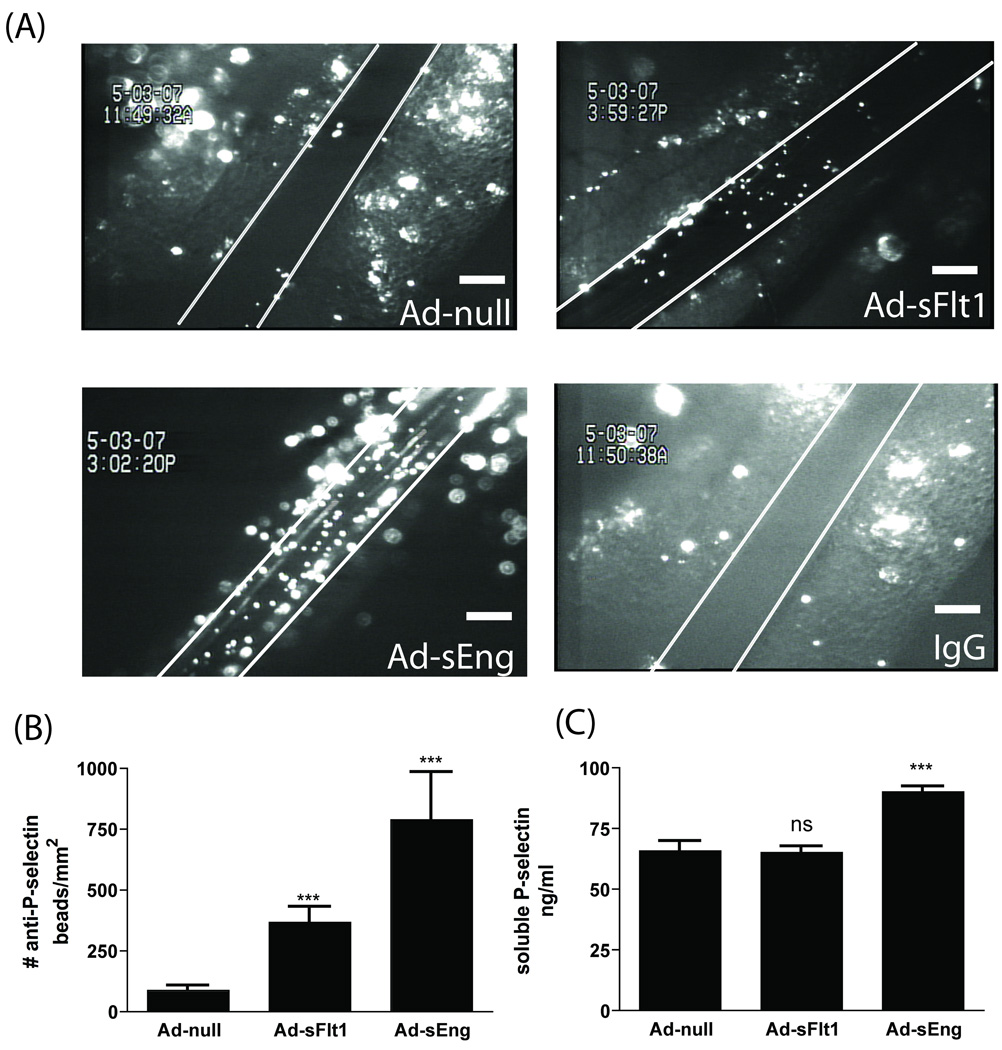
Leukocyte rolling can also be mediated by endothelial surface expression of P-selectin. Injection of anti-P-selectin-conjugated beads (or IgG-conjugated beads as a control) to visualize P-selectin on the endothelial surface revealed a significant increase in P-selectin in both Ad-sFlt1 (367.2 +/−63.0 /µm2, n=12) and Ad-sEng-expressing (789.1 +/−195.0/µm2, n=8) mice compared to Ad-null mice (85.9 +/−21.5 /µm2, n=12) (Figure 3A and 3B). There was virtually no adherence of control-IgG conjugated beads to the venules in any treatment group (Figure 3A). The significant increase in levels of P-selectin on the endothelial cell surface in Ad-sEng mice was accompanied by an increase in sP-selectin (90.12 +/−2.1 ng/ml) when compared to Ad-null injected mice (65.8 +/−3.9 ng/ml) (Figure 3C) that was not seen in the sFlt1-expressing mice (65.1 +/−2.4 ng/ml).
Figure 3.
