Abstract
Objective
The Pneumonia Severity Index (PSI) and CURB-65 predict outcomes in community acquired pneumonia (CAP), but have limitations. Procalcitonin, a biomarker of bacterial infection, may provide prognostic information in CAP. Our objective was to describe the pattern of procalcitonin in CAP, and determine if procalcitonin provides prognostic information beyond PSI and CURB-65.
Methods
We conducted a multi-center prospective cohort study in 28 community and teaching emergency departments. Patients presenting with a clinical and radiographic diagnosis of CAP were enrolled. We stratified procalcitonin levels a priori into four tiers – I: < 0.1; II: ≥ 0.1 to <0.25; III: ≥ 0.25 to < 0.5; and IV: ≥ 0.5 ng/ml. Primary outcome was 30d mortality.
Results
1651 patients formed the study cohort. Procalcitonin levels were broadly spread across tiers: 32.8% (I), 21.6% (II), 10.2% (III), 35.4% (IV). Used alone, procalcitonin had modest test characteristics: specificity (35%), sensitivity (92%), positive likelihood ratio (LR) (1.41), and negative LR (0.22). Adding procalcitonin to PSI in all subjects minimally improved performance. Adding procalcitonin to low risk PSI subjects (Class I–III) provided no additional information. However, subjects in procalcitonin tier I had low 30d mortality regardless of clinical risk, including those in higher risk classes (1.5% vs. 1.6% for those in PSI Class I–III vs. Class IV/V). Among high risk PSI subjects (Class IV/V), one quarter (126/546) were in procalcitonin tier I, and the negative LR of procalcitonin tier I was 0.09. Procalcitonin tier I was also associated with lower burden of other adverse outcomes. Similar results were seen with CURB-65 stratification.
Conclusions
Selective use of procalcitonin as an adjunct to existing rules may offer additional prognostic information in high risk patients.
Keywords (MeSH): Biological markers, procalcitonin, pneumonia, prediction rules
INTRODUCTION
Background
Community-acquired pneumonia (CAP) accounts for 1.3 million hospitalizations in the US each year (1) at a cost of $8.4 billion.(2) It is the most common cause of severe sepsis (3) and infection-related death.(4) Key to the safe and efficient management of CAP is the ability to reliably predict who will fare well or poorly. The Pneumonia Severity Index (PSI) (5) and CURB-65 (6) are clinical rules that identify a subset of individuals at low risk of death who are candidates for outpatient care.(7;8) However, all remaining patients are classified as high risk, usually prompting hospital admission and parenteral antibiotics, even though a large proportion may do well.(9) Thus, there has been considerable interest in the development of rapidly available biomarkers that might confer additional prognostic information.(10)
Importance
Procalcitonin is a calcitonin precursor that is generally elevated in bacterial infections but low in viral infections.(11) Procalcitonin has good discrimination for bacterial infections and sepsis (12–15), and three trials used low procalcitonin levels to withhold antibiotics in emergency department (ED) patients presenting with respiratory illnesses.(16–18) However, two recent meta-analyses concluded that procalcitonin could not reliably differentiate sepsis from non-infectious inflammation in critically ill patients (19), and had only moderate diagnostic performance for identifying bacteremia in ED patients.(20) Furthermore, the prognostic value of procalcitonin measurement beyond existing prediction rules is unclear. Masia et al noted higher procalcitonin levels in patients with high PSI scores, and that higher concentrations were associated with mortality and complications(21), but Beovic et al found no association between procalcitonin and PSI.(22) These single center studies were limited by small sample sizes, and used older procalcitonin assays with low sensitivity.(23)
Goals of This Investigation
Our goal was to determine the prognostic utility of a newer, high sensitivity procalcitonin assay for 30d mortality, and assess its value beyond established clinical prediction rules. We tested this assay within a multi-center, prospective cohort of patients presenting to the ED with a clinical and radiographic diagnosis of CAP. We hypothesized that an early singular procalcitonin measurement would aid risk assessment beyond that available from the PSI and CURB-65.
METHODS
Study design and setting
We conducted a multicenter, prospective cohort study of patients presenting to the EDs of 28 teaching and non-teaching hospitals in southwestern Pennsylvania, Connecticut, southern Michigan, and western Tennessee between November 2001 and November 2003 (GenIMS – Genetic and Inflammatory Markers of Sepsis). A specific aim of GenIMS was to develop and validate risk prediction tools based on information available early in the course of disease. As part of this aim, we sought to determine the prognostic utility of procalcitonin for 30-day mortality.
Selection of participants
Eligible subjects were ≥ 18 years old and had a clinical and radiologic diagnosis of pneumonia as per Fine, et al.(5) We excluded those transferred from another hospital, discharged from a hospital within the prior 10 days, with an episode of pneumonia within the past 30 days, on chronic mechanical ventilation, with cystic fibrosis, with active pulmonary tuberculosis, with a known positive HIV antibody titer, having alcoholism with evidence of end-organ damage, admitted for palliative care, enrolled previously in GenIMS, incarcerated, and pregnant women. We obtained informed consent from the subject or proxy. The Institutional Review Boards of the University of Pittsburgh and all participating sites approved the study.
Data collection and processing
We gathered baseline and sequential clinical information by structured patient or proxy interviews, bedside assessment by study nurses, and structured medical record reviews. Median time from ED admission to day 1 blood sample collection was 1.3 hours. We did not obtain day 1 samples from patients presenting after 11pm or on weekends and holidays for logistical reasons. Study personnel collected blood sample into pyrogen-free vials containing heparin, and separated plasma by centrifugation within one hour. Plasma was frozen and shipped on dry ice to our central laboratory in Pittsburgh. We tracked clinical data and blood samples using unique anonymized identification numbers, merging data only after assay completion. We observed strict data confidentiality and audited clinical data and assays for accuracy, including random chart audits, repeat blood assays, and computer flags for inconsistencies.
Methods of measurement
We measured procalcitonin using a time-resolved, amplified cryptate emission assay (Kryptor PCT, BRAHMS, Hennigsdorf, Germany). The assay has a functional assay sensitivity of 0.06 ng/ml, and at 0.1 ng/ml the coefficient of variation is 10–15%. (24) Study nurses ascertained deaths in hospital. Post discharge deaths were ascertained by telephone and National Death Index (NDI) search. We enrolled subjects between November 2001 and November 2003, locked clinical data in 2004, completed assays in 2005, and petitioned complete NDI data when it became available in 2006.
