Abstract
Most studies of cocaine’s effects on brain activity in laboratory animals are preformed under anesthesia, which could potentially affect the physiological responses to cocaine. Here we assessed the effects of two commonly used anesthetics (α-chloralose and isofluorane) on the effects of acute cocaine (1 mg/kg iv) on cerebral-blood-flow (CBF), cerebral-blood-volume (CBV), and tissue-hemoglobin-oxygenation (StO2) using optical techniques and cocaine’s pharmacokinetics and binding in the rat brain using PET and [11C]cocaine. We showed that acute cocaine at a dose abused by cocaine abusers decreased CBF, CBV and StO2 in rats anesthetized with isoflurane, whereas it increased these parameters in rats anesthetized with α-chloralose. Importantly, in isoflurane-anesthetized animals cocaine-induced changes in CBF and StO2 were coupled whereas for α-chloralose these measures were uncoupled. Moreover, the clearance of [11]cocaine from brain was faster for isoflurance (peak-half-clearance 15.8±2.8 min) than for α-chloralose (27.5±0.6 min) and the ratio of the specific to non-specific binding of [11C]cocaine in brain was higher for isoflurane (3.37 ± 0.32) than for α-chloralose anesthetized rats (2.24 ± 0.4). For both anesthetics cocaine induced changes in CBF followed the fast uptake of [11C]cocaine in brain (peaking at ~ 2.5–4 minutes) but only for isoflurane did the duration of the CBV and StO2 changes correspond to the rate of [11C]cocaine’s clearance from the brain. These results demonstrate that anesthetics influence cocaine’s hemodynamic and metabolic changes in brain and its binding and pharmacokinetics, which highlights the need to better understand the interactions between anesthetics and pharmacological challenges in brain functional imaging studies.
Keywords: cocaine and anesthesia, pharmacodynamic, pharmacokinetics of cocaine, brain imaging, cerebral blood flow, cerebral blood volume and hemoglobin oxygenation of tissue
Introduction
Imaging technologies have enabled the investigation of the pharmacological effects of psychoactive drugs directly in the brain non-invasively. In the case of drugs of abuse imaging studies in laboratory animals enable studies on the effects of acute and chronic drug administration on regional brain function and neurochemistry. Such studies permit interventions that are difficult to conduct on human subjects, including the use of pharmacological manipulations of specific neurotransmitter systems and receptor subtypes, and the evaluation in animals with genetic manipulations (i.e., knockouts). Unfortunately, unlike humans, laboratory animals have to be anesthetized during most neuroimaging studies for ethical reasons (Marota, et al., 2000), to immobilize the animal in order to reduce motion artifacts. Therefore a possible confound is the potential interaction between the anesthetic and the drug tested.
Most of the functional magnetic resonance imaging studies (fMRI) assessing cocaine’s effects on the brain have used anesthetics designed to interfere as little as possible with the fMRI responses. Thus, α-chloralose anesthesia in contrast to volatile anesthetics has been favored for preclinical fMRI studies because of its minimal effects on the neurovascular coupling response (Ueki et al., 1992). However, volatile anesthetics (e.g. isoflurane), in comparison to α-chloralose, are advantageous for experimental studies because of their stability in achieving anesthetic depth with a simple non-invasive induction. However, there is limited information in the literature regarding the influence of anesthetics on the brain’s response to cocaine.
Here, we compared the hemodynamic, metabolic and pharmacokinetic responses to acute cocaine in the rat brain when they were anesthetized with isoflurane (ISO) versus α-chloralose (α-CHLOR). We used laser-doppler-flowmetry (LDF) in parallel with multi-wavelength optical spectroscopy (MWOS) to measure the changes in cerebral blood flow (CBF), cerebral blood volume (CBV) and hemoglobin oxygenation of tissue (StO2) in response to an acute cocaine challenge (1 mg/kg iv). In order to have a temporal reference with which to assess the temporal course of the hemodynamic and metabolic effects of cocaine as a function of anesthesia, we measured the pharmacokinetics (PK) of cocaine in the rat brain using small animal PET and [11C]cocaine in a separate group of animals. We chose a 1 mg/kg intravenous dose of cocaine since in the rodent brain, this dose induces comparable blockade of dopamine transporters (main molecular target for the reinforcing effects of cocaine; Chen et al., 2006) as that induced by typically abused doses of cocaine in cocaine abusers (Gatley et al 1999, Volkow et al., 1999). Isoflurane and α-chloralose were used in separate groups of animals to test our main hypothesis that neural and cerebrovascular responses to cocaine are dependent on the anesthetics used. Specifically, given the fact that α-chloralose, is known to preserve cerebrovascular reactivity and neuronal excitability we hypothesize that cocaine under α-chloralose conditions would enhance cocaine’s neurometabolic effects (in contrast to isoflurane).
Material & Methods
Subjects and experimental protocol
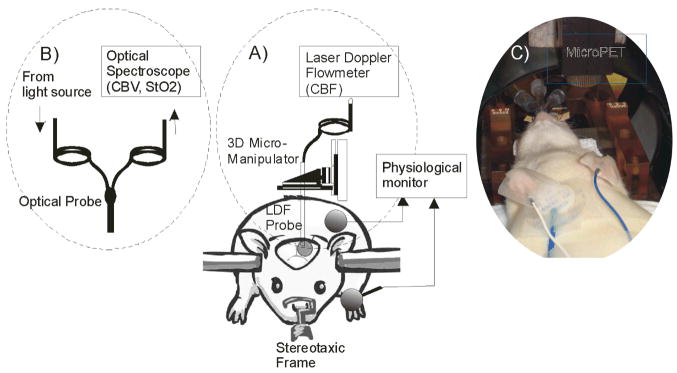
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC). Fourty-three female Sprague-Dawley rats (250–350g; Taconic) were divided into experimental groups as shown in Table 1. All animals were maintained for a specified duration of time prior to experimental drug administration to avoid physiological fluctuations that might be induced by the surgical process, and to minimize any carry-over effects from the anesthesia required for the surgical regimen (e.g., isoflurane). For optical measurements (probe A, B as shown in Figure 1A and 1B), saline (0.1 cc/100 g) or cocaine-hydrochloride bolus (1 mg/kg) was quickly injected (<10s) via the femoral vein followed by 1 cc of saline. For the small animal PET studies (microPET, Figure 1C), the procedure was the same except that a fraction of the cocaine was radiolabeled with carbon-11 to give [11C]-cocaine.
Table 1.
Animal groups and experimental design
| Group | Anesthetic | Detection probe | Measured parameters | Intravenous drug challenge |
|---|---|---|---|---|
| a1 (n=4) | Isoflurane | Optical A, B | CBF | Saline (0.9% NaCl, 0.1cc/100mg) |
| a2 (n=4) | CBV, StO2 | |||
| b1 (n=5) | Optical A, B | CBF | Cocaine hydrochloride (1mg/kg) | |
| b2 (n=6) | CBV, StO2 | |||
| b3 (n=3) | PET C | PK of [11C]-cocaine | [11C]-cocaine† | |
| c1 (n=4) | α–chloralose | Optical A, B | CBF | Saline (0.9% NaCl, 0.1cc/100mg) |
| c2 (n=4) | CBV, StO2 | |||
| d1 (n=5) | Optical A, B | CBF | Cocaine hydrochloride (1mg/kg) | |
| d2 (n=5) | CBV, StO2 | |||
| d3 (n=3) | PET C | PK of [11C]-cocaine | [11C]-cocaine‡ |
903 ± 347 μCi with a mass dose of 0.00039 ± 0.0002 mg/kg cocaine
713 ± 99 μCi with a mass dose of 0.0004 ± 0.00004 mg/kg cocaine
Fig. 1.