(A) VEGF and TGF-β neutralization increased mesenteric venule P-selectin surface expression. Scale = 100 µm. (B) Quantification of (A). (C) Circulating sP-selectin levels increased in sEng expressing mice compared to control (Ad-null). ***p<0.001
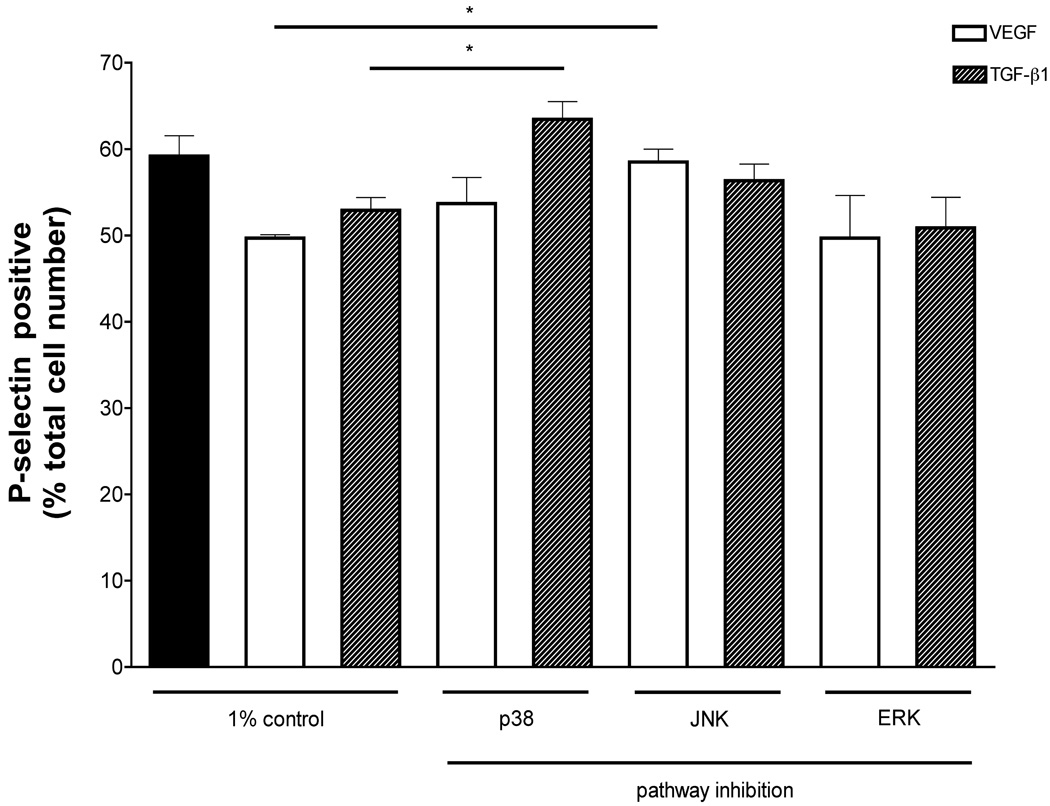
Role of mitogen activated protein (MAP) kinase signaling in the modulation of endothelial surface P-selectin
We examined the role of MAP kinase signaling in increased endothelial surface P-selectin using flow cytometry of endothelial cells in vitro. Addition of VEGF (10 ng/ml) or TGF-β1 (1 ng/ml) to BAEC significantly decreased surface P-selectin (control: 59.18%; VEGF: 49.70%; TGF-β1: 52.90%) (Figure 4). The effect of p38, JNK and ERK inhibition on VEGF- or TGF-β-induced changes in surface P-selectin levels was determined. BAEC were grown in reduced serum +/− VEGF or TGF-β in the absence or presence of 1 µM PD169316, 1 µM SP600125 or 10 µM 3-(2-aminoethyl)-5-((4-ethoxyphenyl) methylene)-2,4-thiazolidinedione hydrochloride, specific inhibitors of p38, JNK and ERK, respectively. PD169316 significantly attenuated TGF-β1-induced changes in BAEC surface P-selectin (63.45%), whereas SP600125 lead to a significant reduction of VEGF-induced changes in BAEC surface P-selectin (58.90%) (Figure 4).
Figure 4.
Role of MAP kinase signaling in endothelial surface P-selection. FACs analysis revealed that VEGF (10 ng/ml) or TGF-β1 (1 ng/ml) significantly decreased surface P-selectin on BAEC. Pharmacological inhibition of p38 prevented TGF-β-induced changes, whereas inhibition of JNK blocked VEGF modulation of BAEC surface P-selectin.