We prospectively assessed severity of illness using PSI.(5) We calculated CURB-65 retrospectively using altered mental status or a new change in Glasgow Coma Scale as proxy measures for confusion.(25) Based on prior studies, we stratified procalcitonin into four tiers - tier I: < 0.1; tier II: ≥ 0.1 to < 0.25; tier III: ≥ 0.25 to < 0.5, and; tier IV: ≥ 0.5 ng/ml.(16–18) We defined a clinically significant positive culture based on published guidelines and prior literature.(26–33) For example, single blood cultures that yielded coagulase negative staphylococci, and sputum cultures that yielded normal oral flora were not counted as clinically significant. We defined severe sepsis as infection plus acute organ dysfunction following international consensus criteria.(34) Acute organ dysfunction was defined as a new Sequential Organ Failure Assessment (SOFA) score of >2 in any of six organ systems.(35)
Outcome measures
Our primary outcome was 30-day mortality, the traditional endpoint used for clinical prediction rules in CAP, including PSI and CURB-65. Secondary outcomes included 90-day mortality, length of stay, and intensive care unit (ICU) admission.
Primary data analysis
We generated descriptive data, comparing initial presentation and outcome measures across procalcitonin tiers. To test for trends across procalcitonin tiers for ordinal variables, we used the Jonckheere-Terpstra trend test. To test for differences across procalcitonin tiers for continuous variables, we used the Kruskal-Wallis test.
To understand the prognostic utility of procalcitonin and the two clinical prediction rules as stand-alone tests, we first generated Kaplan-Meier plots of 30-day mortality by category (tier, class or group). We then generated test characteristics (sensitivity, specificity, negative and positive likelihood ratios [LR]) for each test dichotomized into low and high risk. A positive LR > 10 and a negative LR < 0.1 may be considered to provide strong evidence to rule in or rule out the condition of interest.(36) We defined low risk as Class I–III for PSI, Group 1 for CURB-65, and tier I (<0.1 ng/mL) for procalcitonin, based on previous criteria. (5;18;37)
To understand the value of adding procalcitonin to PSI, we first generated 30-day mortality Kaplan-Meier plots stratified by procalcitonin tier within each PSI Class. Second, we assessed the change in test characteristics for a logistic regression model where procalcitonin was added to a model with PSI alone. This approach assumes both ‘tests’ (PSI and procalcitonin) are performed in all patients. We derived and validated models using a 3:1 random split of the overall cohort, fitting with all possible combinations of PSI, procalcitonin, and the corresponding interaction term. These models were also fit with PSI and procalcitonin treated as categorical variables. Third, because one could selectively order procalcitonin measurement depending on the clinical risk assessment, we assessed the test characteristics of procalcitonin within the clinical low and high risk strata. We used the same approach to determine the prognostic value of adding procalcitonin to CURB-65. Analyses were performed using SAS 9.1 (SAS, Cary, NC).
Sensitivity analyses
To complete sensitivity analyses, we explored the effects of reclassifying PSI Class I–III subjects as high risk if they were hypoxic (5), and excluding patients who were subsequently ruled out for CAP during their hospitalization. Due to the large number of variables for the analysis stratifying and comparing the baseline presentation of high-risk patients by procalcitonin tier I vs. tiers II–IV, we chose a significance level of p < 0.01. For all other analyses, we assumed significance at p < 0.05.
RESULTS
Characteristics of study subjects
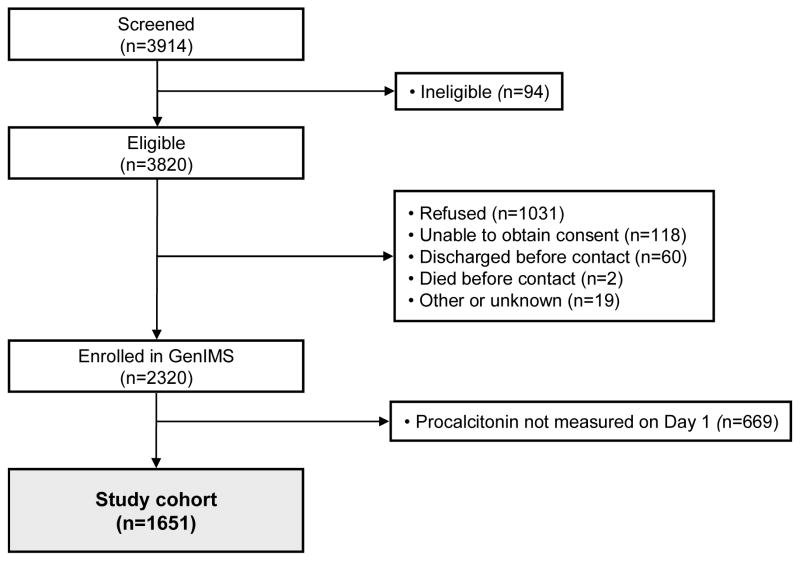
Of the 2320 subjects enrolled in GenIMS, 1651 (71.2%) had a day 1 procalcitonin drawn and formed the study cohort. PSI was measured in the ED in 1384 (83.8%) of the 1651 study cohort subjects (Figure 1). The study cohort was predominantly white, underlying disease was common, approximately half were identified as high risk by the PSI and CURB-65 clinical risk rules, and most were admitted to hospital. Very few subjects identified in the ED as having CAP were subsequently “ruled out for CAP” by the inpatient clinical team (Table 1). Severe sepsis developed in one quarter, and 30-day mortality was 6.4% overall (Table 2), and 15.0% and 11.3% in those labeled as high risk by PSI and CURB-65 (Appendix Tables 1 and 2).
Figure 1. Flow diagram of study.
Table 1.
Study cohort demographic and clinical characteristics (n = 1651)
| Value | |
|---|---|
| Age (years), mean (SD) | 65.0 (18.5) |
| Sex (male), n (%) | 860 (52%) |
| Race, n (%) | |
| White | 1336 (81%) |
| Black | 227 (14%) |
| Other | 88 (5%) |
| Prior antibiotics, n (%) | 271 (16%) |
| Pre-enrollment duration of symptoms, mean (SD), median | 4.8 (14.5), 3.0 |
| Charlson co-morbidity index | |
| Mean (SD), median | 1.69 (2.12), 1.0 |
| Index > 0, n (%) | 1094 (66%) |
| PSI* | |
| Mean (SD), median | 82.0 (34.3), 80.5 |
| Class, n (%) | |
| I, II | 541 (39%) |
| III | 297 (21%) |
| IV | 419 (30%) |
| V | 127 (9%) |
| CURB-65 | |
| Mean (SD), median | 1.57 (1.19), 1.0 |
| Group, n (%) | |
| 1 | 826 (50%) |
| 2 | 421 (25%) |
| 3 | 404 (24%) |
| ED discharges, n (%) | 265 (16%) |
| Ruled out for CAP, n (%) | 94 (6%) |
SD – standard deviation, PSI - Pneumonia Severity Index, ED – emergency department, CAP – community acquired pneumonia
PSI was measured in the ED in 1384 (83.8%) subjects. There were no significant differences between subjects who did, and did not, have a PSI measured.