Schematic illustration of the experimental setup used for studies of changes in A). cerebral blood flow (CBF) and B). cerebral blood volume (CBV) and tissue hemoglobin oxygenation (StO2). C) pharmacokinetics (PK) of cocaine in the rat brain non-invasively using microPET imaging.
Animal preparation
Each animal was initially anesthetized with ~3% isoflurane, orally intubated, and mechanically ventilated (Harvard Apparatus, Inspira asv) during the surgery. Anesthesia was then maintained with 1.8–2.0% isoflurane in a 60–70% O2/air mixture. The femoral artery was cannulated for continuous arterial-blood-pressure monitoring and the femoral vein was catheterized for administration of drugs. For optical measurement, the anesthetized rat was then positioned in a stereotaxic frame (Kopf, Frame no 9) and a left craniotomy of ~2.5×1.5 mm2 was made above the area of the somatosensory cortex. Fig. 1 illustrates the experimental animal setup. The electrocardiogram (ECG), intra-arterial-blood-pressure, respiratory rate and body-temperature were continuously recorded (Module 224002, Small Animal Instr. Inc.). Blood gases were monitored regularly to keep PCO2 in the range of 30–45 mmHg during the experiments (Model 700, Radiometer, Copenhagen). In the isoflurane-anesthetized rats (Group a&b), all animals were maintained with an inspiratory isoflurane concentration of 1.8–2% during the experiments. In Group c&d rats, the isoflurane was discontinued after the surgery when α-chloralose had been implemented (i.e. an initial i.v. bolus of 50mg/kg followed by a maintenance infusion of ~25 mg/kg/hr).
Optical measurements of CBF, CBV and StO2 from the cortex of rat brains
Both Laser Doppler Flowmeter (LDF) and optical multi-wavelength spectroscopy systems (OMWS) were used in parallel to study the changes of cerebral blood flow (CBF), cerebral blood volume (CBV) and tissue oxygenation (StO2) in response to saline or cocaine challenges under the different anesthetics (Table 1).
As shown in Fig. 1A) a small fiber optical probe of LDF (ϕ=0.8 mm, MP3 Moor Instruments) was perpendicularly mounted above the brain and positioned to gently touch the exposed cortex using a 3-dimentional micromanipulator (MM1-3, WPI). The near-infrared laser (λ=785 nm) was delivered to the brain tissue through an illumination fiber and the backscattered photons that carry the information of the microvasular perfusion were collected by two detection fibers within the fiber bundle (Moor Instruments, Axminster, UK). The theoretical background for detection of CBF is summarized in Appendix A below. When mounting the Doppler probe onto the cortical area, attention was paid to avoid local compression of the underlying cerebral vessels (arteriolae and venules) present most superficially on the cortex to preserve a normal CBF response to the pharmacological challenge.
Fig. 1B) shows a catheter-based optical multi-wavelength spectroscopy (MWOS) probe designed to detect the changes of the local cerebral blood volume (CBV) and tissue hemoglobin oxygenation (StO2) of the cortical response to cocaine/vehicle administration. The OMWS system has been described previously (Du et al., 2005). Briefly, it consisted of a xenon lamp, a monochromater and a photoncounting detector. The lamp was connected to the computer-controlled monochromator to select the incident lights of 555nm and 572nm by time-sharing to sequentially deliver the light onto the brain surface through one arm of a Y-shaped bifurcated fiber bundle (Fig. 1B). The diffusive reflectance from the brain tissue was collected by the fiber tip of the common leg (ϕ=3mm) and detected by a photon detector. The changes in CBV and StO2 can be separately distinguished from the reflectance measured by summing and subtracting the optical densities of the signals at these two wavelengths. The theoretical background for deriving these optical parameters has been previously described (Du et al., 2005), and here we simplified as in Appendix B.
Rodent PET Scanning
Six rats were scanned with [11C]-cocaine as listed in Table 1 (Group b3 & d3). Three of them (Group b3) were anesthetized by isoflurane and the rest of the rats (Group d3) were anesthetized by α-chloralose. For each rat, the surgery procedure was the same as that applied for the optical measurements described above, except that the craniotomy was not performed. After the surgery, the animals were positioned in the center of the field of view of the microPET, and the femoral vein was used for radiotracer administration. [11C]-Cocaine was synthesized as previously reported (Farde et al., 1988, Fowler et al., 1989). Specific activities at the time of injection are given in Table 1. Imaging was performed using a microPET R4 tomograph (Concorde Microsystems, Knoxville, TN), which has a transaxial resolution of 2.0 mm full width at half maximum (FWHM) with an image field of view of 11.5 cm. Dynamic emission scans began simultaneously with intravenous radioligand administration and ran for 40 minutes. Early microPET data were binned in 15 to 60 second intervals, with later data binned every 3 min. The binning produced 30 time frames (4 × 15s, 3 × 20s, 6 × 60s, 17 × 180s) and included subtraction of random coincidences collected in a delayed time window. The resulting sinogram data was rebinned using Fourier rebinning and reconstructed with 2-dimensional filtered backprojection (FBP) with a ramp filter (Nyquist cutoff) and software provided by the manufacturer. Image pixel size in FBP reconstructed images was 0.85 mm transaxially with a 1.21 mm slice thickness.
Data acquisition and analysis
Both LDF and MWOS were used to optically detect the changes of CBF, CBV and StO2 from the cortical brain of the rats in response to saline or cocaine administrations as described above. Also, the microPET was used to characterize the pharmacokinetics (PK) of cocaine in the rat brain using [11C]-Cocaine. During all experiments, the physiology of the rat was monitored, and the time courses of the mean-arterial-blood-pressure (MABP) and heart-rate (HR), were recorded in real time from 10 min before until 30 min after saline, cocaine or [11C]-Cocaine administration. The sampling rates were 0.1s for CBF and 0.05s for CBV and StO2 recordings per data point, respectively. The data of the CBF, CBV and StO2 were further rebinned to 0.2s intervals for the time-course figures for clarity of display. In order to compare changes in CBF, CBV and StO2 between vehicle and cocaine-challenged animals, each time course was normalized to the mean of the 10-min baseline period prior to the challenges and thus expressed as a percentage change of the baseline ± SEM.
For microPET studies, Regions of Interest (ROI) were selected in three brain regions: somatosensory cortex (the region from which optical measures were recorded), striatum (the region with the highest specific to non-specific ratio in the brain) and the cerebellum (the region where cocaine binding is mostly non-specific). ROI analysis was performed using PMOD software (www.pmod.com) on FBP reconstructed images. ROIs for PET studies were chosen based on previous guidelines provided for primates by Black et al. (2004) and recently described for rodent PET data (Dalley et al., 2007; Schiffer et al., 2009). Rather than outlining the entire structure on the MRI template, this approach accommodates some limitations specific to PET, minimizing the effects of spillover by using spheres (2.0 mm diameter) placed at the stereotaxic center of each region. The stereotaxic center of each ROI was chosen based on coordinates in Paxinos and Watson (Paxinos & Watson, 1998) stereotaxic space. The center of the somatosensory ROI coincided with the coordinates of the optical probe (2.0 mm posterior, ±2.0 mm lateral and 2.0 mm ventral to bregma) such that the striatal ROI had a center at +0.5 mm anterior, ±2.5 mm lateral and 5.0 mm ventral from bregma and the center of the cerebellar ROI was located at 12.5 mm posterior and 6.0 mm ventral to bregma on the midline.
