Abstract
The melanocortin receptor (MCR) subtype family is a member of the GPCR superfamily and each of them has a different pharmacological profile regarding the relative potency of the endogenous and synthetic melanocortin peptides. α-MSH and ACTH are endogenous nonselective agonists for MC1R, MC3R, MC4R and MC5R. In this study, we examined the role of Phe7 in ACTH on Human (h)MC1R, MC3R and MC4R binding and signaling. Our results indicate that substitution of the Phe7 with DNal (2’)7 in ACTH1-24 has different pharmacological profile from that of substitution of the Phe7 with DNal (2’)7 in MSH at hMC1R, hMC3R and hMC4R. NDNal (2’)7-ACTH1-24 is an agonist at hMC3R and hMC4R which did not switch peptide from agonist to antagonist at hMC3R and hMC4R. Further experiments indicate that NDNal (2’)7-ACTH1-17 is the minimal peptide required for hMC3R and hMC4R activation. Single amino acid substitution studies of DNal (2’)7 ACTH1-17 indicate that the amino acid residues 15, 16 and 17 in NDNal (2’)7-ACTH1-17 are crucial for hMC3R and hMC4R activation. Substitutions of these amino acid residues reduced or abolished agonist activity at hMC3R and hMC4R. Conformational studies revealed a new β-turn (-Arg8-Trp9-Gly10-Lys11-) in NDNal (2’)7-ACTH1-17, compared to the β-turn like structure at NDP-α-MSH (–His6-DPhe7-Arg8-Trp9-). Our results suggest that NDP-α-MSH and NDNal (2’)7-ACTH1-17 does not share the same binding site; highly basic C terminal fragment Lys15-Lys16-Arg17 of NDNal (2’)7-ACTH1-17 induced a new β-turn and this shift contributed the selective agonist activity at hMC3R and hMC4R.
Keywords: POMC, MCR, agonist, GPCR, receptor activation
The five known subtypes of human melanocortin receptors (hMC1-5R) are members of the super-family of seven transmembrane G-protein-coupled receptors (GPCRs) expressed in various tissues, including skin (hMC1R) (1, 2) the adrenal cortex (hMC2R) (3, 4), and throughout the central nervous system (hMC3R, hMC4R, hMC5R) (5). The melanocortin system has received much attention in recent years due to its involvement in a large number of important physiological functions, such as skin pigmentation (1, 2), control of the immune system, erectile function(6), blood pressure and heart rate, control of feeding behavior and energy homeostasis(7, 8), modulation of aggressive/defensive behavior and mediation of pain (9–12). The endogenous melanocortin agonists include α-, β-, γ-melanocyte-stimulating hormones (MSH), and adrenocorticotropin (ACTH), while agouti signaling protein and agouti-related protein have been identified as the endogenous antagonists (13, 14).
A considerable effort has been made toward the development of highly potent hMC3R, hMC4R-selective agonists and antagonists due to the involvement of these receptors in the regulation of feeding and sexual behavior (15–23). Most of the design and SAR work are based on MSH and its analogue. Almost all of the peptide agonists possess a β-turn like structure in their binding pharmacophore (24–28). Comparatively little attention has been given to ACTH, owing to the dearth of specific evidence on their physiological functions in the melanocortin system and its larger size not being appropriate for the drug development. Nevertheless, ACTH is active at hMCRs and its structure information of the pharmacophore will be able to lead to drug design and the selective cell signaling. Earlier reports have demonstrated that ACTH stimulation of MC2Rs in the adrenal cortex, especially in the zona fasciculate, results in the secretion of the glucocorticoids cortisol and corticoserone (29). Lately, it has been reported that ACTH plays an important physiological role in stimulating fetal immune response which will be important for the study of cancer.
Several approaches to the design of hMC3R-selective agonists and antagonists have been described in the literature. Among the natural melanocyte-stimulating hormones, γ-MSH exhibits substantial hMC3R selectivity, whereas α-MSH and β-MSH show little selectivity for any specific receptor subtype (30, 31). A D-amino acid scan of the γ-MSH sequence revealed the importance of position 8 in hMC3R selectivity, and led to the discovery of a highly selective hMC3R agonist (30). A DNal(2’) scan of linear α-MSH let to the discovery of a potent hMC3R/hMC5R antagonist and hMC4R agonist H-Tyr-Val-Nle-Gly-His-D-Nal(2’)-Arg-Trp-Asp-Arg-Phe-Gly-NH2 (PB-II-94) (32). Structure-activity relationships of γ-MSH have yielded linear peptide analogues with enhanced potency and selectivity, most notably, the non-selective super-agonist Ac-Tyr-Val-Nle-Gly-His-D-Phe-Arg-Trp-Asp-Arg-Phe-Gly-NH2 (Ac-NDP-γ-MSH-NH2) (31), and recently, Hruby’s laboratory has produced several potent and selective hMC3R agonists and hMC3R/hMC5R antagonists by placing a bulky hydrophobic Nle residue next to the melanocortin pharmacophore Xaa-Phe-Arg-Trp in a cyclic γ-MSH-derived template (33). Some cyclic α-MSH templates have also been described, where increased selectivity in hMC3R agonists and antagonists was observed. Thus, Kavarana et al have found that enhancing the hydrophobic properties of the cyclic α-MSH analogues and increasing the peptide macrocycle size resulted in improved hMC3R selectivity (34). Furthermore, Grieco et al. have shown that certain dihedrally constrained amino acid substitutions at position 6 of Ac-Nle4-c[Asp5, D-Nal(2’)7, Lys10]α-MSH(4–10)-NH2 (SHU9119) led to potent and highly hMC3R- and hMC4R-selective antagonists (24, 35). Balse-Srinivasan et al. have reported a series of cyclic disulfide α-MSH/β-MSH hybrid peptides with highly selective hMC3R (Ac-c[Pen-Glu-His-D-Nal(2’)-Arg-Trp-Cys]-Pro-Pro-Lys-Asp-NH2) and hMC5R (Ac-c[Cys-Glu His-D-Phe-Arg-Trp-D-Cys]-Pro-Pro-Lys-Asp-NH2) antagonists (36).
The novel drug discovery study is not only based on the ligand structure information, but also based on the receptor structure information. The melanocortin receptors consist of a single polypeptide featuring seven α-helical transmembrane domains (TMs), an extracellular N-terminus, three extracellular loops, three intracellular loops and an intracellular C-terminus. Many structural features conserved in other G-protein-coupled receptors are found in the melanocortin receptors (37, 38). However, the melanocortin receptors lack several features found in most G-protein-coupled receptors; one or two cysteine residues in the first and second extracellular loops and proline found in the fourth and fifth TMs. This receptor structural information will be critical for finding the residues or domains which contribute the selectivity of melanocortin system. Extensive studies have been performed using receptors chimeras, multiple site directed mutagenesis (16, 39–44). In this study, we described a series of ACTH analogues possessing a DNal (2’)7 which have been designed to further pursue SAR trends leading to hMC3R, hMC4R agonist selectivity from both of ligand structure and receptor structure studies.