Table 2.
Initial presentation and outcomes, by procalcitonin tier
| All | Procalcitonin tier (ng/ml) | |||||
|---|---|---|---|---|---|---|
| I (< 0.1) |
II (≥ 0.1; < 0.25) |
III (≥ 0.25; < 0.5) |
IV (≥ 0.5) |
|||
| N (%) | p-value* | 1651 (100%) | 542 (32.8%) | 356 (21.6%) | 169 (10.2%) | 584 (35.4%) |
| Initial presentation | ||||||
| SIRS criteria, n (%) | ||||||
| SIRS by white blood cell | .0018 | 1475 (96%) | 449 (93%) | 317 (96%) | 157 (97%) | 552 (97%) |
| SIRS by temperature | <.0001 | 766 (47%) | 155 (29%) | 169 (48%) | 87 (52%) | 355 (61%) |
| SIRS by respiratory | .1292 | 1605 (97%) | 532 (99%) | 341 (96%) | 166 (99%) | 566 (97%) |
| SIRS by heart rate | <.0001 | 1138 (76%) | 312 (67%) | 239 (73%) | 116 (74%) | 471 (85%) |
| Total # of SIRS criteria met, mean (SD) | <.0001 | 3.02 (0.87) | 2.67 (0.85) | 2.99 (0.87) | 3.11 (0.82) | 3.33 (0.78) |
| Pneumonia Severity Index | ||||||
| Mean (SD) | <.0001 | 82.0 (34.3) | 71.2 (30.9) | 83.9 (33.7) | 86.8 (32.9) | 89.7 (35.5) |
| Class, n (%) | ||||||
| I, II | <.0001 | 541 (39%) | 224 (48%) | 109 (37%) | 52 (35%) | 156 (33%) |
| III | 297 (21%) | 112 (24%) | 66 (22%) | 28 (19%) | 91 (19%) | |
| IV | 419 (30%) | 110 (24%) | 91 (31%) | 55 (37%) | 163 (34%) | |
| V | 127 (9%) | 16 (3%) | 29 (10%) | 13 (9%) | 69 (14%) | |
| CURB-65 | ||||||
| Mean (SD), median | <.0001 | 1.57 (1.19), 1.0 | 1.16 (1.06), 1.0 | 1.56 (1.15), 2.0 | 1.73 (1.19), 2.0 | 1.91 (1.22), 2.0 |
| Group, n (%) | ||||||
| 1 | <.0001 | 826 (50%) | 361 (67%) | 172 (48%) | 70 (41%) | 223 (38%) |
| 2 | 421 (25%) | 111 (20%) | 99 (28%) | 48 (28%) | 163 (28%) | |
| 3 | 404 (24%) | 70 (13%) | 85 (24%) | 51 (30%) | 198 (34%) | |
| Prior antibiotics, n (%) | .0707 | 271 (16%) | 103 (19%) | 64 (18%) | 23 (14%) | 81 (14 %) |
| Outcomes, n (%) | ||||||
| 30-day mortality | <.0001 | 106 (6.4%) | 8 (1.5%) | 30 (8.4%) | 16 (9.5%) | 52 (8.9%) |
| 90-day mortality | <.0001 | 161 (9.8%) | 24 (4.4%) | 41 (11.5%) | 26 (15.4%) | 70 (12.0%) |
| Hospital LOS (admitted patients), mean (SD), median | <.0001 | 6.2 (5.3), 5.0 | 5.1 (4.4), 4.0 | 6.3 (5.4), 5.0 | 6.9 (5.2), 5.0 | 7.0 (5.9), 5.5 |
| ICU admission | <.0001 | 215 (13%) | 42 (8%) | 48 (13%) | 20 (12%) | 105 (18%) |
| ED discharges | <.0001 | 265 (16%) | 137 (25%) | 62 (17%) | 16 (9%) | 50 (9%) |
| Ruled out for CAP | .040 | 94 (6%) | 38 (7%) | 24 (7%) | 6 (4%) | 26 (4%) |
| Clinically significant culture | <.0001 | 215 (13%) | 34 (6%) | 29 (8%) | 22 (13%) | 130 (22%) |
| Severe sepsis | <.0001 | 423 (26%) | 91 (17%) | 99 (28%) | 51 (30%) | 182 (31%) |
| Severe sepsis on Day 1 | <.0001 | 202 (12%) | 33 (6%) | 43 (12%) | 26 (15%) | 100 (17%) |
| Mechanical ventilation | .0002 | 89 (5%) | 14 (3%) | 23 (6%) | 6 (4%) | 46 (8%) |
SIRS – systemic inflammatory response syndrome, SD – standard deviation, ED – emergency department, CAP – community acquired pneumonia, ICU – intensive care unit, LOS – length of stay
Test for trend across procalcitonin tiers, using the Jonckheere-Terpstra test for trend for ordinal variables, and test for differences across procalcitonin tiers using the Kruskal-Wallis test for continuous variables.
Appendix Table 1.
Procalcitonin, PSI, mortality and severe sepsis.
| PSI Class I, II | |||||
|---|---|---|---|---|---|
| Procalcitonin tier (ng/ml) | N | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) |
| < 0.1 | 224 | 2 (1%) | 3 (1%) | 15 (7%) | 4 (2%) |
| ≥ 0.1; < 0.25 | 109 | 0 (0%) | 0 (0%) | 11 (10%) | 5 (5%) |
| ≥ 0.25; < 0.5 | 52 | 2 (4%) | 2 (4%) | 9 (17%) | 4 (8%) |
| ≥ 0.5 | 156 | 1 (1%) | 1 (1%) | 19 (12%) | 10 (6%) |
| TOTAL | 541 | 5 (1%) | 6 (1%) | 54 (10%) | 23 (4%) |
| PSI Class III | |||||
| Procalcitonin tier (ng/ml) | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) | |
| < 0.1 | 112 | 3 (3%) | 8 (7%) | 11 (10%) | 3 (3%) |
| ≥ 0.1; < 0.25 | 66 | 2 (3%) | 3 (5%) | 20 (30%) | 6 (9%) |
| ≥ 0.25; < 0.5 | 28 | 0 (0%) | 1 (4%) | 4 (14%) | 0 (0%) |
| ≥ 0.5 | 91 | 2 (2%) | 3 (3%) | 22 (24%) | 9 (10%) |
| TOTAL | 297 | 7 (2%) | 15 (5%) | 57 (19%) | 18 (6%) |
| PSI Class IV | |||||
| Procalcitonin tier (ng/ml) | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) | |
| < 0.1 | 110 | 2 (2%) | 11 (10%) | 42 (38%) | 13 (12%) |
| ≥ 0.1; < 0.25 | 91 | 13 (14%) | 15 (16%) | 33 (36%) | 14 (15%) |
| ≥ 0.25; < 0.5 | 55 | 10 (18%) | 16 (29%) | 25 (45%) | 15 (27%) |
| ≥ 0.5 | 163 | 19 (12%) | 31 (19%) | 62 (38%) | 34 (21%) |
| TOTAL | 419 | 44 (11%) | 73 (17%) | 162 (39%) | 76 (18%) |
| PSI Class V | |||||
| Procalcitonin tier (ng/ml) | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) | |
| < 0.1 | 16 | 0 (0%) | 0 (0%) | 8 (50%) | 8 (50%) |
| ≥ 0.1; < 0.25 | 29 | 11 (38%) | 16 (55%) | 19 (66%) | 9 (31%) |
| ≥ 0.25; < 0.5 | 13 | 4 (31%) | 5 (38%) | 5 (38%) | 3 (23%) |
| ≥ 0.5 | 69 | 23 (33%) | 27 (39%) | 44 (64%) | 29 (42%) |
| TOTAL | 127 | 38 (30%) | 48 (38%) | 76 (60%) | 49 (39%) |
Appendix Table 2.