Statistical analysis was performed on the percentage changes for CBF, CBV and StO2, and on the absolute measurements of the physiology data (MABP, HR). For the small animal PET data, statistical analysis was performed on the [11C]-cocaine uptake data (relative to the injected dose) as well as the specific binding data (striatal to cerebellar ratios). Intragroup differences were analyzed with a paired sample Student’s t test, and intergroup differences were analyzed with a two-sample unpaired t test. P<0.05 (two sided) was considered significant.
Results
Distribution and Pharmacokinetics of cocaine in rat brain
Fig. 2a)-c) show the images for the distribution of [11C]cocaine in brain superimposed on an MRI atlas (Fig. 2a, Schweinhardt et al., 2003) for the isoflurane (Fig. 2b) and α-chlorose (Fig. 2c) that were used to obtain cocaine’s distribution and pharmacokinetics. Fig. 2d shows the pharmacokinetics of cocaine in the somatosensory cortex of the rat brain when anesthetized with either isoflurane or α-chloralose. Cocaine entered the brain very rapidly with peak uptake of 2.5–4.0 minutes after its intravenous administration (Fig 2d). The time of the half-peak clearance of cocaine from brain (i.e., t(PK1/2Max)) was 15.8±2.8 min for isoflurane and 27.5±0.6 min for α-chloralose anesthetized rats. This indicates that the time of cocaine clearance from the brain is affected by the anesthetic used and it is slower when using α-chloralose than isoflurane.
Fig. 2.
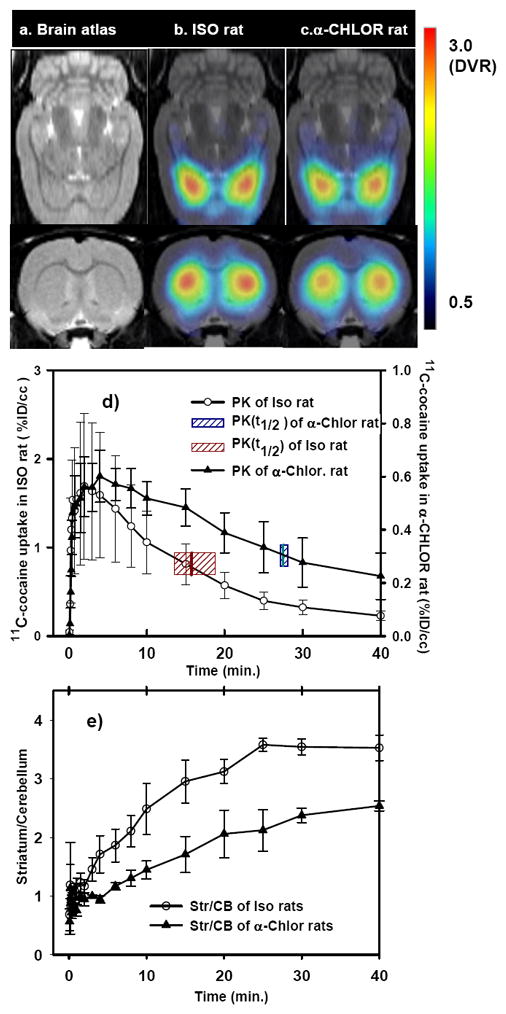
Distribution of [11C]-cocaine superimposed on MRI atlas (a) for the isoflurane (b, n=3) and α-chloralose (c, n=3) used in the animals. Time activity of [11C]-cocaine binding in the somatosensory cortex of rat brains (d) and the time course of the ratio of [11C]-cocaine between the striatum and cerebellum (e). The shadow bars in d) represent the half-peak clearance of [11C]-cocaine from the brains. Peak uptake of [11C]-cocaine (t(PKmax)) occurred at 2.5 ± 1.1 min for ISO- and 4.0 ± 1.6 min for α-CHLOR-anesthetized animals; The half-peak clearance of cocaine from brain (t(PK1/2max)) was 15.8 ± 2.8 min for ISO- and 27.5 ± 0.6 min for α-CHLOR-anesthetized animals. The ¼ peak- clearance times (t(PK1/4Max)) were 22.9±5.6 min and 37.6±8.1 min, from animals anesthetized with isoflurane and α-chloralose, respectively.
Similarly, the striatum to cerebellum ratio, which is used to quantify the relative binding of cocaine to dopamine transporters (main cellular targets of cocaine’s rewarding effects), was affected by the anesthetic used and was significantly higher in the isoflurane than in the α-chloralose anesthetized rats (3.37 ± 0.32 and 2.24 ± 0.4, respectively; p < 0.05). This indicates that the binding of cocaine in the rodent brain was also affected by the anesthetic used.
Changes in CBF, CBV and StO2 of the brain in response to cocaine in ISO-anesthetized rats
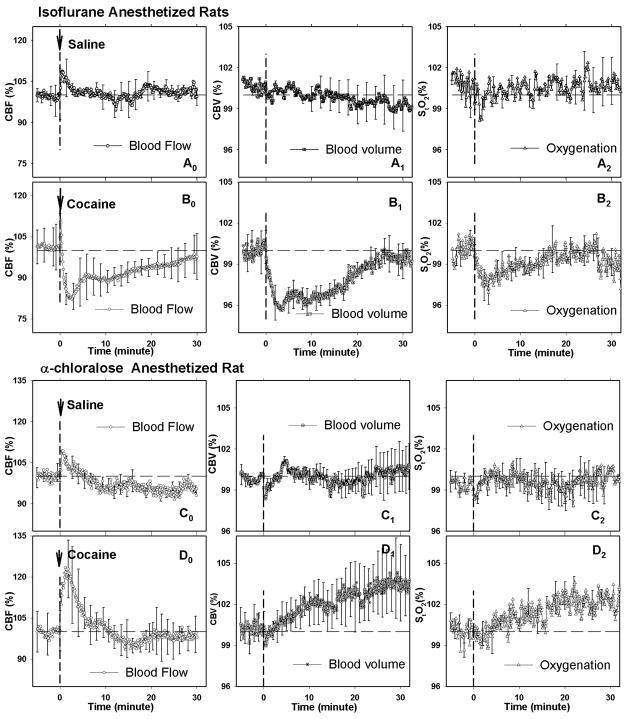
Fig. 3A, B show the temporal profiles of CBF, CBV and StO2 changes in the ISO-anesthetized animals following a bolus of saline (i.e., vehicle) or cocaine administration. As shown in Fig. 3 A0-A2, the saline injection did not produce significant CBF, CBV and StO2 changes, whereas cocaine induced fast decreases of these signals with peak effects observed 3–4 min post- injection, which corresponds with the peak uptake of cocaine in rat brain (i.e., t(PKmax) = 2.5±1.1 min). The maximal decrease in CBF, CBV and StO2 were 17.5 ± 4.7% (p<0.003), 4.2 ±1.2% (p<0.005) and 3.1±0.9% (p<0.02), respectively. These signals returned to baseline values between 16 to 25 min following the cocaine challenge, thus approximately corresponding with the time for half-peak clearance of cocaine from brain (i.e., t(PK1/2Max)= 15.8±2.8 min). Collectively these results indicate that the decreases of CBF, CBV and StO2 following the cocaine injection in ISO-anesthetized rats were induced by the pharmacological effect of cocaine (and/or its interaction with isoflurane but not due to isoflurane by itself). Further, it followed the temporal course of the uptake of [11C]-cocaine in the rat brain.
Fig. 3.
Time courses of changes in CBF, CBV and StO2 obtained from the cortical brain of the rats with ISO anesthetic by following saline (vehicle, Ao-A2), cocaine (Bo-B2), and those obtained from the cortical brain of the rats with α-CHLOR anesthetic by following saline (vehicle, Co-C2) and cocaine (Do-D2). Data are presented as relative changes from the baseline (100%) and the vertical dashed lines in each graph represent the time of intravenous drug administration.