Experimental Section
Materials
All MSH and ACTH peptides were made by Genscript Inc.(Piscataway, NJ). To avoid of oxidization, all of ACTHs the 4th position of Met were replace with Norleucine. The peptide sequence is shown in Table 1. 3-Isobutyl-methylxanthine (IBMX) is from Sigma, and [125I] NDP-α-MSH is from Perkin-Elmer Life Sciences (Boston, MA). The HEK-293 cell line was purchased from ATCC (Manassas, VA) and DMEM, lipofectamine from Life Technologies (Rockville MD).
Table 1.
Sequences of the substituted ACTH peptides
| NDP-α-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-Trp-Gly-Lys-Pro-Val-NH2 |
| NDNal(2’)7-α-MSH | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val- NH2 |
| NDPhe7-ACTH1-24 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DPhe-Arg-T rp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-AA18-24- NH2 |
| NDNal(2’)7-ACTH1-24 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg-AA18-24- NH2 |
| N-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
| NDNal(2') 7-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
| NDNal(2') 7-ACTH1-14 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly- NH2 |
| NDNal(2') 7-ACTH1-15 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys- NH2 |
| NDNal(2') 7-ACTH1-16 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys- NH2 |
| NDNal(2') 7-Nle15-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Nle-Lys-Arg- NH2 |
| NDNal(2') 7-Nle16-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Nle-Arg- NH2 |
| NDNal(2') 7-Nle17-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Nle- NH2 |
| NAla7-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-Ala-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
| NDNal(2') 7-Ala8-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Ala-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
| NDNal(2') 7-Ala9-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-DNal(2')-Arg-Ala-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
| NDNal(2') 7-Ala7,8,9-ACTH1-17 | Ac-Ser-Tyr-Ser-Nle-Glu-His-Ala-Ala-Ala-Arg-Trp-Gly-Lys-Pro-Val-Gly-Lys-Lys-Arg- NH2 |
Site-directed mutagenesis of human melanocortin receptors
Single mutation was constructed using the Quick-Change Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The entire coding region of the mutated receptors was sequenced to confirm that the desired mutation sequences were present and that no sequence errors had been introduced by University of Alabama at Birmingham Sequence Core. The mutant receptors were then subcloned into the eukaryotic expression vector pCDNA 3.1 (Invitrogen; Carlsbad, CA).
Cell culture and transfection
The HEK-293 cell line was utilized in this study. The cells were cultured in DMEM medium containing 10% bovine fetal serum and HEPES. Cells at 80% confluence were washed twice, and the receptor constructs were transfected into cells using lipofectamine (Life Technologies, Rockville MD). The permanently transfected clonal cell lines were selected by resistance to the neomycin analogue G418.
Binding assays
Binding experiments were performed using the conditions previously described (16). Briefly, after removal of the media, cells were incubated with non-radioligand from 10−10 to 10−6M in 0.5 mL MEM containing 0.2% BSA and 2 × 105 cpm of 125I-NDP- α -MSH for one hour. The binding reactions were terminated by removing the media and washing the cells twice with MEM containing 0.2% BSA. The cells were then lysed with 0.2 N NaOH, and the radioactivity in the lysate was quantified in an analytical gamma counter (PerkinElmer, Shelton, CT). Nonspecific binding was determined by measuring the amount of 125I-label bound on the cells in the presence of excess 10−6 M unlabeled ligand. Specific binding was calculated by subtracting nonspecifically bound radioactivity from total bound radioactivity. Binding data are reported as IC50. Ki values for ligands were calculated using the equation Ki = Kd = IC50 – [radioligand] (45).
cAMP assay
Cellular cAMP generation was measured using a competitive binding assay kit (TRK 432, Amersham, Arlington Heights, IL). Briefly, cell culture media was removed, and cells were incubated with 0.5 mL Earle’s Balanced Salt Solution (EBSS), containing the melanocortin agonist (10−10−10−6 M), for one hour at 37°C in the presence of 10−3 M isobutylmethylxanthine. The reaction was stopped by adding ice-cold 100% ethanol (500µl/well). The cells in each well were scraped, transferred to a 1.5 mL tube, and centrifuged for 10 min at 1900 × g, and the supernatant was evaporated in a 55°C water bath with pre-purified nitrogen gas. cAMP content was measured as previously described, according to instructions accompanying the assay kit (46).
Receptor expression by using FACs. To determine whether the receptor proteins are expressed at the cell surface, we generated a chimeric hMC4R with the N terminal fusion to the FLAG (Flag-hMC4R). The FLAG protein is an eight amino acid peptide (Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Lys) and FLAG-tagged receptor expression vectors have been widely used to purify receptor protein and to determine the protein expression (47). Cells transfected with the receptors were harvested using 0.2% EDTA and washed twice with phosphate buffer saline (PBS). Aliquots of 3×106 cells were centrifuged and fixed with 3% paraformaldehyde in PBS (pH 7.4). The cells were incubated with 50 µL of 10 µg/mL murine anti-FLAG M1 monoclonal antibody (Sigma, catalog No. 316) in incubation buffer for 45 minutes. Under this condition the primary antibody binds only to receptors located at the cell surface. The cells were collected by centrifugation and washed three times with incubation buffer. The cell pellets were suspended in 100 µL of incubation buffer containing CY™3-conjugated Affinity Pure Donkey Anti-Mouse Ig G (ImmunoResearch Lab, Inc., West Grove, PA) and incubated at room temperature for 30 minutes. Flow cytometry was performed on a fluorescence-activated cell sorter (Becton Dickinson FACStar plus six parameter cytometer/sorter with a dual Argon ion laser, San Jose, California). The results were analyzed using the software CellQuest (Beckton-Dickinson Immunocytometry Systems, San Jose, California).
Computational Procedures
Molecular modeling experiments employed MacroModel version 9.1 equipped with Maestro 7.5 graphical interface (Schrödinger, LLC, New York, NY, 2005) installed on a Linux Red Hat 9.0 system, and were performed as previously described (33). Peptide structures were built into extended structures with standard bond lengths and angles, and they were minimized using the OPLS 2005 force field and the Polak-Ribier conjugate gradient (PRCG). Optimizations were converged to a gradient RMSD less that 0.05 kJ/Å mol or continued until a limit of 50,000 iterations was reached. Aqueous solution conditions were simulated using the continuum dielectric water solvent model (GB/SA). Extended cut-off distances were defined at 8Å for Van der Waals, 20Å for electrostatics and 4 Å for H-bonds.