Effects of VEGF and TGF-β neutralization on other markers of endothelial activation
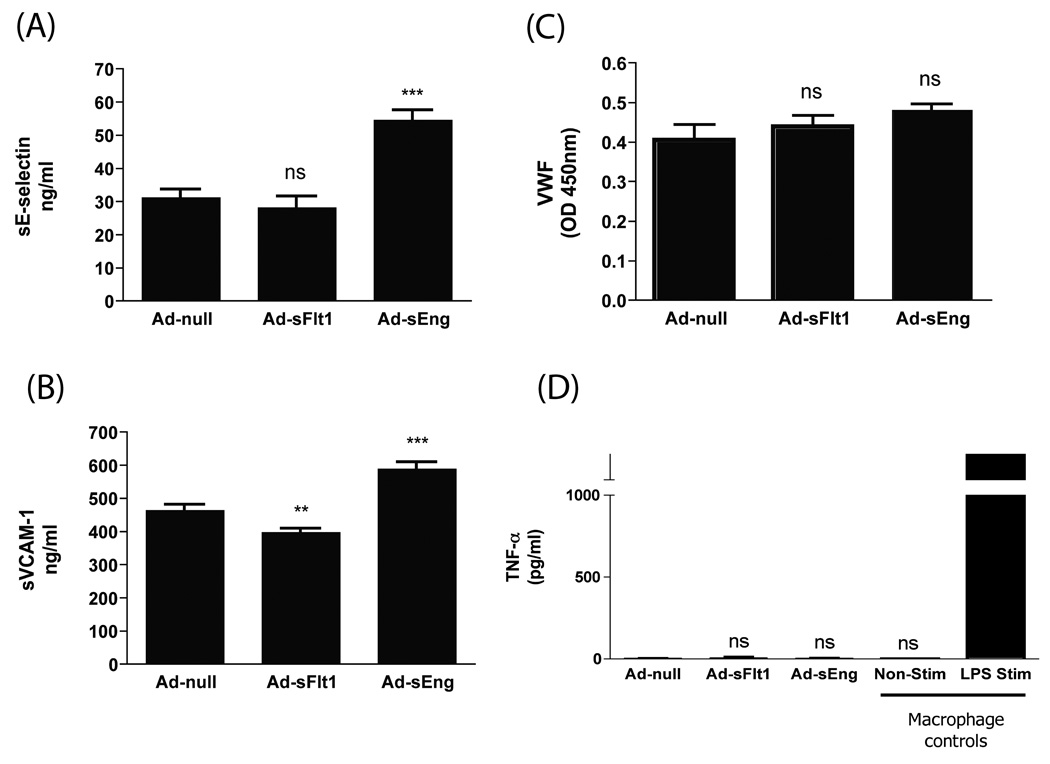
Endothelial activation leads to surface expression of P-selectin and release of VWF into the plasma, and can be succeeded by expression of E-selectin and VCAM-1 as inflammation progresses.3, 22 We therefore examined the plasma levels of sE-selectin, sVCAM-1 and VWF in Ad-sFlt1- and Ad-sEng-expressing mice. When compared to Ad-null mice (31.06 +/−2.473 ng/ml), sE-selectin levels in Ad-sFlt1-expressing mice (28.11 +/−3.323 ng/ml) were unchanged (Figure 4A). However, sE-selectin levels were significantly increased in Ad-sEng-expressing mice (54.37 +/−3.069 ng/ml) (Figure 5A). Similarly, sVCAM-1 was elevated in Ad-sEng-expressing mice (587.7 +/−20.53 ng/ml), compared to control Ad-null mice (462.7 +/−16.84 ng/ml) (Figure 5B). In contrast, circulating sVCAM-1 was slightly decreased in Ad-sFlt1-expressing mice compared to control (396.6 +/−11.63 ng/ml) (Figure 5B). No changes were apparent in circulating VWF in Ad-sFlt1-expressing (0.437 +/−0.028 ng/ml, n=3) or Ad-sEng-expressing mice (0.506 +/−0.032 ng/ml, n=6) compared to Ad-null mice (0.403 +/−0.046 ng/ml, n=7) (Figure 5C), though a trend of increasing VWF in Ad-sEng-expressing mice was noted. TNF-α levels were not increased in either Ad-sEng- or Ad-sFlt1-expressing mice compared to Ad-null (Figure 5D). Controls for the TNF-α assay were culture media from non-stimulated and LPS-stimulated macrophages, which released more than 1500 pg/ml TNF-α.
Figure 5.