Changes in CBF, CBV and StO2 of the brain in response to cocaine in α-CHLOR -anesthetized rats
In contrast to the ISO-anesthetized rats, cocaine elicited an increase in CBF, CBV and StO2 in rats anesthetized with α-chloralose, as shown in Fig. 3 Do-D2. CBF rapidly increased and reached a maximum of 122.9 ± 10.5% (p< 0.01) within 3–4 min after the cocaine challenge, which corresponded with peak cocaine uptake in somatosensory cortex of the brain (i.e., t(PKmax) = 4.0±1.6 min). Also, cocaine elicited a slow and prolonged increase in CBV and StO2 with a peak of ~2–3% (p<0.05) above their respective baselines at ~ 25 min after the cocaine injection, which corresponds to a time when significant clearance of cocaine from brain has already occurred (t(PK1/2Max)= 27.5±0.6 min) and when motor effects from acute administration of cocaine to rodents has almost returned back to baseline levels (Wang et al., 2001). In contrast, CBF increased immediately after cocaine injection and returned to baseline at ~10–13 mins, which is when peak motor activating effects of cocaine occur when given to rodents (Wang et al., 2001). As CBF, CBV and StO2 did not change in response to the saline challenge (Fig. 3 Co-C2), this indicates that the increases of CBF, CBV and StO2 following the cocaine injection in the α-chloralose-anesthetized rats were induced by the pharmacological effect of cocaine (and/or its interaction with α-chloralose), but not due to α-chloralose by itself.
Physiological responses to cocaine in ISO- and α-CHLOR-anesthetized rats
Blood gas parameters including pH and pCO2 were measured at baseline (i.e., 10 min prior to cocaine injection) and 30 min after the cocaine injection. For ISO-anesthetized rats, the mean pH values were 7.30 ± 0.07 and 7.32 ± 0.03, and the mean pCO2 were from 36.6 ± 1.5 and 34.3 ± 1.5 before and after cocaine administration. Similar results were obtained with α-CHLOR- anesthetized rats, i.e., pH was 7.43 ± 0.07 and 7.37 ± 0.08, and pCO2 was 37.1 ± 6.1 and 33.8 ± 4.8, for baseline and after cocaine measurements, respectively. This indicates there were no significant alterations in blood gas parameters between anesthetic agents (p>0.05), and suggests that alterations in CBF, CBV and StO2 following cocaine administration were not secondary to general systemic effects induced by the anesthesia and/or insufficient ventilation but rather were likely to be the consequence of direct drug actions (cocaine + anesthetic) upon the cerebral vasculature and cellular activities.
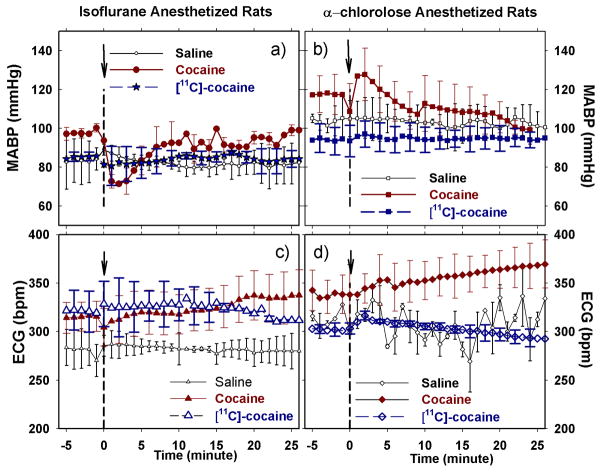
Fig. 4 summarizes the changes in MABP and heart-rate (measured by ECG) in the ISO-anesthetized and the α-CHLOR-anesthetized rats. As shown in Fig. 4a, cocaine caused slightly transient decreases in MABP form 97.0 ± 2.2 to 71.4 ± 4.3 mmHg in the ISO-anesthetized rats (group b1-b2), whereas it induced a brief increase in MABP from 118.6 ± 13 to 127.8 ± 10 mmHg in the α-CHLOR-anesthetized rats (group d1-d2) within 2–4 min after the cocaine injection (Fig. 4b). Both of these changes returned to baseline levels 5–8 min after the cocaine challenge. Cocaine also increased HR slightly in both ISO- and α-CHLOR-anesthetized rats, from 304.3 ± 19 to 337.6 ± 25 beat/min, and from 338.1 ± 22 to 372.1 ± 19 beat/min, respectively. The saline and [11C]-cocaine injections did not change MABP or HR significantly in either ISO- or α-CHLOR-anesthetized rats (Fig. 4a–d). The lack of an effect of [11C]-cocaine reflects the fact that it was given at tracer doses that are devoid of pharmacological effects.
Fig. 4.
Changes of mean arterial blood pressure (MABP), heart rate (ECG) as a function of time in response to 1mg/kg cocaine, vehicle and [11C]-cocaine in isoflurane-anesthetized rats (A and C), and in α-chloralose anesthetized rats (B and D). Data are presented as a mean ± SEM.
The Cocaine-induced changes in StO2 as a function of changes in CBF, and CBV
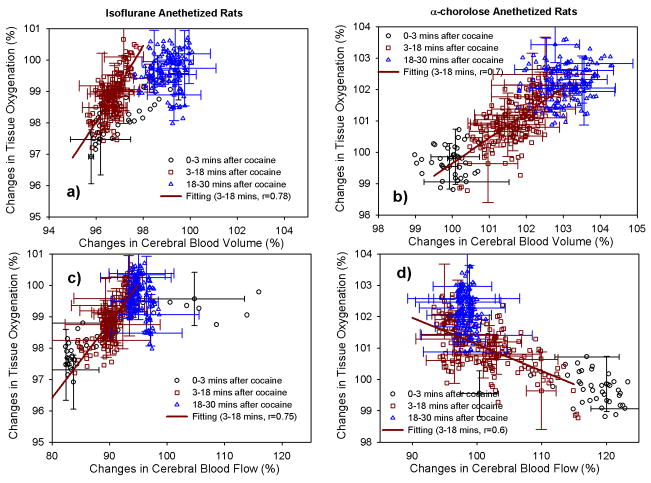
Fig. 5 shows the scatter plots of the cocaine-induced changes in StO2 as a function of the changes in CBV and CBF under ISO- or α-CHLOR anesthetics. The data was divided into three time periods based on cocaine’s pharmacokinetic in the brain: t1 = 0–3 mins (i.e., ≤ t(PKMax), presents the period of cocaine’s uptake into the brain; t2 = 3–18 mins (i.e., t(PKMax)≤ t2 ≤ t(PK1/2Max)) represents the period of high level of cocaine binding in brain; and t3 = 18–30 mins (i.e., t3 ≥ t(PK1/2Max)) represents the time period of cocaine’s clearance from brain. The correlations of StO2 versus CBV and StO2 versus CBF were analyzed during t2 period for both animal groups anesthetized by ISO- and α-CHLOR anesthetic drugs, respectively. As we can see in Fig. 5a) and 5b), cocaine induced about 3–4% changes in the amplitudes of StO2 and CBV in both ISO- and α-CHLOR anesthetized animal brains. The mean cross-correlations of the signals between StO2 and CBV are 0.78 and 0.7, respectively in the ISO- and α-CHLOR anesthetized rat brains. This indicates that there was a positive correlation between StO2 and CBV, which was independent of the anesthetics applied. Fig. 5c) and 5d) show the StO2 changes as a function of CBF under the ISO- and α-CHLOR anesthetics. The amplitude of cocaine-induced CBF decreased by ~20% in ISO- anesthetized rat brain (Fig. 3Bo) but increased by ~22% in α-CHLOR-anesthetized rats (Fig. 3D0). However, cocaine induced a positive correlation between StO2 and CBF in ISO-anesthetized rats (r=0.75, Fig. 5c), and a negative correlation in α-CHLOR-anesthetized rats (r=−0.6, Fig. 5d).