Conformational profiles of the peptides were investigated by the hybrid Monte Carlo/Low Frequency Mode (MCMM/LMCS)(48) procedure as implemented in Macromodel using the energy minimization parameters as described above. MCMM torsional variations and Low Mode parameters were set up automatically within Maestro graphical user interface. A total of 20,000 search steps were performed and the conformations with energy difference of 50 kJ/mol from the global minimum were saved. Interatomic dihedral angles were measured for each peptide analogue using the Maestro graphical user interface, and they are described in Table 6.
Statistical analysis
Each experiment was performed in duplicate three separate times. The mean value of the dose-response data of binding and cAMP production was fitted to a sigmoidal curve with a variable slope factor using non-linear squares regression analysis (Graphpad Prism, Graphpad Software, San Diego, CA). Data are expressed as mean ± SEM. Statistical significance was assessed by one-way ANOVA with P <0.05.
Results
DPhe7- and DNal (2’)7-ACTH analogue binding and activity
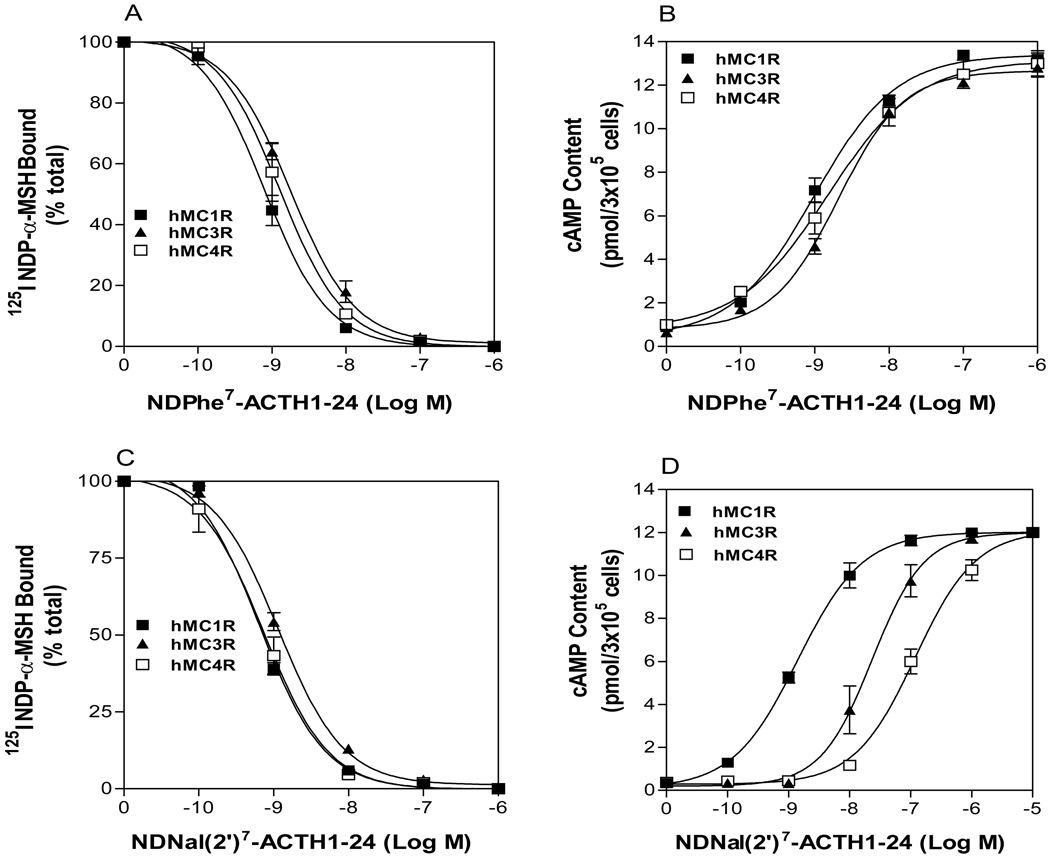
It is well known that the amino acid Phe7 in MSH is critical for agonist activity at the MC3R and the MC4R. Substitution of Phe7 in MSH with DPhe7 results in increase of agonist activity but replacement of DPhe7 with DNal(2’)7 caused ligand to lose agonist activity at the MC3R and the MC4R (28). To determine whether Phe7 in ACTH is also crucial for hMC3R and hMC4R binding and activation, we examined the substitutions of DPhe7 in ACTH on receptor binding. NDPhe7-ACTH dose-dependently displaces 125I NDP-α-MSH binding at the hMC3R and hMC4R (Figure 1A). The binding affinities of NDPhe7-ACTH analogues are higher than that of NPhe7-ACTH analogues. Consistent with binding data, NDPhe7-ACTH dose-dependently increased cAMP generation at hMC3R and hMC4R (Figure 1B). NDPhe7-ACTH has high agonist potency compared to that of NPhe7-ACTH. The role of DPhe7 in ACTH is similar to that of NDP-α-MSH and their IC50 and EC50 are shown in Table 2.
Figure 1.
Binding affinity and potency of NDP-α-MSH and ACTH(1–24) analogues at the wild-type hMC1R, hMC3R and hMC4R. Panel A and C show that the effect of ACTH analogue on 125I-ACTH binding at hMC1R, hMC3R and hMC4R. Panel B and D show the effect of ACTH analogue on total cAMP accumulation at hMC1R, hMC3R and hMC4R (n ≥ 3; see Table 2 for Ki and EC50 values).
Table 2.
Binding affinities and potencies of NDP-α-MSH and ACTH analogues at hMC1R, hMC3R and hMC4R.
| hMC1R | hMC3R | hMC4R | ||||
|---|---|---|---|---|---|---|
| Ki(nM) | EC50(nM) | Ki(nM) | EC50(nM) | Ki(nM) | EC50(nM) | |
| NDP-α-MSH | 0.4 ± 0.03 | 0.7 ± 0.1 | 3.5 ± 1.0 | 1.8 ± 0.1 | 2.1 ± 0.2 | 1.0 ± 0.3 |
| NDNal(2’)7-α-MSH | 1.2 ± 0.3 | 1.8 ± 0.5 | 5.5 ± 1.0 | NA | 3.4±0.3 | NA |
| NDPhe7-ACTH1-24 | 0.5 ± 0.2 | 1.0 ± 0.2 | 6.1 ± 0.6 | 0.6 ± 0.1 | 3.9 ± 0.4 | 1.3 ± 0.2 |
| NDNal(2’)7-ACTH1-24 | 0.8 ± 0.3 | 2.7 ± 0.5 | 5.9 ± 0.8 | 83 ± 15 | 3.8 ± 0.1 | 134 ± 32 |
NA: no activity (at 10−6M)
To determine whether DNal (2’)7 in ACTH is also crucial for hMC3R and hMC4R activation, NDNal (‘2)7-ACTH was synthesized and tested. Our results indicate that NDNal (2’)7-ACTH1-24 dose-dependently displaced 125I-ACTH binding at the hMC3R and the hMC4R and its Ki was showed in Table 2. Surprisingly, our results indicate that NDNal (2’)7-ACTH1-24 remains an agonist at hMC3R and hMC4R. Unlike NDNal (2’)7-α-MSH, NDNal (2’)7-ACTH1-24 was able to induce cAMP production at hMC3R and hMC4R although NDNal (2’)7-ACTH1-24 has significantly decreased potency (near 100 fold) compared to NDP-α-MSH at hMC3R and hMC4R. It suggests that DNal (2’)7-ACTH1-24 has different binding sites compared to that of DNal (2’)7 -α-MSH.