Effect of VEGF and TGF-β neutralization on circulating adhesion molecules. (A) E-selectin, (B) VCAM-1, (C) VWF and (D) TNF-α. Neutralization of TGF-β increased circulating E-selectin and VCAM-1. Neutralization of VEGF decreased circulating VCAM-1. **p<0.001; ***p<0.0001
Effect of VEGF and TGF-β neutralization on endothelial autoregulation
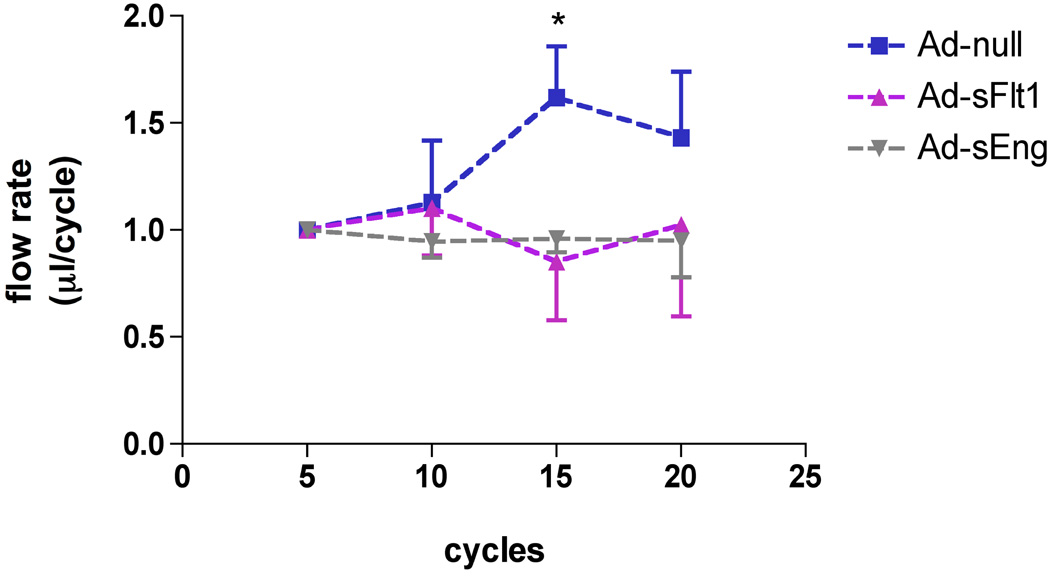
To indirectly determine the effect of VEGF and TGF-β neutralization on endothelial nitric oxide (NO) formation, we measured the peripheral blood flow autoregulatory response.23 Flow rates in the tail-vein were measured in response to intravascular injection of ACh, an endothelium-dependent vasodilator that increases local endothelial release of NO. As expected, tail vein flow rates were transiently increased following ACh in Ad-null mice (1.619 +/−0.107 ml/cycle, n=5) (Figure 6) as compared to both baseline flow rates (pre-injection) or to saline injection (data not shown). In contrast, there was no change in blood flow rates in response to ACh in Ad-sFlt1- (0.852 +/−0.112 ml/cycle, n=4) or Ad-sEng-expressing mice (0.960 +/−0.032 ml/cycle; n=4) (Figure 6). This impaired autoregulation suggests that VEGF or TGF-β signaling is necessary to maintain normal vascular autoregulation.
Figure 6.
Effect of TGF-β or VEGF neutralization on endothelial autoregulation. Blood flow rates in mice tail vein were measured in response to ACh. In Ad-null mice, ACh increased tail-vein blood flow rates 11–15 cycles post-injection, whereas blood flow rates were unchanged in Ad-sFlt1 or Ad-sEng-expressing mice. *p<0.01
DISCUSSION
The quiescent vascular endothelium maintains a non-thrombogenic and non-inflammatory surface. At the same time, the endothelium plays an important regulatory role in the inflammatory response by expressing factors that modulate the surface adherence of leukocytes and platelets.24 There is increasing evidence that VEGF and TGF-β are required for endothelial stability in vivo.4, 5, 15 In this study, we demonstrate that VEGF and TGF-β are important modulators of endothelial activation. Systemic VEGF neutralization resulted in increased numbers of rolling leukocytes, as well as an elevated number of peripheral blood leukocytes. These results are consistent with the previous observation that administration of VEGF attenuates leukocyte-endothelial interactions in an experimental model of endothelial activation25 and with the systemic inflammation associated with preeclampsia where circulating sFlt1 is elevated.15 Similarly, patients with age-related macular degeneration treated intravitreally with the anti-VEGF drug, Avastin, display an enhanced local inflammatory response and increased leukocyte infiltration of choroidal neovessels.26
In contrast to these descriptions of VEGF’s anti-inflammatory actions, several reports describe that VEGF treatment of endothelial cells in culture leads to induction of endothelial adhesion factors.27–29 Transgenic overexpression of VEGF in the epidermis of mice can induce a psoriasis-like phenotype in a mouse model of inflammation.30 Whereas circulating levels of VEGF are reportedly in the range of 10–70 pg/ml,31–33 tissue culture studies utilize 1–25 ng/ml VEGF. The transgenic over-expression of VEGF likely leads to relatively high VEGF levels and may explain why the effects are more similar to the pro-inflammatory actions seen in the tissue culture studies.