Procalcitonin, CURB-65, mortality and severe sepsis.
| CURB-65 Group 1 | |||||
|---|---|---|---|---|---|
| Procalcitonin tier (ng/ml) | N | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) |
| < 0.1 | 361 | 4 (1%) | 8 (2%) | 35 (10%) | 12 (3%) |
| ≥ 0.1; < 0.25 | 172 | 3 (2%) | 4 (2%) | 28 (16%) | 11 (6%) |
| ≥ 0.25; < 0.5 | 70 | 2 (3%) | 2 (3%) | 16 (23%) | 5 (7%) |
| ≥ 0.5 | 223 | 4 (2%) | 6 (3%) | 39 (17%) | 16 (7%) |
| TOTAL | 826 | 13 (2%) | 20 (2%) | 118 (14%) | 44 (5%) |
| CURB-65 Group 2 | |||||
| Procalcitonin tier (ng/ml) | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) | |
| < 0.1 | 111 | 4 (4%) | 9 (8%) | 33 (30%) | 11 (10%) |
| ≥ 0.1; < 0.25 | 99 | 8 (8%) | 13 (13%) | 26 (26%) | 11 (11%) |
| ≥ 0.25; < 0.5 | 48 | 5 (10%) | 11 (23%) | 16 (33%) | 9 (19%) |
| ≥ 0.5 | 163 | 10 (6%) | 17 (10%) | 46 (28%) | 17 (10%) |
| TOTAL | 421 | 27 (6%) | 50 (12%) | 121 (29%) | 48 (11%) |
| CURB-65 Group 3 | |||||
| Procalcitonin tier (ng/ml) | 30d mortality (%) | 90d mortality (%) | Severe sepsis (%) | Severe sepsis on Day 1 (%) | |
| < 0.1 | 70 | 0 (0%) | 7 (10%) | 23 (33%) | 10 (14%) |
| ≥ 0.1; < 0.25 | 85 | 19 (22 %) | 24 (28%) | 45 (53%) | 21 (25%) |
| ≥ 0.25; < 0.5 | 51 | 9 (18%) | 13 (25%) | 19 (37%) | 12 (24%) |
| ≥ 0.5 | 198 | 38 (19%) | 47 (24%) | 97 (49%) | 67 (34%) |
| TOTAL | 404 | 66 (16%) | 91 (23%) | 184 (46%) | 110 (27%) |
The mean procalcitonin level at presentation was 3.4 ng/mL, but levels were broadly spread (SD: 16.5), such that 542 subjects (32.8%) were in tier I, 356 (21.6%) in tier II, 169 (10.2%) in tier III, and 584 (35.4%) in tier IV. Higher procalcitonin tiers were associated with more clinical signs of infection and a worse course and outcome (Table 2). For example, subjects in the lowest tier had the lowest severity scores, lowest likelihood of developing clinically significant cultures, and lowest rates of severe sepsis, mechanical ventilation, ICU admission, and death. Lowest procalcitonin tier patients were also the most likely to be discharged from the ED and, if admitted, to be subsequently ruled out for CAP (Table 2). Among subjects who developed severe sepsis, those in the lowest procalcitonin tier were less likely to develop central nervous (5.3% vs. 24.2%; p = 0.0001), cardiovascular (4.0% vs. 11.0%; p = 0.08), and ≥ 3 organ (1.3% vs. 16.5%; p = 0.0001) system dysfunction, and had more organ failure-free days in the first 30 days (25.5 vs. 18.5; p < 0.0001), compared to those in procalcitonin tiers II–IV.
Main Results
As stand-alone tests, the PSI, CURB-65 and procalcitonin had similar characteristics. For each, 30-day mortality generally rose with rising category (Figures 2 and 3, Table 2). In addition, after dichotomizing subjects as ‘high’ or ‘low’ risk, each test alone had moderate specificity (35–64%), high sensitivity (87–92%), low positive LRs (1.41–2.43) and modest negative LRs (0.20–0.23) (Table 3). Simply adding the procalcitonin test result to the PSI in all subjects led to only minimal improvement in test performance. In the derivation cohort, sensitivity increased from 71.8% to 76.1%, specificity from 77.3% to 79.8%, and area under the receiver operating curve from 0.83 to 0.85. Similarly, in the validation cohort, sensitivity and specificity changed from 78.0% to 78.3% and from 75.2% to 78.9%. Results were similar when adding procalcitonin to CURB-65.
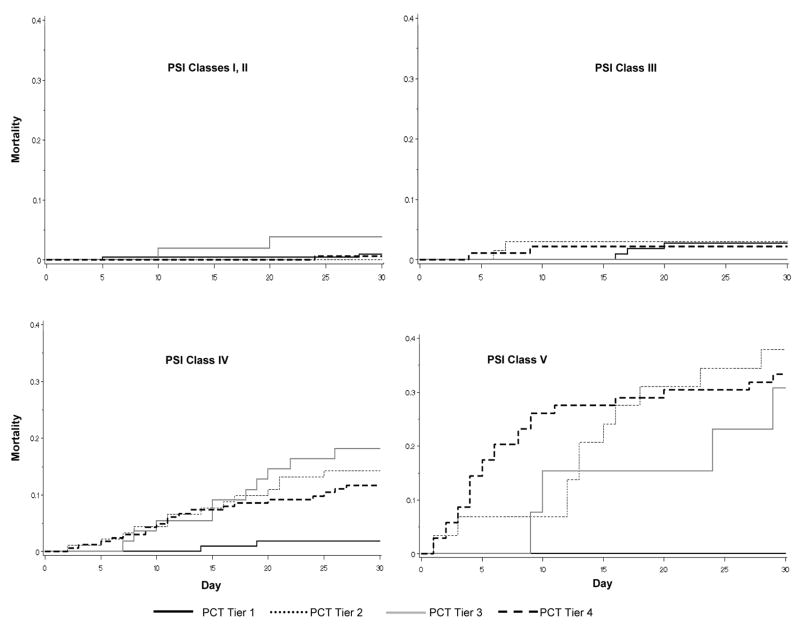
Figure 2. Kaplan-Meier survival curves, by PSI Class and procalcitonin tier.