Truncated DNal (2’)7-ACTH analogue binding and activity
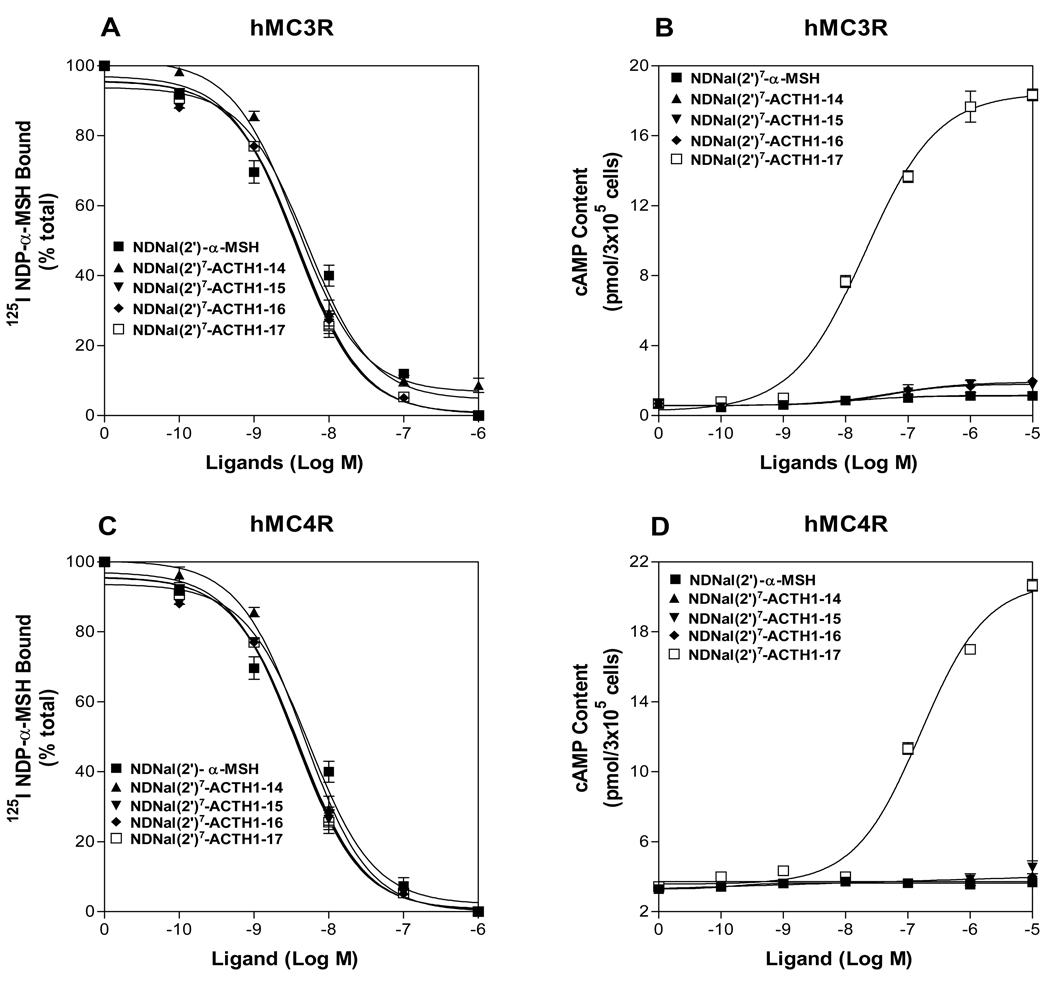
To further investigate which region of ACTH is crucial for DNal (2’)7-ACTH activity, several truncated DNal (2’)7-ACTH analogues were synthesized and tested. The sequences of the tested peptides are shown in Table 1. Our results demonstrated that the activities of these analogues are different at these MCRs. NDNal (2’)7-ACTH1-17 is the least sequence which can activate hMC3R and hMC4R (Figure 2). If the length of DNal (2’)7-ACTH analogues is less than 17 amino acids, the truncated peptides lose their agonist activities at the hMC3R and the hMC4R. Their Ki and EC50 are shown in Table 3.
Figure 2.
Binding affinity and potency of the truncated ACTH fragments at the wild-type hMC3R and hMC4R. Panel A and C show that the effects of the truncated ACTH fragments on 125I-NDP-α-MSH binding at hMC3R and hMC4R. Panel B and D show the effects of the truncated ACTH fragments on total cAMP accumulation at hMC3R and hMC4R (n ≥ 3; see Table 3 for Ki and EC50 values).
Table 3.
The affinities and potencies of the truncated ACTH analogues at hMC1R, hMC3R and hMC4R.