Neutralization of TGF-β led to an increase in the number of leukocytes rolling on the EC surface, and a decreased leukocyte velocity. Consistent with these findings, TGF-β1 treatment of cultured endothelial cells led to decreased leukocyte adhesion34, 35 and increased TGF-β1 in tumor microvessels is associated with decreased leukocyte-endothelial interactions.36, 37 Similarly, TGF-β1 null mice display marked immune cell infiltration in the heart, lung, liver, stomach, the CNS and pancreas,38 and mice lacking the TGF-β1 activators, thrombospondin-1 or the integrin β6 subunit, display a similar phenotype with multi-organ inflammation and infiltration.39, 40
Since neither platelet nor leukocyte activation was detected with VEGF and TGF-β neutralization, activation appears to be at the level of the endothelium. Increased levels of TNF-α were not detected in mice overexpressing sEng or sFlt1, indicating the observed endothelial activation is not due to adenoviral-induced inflammation. Rather, these results reflect a role for basal levels of endothelial VEGF and TGF-β signaling in the maintenance of endothelial quiescence.
TGF-β neutralization also led to a decreased number of circulating platelets, suggesting that circulating TGF-β may contribute to the maintenance of platelet numbers. Previous accounts demonstrate both inhibitory and stimulatory effects of TGF-β1 on megakaryopoiesis, depending on growth factor concentration and presence of other growth factors.41, 42 Interestingly, the adhesive interactions of megakaryocytes with the vessel wall also impacts megakaryopoiesis in the bone marrow, with P-selectin but not E-selectin deletion resulting in expansion of megakaryocyte progenitors and immature megakaryoblasts, and with enhanced release of TGF-β1,43 suggesting a role for TGF-β1 in platelet production. Finally, we have previously observed microthrombi in the microvasculature of the choroid plexus of mice expressing sFlt1, raising the possibility that the reduced platelet number could also be due to increased consumption and/or clearance.
Under normal conditions, leukocytes interact minimally with the endothelium. However, upon endothelial activation, surface expression of adhesion molecules results in leukocyte-endothelial interaction, leading to leukocyte extravasation. Neutralization of VEGF or TGF-β led to increased endothelial surface P-selectin levels and increased leukocyte rolling. Changes in the plasma concentration of soluble adhesion molecules reflect altered cell surface turnover and proteolytic cleavage. P-selectin is found in both EC and platelets and activation of either can contribute to an increase in soluble P-selectin in plasma.44 The elevated sP-selectin observed with TGF-β neutralization was likely derived from EC as P-selectin levels on platelets were unchanged. On the other hand, despite increased EC surface P-selectin in sFlt-1 expressing mice, sP-selectin levels were unchanged. This finding can be explained by the reported role of VEGF in the shedding of E-selectin.27 Despite increased endothelial P-selectin in mice overexpressing sFlt1 and sEng, we did not note any thrombosis in the mesenteric venules, however we have previously reported microthrombosis in the choroid plexus of mice expressing sFlt1.4
TGF-β1 has been reported to suppress neutrophil recruitment in vitro via decreased endothelial E-selectin35, 45 and induction of E-selectin is blocked by pretreatment of endothelial cells with TGF-β1.46 The observed increase in sE-selectin in sEng-expressing mice corroborates these observations and provides insight into the mechanism by which baseline circulating TGF-β contributes to maintaining the endothelium in a quiescent state. The presence of circulating TGF-β1 (<5 ng/ml in mice47 and ∼170 pg/ml in humans48), suggests a role for a basal level of TGF-β1 in maintaining endothelial homeostasis.
Numerous studies describe the role of the MAP kinase pathways (p38, JNK, ERK) in VEGF or TGF-β signaling in endothelial cells, however the relative contribution of MAPKs in maintaining an anti-inflammatory endothelium has not been described. We demonstrate that basal levels of VEGF limit P-selectin on the endothelial surface via the JNK pathway, but not the p38 or ERK pathway, whereas TGF-β1 acts through the p38 pathway, but not ERK or JNK.
Endothelial production of NO plays a central role in maintaining the endothelium in a non-thrombogenic and non-activated state.49 NO synthesis in the endothelium is mediated by the endothelial nitric oxide synthase (eNOS). VEGF and TGF-β receptors interact with eNOS in the caveolae of normal EC to regulate eNOS enzymatic activity.50, 51 Imbalance of NO leads to deficient arteriolar dilation so that the impaired EC autoregulation observed upon VEGF and TGF-β neutralization is likely mediated via reduced eNOS expression. Consistent with our observations, experimental systemic NO inhibition has been shown to lead to endothelial activation and leukocyte adhesion, effects that were reversible with VEGF administration.52 Recent evidence suggests infusion of ACh can also lead to vasodilation by inducing formation of prostaglandins and P450 eicosanoids,53, 54 though the vasodilatory effects of P450 eicosanoids are potentially through induction of NO release from ECs.55 Since both VEGF and TGF-β1 induce EC to release NO, and since previous studies demonstrate that NO prevents leukocytes adhering to the EC surface,56 we speculated that the increased endothelial P-selection observed in the sFlt1- and sEng-expressing mice may be due to reduced endothelial NO production. However, addition of the NO inhibitor L-NAME did not block the ability of VEGF or TGF-β1 to limit EC surface P-selectin in vitro (data not shown), suggesting that NO production downstream of VEGF or TGF-β1 is not the mechanism limiting P-selectin at the endothelial membrane.