In PSI Class I–III, mortality was low, and stratification by procalcitonin tier did not provide additional information. In PSI Class IV/V, patients with a procalcitonin < 0.1 ng/mL had the lowest 30-day mortality. PSI – Pneumonia Severity Index. PCT – procalcitonin.
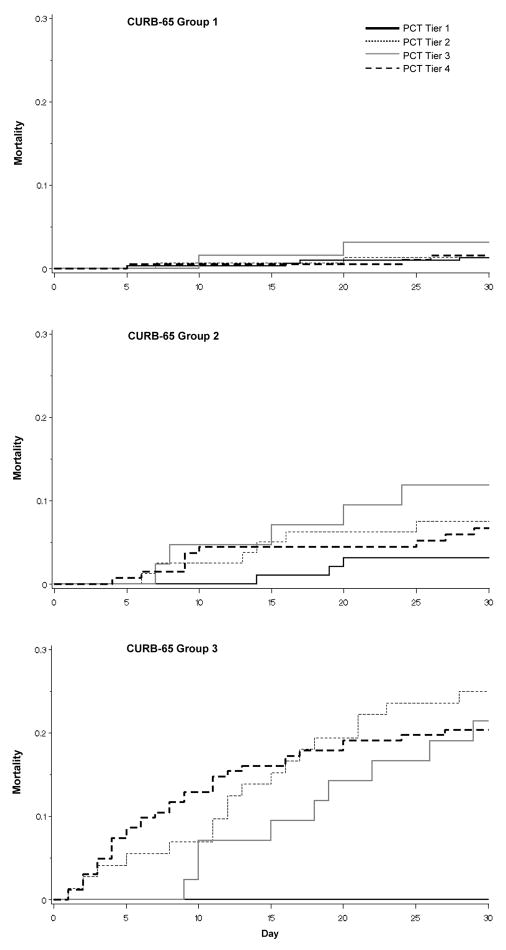
Figure 3. Kaplan-Meier survival curves, by CURB-65 Group and procalcitonin tier.
In CURB-65 Group 1 patients, mortality was low, and and stratification by procalcitonin tier did not provide additional information. In CURB-65 Groups 2/3, patients with a procalcitonin < 0.1 ng/mL had the lowest 30-day mortality. PCT – procalcitonin.
Table 3.
Test characteristics of alternative risk assessment strategies for 30-day mortality
| Risk assessment strategy | N | Positive likelihood ratio (95% CI) |
Negative likelihood ratio (95% CI) |
Specificity | Sensitivity |
|---|---|---|---|---|---|
| Using each test alone in all patients | |||||
| PSI* | 1384 | 2.43 (2.18 – 2.70) | 0.20 (0.12 – 0.34) | 0.64 | 0.87 |
| CURB-65† | 1651 | 1.85 (1.70 – 2.02) | 0.23 (0.14 – 0.39) | 0.53 | 0.88 |
| Procalcitonin‡ | 1651 | 1.41 (1.32 – 1.51) | 0.22 (0.11 – 0.43) | 0.35 | 0.92 |
| Using procalcitonin in low-risk patients‡ | |||||
| PSI Class I–III | 838 | 0.97 (0.60 – 1.58) | 1.04 (0.53 – 2.04) | 0.40 | 0.58 |
| CURB-65 Group 1 | 826 | 1.23 (0.85 – 1.78) | 0.70 (0.31 – 1.59) | 0.44 | 0.69 |
| Using procalcitonin in high-risk patients‡ | |||||
| PSI Class IV/V | 546 | 1.33 (1.25 – 1.42) | 0.09 (0.02 – 0.36) | 0.27 | 0.98 |
| CURB-65 Group 2/3 | 825 | 1.26 (1.19 – 1.34) | 0.18 (0.07 – 0.47) | 0.24 | 0.96 |
PSI Class IV/V vs. Class I–III
CURB-65 Group 2/3 vs. Group 1
Procalcitonin ≥; 0.1 ng/ml vs. procalcitonin < 0.1 ng/ml
PSI – Pneumonia Severity Index
Mortality at 30 days was very low among subjects identified as low risk by the clinical prediction rules (1.4% for PSI Class I–III and 1.6% for CURB-65 Group 1) and there was no additional prognostic advantage when stratified by procalcitonin tier (Figures 2 and 3, Table 3). However, among those identified as high risk by PSI, 23.1% (126/546) had a procalcitonin level in tier I. In this subgroup, only two subjects died by day 30, yielding a mortality rate of 1.6%, similar to that of the low risk subjects. In contrast, mortality was higher in the PSI Class IV/V subjects who had a procalcitonin tier >I (19.0% [80/420], p < 0.0001) (Figure 2). The negative LR for a low procalcitonin within clinically high risk subjects was 0.09 (Table 3). Results were similar with CURB-65: 21.9% (181/825) of CURB-65 Group 2/3 subjects had a procalcitonin level in tier I, and mortality was 2.2% (4/181) vs. 13.8% (89/644) for subjects with procalcitonin levels in tier I vs. tiers II–IV (p < 0.0001), yielding a negative LR for a low procalcitonin of 0.18 (Figure 3 and Table 3).
Secondary and sensitivity analyses
We explored possible reasons for the large mortality difference by procalcitonin tier among subjects identified clinically as high risk. Some clinical signs were worse for those with higher procalcitonin tiers in comparison to those in tier I, but differences were generally modest, and many other aspects of clinical presentation were similar. For example, those in procalcitonin tiers II–IV were more likely to have renal impairment, altered mental status, fever, and leukocytosis, but no more likely to be hypoxic, tachypneic, tachycardic, hypotensive, thrombocytopenic or have abnormal blood glucose or sodium. There were also no differences in age, race, sex, Charlson co-morbidity, functional status, duration of symptoms, or domicile (nursing home vs. home).