| hMC1R | hMC3R | hMC4R | ||||
|---|---|---|---|---|---|---|
| Ki (nM) | EC50 (nM) | Ki(nM | EC50 (nM) | Ki(nM | EC50 (nM) | |
| NDNal(2')7-α-MSH | 0.92 ± 0.1 | 0.63 ±0.03 | 5.3 ± 1.0 | NA | 1.6 ± 0.3 | NA |
| NDNal(2')7-Nle4-ACTH1-14 | 0.83 ± 0.2 | 0.67 ± 0.01 | 5.6 ± 0.9 | NA | 1.8 ± 0.2 | NA |
| NDNal(2')7-Nle4-ACTH1-15 | 0.90 ± 0.2 | 0.71 ±0.05 | 5.7 ± 1.5 | NA | 1.3 ± 0.5 | NA |
| NDNal(2')7-Nle4-ACTH1-16 | 0.89 ± 0.16 | 0.65 ± 0.06 | 5.1 ± 1.6 | NA | 1.8 ± 0.2 | NA |
| NDNal(2')7-Nle4-ACTH1-17 | 0.88 ± 0.17 | 0.58 ± 0.07 | 5.3 ± 1.1 | 95 ± 7 | 2.1 ± 0.6 | 184 ± 17 |
| NDNal(2')7-Nle15-ACTH1-17 | 0.95 ± 0.13 | 0.5 ± 0.03 | 5.8 ± 0.8 | NA | 1.4 ± 0.3 | NA |
| NDNal(2')7-Nle16-ACTH1-17 | 0.99 ±0.15 | 0.45 ±0.05 | 5.4 ± 1.4 | NA | 1.5 ± 0.4 | NA |
| NDNal(2')7-Nle17-ACTH1-17 | 0.96 ± 0.13 | 0.56 ± 0.03 | 5.5 ± 0.9 | NA | 1.7 ± 0.3 | NA |
| Ala7-ACTH1-17 | 37.8 ± 5.2 | 7.8 ± 1.2 | >103 | 165 ± 15 | >103 | 257 ± 32 |
| NDNal(2')7-Ala8-ACTH1-17 | 10.9 ± 2.3 | 9.5 ± 3.0 | >103 | 293 ± 2 6 | >103 | 335 ± 27 |
| NDNal(2')7-Ala9-ACTH1-17 | 21.5 ± 4.7 | 16.5 ± 2.7 | >103 | NA | >103 | NA |
| NDNal(2')7-Ala7,8,9-ACTH1-17 | >103 | NA | >103 | NA | >103 | NA |
NA: No activity (at 10 −6M).
Single amino acid substitution in DNal (2’)7-ACTH1-17 analogues binding and activity
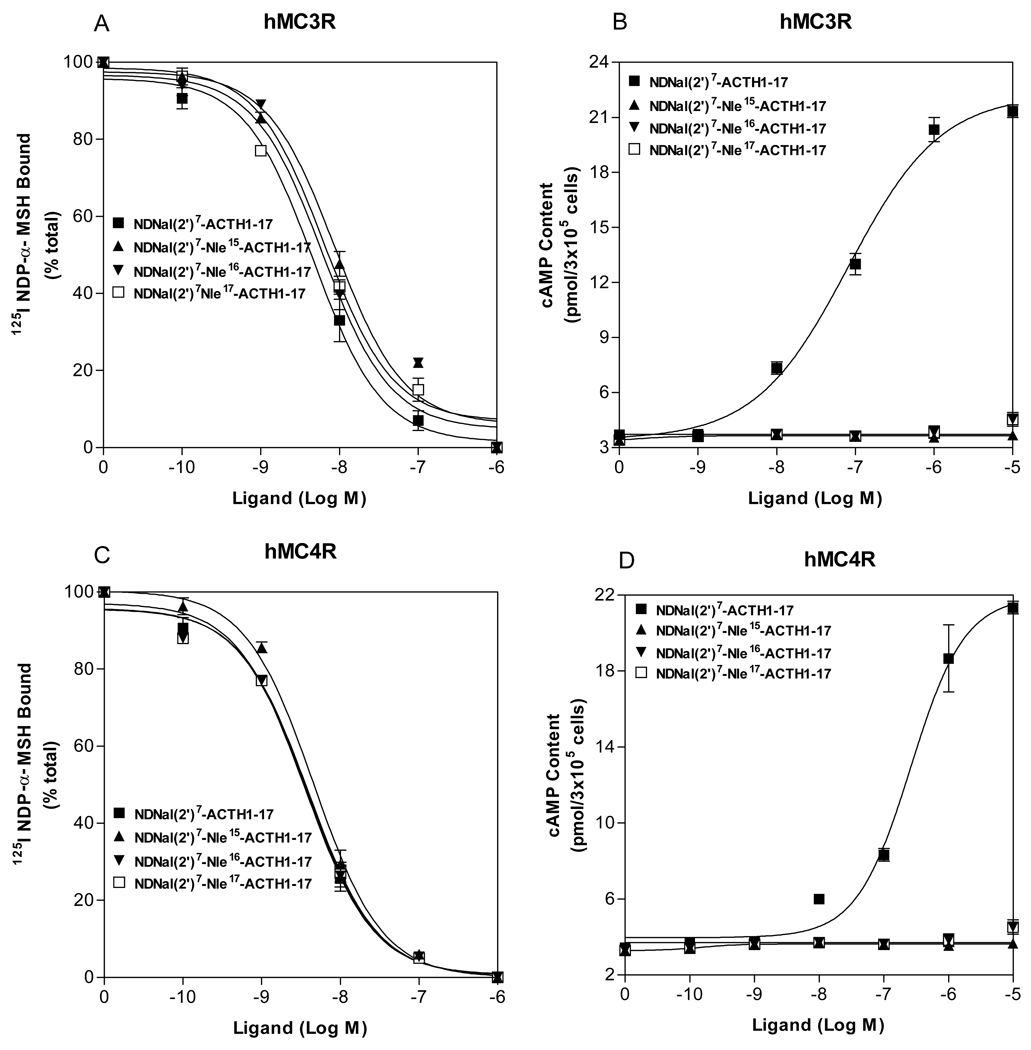
The first 13 amino acid residues of ACTH are identical to that of α-MSH. DNal (2’)7-ACTH1-17 has only four more amino acids than that of DNal (2’)7-NDP-α-MSH. The fact that DNal (2’)7-ACTH1-17 is an agonist at hMC3R and hMC4R implies that the residues Lys15-Lys16-Arg17 are important for DNal (2’)7-ACTH1-17 agonist activity. To evaluate the roles of the amino acid 15, 16 and 17 in DNal (2’)7-ACTH1-17 on hMC3R and hMC4R activation, these residues were replaced with norleucine individually (Table 1) and the effects of these peptides on cAMP production at hMC3R and hMC4R were examined. As shown in Figure 3, our results indicate that either one of the residue 15, 16 and 17 substitutions in DNal(2’)7-ACTH1-17 resulted in the loss of the peptide agonist activity at the hMC3R and the hMC4R, implying that these residues are essential for DNal(2’)7-ACTH1-17 activity.
Figure 3.
Binding affinity and potency of ACTH analogues at the wild-type hMC3R and hMC4R. Panel A and C show that the effects of ACTH analogue on 125I-NDP-α-MSH binding at hMC3R and hMC4R. Panel B and D show the effects of ACTH analogue on total cAMP accumulation at hMC3R and hMC4R (n ≥ 3; see Table 3 for Ki and EC50 values).