Preeclampsia, which is characterized by high levels of circulating sFlt-1 and sEng, is associated with an increase in cytokine levels, endothelial cell damage,57 platelet activation,58 as well as elevations in P-selectin,59, 60 ICAM-1, VCAM-1 and E-selectin.61 In addition, plasma from patients with preeclampsia significantly increases VCAM-1 expression62 and leukocyte adhesion to endothelial cells in vitro.9, 63 In our model of systemic adenoviral expression of sEng and sFlt, concentrations of sEng and sFlt are similar to those reported in the serum of preeclampsia patients,64, 65 and thus our observations support the increasing evidence that inhibition of VEGF and TGF-β1 mediates much of the pathology in preeclampsia.
Endothelial dysfunction is the basis of many cardiovascular pathologies. Our data suggest that basal circulating levels of VEGF and TGF-β contribute to vascular stability by maintaining the endothelium in a quiescent state. Understanding the molecular mechanisms that underlie endothelial dysfunction following VEGF or TGF-β neutralization in vivo will provide insight into the role of these growth factors in vascular homeostasis and may provide strategies to circumvent side effects associated with therapies targeting VEGF or TGF-β.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grant EY05318 to PAD, POI HL066105 to DDW and Knights Templar Eye Foundation award to TEW.
We thank Mrs. Christine Bagley for her editorial assistance with the preparation of the manuscript.
Footnotes
The authors have no conflicting financial interests.
REFERENCES
- 1.Lentsch AB, Ward PA. Regulation of inflammatory vascular damage. J Pathol. 2000;190:343–348. doi: 10.1002/(SICI)1096-9896(200002)190:3<343::AID-PATH522>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 3.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, Matharu KS, Karumanchi SA, D’Amore PA. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 6.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006;33:S26–S34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 9.Ryu S, Huppmann AR, Sambangi N, Takacs P, Kauma SW. Increased leukocyte adhesion to vascular endothelium in preeclampsia is inhibited by antioxidants. Am J Obstet Gynecol. 2007;196(400):e401–e407. doi: 10.1016/j.ajog.2006.12.023. discussion 400 e407–408. [DOI] [PubMed] [Google Scholar]
- 10.Roncone D, Satoskar A, Nadasdy T, Monk J, Rovin B. Proteinuria in a patient receiving anti-VEGF therapy for metastatic renal cell carcinoma. Nat Clin Pract Nephrol. 2007;3:287–293. doi: 10.1038/ncpneph0476. [DOI] [PubMed] [Google Scholar]
- 11.Bollée G, Patey N, Cazajous G, Robert C, Goujon JM, Fakhouri F, Bruneval P, Noël LH, Knebelmann B. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2008 doi: 10.1093/ndt/gfn657. [DOI] [PubMed] [Google Scholar]
- 12.Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. 2007;28:192–209. doi: 10.1016/j.mam.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Agrotis A, Kalinina N, Bobik A. Transforming growth factor-beta, cell signaling and cardiovascular disorders. Curr Vasc Pharmacol. 2005;3:55–61. doi: 10.2174/1570161052773951. [DOI] [PubMed] [Google Scholar]
- 14.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SK, Yang WS, Lee SK, Ahn H, Park JS, Hwang O, Lee JD. TGF-beta(1) down-regulates inflammatory cytokine-induced VCAM-1 expression in cultured human glomerular endothelial cells. Nephrol Dial Transplant. 2000;15:596–604. doi: 10.1093/ndt/15.5.596. [DOI] [PubMed] [Google Scholar]
- 18.Zhu ZS, Wang JM, Chen SL. Mesenteric artery remodeling and effects of imidapril and irbesartan on it in spontaneously hypertensive rats. World J Gastroenterol. 2004;10:1471–1475. doi: 10.3748/wjg.v10.i10.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geerts AM, De Vriese AS, Vanheule E, Van Vlierberghe H, Mortier S, Cheung KJ, Demetter P, Lameire N, De Vos M, Colle I. Increased angiogenesis and permeability in the mesenteric microvasculature of rats with cirrhosis and portal hypertension: an in vivo study. Liver Int. 2006;26:889–898. doi: 10.1111/j.1478-3231.2006.01308.x. [DOI] [PubMed] [Google Scholar]
- 20.Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayadas TN, Johnson RC, Rayburn H, Hynes RO, Wagner DD. Leukocyte rolling and extravasation are severely compromised in P selectin-deficient mice. Cell. 1993;74:541–554. doi: 10.1016/0092-8674(93)80055-j. [DOI] [PubMed] [Google Scholar]