Fig. 5.
Changes of tissue oxygenation (StO2) as a function of changes in cerebral blood volume CBV (a, b) and blood flow CBF (c, d) following the cocaine administration within the cortical brains of the rats anesthetized by using ISO or α-CHLOR. The cross-correlation was analyzed using a linear fitting for StO2 versus CBV, StO2 versus CBF during the typical period of the cocaine binding into the brain (i.e., t= t1+t2 = 0–18 mins; t ≤ t(PK1/2max)). r represents the correlation coefficient of the linear fitting.
Discussion
In the present study, multi-modality optical techniques were used to separately distinguish changes in CBF, CBV and StO2 in the rodent brain in response to an acute i.v. cocaine challenge in rats anesthetized with different agents along with concomitant physiological measurements. MicroPET was also used to measure the PK of cocaine in the rodent brain as a temporal reference with which to compare the hemodynamic and metabolic effects of cocaine and to assess cocaine’s binding in the rat brain. The main findings from this study can be summarized as follows:
Acutely, 1mg/kg cocaine induced significant changes in CBF, CBV, StO2, which depended on the anesthetic used: In the ISO-anesthetized rats cocaine decreased these parameters, whereas in the α-CHLOR-anesthetized rats it increased them;
Cocaine’s PK in brain showed fast uptake in the animals anesthetized with both ISO-and α-CHLOR (peak uptake occurring 2.5–4 minutes after administration). However cocaine’s clearance from brain differed significantly as a function of anesthetic; with half peak clearances of [11C]-cocaine (t(PK1/2Max)) of 15.8 ± 2.8 min for ISO and 27.5 ± 0.6 min for α-CHLOR. The fast changes in CBF corresponded well with the fast uptake of [11C]-cocaine into the brain for both anesthetics. In contrast cocaine’s induced changes in CBV and StO2 were associated with [11C]-cocaine’s PK in brain for ISO (CBV and StO2 returned to baseline by 25 and 16 min respectively) but not for α-CHLOR (CBV and StO2 peak until 25 min).
The specific to non-specific binding ratio of cocaine was also influenced by the anesthetic and was higher for ISO (3.37 ± 0.32) than for α-CHLOR (2.24 ± 0.4) at 30 min post-administration of [11C]-cocaine.
Cocaine increased heart rate in both ISO- and α-CHLOR anesthetized rats. However, a transient decrease of MABP was observed in ISO-anesthetized rats while cocaine transiently increased MABP in α-CHLOR-anesthetized rats. The MABP changes were modest (i.e., ΔMABP≈25mmHg and 9mmHg for ISO- and α-CHLOR anesthetized rats, respectively), and short lasting (<8-min), in contrast to the longer lasting effects of cocaine on CBF, CBV and StO2 changes observed for both anesthetics.
Anesthesia and cocaine pharmacokinetics
Our results showed that cocaine elicited fast (peak effects at ~3–4 min) and intermediate lasting (~16–26min) decreases in CBF, CBV and StO2 when the rats were anesthetized with ISO and shorter lasting increases in CBF (≤ 10 min) and longer lasting (> 25 min) increases in CBV and StO2 when the rats were anesthetized with α-CHLOR. The microPET experiments allowed us to compare these cerebral dynamical responses measured with optics with cocaine’s PK profile in the brain using the same two anesthetics. Interestingly, in spite of the fact that the cerebral responses to cocaine were opposite (i.e. negative in ISO and positive in a-CHLOR) they both paralleled the fast uptake of [11C]-cocaine in the brain. For ISO the CBF, CBV and StO2 peaked when the concentration of cocaine in brain was highest (t(PKMax)=2.5±1.1 mins) whereas for α-CHLOR peak of CBF corresponded to the peak of cocaine concentrations in the brain (t(PKMax)=4.0±1.6 mins) but the duration of the changes in CBV and StO2 were longer than that predicted by the rate of [11C]cocaine’s clearance from the brain. These results do suggest that that the rapid changes (regardless of the direction of the dynamic responses) are directly associated with cocaine’s effects as it enters the brain.
The long-lasting responses of CBV and StO2 to cocaine differed for both anesthetics as did the brain PK’s of cocaine for the two anesthetic agents (t(PK1/2Max)=15.8±2.8 mins and 27.5±0.6 mins for Iso- and α-CHLOR, respectively). However, the PK differences were not sufficient to account for the much longer duration of α-CHLOR effects on CBV and StO2, which were still elevated at 30 minutes when measurements were terminated. The longer lasting effects with α-CHLOR than with ISO do not correspond to the higher specific to non-specific binding observed with ISO than with α-CHLOR, which would predict greater dopamine increases when cocaine is given with ISO than with α-CHLOR (Fig. 2e). Rather these long lasting changes with α-CHLOR are likely to reflect an interaction between cocaine and α-CHLOR, which might be associated with the increases of intracellular calcium in response to cocaine observed by several investigators (Lu et al., 2007, Hu et al., 2007, Du et al, 2006).
Influence of peripheral hemodynamic changes on cocaine’s effects on CBF, CBV, StO2
In α-CHLOR anesthetized rats the MABP increased slightly form 118.6 ± 13 to 127.8 ± 10 mmHg (i.e., ΔMABP ~ 9mmHg) within 2–4 min and returned to baseline 5–8 min after the cocaine challenge and peripheral hemodynamics are therefore not likely to have contributed to any of the observed effects on CBF, CBV and StO2 induced by cocaine. In the ISO-anesthetized rats, the MABP decreased in response to cocaine but the hypotension was modest (MABP > 70mmHg) and short lasting (< 8 min, which suggests that neither the immediate nor the longer lasting cocaine-induced decreases in CBF, CBV and StO2 (>8 min) were likely to be driven by failure of cerebral autoregulation (Gozzi et al., 2007) at any time-point following the acute cocaine challenge.
Impact of anesthetics on neurovascular responses to cocaine
In our experiments, the changes in CBF and CBV appeared to be tightly coupled to changes in StO2 in rats anesthetized by ISO; in contrast, in α-CHLOR anesthetized rats the CBV and StO2 responses were prolonged and more sustained than that of CBF, indicating a decoupling of CBF and StO2 following the acute intravenous cocaine challenge. The dissimilar neurovascular profiles in response to acute cocaine of the two anesthetics can be partly explained by their different actions on the cerebral vasculature and tone. Isoflurane vasodilates blood vessels in the brain and increases baseline CBF in comparison to the awake state (130–150 ml/100g/min, 1 MAC ISO, Maekawa et al., 1986, Lenz et al, 1999) whereas α-chloralose does not (e.g., 70–90 ml/100g/min, Ueki et al., 1998, Lee et al., 2001). Such CBF baseline difference during anesthesia are known to affect the magnitude of electrical-stimulation-induced CBF responses (Masamoto, et al., 2007). For example, in ISO anesthetized animals where the baseline CBF is elevated compared to the awake state the stimulus-evoked CBF responses as well as the amplitude of cortical evoked field potentials are lower when compared to those elicited during α-chloralose anesthesia (Masamoto et al., 2007). Thus, differences in the anesthetic-dependent resting CBF and neuronal activity patterns (Ranft et al., 2004) will also affect the hemodynamic responses to acute cocaine and explain the different cerebrovascular and metabolic profiles observed here.