Within clinical high risk subjects, those in procalcitonin tier I also had lower 90-day mortality (PSI: 9% vs. 26%, p < .0001; CURB-65: 9% vs. 19%, p=.0004) and, in those identified as high risk by CURB-65, shorter length of stay (6.7d vs. 7.9d, p=.002) and reduced likelihood of ICU admission (13% vs. 21%, p=.01), compared to those in procalcitonin tiers II–IV. Neither excluding subjects who ruled out for CAP, nor reclassifying hypoxic PSI Class I–III subjects as ‘high risk’ significantly changed our results.
LIMITATIONS
We chose 30-day mortality as the primary outcome, following the methodology of the original PSI and CURB-65 studies. Other outcomes are important, and not necessarily correlated with mortality. Nevertheless, subjects with a low procalcitonin appeared to have lower rates of many other outcomes, including mortality measured at different timepoints (Appendix Tables 1 and 2). Second, although procalcitonin tier I was consistently associated with a low mortality, a “dose-response” was not seen across procalcitonin tiers II–IV, with similar 30-day mortality rates across these tiers (range: 8.4% – 9.5%) (Table 2). Thus, it is important to emphasize that only the lowest range of procalcitonin is associated with low mortality. Third, CAP is a clinical diagnosis with inherent subjectivity. A recent acute bronchitis review noted that a procalcitonin < 0.1 ng/ml may be able to safely discriminate between acute bronchitis and CAP, but that more data were needed. (38) This raises the possibility that some of our cohort with a low procalcitonin, although diagnosed clinically and radiographically with CAP by their treating physicians, may not actually have had pneumonia. However, all GenIMS patients met the CAP criteria of Fine et al (5), an expected, low percentage of patients were later deemed not to have CAP, this percentage did not markedly vary by procalcitonin tier (range: 4% – 7%), and eliminating these patients from our analyses had minimal effect. Lastly, as with PSI, CURB-65, or any clinical decision adjunct, procalcitonin must be interpreted in the context of the individual patient, and clinical judgment is always necessary.
DISCUSSION
Over a century ago, it was observed that “…the result of a laboratory test should have, in a given case, the same value as a cardinal symptom or an approved clinical sign….many … forget this, and fail to correlate the laboratory findings with the clinical findings.”(39) We also observed that used alone, procalcitonin performed similarly to existing clinical prediction rules, but that indiscriminately adding procalcitonin to all patients, regardless of clinical risk category, provided little additional information. However, clinical prediction rules have two important limitations – physicians may misapply or not remember them, and within a given risk category there can be a significant range in outcome. We therefore sought to determine if procalcitonin could address these concerns, first as a stand-alone test, and then layered on top of clinical risk assessment.
Our main finding was that in a large, contemporaneous cohort of patients diagnosed in the ED with CAP, patients with a procalcitonin < 0.1 ng/ml had a low 30-day mortality rate, even in patients defined as high-risk by established clinical risk prediction rules. Thus, adding procalcitonin to the assessment of high clinical risk patients significantly improved the ability to rule out the likelihood of death. There are, however, important caveats to these observations.
First, although a procalcitonin < 0.1 ng/ml in high-risk subjects had a very low LR for death, the relatively wide 95% confidence interval merits caution. Second, a good outcome for a high-risk subject could either be because the risk prediction tool was inadequately discriminant or because the ensuing care averted an adverse outcome. Thus, a retrospective identification of a rule or test with potentially valuable test characteristics should be followed up by prospective assessment of its impact on clinical decision-making and outcomes. The potential of the PSI was not fully understood until Marrie et al and Yealy et al demonstrated that it could help physicians safely withhold hospital admission and intravenous antibiotics.(37;40) We similarly recommend that procalcitonin as an adjunct to clinical tools be tested prospectively before wider use.
Our primary goal was to determine how procalcitonin might enhance existing CAP prediction rules and decision making. We recognize though that physicians often do not explicitly calculate PSI in daily clinical practice. However, the same factors that comprise PSI and other prediction rules also go into the bedside clinical judgment many physicians use to guide their decisions. Our results therefore suggest that procalcitonin may aid decision making in high risk patients, defined explicitly or implicitly with the PSI or a similar tool. Most importantly, we again emphasize that procalcitonin should never be used in isolation to make clinical decisions, and does not replace physician assessment.
Current CAP guidelines recommend that clinically high risk patients be hospitalized, and that ICU admission be considered for patients in the highest risk categories. (7;41;42) Of interest, Marrie et al also observed that many clinical high-risk patients might be safely managed at home.(43) Our data suggest that procalcitonin may aid in identifying PSI/CURB 65 high risk patients who will rarely suffer mortal and other complications. Thus, there could be considerable benefits, both in terms of conserved resources and antibiotic management, if one could better stratify high risk patients. Prospective studies are needed to determine if procalcitonin can improve physician management decisions and outcomes in high risk patients.
However, two clinically important questions merit emphasis. First, is a low procalcitonin in a high risk patient clinically obvious, and only a “costly surrogate measure of health status”? (44) We believe not, since these patients did not appear different than other high risk patients across a wide range of baseline variables, suggesting that a low procalcitonin is not particularly obvious at the bedside. Second, why did those patients with high clinical risk scores, yet low procalcitonin levels, do so well? We found that a low procalcitonin was associated with shorter length of stay, lower proportions of mechanical ventilation and ICU admission, and a more benign severe sepsis phenotype. This suggests that while CAP patients with high clinical risk scores often already have severe sepsis at ED presentation or develop it later, that a low procalcitonin portends a less severe course, potentially explaining the associated low mortality.
Of note, our observational study design does not allow us to address whether procalcitonin can be useful in guiding antibiotic treatment, based on ability to determine bacterial infection. Instead, our work focused on procalcitonin and links to adverse outcomes in those already diagnosed with CAP. We recognize however that these two domains, while theoretically distinct, are practically intermingled. For example, patients with CAP that have positive blood cultures tend to fare worse, and low procalcitonin is associated with both low positive culture rates and low mortality.
We conclude that in a large, multicenter CAP cohort, patients in the lowest procalcitonin tier (≤ 0.1 ng/ml) were at a low risk of death, regardless of clinical risk. Used indiscriminately, procalcitonin provided little additional information over PSI and CURB-65 risk assessment. However, a selective two-tiered approach of first performing a clinical risk assessment, and then obtaining procalcitonin only in those judged to be high-risk, offers potentially important value.