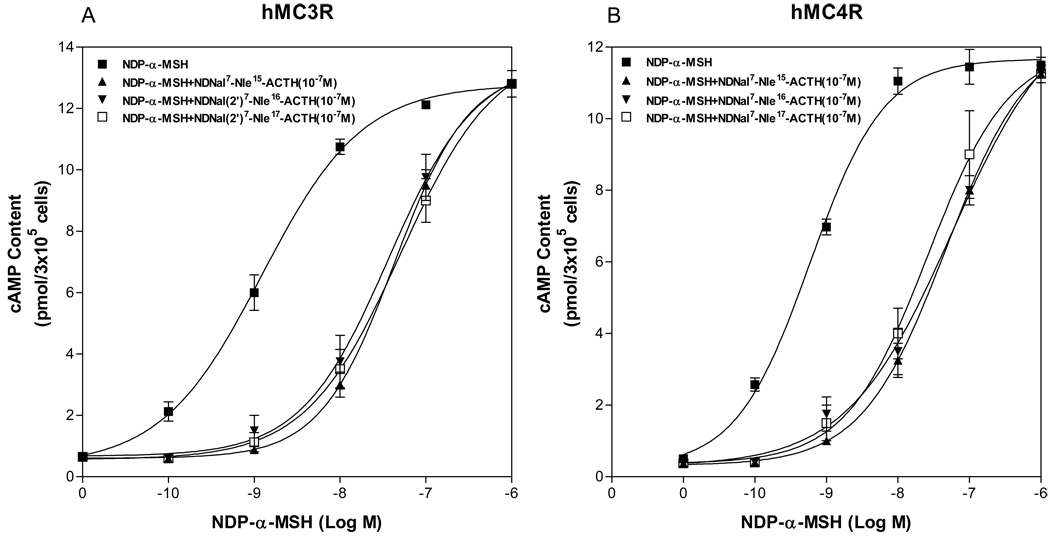
Although these peptides lose agonist activities at the hMC3R and the hMC4R, whether they still possess antagonist activity at the hMC3R and the hMC4R is unknown. We examined the abilities of the peptides, NDNal (2’)7-Nle15-ACTH1-17, NDNal (2’)7-Nle16-ACTH1-17 and NDNal (2’)7-Nle17-ACTH1-17, to inhibit NDP-α-MSH stimulated cAMP generation at hMC3R and hMC4R. The cells expressing hMC3R or hMC4R were incubated with the concentration of these peptides (10−7M) and NDP-α-MSH (10−10M – 10−6M). cAMP assays were performed and these peptide antagonist activities were determined. As shown in Figure 4, our results indicate that introduction of the peptides resulted in a right shift of NDP-α-MSH induced dose response curve, suggesting that these peptides potently inhibit NDP-α-MSH stimulated cAMP production at hMC3R and hMC4R.
Figure 4.
Effects of the mutated ACTH analogues on NDP-α-MSH mediated cAMP production. (A) Effect of the mutated ACTH analogues on NDP-α-MSH mediated cAMP production at hMC3R. (B) Effect of the mutated ACTH analogues on NDP-α-MSH mediated cAMP production at hMC4R.
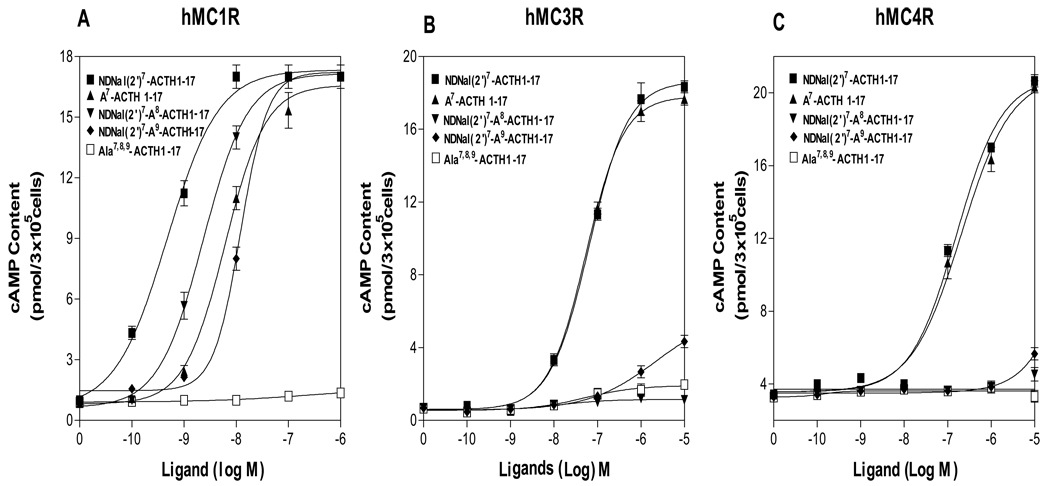
To determine whether the core sequence “Phe-Arg-Trp” in NDNal(2’)7-ACTH are also important for the hMC3R and the hMC4R activation, the peptide residues, Phe7, Arg8 and Trp9 in DNal(2’)7-ACTH1-17 were individually or substituted with alanine and their activities were tested at hMC1R, hMC3R and hMC4R. Our results show that NAla7-ACTH1-17 has similar potency compared to that of NDNal (2’)7-ACTH1-17, which indicate that Phe7 is not the key residue for the agonist activation at the hMC3R and hMC4R. Whereas, NDNal (2’)7-Ala8-ACTH1-17 and NDNal (2’)7-Ala9-ACTH1-19 resulted in the loss of the agonist activity at hMC3R and hMC4R (Figure 5), suggesting that the residues ‘Arg8-Trp9’ are crucial for DNal (2’)7-ACTH agonist activity at hMC1R, hMC3R and hMC4R.
Figure 5.
Binding affinities and potencies of ACTH analogues at the wild-type hMC1R, hMC3R and hMC4R. Panel A shows that the effects of ACTH analogues on total cAMP accumulation at hMC1R. Panel B shows the effects of ACTH analogues on total cAMP accumulation at hMC3R. Panel C shows that the effects of ACTH analogues on total cAMP accumulation at hMC4R. (n ≥ 3; see Table 3 for Ki and EC50 values).