In our study, acute cocaine induced a decrease in CBF, CBV and StO2 in ISO anesthetized animals. Furthermore, recently we used laser-speckle imaging and also observed decreases of cortical blood flow in tissue in response to an acute cocaine challenge in ISO-anesthetized animals (Luo et al., 2009). Most recently, by using 3D Doppler Optical Coherent tomography (DOCT), a technique that permits to quantitatively image CBF and vascular profiles (Yuan et al., 2009) we visualized what appears to be cocaine-induced cortical vasoconstriction in ISO-anesthetized rat brain (data not shown). Our current results are in agreement with the previous finding of a negative BOLD signal on the immediate cortical surface (Schmidt et al., 2006) in ISO-anesthetized rats that used a similar experimental condition (ISO=1.1%, cocaine hydrochloride of 1mg/kg, i.v.). However, in another study with halothane (0.7%), which is also a vasodilatory gaseous anesthetic agent, 1mg/kg cocaine was found to elicit widespread increases in regional CBV using contrast MRI (Marota et al., 2000). This discrepancy might be due to differences in experimental/physiological conditions including MABP levels, state of paralysis and potential transient malfunction of cerebral autoregulation. (Gozzi et al. (2007). In addition, potential interactions of either of the two anesthetics with cocaine’s binding to dopamine transporters must also be taken into account. For example, Eckenhoff et al. and others have shown a competitive interaction for binding to dopamine transporters between cocaine and halothane, but not ISO in rat brain synaptosomes in vitro (Eckenhoff and Fagan, 1994; EI-Maghrabi and Eckenhoff, 1993).
In contrast to ISO, α-CHLOR is considered a hypnotic drug with no analgesic properties. It has been widely used for rodent fMRI studies because it 1) preserves metabolic coupling for somatosensory stimulation (Ueki et al., 1992, Silva et al.,1999); 2) provides a normal CBF baseline close to that measured in the awake state when compared with ISO (Masamoto et al., 2007) and 3) preserves cerebrovascular reactivity (Bonvento et al., 1994). However, the action of α-CHLOR is poorly understood but involves potentiation of γ-aminobutyric acid (GABA)- induced currents via enhancement of GABAA receptor activity. We previously reported that cocaine increases the intracellular calcium concentration [Ca2+]I in the brain for both ISO- and α-CHLOR rats [Du et al., 2006]. The time course of [Ca2+]I increases observed with either of the two anesthetics are similar to the specific to non-specific binding ratio profile of cocaine within the brain (Fig. 2e). For example, we previously demonstrated that the [Ca2+]I gradually increased and peaked at 30–40 min after the cocaine challenge (1mg/kg) in the ISO-anesthetized rat brain, which corresponds to the time period of the greatest specific to non-specific binding ratio (~30min, see Fig. 2e) observed here. Similarly for the α-CHLOR anesthetized rat brain cocaine induced increases in [Ca2+]I were longer lasting than with ISO and were still elevated 1 hr after cocaine’s administration. Since [Ca2+]I levels affect neuronal excitability (Verkhratsky et al., 2006), this suggests that the long-lasting changes in CBV and StO2 in α-CHLOR animal brains may be caused by the neuronal effects of cocaine i.e. reflecting neurometabolic changes induced by cocaine. To further explore this statement we estimated the cocaine-induced changes in the local rate of oxygen consumption (CMRO2) based on the measured changes in CBF, CBV and StO2 obtained in α-CHLOR animal group. Our calculations confirmed an increase of CMRO2 (Eqs. A7 & A12, Appendix C), supporting the notion of increased neuronal ‘activity’ possibly related to changes in [Ca2+]I. Indeed, Schmidt et al.(2006) have also shown a positive offset of ΔCMRO2 at ΔCBF=0 in rat brain induced by cocaine in their MRI pharmacological studies. Further, Devonshire et al., (2004) previously reported that both hemodynamic and field potential responses to 6 minutes of sensory stimulation (Whisker) were significantly enhanced after acute cocaine administration. Cocaine’s ability to increase [Ca2+]I in cortical tissue could underlie this enhanced cortical activation with stimulation.
CBF and CBV
Cocaine-induced temporal uncoupling between of CBF and CBV is well recognized (Choi et al., 2006, Schmidt et al., 2006, Luo et al., 2009). The increases in CBF in response to cocaine observed in the α-CHLOR anesthetized animals are in agreement with the previous findings by Stein and Fuller et al., (1992), who reported CBF increases peaking at ~2 min in awake rats after cocaine administration (1mg/kg iv) using the [14C]iodoantipyrine CBF technique. A similar CBF response was also observed by Colin et al.(2007) in the motor cortex using laser Doppler technique, who reported a rapid increase of CBF peaking at ~ 4 min and returning to baseline with in 15 min following an acute cocaine challenge. With α-CHLOR, we found that CBV and StO2 increased slowly and peaked at ~25 min after cocaine’s injection, which is longer than the duration of the CBF increases (<10 min) as well as those reported with [14C]iodoantipyrine in the awake animals. This ‘uncoupling’ phenomenon between CBV and CBF was also reported by Ceolin et al., (2007), who observed that the rCBV response to cocaine (1 mg/kg, i.v.) was more sustained than that of Laser Doppler flow in the motor cortex of the rats anesthetized by ~1% of halothane. Although the mechanism underlying these temporal differences in the hemodynamic profiles are unclear, we can not rule out the influence of the penetration depth mismatch between LDF and MWOS probes used for CBF and CBV measurements in our studies on the ‘decoupling’ events. The light of LDF probe is at near-infrared range (λ=785nm), it can propagate ~3–4 mm beyond the cortical surface (Luo et al., 2009), which is 8–10 folds deeper than the visible lights (λ=555nm and 572nm) used in the MWOS probe (~0.4–0.5mm, Dunn et al., 2005). As a result the LDF probe collects photons remitted from both cortical (e.g., somatosensory) and subcortical (e.g., striatum) brain regions (Watson, 1982), whereas the MWOS detected the signal only from the superficial cortex (e.g., somotosensory) where the large draining veins appear that take much longer to return to basal state (Silva et al., 2007).
Conclusion
This study documents the potential perturbations caused by anesthetics on cerebral hemodynamics (CBF, CBV) and hemoglobin oxygenation of tissue (StO2) in response to intravenous cocaine, and on cocaine’s PK and its specific to non-specific binding in brain. The opposite hemodynamic changes induced by cocaine with ISO- and α-CHLOR within the cortical brain are likely to be associated with the anesthetic-dependent “state” of the vasculature as well as differences in neurometabolic profiles and neuronal activity patterns. The findings from our study documenting significant interactions between anesthetics and cocaine’s pharmacological and pharmacokinetic effects may be clinically relevant. Specifically, clinical and preclinical studies have documented that cocaine’s toxic effects are significantly accentuated by alcohol administration (Boag and Havard, 1985); inasmuch as alcohol possess anesthetic effects (Goldstein, 1984, Busse and Riley, 2003) this enhanced toxicity could reflect these interactions.
Finally, for future studies focusing on characterizing cocaine’s acute and/or chronic actions on brain function including cerebral hemodynaics it is clear that data derived from animal models must be interpreted according to the anesthetic used. If a major study goal is to draw conclusions which can be inferred to the non-anesthetized condition an anesthetic like α-CHLOR which more closely mimics the conditions of the awake brain should be used. However, in situations where this is not possible (e.g. animal models which require long-term follow up) and a gaseous anesthetic is considered necessary and convenient the derived data will be flawed from the point of view of cerebral vascular reactivity and tone which are severely altered by these compounds. Our study highlights the importance of developing imaging instruments or experimental strategies that can be used to measure the effects of cocaine actions in non-anesthetized animals. Our study also confirms that multimodal optical measurements can be used to separately distinguish the changes in CBF, CBV and StO2 of the brain, thus providing with a complementary tool to fMRI to investigate the differential contribution of CBF, CBV and StO2 to the BOLD response as well as the influence of anesthetics in pharmacological studies.