Acknowledgments
Source of support: NIGMS R01 GM061992
We thank the patients for their participation in this study. We are also indebted to the investigators, coordinators, clinical, laboratory and research personnel at each of the participating hospitals for their efforts. In addition to the authors, the following individuals and institutions participated in the GenIMS study:
Co-Investigators
University of Pittsburgh, Pittsburgh: Mark S. Roberts, MD; Mitchell P. Fink, MD; Michael J. Fine, MD; Kelly A. Wood, MD, MHS; Kenneth Kalassian, MD; Russell L. Delude, PhD; Haichao Wang, PhD; Thomas Auble, PhD; Andrew Schaefer, PhD; Michael R. Pinsky, MD; Eleanor Finegold, PhD; Greg Cooper, MD, PhD; Vincent Arena, PhD; Alex Krichevsky, DVM, PhD
Norwalk Hospital, Norwalk: Jonathan M. Fine, MD
New York University, New York: Kevin J. Tracey, MD, FACS
Site Investigators
Pennsylvania
Jefferson Hospital/SHHS, Pittsburgh: Christopher Dooley, MD; Jacqueline Anderson, RN; David Laman, MD; Mahapareh Mostoufi, MD
Mercy Hospital, Pittsburgh: Bruce A. MacLeod, MD; Susan Rolniak, RN, CRNP; Pat Riley, MD; Ken Greer, MD; Dennis Borochovitz, MD
Sewickley Valley Hospital, Sewickley: Frank Gaudio, MD; Greg Maggi, RN; Mindy Hufnagel
St. Clair Memorial Hospital, Pittsburgh: Christopher DeLuca, MD; Arlene Grogan, RN; Stephen Basheda, DO; Martha Clark, MD
UPMC Braddock, Braddock: Richard Heath, MD; Sandra Casey, RN; Emily Yee, MD; Charles Krifcher, MD
UPMC Horizon Health System (Greenville), Greenville and UPMC Horizon Health System (Shenango), Ferrell: Jeffrey Moldovan, DO; Amy Pagano, MSN; Janet Moldovan, RN, MSN
UPMC Lee Regional, Johnstown: Sandy Ergas, MD; Karen Betcher, RN; Ed Rocker
UPMC McKeesport, McKeesport: Rani K. Kumar, MD; Linda Hewitt, RN; Linda Knestaut, RN; Rahut Chaudhry, MD; Ray Probst
UPMC Passavant, Pittsburgh: William G. Kristan, MD; Carol Hewlett, RN; Linda Campbell, RN; Mark Provenzano, MD; Joe Kuzma
UPMC Presbyterian, Pittsburgh: Theodore R. Delbridge, MD, MPH; Mary Ann Murcek, RN; Alan Wells, MD
UPMC Shadyside, Pittsburgh: Jerold Solot, DO; Joel Weinberg, MD; Linda Waddell, RN; Michael Becich, MD
UPMC South Side, Pittsburgh: Joel Rosenbloom, DO; Carol Joyce, RN; C. Vaugh Strimlan, MD; Sukamal Khasnabis, MD
UPMC St. Margaret, Pittsburgh: James Nicholas, MD; Ann Morris, RN; Nancy Gorsha, RN; Mitchell Patti, MD; Jagjit Singh, MD; Meredith Naples
The Western Pennsylvania Hospital, Pittsburgh: Thomas P. Campbell, MD; Diana Morrow, RN; Paul C. Fiehler, MD; Stanley Geyer, MD; Bud Jozwiak
Connecticut
Bridgeport Hospital, Bridgeport: Michael Werdmann, MD; Kathy Nunnink, RN, BSN, CCRN; Herbert Scherzer, MD; Larry H. Bernstein, MD; Raymond Haddad, MD
Hartford Hospital, Hartford: Robert Grant, MD; Caryn St. Clair, RN; Beverly Reynholds, RN, Roseanne Papa, RN,
Milford Hospital, Milford: Jay Walshon, MD; Tina Null, RN; Lloyd Friedman, MD; Suri Pappu; Will Jones
New Britain General, New Britain: Louis Graff, MD; Michael McNamme, MD; Barry Jacobs, MD; Stephen Wolf, MD; David Buono, MD; Nancy Bennett, RN
Norwalk Hospital, Norwalk: Jonathan Fine, MD; Michael Carius, MD; Saraswathi Nair, MD; Christine Belden, RN; Leonard Scinto
St. Mary’s Hospital, Waterbury: Steve Holland, MD; Eleanor Flynn, RN; Edie Kalan, RN; Rose Riordan
Yale University Hospital, New Haven: Linda C. Degutis, DrPH; Nancy Olson, RN, MS; Mark Siegel, MD; Peter Jatlow, MD
Michigan
Henry Ford Health System, Detroit: Emanuel P. Rivers, MD, MPH; Kant Shah, MD
Detroit Receiving/Wayne State, Detroit: Robert Welch, MD; Denise Waselewsky, RN; James Kruse, MD; Kristen Bilicki
Sinai-Grace, Detroit: Robert Dunne, MD; Denise Waselewsky, RN; Kristen Bilicki
Tennessee
Methodist Healthcare, Memphis: Richard Wunderink, MD; Carol Jones, RN, BSN; Lori Kessler, RN
Others
University of Pittsburgh, Pittsburgh: Tammy L. Young; Gerard Nau, MD; Malik Rahim, MD; Michael Coughlin, MS, PhD; Margaret V. Bowman, RN; Tricia A. Powell, RN; LeeAnn Mandich, RN; Heather Sterling, RN; Karen Greenwald, RN; Tracy Hoteck, RN; Susan Hebda, RRT; Mark Cohen; Marcia McCaw, RN; Heather Woods, RN; Michael Martino, MD; Melinda Carter; Angela Darnley; Angel Shaufl; Jodi Gigler; Javier Martinez, PhD; Xia Tang, MSIS
University of Pennsylvania, Philadelphia: Jason Christie, MD, PhD
North Shore–LIJ Research Institute, Manhasset: Annette Lee, PhD
Yale University School of Medicine, New Haven: Courtney McDonald, BS
Footnotes
The work was performed at the CRISMA Laboratory, Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA and the participating sites. The study was presented at the October 2006 American College of Emergency Physicians Research Forum in New Orleans, LA.