- 22.Ley K, Laudanna C, Cybulsky M, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 23.Henry E, Newby DE, Webb DJ, O’Brien C. Peripheral endothelial dysfunction in normal pressure glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1710–1714. [PubMed] [Google Scholar]
- 24.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 25.Scalia R, Booth G, Lefer DJ. Vascular endothelial growth factor attenuates leukocyte-endothelium interaction during acute endothelial dysfunction: essential role of endothelium-derived nitric oxide. Faseb J. 1999;13:1039–1046. doi: 10.1096/fasebj.13.9.1039. [DOI] [PubMed] [Google Scholar]
- 26.Tatar O, Yoeruek E, Szurman P, Bartz-Schmidt KU, Group TBS, Adam A, Shinoda K, Eckardt C, Boeyden V, Claes C, Pertile G, Scharioth GB, Grisanti S. Effect of bevacizumab on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2008;126:782–790. doi: 10.1001/archopht.126.6.782. [DOI] [PubMed] [Google Scholar]
- 27.Stannard AK, Khurana R, Evans IM, Sofra V, Holmes DI, Zachary I. Vascular endothelial growth factor synergistically enhances induction of E-selectin by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 2007;27:494–502. doi: 10.1161/01.ATV.0000255309.38699.6c. [DOI] [PubMed] [Google Scholar]
- 28.Kim I, Moon SO, Park SK, Chae SW, Koh GY. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circulation Research. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 29.Kim I, Moon SO, Kim SH, Kim HJ, Koh YS, Koh GY. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001;276:7614–7620. doi: 10.1074/jbc.M009705200. [DOI] [PubMed] [Google Scholar]
- 30.Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- 31.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz V, Vilanueva H, Raskopf E, Hilbert T, Barajas M, Dzienisowicz C, Gorschlüter M, Strehl J, Rabe C, Sauerbruch T, Prieto J, Caselmann W, Qian C. Increased VEGF levels induced by anti-VEGF treatment are independent of tumor burden in colorectal carcinomas in mice. Gene Ther. 2006;13:1198–1205. doi: 10.1038/sj.gt.3302772. [DOI] [PubMed] [Google Scholar]
- 34.Gamble JR, Vadas MA. Endothelial cell adhesiveness for human T lymphocytes is inhibited by transforming growth factor-beta 1. J Immunol. 1991;146:1149–1154. [PubMed] [Google Scholar]
- 35.Gamble JR, Khew-Goodall Y, Vadas MA. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J Immunol. 1993;150:4494–4503. [PubMed] [Google Scholar]
- 36.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 37.Gohongi T, Fukumura D, Boucher Y, Yun CO, Soff GA, Compton C, Todoroki T, Jain RK. Tumor-host interactions in the gallbladder suppress distal angiogenesis and tumor growth: involvement of transforming growth factor beta1. Nat Med. 1999;5:1203–1208. doi: 10.1038/13524. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni AB, Ward JM, Yaswen L, Mackall CL, Bauer SR, Huh CG, Gress RE, Karlsson S. Transforming growth factor-beta 1 null mice. An animal model for inflammatory disorders. Am J Pathol. 1995;146:264–275. [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 40.Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr, Sheppard D. Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol. 1996;133:921–928. doi: 10.1083/jcb.133.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson H, Williams N, Westcott KR, Green R. Differential effects of transforming growth factor-beta 1 on distinct developmental stages of murine megakaryocytopoiesis. J Cell Physiol. 1994;161:312–318. doi: 10.1002/jcp.1041610216. [DOI] [PubMed] [Google Scholar]
- 42.Kuter DJ, Gminski DM, Rosenberg RD. Transforming growth factor beta inhibits megakaryocyte growth and endomitosis. Blood. 1992;79:619–626. [PubMed] [Google Scholar]
- 43.Banu N, Avraham S, Avraham HK. P-selectin, and not E-selectin, negatively regulates murine megakaryocytopoiesis. J Immunol. 2002;169:4579–4585. doi: 10.4049/jimmunol.169.8.4579. [DOI] [PubMed] [Google Scholar]
- 44.Dole VS, Bergmeier W, Patten IS, Hirahashi J, Mayadas TN, Wagner DD. PSGL-1 regulates platelet P-selectin-mediated endothelial activation and shedding of P-selectin from activated platelets. Thromb Haemost. 2007;98:806–812. [PubMed] [Google Scholar]