Acknowledgments
This work was supported in part by NIH (1K25DA021200) and NYSTAR. The experiments were conducted in Brookhaven National Laboratory supported by Department of Energy (Contract DE-AC02-98CH10886). The authors thank to Yingtain Pan, Jim Ma and Mario Rebecchi for valuable discussions and to David Smith for the assistance on the physiological monitoring.
Abbreviations
- CBF
cerebral blood flow
- ΔCBF
change in CBF
- CBV
cerebral blood volume
- CMRO2
local rate of oxygen consumption
- fMRI
functional magnetic resonance imaging
- HR
heart rate
- ISO
isoflurane
- LDF
Laser Doppler Flowmetry
- MABP
mean arterial blood pressure
- MWOS
Multi-wavelength Optical Spectroscopy
- PK
pharmacokinetics
- ROI
region of interest
- StO2
hemoglobin oxygenation of tissue
- t(PK1/2Max)
half-peak clearance time of cocaine within the brain
- α-CHLOR
α-chloralose
Appendix A: CBF detection using Laser Doppler Flowmetry
Local CBF in the rat cortex was detected with LDF, a technique that allows recording of changes in local CBF continuously. The principles of LDF are based on the Doppler effect of moving red blood cells (rbc) within tissue, and the magnitude of the Doppler frequency shift is given by the general Doppler equation (Barnett et al., 1990).
| (A1) |
where ν is rbc velocity; n is tissue refractive index; θ is the angle between colliding photons and rbcs and λ is the optical wavelength applied. Here, LDF was performed using a MoorLab (Moor Instruments, Axminster, UK). The mean blood flow obtained from tissues or an organ such as the brain, is often defined as ‘blood flux’ and can be expressed as (refs)
| (A2) |
where, ω= 2π Δf and k is a systematic constant and P(ω) is the power density spectrum of the photon current detected at the frequency of ω · ω1~ω2 is the frequency bandwidth, and a wideband of 20–15 kHz was used during the experiments to provide a linear dynamic recording of blood flow; <I2dc> is the mean square of the DC current of the photonmultiplier to normalize the variation of laser source.
Appendix B: CBV and StO2 detections using dual-wavelength optical spectroscopy
The changes in the cerebral blood volume ΔCBV and the oxygenated hemoglobin oxygenation StO2 Δ[HbO] can be separately distinguished from the reflectance measured by summing and subtracting the optical densities of the signals at these two wavelengths, i.e.,
| (A3) |
| (A4) |
where ΔOD = ODafter − ODbefore is the change in optical density before and after drug administration that was associated with the changes in hemoglobin concentrations; λ1= 555nm and λ2= 572nm; εHb and εHbo represent the extinction coefficients of the deoxygenated-and oxygenated hemoglobin, which are constant; B is a pathlength factor that accounts for increases in the photon pathlength caused by tissue scattering and L is the distance between where the light enters the tissue and where the detected light exits the tissue. B and L are assumed not to be changed during the experiments. Therefore, Δ[CBV] and Δ[HbO] (i.e., referring to the changes in hemoglobin oxygenation ΔStO2) represent the changes in blood volume and oxygenated-hemoglobin concentration induced by a given drug such as cocaine.
Appendix C: Evaluation of CMRO2 changes from CBF, CBV and StO2 data obtained from rats anesthetized by α-CHLOR
According to Eq.12. of Hoge et. al. (1999), the changes in HbR (i.e., [dHb]) depend on changes in blood flow (CBF) & oxygen consumption (CMRO2), given by (Mayhew, et al, 2000):
| (A5) |
The changes in CMRO2 can be calculated from the changes in CBF, total hemoglobin (HbT), and deoxy hemoglobin (HbR) using the relationship (Jone et al., Eq. 3, NeuroImage 13, 1002–1015–2001; Dunn et al., Eq.2, NeuroImage 27, 279–290, 2005):
| (A6) |
Where the subscript ‘o’ indicates baseline values, the parameters γr & γt are vascular weighting constants which take into account that the measured changes in hemoglobin are combinations of venous, and arterial quantities. Due to the superficial measurement of optical images in the brain, γr & γt can be set to 1 (Dunn et al, 2005).
When CBF returns to baseline, i.e. ΔCBF=0, and CMRO2 can be increased only if:
| (A7) |
In this condition, eq.(A6) above becomes:
| (A8) |
or,
| (A9) |
because ΔCBF=0 and γr =γt =1 as described above. Assuming that HbRo= 40 μM, and HbTo=100μM. (Dunn et al., 2005, Mayhew et al., 2000), we will have
| (A10) |
As ΔHbT = ΔHbR+ΔHbO, eq.(A10) can be expressed as
| (A11) |
We experimentally determined the ΔHbT (i.e, ΔCBV, Eq.3 in Appendix B) and ΔHbO (Eq.4, Appendix B). At the time of maximal ΔHbO (i.e., tmax=25.6 min), we had ΔHbT(tmax) =4.2±0.02 and ΔHbO(tmax)= 2.1±0.03 (Fig. 3D1 & D2). By substituting these values into eq.(A11), we get,
| (A12) |
which means that our data empirically fit eq.(A11), so does eq.(A7). In other words, CMRO2 is indeed increased in the α-CHLOR anesthetized rats, even though ΔCBF = 0.