Reprints not available from the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Adv Data. 2006;(371):1–19. [PubMed] [Google Scholar]
- 2.Niederman MS, McCombs JS, Unger AN, et al. The cost of treating community-acquired pneumonia. Clinical Therapeutics. 1998;20(4):820–837. doi: 10.1016/s0149-2918(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan V, Angus DC, Griffin MF, et al. Hospitalized community-acquired pneumonia in the elderly: Age- and sex-related patterns of care and outcome in the United States. Am J Respir Crit Care Med. 2002;165(6):766–772. doi: 10.1164/ajrccm.165.6.2103038. [DOI] [PubMed] [Google Scholar]
- 5.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 6.Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American College of Emergency Physicians. Clinical policy for the management and risk stratification of community-acquired pneumonia in adults in the emergency department. Ann Emerg Med. 2001;38(1):107–113. doi: 10.1067/mem.2001.115880. [DOI] [PubMed] [Google Scholar]
- 8.Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med. 2005;143(12):881–894. doi: 10.7326/0003-4819-143-12-200512200-00006. [DOI] [PubMed] [Google Scholar]
- 9.Angus DC, Marrie TJ, Obrosky DS, et al. Severe community-acquired pneumonia: Use of intensive care and evaluation of the American Thoracic Society and British Thoracic Society criteria. Am J Respir Crit Care Med. 2002;166(5):717–723. doi: 10.1164/rccm.2102084. [DOI] [PubMed] [Google Scholar]
- 10.Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol. 2006;24(8):971–983. doi: 10.1038/nbt1235. [DOI] [PubMed] [Google Scholar]
- 11.Christ-Crain M, Muller B. Procalcitonin in bacterial infections--hype, hope, more or less? Swiss Med Wkly. 2005;135(31–32):451–460. doi: 10.4414/smw.2005.11169. [DOI] [PubMed] [Google Scholar]
- 12.Luzzani A, Polati E, Dorizzi R, et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 13.Casado-Flores J, Blanco-Quiros A, Asensio J, et al. Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med. 2003;4(2):190–195. doi: 10.1097/01.PCC.0000059420.15811.2D. [DOI] [PubMed] [Google Scholar]
- 14.Caterino JM, Scheatzle MD, Forbes ML, et al. Bacteremic elder emergency department patients: procalcitonin and white count. Acad Emerg Med. 2004;11(4):393–396. doi: 10.1197/j.aem.2003.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Uzzan B, Cohen R, Nicolas P, et al. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 16.Christ-Crain M, Stolz D, Bingisser R, et al. Procalcitonin-Guidance of Antibiotic Therapy in Community-Acquired Pneumonia - A Randomized Trial. Am J Respir Crit Care Med. 2006 doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 17.Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131(1):9–19. doi: 10.1378/chest.06-1500. [DOI] [PubMed] [Google Scholar]
- 18.Christ-Crain M, Jaccard-Stolz D, Bingisser R, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363(9409):600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 19.Tang BM, Eslick GD, Craig JC, et al. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. Lancet Infect Dis. 2007;7(3):210–217. doi: 10.1016/S1473-3099(07)70052-X. [DOI] [PubMed] [Google Scholar]
- 20.Jones AE, Fiechtl JF, Brown MD, et al. Procalcitonin test in the diagnosis of bacteremia: a meta-analysis. Ann Emerg Med. 2007;50(1):34–41. doi: 10.1016/j.annemergmed.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Masia M, Gutierrez F, Shum C, et al. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128(4):2223–2229. doi: 10.1378/chest.128.4.2223. [DOI] [PubMed] [Google Scholar]
- 22.Beovic B, Kreft S, Osredkar J, et al. Serum procalcitonin levels in patients with mild community-acquired pneumonia. Clin Microbiol Infect. 2005;11(12):1050–1051. doi: 10.1111/j.1469-0691.2005.01285.x. [DOI] [PubMed] [Google Scholar]
- 23.Nylen E, Muller B, Becker KL, et al. The future diagnostic role of procalcitonin levels: the need for improved sensitivity. Clin Infect Dis. 2003;36(6):823–824. doi: 10.1086/368088. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach G, Rau B, Debard AL, et al. Multicenter evaluation of a new immunoassay for procalcitonin measurement on the Kryptor System. Clin Chem Lab Med. 2004;42(4):440–449. doi: 10.1515/CCLM.2004.077. [DOI] [PubMed] [Google Scholar]
- 25.Aujesky D, Auble TE, Yealy DM, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118(4):384–392. doi: 10.1016/j.amjmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Richter SS, Beekmann SE, Croco JL, et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory-based algorithm. J Clin Microbiol. 2002;40(7):2437–2444. doi: 10.1128/JCM.40.7.2437-2444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinstein MP. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol. 2003;41(6):2275–2278. doi: 10.1128/JCM.41.6.2275-2278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metersky ML, Ma A, Bratzler DW, et al. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169(3):342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 29.Graham JC, Galloway A. ACP Best Practice No 167: the laboratory diagnosis of urinary tract infection. J Clin Pathol. 2001;54(12):911–919. doi: 10.1136/jcp.54.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayhall C, editor. Hospital epidemiology and infection control. Philadelphia: Lippincott Williams & Wilkins; 2004. Surveillance of nosocomial infections. Appendix A-1. CDC definitions of nosocomial infections; pp. 1659–1702. [Google Scholar]
- 31.Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000;31(2):347–382. doi: 10.1086/313954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niederman MS, Mandell LA, Anzueto A, et al. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163(7):1730–1754. doi: 10.1164/ajrccm.163.7.at1010. [DOI] [PubMed] [Google Scholar]
- 33.Granato PA. Pathogenic and indigenous microorganisms of humans. In: Murray PR, editor. Manual of Clinical Microbiology. Washington DC: American Society for Microbiology Press; 2003. [Google Scholar]
- 34.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29(4):530–538. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL, Sakr Y, Sprung CL, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 36.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329(7458):168–169. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yealy DM, Auble TE, Stone RA, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Ann Intern Med. 2005;143(12):881–894. doi: 10.7326/0003-4819-143-12-200512200-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wenzel RP, Fowler AA., III Acute Bronchitis. N Engl J Med. 2006;355(20):2125–2130. doi: 10.1056/NEJMcp061493. [DOI] [PubMed] [Google Scholar]
- 39.Reiling J. JAMA 100 years ago - The laboratory in diagnosis. Journal of the American Medical Association. 2007;297(5):538.2–7. Ref Type: Journal (Full) [Google Scholar]
- 40.Marrie TJ, Lau CY, Wheeler SL, et al. A controlled trial of a critical pathway for the treatment of community acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA. 2000;283(6):749–755. doi: 10.1001/jama.283.6.749. [DOI] [PubMed] [Google Scholar]
- 41.Macfarlane JT, Boldy D. 2004 update of BTS pneumonia guidelines: what’s new? Thorax. 2004;59(5):364–366. doi: 10.1136/thx.2004.024992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 (Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marrie TJ, Huang JQ. Admission is not always necessary for patients with community-acquired pneumonia in risk classes IV and V diagnosed in the emergency room. Can Respir J. 2007;14(4):212–216. doi: 10.1155/2007/451417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schelbert E. Prognostic value of B-type natriuretic peptide in unstable coronary artery disease. JAMA. 2006;295(16):1895–1896. doi: 10.1001/jama.295.16.1895-a. [DOI] [PubMed] [Google Scholar]