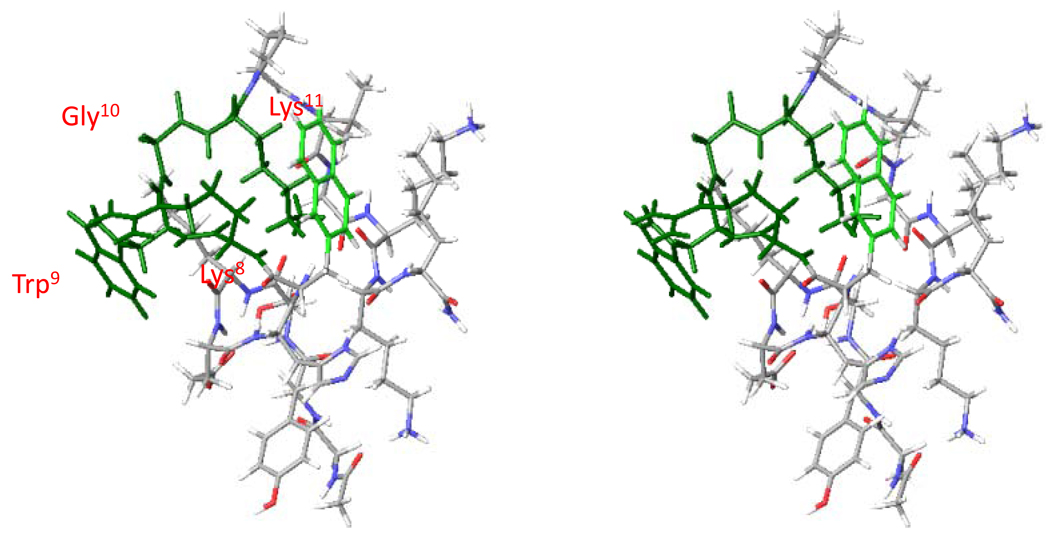
Molecular modeling data shown that in the lowest energy conformation of NDNal(2’)7-ACTH1-17, there is a β-turn like structure -Arg8-Trp9-Gly10-Lys11- in NDNal(2’)7-ACTH (Figure 6). In this turn Arg8 and Trp9 become the most important binding residues and Phe7 is not involved the turn. Compared to the β-turn like structure at NDP-α-MSH –His6-DPhe7-Arg8-Trp9-, the β – turn is shifted. This shift might contribute the agonist activity at hMC3R and hMC4R for the DNal (2’)7-ACTH. Table 4 shows both the backbone dihedral angle conformation and side chain dihedral angle conformation.
Figure 6.
Stereo view of the NDNal(2’)7-ACTH1-17. The picture was obtained by MCMM/LMCS (Monte Carlo Multiple Minima-Low Frequency Mode)-OPLS 2005. Green color show a new β-turn like structure at -Arg8-Trp9-Gly10-Lys11-.
Table 4.
Backbone Torsion Angles (deg) for the Global Minima of Selected NDNal(2')7-ACTH1-17
| No. | His6 |
D-Phe7/D- DNal(2’)7 |
Arg8 |
Trp9 |
Gly10/Lys10 |
Lys11 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ϕ | ψ | ϕ | ψ | ϕ | ψ | ϕ | ψ | ϕ | ψ | ϕ | ψ | |
| NDNal(2’)7-ACTH1-17 | −144 | 144 | −85 | −183 | −156 | 140 | −85 | 131 | 116 | −8 | −70 | 152 |
| MT-II | −108 | 109 | 84 | 0 | −122 | 90 | −77 | 108 | −101 | 103 | ||
| SHU9119 | −90 | 49 | 82 | −6 | −99 | 117 | −79 | 111 | −90 | −68 | ||
This table is based on MCMM/LMCS-OPLS2005 calculations and compared with NMR structures of MTII and SHU9119.
Substitution of the TM3 residue of the hMC1R, hMC3R and hMC4R on DNal (2’)-ACTH1-17 Activity
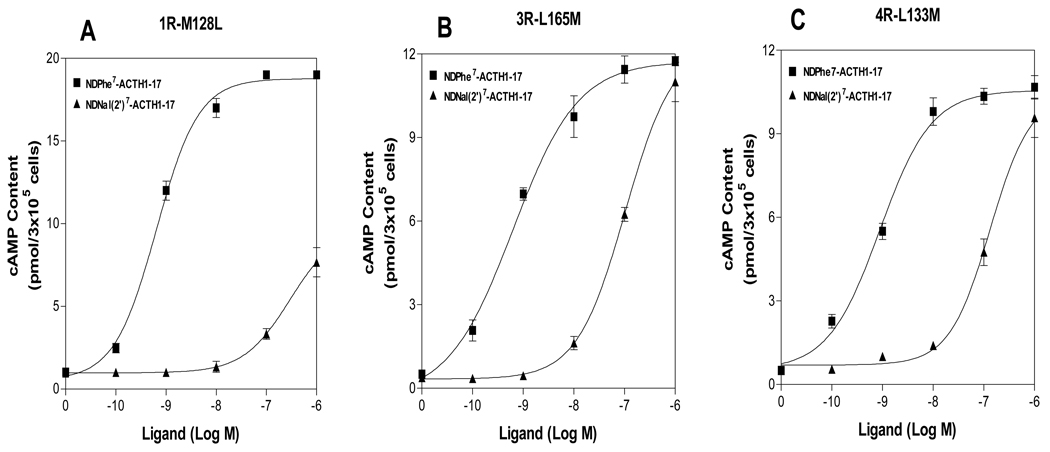
The transmembrane domain 3 of the melanocortin receptors has been identified to play an important role in ligand selectivity for receptor activation. The residues L165 and L133 in TM3 of the hMC3R and hMC4R have been identified to be crucial for SHU9119 selectivity. Substitutions of these residues of the hMC3R and hMC4R with the corresponding region of hMC1R switched SHU9119 from an antagonist to an agonist (42). To determine whether these residues are also responsible for NDNal (2’)7-ACTH1-17 specific activities at the hMC1R, hMC3R and hMC4R, we tested this new compound on these previously made mutant receptors. We tested L165M of hMC3R and L133M of hMC4R and examined the effects of the mutations on NDNal (2’)7-ACTH1-17 agonist activity. As shown in Figure 7, our results indicate that L165M and L133M did not dramatically increase NDNal (2’)7-ACTH1-17 agonist potency, suggesting that these residues are not crucial NDNal (2’)7-ACTH1-17 agonist activity. To determine whether the corresponding residue in hMC1R is also responsible for NDNal (2’)7-ACTH1-17 specific activity, we substituted the amino acid residue M128 in TM3 of the hMC1R with methione. Cells expressing M128L were treated with NDNal (2’)7-ACTH1-17. Ligand agonist potency was evaluated. Our results indicate that unlike L156M and L133M in hMC3R and hMC4R, M128L did significantly decrease NDNal (2’)7-ACTH1-17 agonist potency, suggesting that this residue is crucial for NDNal (2’)7-ACTH1-17 activity (Table 5).
Figure 7.
Potency of NDNal (2’)7-ACTH1-17 at the mutations, M128L in hMC1R, L165M in hMC3R and L133M in hMC4R. Panel A shows that the effects of NDPhe7-ACTH1-17 and NDNal (2’)7-ACTH1-17 to stimulate cAMP production at M128L of the hMC1R. Panel B shows that the effects of DPhe7-ACTH1-17 and NDNal (2’)7-ACTH1-17 to stimulate cAMP production at L165M of the hMC3R. Panel C shows that the effects of NDPhe7-ACTH1-17 and NDNal (2’)7-ACTH1-17 to stimulate cAMP production at L133M of the hMC4R. Data points represent the mean ± SEM of at least three independent experiments.