- 45.Thornhill MH, Haskard DO. IL-4 regulates endothelial cell activation by IL-1, tumor necrosis factor, or IFN-gamma. J Immunol. 1990;145:865–872. [PubMed] [Google Scholar]
- 46.Melrose J, Tsurushita N, Liu G, Berg EL. IFN-gamma inhibits activation-induced expression of E- and P-selectin on endothelial cells. J Immunol. 1998;161:2457–2464. [PubMed] [Google Scholar]
- 47.Kanzler S, Lohse AW, Keil A, Henninger J, Dienes HP, Schirmacher P, Rose-John S, zum Büschenfelde KH, Blessing M. TGF-beta1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol. 1999;276:G1059–G1068. doi: 10.1152/ajpgi.1999.276.4.G1059. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson EE, Werr J, Guo Y, Thoren P, Lindbom L. Direct observations in vivo on the role of endothelial selectins and alpha(4) integrin in cytokine-induced leukocyte-endothelium interactions in the mouse aorta. Circ Res. 2000;86:526–533. doi: 10.1161/01.res.86.5.526. [DOI] [PubMed] [Google Scholar]
- 49.De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, Jr, Shin WS, Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonveaux P, Martinive P, DeWever J, Batova Z, Daneau G, Pelat M, Ghisdal P, Gregoire V, Dessy C, Balligand JL, Feron O. Caveolin-1 expression is critical for vascular endothelial growth factor-induced ischemic hindlimb collateralization and nitric oxide-mediated angiogenesis. Circ Res. 2004;95:154–161. doi: 10.1161/01.RES.0000136344.27825.72. [DOI] [PubMed] [Google Scholar]
- 52.Furspan PB, Chatterjee S, Mayes MD, Freedman RR. Cooling-induced contraction and protein tyrosine kinase activity of isolated arterioles in secondary Raynaud’s phenomenon. Rheumatology (Oxford) 2005;44:488–494. doi: 10.1093/rheumatology/keh517. [DOI] [PubMed] [Google Scholar]
- 53.Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M, Huang Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circulation Research. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- 54.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 55.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, Weldon SM, da Costa Goncalves ACh, Huang Y, Luft FC, Gollasch M. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 56.Willam C, Schindler R, Frei U, Eckardt KU. Increases in oxygen tension stimulate expression of ICAM-1 and VCAM-1 on human endothelial cells. Am J Physiol. 1999;276:H2044–H2052. doi: 10.1152/ajpheart.1999.276.6.H2044. [DOI] [PubMed] [Google Scholar]
- 57.Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 58.Redman CW. Platelets and the beginnings of preeclampsia. N Engl J Med. 1990;323:478–480. doi: 10.1056/NEJM199008163230710. [DOI] [PubMed] [Google Scholar]
- 59.Bosio PM, Cannon S, McKenna PJ, O’Herlihy C, Conroy R, Brady H. Plasma P-selectin is elevated in the first trimester in women who subsequently develop pre-eclampsia. Bjog. 2001;108:709–715. doi: 10.1111/j.1471-0528.2001.00170.x. [DOI] [PubMed] [Google Scholar]
- 60.Halim A, Kanayama N, el Maradny E, Nakashima A, Bhuiyan AB, Khatun S, Terao T. Plasma P selectin (GMP-140) and glycocalicin are elevated in preeclampsia and eclampsia: their significances. Am J Obstet Gynecol. 1996;174:272–277. doi: 10.1016/s0002-9378(96)70407-6. [DOI] [PubMed] [Google Scholar]
- 61.Krauss T, Kuhn W, Lakoma C, Augustin HG. Circulating endothelial cell adhesion molecules as diagnostic markers for the early identification of pregnant women at risk for development of preeclampsia. Am J Obstet Gynecol. 1997;177:443–449. doi: 10.1016/s0002-9378(97)70213-8. [DOI] [PubMed] [Google Scholar]
- 62.Endresen MJ, Morris JM, Nobrega AC, Buckley D, Linton EA, Redman CW. Serum from preeclamptic women induces vascular cell adhesion molecule-1 expression on human endothelial cells in vitro: a possible role of increased circulating levels of free fatty acids. Am J Obstet Gynecol. 1998;179:665–670. doi: 10.1016/s0002-9378(98)70061-4. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Adair CD, Weeks JW, Lewis DF, Alexander JS. Increased neutrophil-endothelial adhesion induced by placental factors is mediated by platelet-activating factor in preeclampsia. J Soc Gynecol Investig. 1999;6:136–141. doi: 10.1016/s1071-5576(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 64.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–1085. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 65.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.