References
- Boag F, Havard CWH. Cardiac arrhythmia and myocardial ischemia related to cocaine and alcohol consumption. Postgrad Med J. 1985;61:997–999. doi: 10.1136/pgmj.61.721.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvento G, Charbonne R, Correze JL, Borredon J, Seylaz J, Lacombe P. Is alpha-chloralose plus halothane induction a suitable anesthetic regimen for cerebrovascular research? Brain Res. 1994;665(2):213–21. doi: 10.1016/0006-8993(94)91340-4. [DOI] [PubMed] [Google Scholar]
- Busse GD, Riley AL. Effects of alcohol on cocaine lethality in rats: acute and chronic assessments. Neurotoxicol Teratol. 2003 May-Jun;25(3):361–4. doi: 10.1016/s0892-0362(02)00351-3. [DOI] [PubMed] [Google Scholar]
- Ceolin L, Schwarz AJ, Gozzi A, Reese T, Bifone A. Effects of cocaine on blood flow and oxygen metabolism in the rat brain: Implications for phMRI. Magn Reson Imaging. 2007;25(6):795–800. doi: 10.1016/j.mri.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A . 2006 Jun 13;103(24):9333–8. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JK, Chen YI, Hamel E, Jenkins BG. Brain hemodynamic changes mediated by dopamine receptors: Role of the cerebral microvasculature in dopamine-mediated neurovascular coupling. Neuroimage. 2006;30:700–712. doi: 10.1016/j.neuroimage.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devonshire IM, Berwick J, Jones M, Martindale J, Johnston D. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. Neuroimage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Du C, Koretsky AP, Izrailtyan I, Benveniste H. Simultaneous detection of blood volume, oxygenation, and intracellular calcium changes during cerebral ischemia and reperfusion in vivo using diffuse reflectance and fluorescence. J Cereb Blood Flow Metab. 2005;25:1078–1092. doi: 10.1038/sj.jcbfm.9600102. [DOI] [PubMed] [Google Scholar]
- Du C, Yu M, Volkow ND, Koretsky AP, Fowler JS, Benveniste H. Cocaine increases intracellular concentration of calcium in brain independently of its cerebrovascular effects, J. of Neuroscience. 2006;26(45):11522–31. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Devor A, Dale AM, Boas DA. Spatial extent of oxygen metabolism and hemodynamic changes during functional activation of the rat somatosensory cortex. 2005;27(2):279–90. doi: 10.1016/j.neuroimage.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Fagan D. Inhalation anesthetic competition at high-affinity cocaine binding sites in rat brain synaptosomes. British J of Anaesthesia. 1994;73:820–825. doi: 10.1093/bja/73.6.820. [DOI] [PubMed] [Google Scholar]
- EI-Maghrabi EA, Eckenhoff RG. Inhibition of dopamine transport in rat brain synaptosomes by volatile anesthetics. Anesthesiology. 1993;78:750–756. doi: 10.1097/00000542-199304000-00019. [DOI] [PubMed] [Google Scholar]
- Farde L, Pauli S, Hall H, Eriksson L, Halldin C, Hogberg T, Nilsson L, Sjogren I, Stone-Elander S. Stereoselective binding of 11C-raclopride in living human brain--a search for extrastriatal central D2-dopamine receptors by PET. Psychopharmacology. 1988;94:471–478. doi: 10.1007/BF00212840. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, MacGregor RR, Hitzemann R, Logan J, Bendriem B, Gatley SJ, Christman D. Mapping cocaine biding sites in human and baboon brain in vivo. Synapse. 1989;16:371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl) 1999 Sep 1;146(1):93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. The effects of drugs on membrane fluidity. Annu Rev Pharmacol Toxicol. 1984;24:43–64. doi: 10.1146/annurev.pa.24.040184.000355. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Leolin L, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. A Multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magn Reson Med. 2007;25:826–833. doi: 10.1016/j.mri.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42:849–63. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hu XT. Cocaine withdraw and neuro-adaptations in ion channel function. Mol Neurobiol. 2007;35(1):95–112. doi: 10.1007/BF02700626. [DOI] [PubMed] [Google Scholar]
- Jones M, Berwick J, Johnston D, Mayhew J. Concurrent optical imaging spectroscopy and laser-Doppler flowmetry: the relationship between blood flow, oxygenation, and volume in rodent barrel cortex. NeuroImage. 2001;13:1002–1015. doi: 10.1006/nimg.2001.0808. [DOI] [PubMed] [Google Scholar]
- Lee SP, Duong TQ, Yang G, Iadecola C, Kim SG. Relative changes of cerebral arterial and venous blood volumes during increased cerebral blood flow:implications for BOLD fMRI. Magn Reson Med. 2001;45:791–800. doi: 10.1002/mrm.1107. [DOI] [PubMed] [Google Scholar]
- Lenz C, Frietsch T, Futterer C, Rebel A, van Ackern K, Kuschinsky W, Waschke KF. Local coupling of cerebral blood flow to cerebral gluocose metabolism during inhalational anesthesia in rats:desflurane versus isoflurane. Anesthesiology. 1999;91:1720–23. doi: 10.1097/00000542-199912000-00025. [DOI] [PubMed] [Google Scholar]
- Luo F, Schmidt KF, Fox GB, Ferris CF. Differential responses in CBF and CBV to cocaine as measured by fMRI: Implications for pharmacological MRI signals derived oxygen metabolism assessment. J Psychiatr Res. 2009 doi: 10.1016/j.jpsychires.2008.11.009. In press. [DOI] [PubMed] [Google Scholar]
- Luo ZC, Yuan ZJ, Tully M, Pan YT, Du C. Quantification of cocaine-induced cortical blood flow changes using laser speckle contrast imaging and Doppler OCT. Applied Optics. 2009;48(10):D247–55. doi: 10.1364/ao.48.00d247. [DOI] [PubMed] [Google Scholar]
- Lu H, Xi ZX, Gitajn L, Rea W, Yang Y, Stein EA. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI) PNAS. 2007;104(7):2489–94. doi: 10.1073/pnas.0606983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maekawa T, Tommasino C, Shapiro HM, Keifer-Goodman J, Kohlenberger RW. Local cerebral blood flow and gluocose utilization during isoflurane anesthesia in the rat. Anesthesiology. 1986;65:144–151. doi: 10.1097/00000542-198608000-00003. [DOI] [PubMed] [Google Scholar]
- Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in Rat. Neuroimage. 2000;11:13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular and BOLD signals in isoflurane-anethetized rat somatosensory cortex. Cerebral Cortex. (17) 2007 April;:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Mayhew J, Johnston D, Berwick J, Jones M, Coffey P, Zheng Y. Spectroscopic analysis of neural activity in brain: increased oxygen consumption following activation of barrel cortex. Neuroimage. 2000;2(6):664–75. doi: 10.1006/nimg.2000.0656. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Academic Press Australia; 1986. [Google Scholar]
- Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgansberger W, Kochs E, Eder M, Hapfelmeier G. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur J Neurosci. 2004;20(5):1276–80. doi: 10.1111/j.1460-9568.2004.03603.x. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Liebling CN, Reiszel C, Brodie JD, Dewey SL. Cue-induced dopamine release predicts cocaine preference: PET studies in freely moving rodents. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.5221-08.2009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KF, Febo M, Shen Q, Luo F, Sicard KM, Ferris CF, Stein EA, Duong TQ. Hemodynamic and metabolic changes induced by cocaine in anesthetized rat observed with multimodal functional MRI. Psychopharmacology (Berl) 2006;185:479–486. doi: 10.1007/s00213-006-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. Cereb Blood Flow Metab. 1999;19:871–79. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Silva AC, Koretsky AP, Duyn JH. Functional MRI impulse response for BOLD and CBV contrast in rat somatosensory cortex. Magnetic Resonance in Medicine. 2007;57:1110–1118. doi: 10.1002/mrm.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein EA, Fuller SA. Selective effects of cocaine on regional cerebral blood flow in the rat. J Pharmacol Exp Ther. 1992 Jul;262(1):327–34. [PubMed] [Google Scholar]
- Ueki M, Linn F, Hossmann KA. Functional activation of cerebral blood flow and matabolism before and after global ischemia of rat brain. J Cereb Blood Flow Metab. 1998;8:486–94. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- Ueki M, Mies G, Hossmann KA. Effect of apha-chloralose, halothane, pentobarbital and nitrous oxide anesthesia on metabolic coupling in somatosensory cortex of rat. Acta Anaesthesiol Scand. 1992;36:318–22. doi: 10.1111/j.1399-6576.1992.tb03474.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, Gatley SJ, Logan J, Wong C, Gifford A, Ding YS, Hitzemann R, Pappas N. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65(1):PL7–12. doi: 10.1016/s0024-3205(99)00225-8. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Calcium ions and integration in neural circuits. Acta Physiol (Oxf) 2006;187:357–69. doi: 10.1111/j.1748-1716.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- Wang Q, Simpao A, Sun L, Falk JL, Lau CE. Contribution of the active metabolite, norcocaine, to cocaine’s effects after intravenous and oral administration in rats: pharmacodynamics. Psychopharmacology (Berl) 2001 Jan;153(3):341–52. doi: 10.1007/s002130000568. [DOI] [PubMed] [Google Scholar]
- Yuan ZJ, Luo ZJ, Du C, Pan YT. A digital frequency ramping method for enhancing Doppler flow imaging in Fourier-domain optical coherence tomography. Optics Express. 2009;17(5):3951–63. doi: 10.1364/oe.17.003951. [DOI] [PubMed] [Google Scholar]