Table 5.
The potencies of NDP-α-MSH and NDNal(2')-A-CTH1-17 analogues at the mutations of the hMC1R, hMC3R and hMC4R.
| EC50 (nM) | |||
|---|---|---|---|
| hMC1R-M128L | hMC3R-L165M | MC4R-L133M | |
| NDP-α-MSH | 0.63 ± 0.1 | 3.2 ± 0.2 | 1.9 ± 0.2 |
| DPhe7-ACTH1-17 | 0.71 ± 0.3 | 3.7 ± 0.1 | 2.3 ± 0.3 |
| NDNal(2')7-ACTH1-17 | >103 | 78 ± 14 | 121 ± 11 |
Discussion
ACTH is endogenous agonist for all MCRs. Its first 13 amino acid residue is identical to that of α-MSH. It is generally believed that ACTH shares similar binding site with a-MSH at MC3R and MC4R. However, our current study demonstrates that NDNal (2’)7-ACTH1-17 has different binding sites at hMC3R and hMC4R. NDNal(2’)7-ACTH1-17, which has four additional amino acid residues compared to that of NDNal(2’)7-α-MSH, is able to induce cAMP production at hMC3R and hMC4R, whereas, NDNal(2’)7-α-MSH can not. This implies that the four extra amino acids at the C terminal of NDNal (2’)7-ACTH1-17 play an important role for the agonist selectivity. Our further works support this hypothesis. We found that substitution of amino acid residue Lys15, Lys16 and Arg17, one at a time, of peptide results in the loss of analogue agonist activity at hMC3R and hMC4R, proving that these residues are important for receptor activation. Adding four amino acid residues to NDNal (2’)7- α-MSH switches peptide from antagonist to agonist, suggesting that NDNal (2’)7-ACTH1-17 may have different binding sites at hMC3R and hMC4R compared to NDP-α-MSH.
The intriguing biological results triggered the further conformation studies. As mentioned earlier, SAR studies have identified that the residue Phe7 in α-MSH is responsible for the MC3R and the MC4R activation but not in the case of NDNal7-ACTH activation at the MC3R and the MC4R. The Arg8-Trp9 plays a critical role for the NDNal7-ACTH activation at the MC3R and the MC4R. The global minimum search has been performed. The lowest energy conformation of the NDNal7-ACTH1-17 shows that there is a new β-turn like structure at -Arg8-Trp9-Gly10-Lys11- (Figure 6). Compared to the β-turn like structure of NDP-α-MSH –His6-DPhe7-Arg8-Trp9-, the new β –turn is shifted and the Phe7 is not involved in the new β –turn. Table 6 show the backbone torsion angles (deg) for the global minima of selected NDNal (2’)7-ACTH1-17. It is note that DNal (2’)7 is completely twisted 180 (deg) but Arg8 and Trp9 remain very close to the backbone conformation (Table 6). The biological data further proved that without Phe7 NAla7-ACTH1-17 is still active potent agonist at hMC3R and hMC4R. Therefore, the new β–turn shift might be contribute the agonist activity at hMC3R and hMC4R
To further pursue how the peptide forms the new β–turn, we analyzed the structure of NDNal7-ACTH1-17. The highly basic residues of Lys15, Lys16 and Arg17 at the C-terminal of NDNal (2’)7-ACTH1-17 formed a few more hydrogen bonds and very hydrophilic surface. This forced the β–turn shifted. Without these three residues, the β–turn will be -His6-DPhe7-Arg8-Trp9- as NDP-α-MSH is. The mutation of these basic amino acids resulted in the dramatic loss of the potency at hMC3R and hMC4R. Therefore, strong electrostatic residues at C terminal cause the peptide folding in a different way which led new β –turn, and this β –turn -Arg8-Trp9-Gly10-Lys11- brought Trp9 up as the sole hydrophobic aromatic amino acid that contribute to receptor activation.
Our newly discovered results are consistent with many earlier works done by us and others. Earlier publications have shown the importance of Trp8 for the hMC3R selectivity. D-Trp8-γ-MSH increases the agonist selectivity at the hMC3R. Recently, new evidence also indicates that deletion of Trp9 of the α-MSH is still an agonist at MC1R but losses its activity at hMC3R and hMC4R (49). Replacement of Arg8 with synthetic amino acid selectively binds to MC5R and results in significantly decrease of ligand binding affinity and potency at the other MCRs, suggesting that melanocortin receptor subtype has different binding sites for different ligands. Further experiments indicate that modification of the residue Phe of the NDP-α-MSH resulted in potent antagonist/partial agonists at the MC3R and full, potent agonists at the MC4R, suggesting that the molecular mechanism of antagonism at the MC3R is different from that of the MC4R.
Activation of GPCRs has been proposed to involve the rotation of TM domains with outward movement of their cytoplasmic ends (50). This theoretically would enable G proteins to interact with some of the intracellular loops as well as the C terminal tail of GPCRs. Some previous MCR studies support this theory since mutations of some TM residues of MCRs can result in inactivation of MCRs (39, 40). Previous finding indicates that the residue leucine in position 165 in hMC3R and 133 of hMC4R is critical for SHU9119 antagonism. The substitutions of theses residue with methionine made SHU9119 from antagonist to agonist (L165M in hMC3R and L133M in hMC4R) (42). However, our current study indicate that the residues leucine in position 165 in hMC3R and 133 in hMC4R are not crucial for NDNal(2’)7-ACTH1-17 activity, supporting that NDNal (2’)7-ACTH1-17 has different binding sites at hMC3R and hMC4R compared to NDNal (2’)7-α-MSH or SHU9119. The amino acid residues Lys15-Lys16-Arg17 in NDNal (2’)7-ACTH1-17 compensate for the loss of the effect of Phe7 in MSH on receptor activation. However, the corresponding residue M128 in hMC1R is crucial for NDNal (2’)7-ACTH1-17 binding and signaling, suggesting that the ligand binding pockets for NDNal (2’)7-ACTH1-17 at hMC1R, hMC3R and hMC4R are different.
Finally, we concluded here that NDNal (2’)7-ACTH1-17 is an agonist at hMC3R and hMC4R;NDP-α-MSH and DNal (2’)7-ACTH1-17 do not share the same binding site. Arg8 and Trp9 in the NDNal (2’)7-ACTH1-17 are critical for the hMC3R and hMC4R agonist activity. This provides further evidence of the importance of Trp at hMC3R and hMC4R activity. We have identified that the residue Lys15-Lys16-Arg17 of NDNal (2’)7-ACTH1-17 are important for NDNal (2’)7-ACTH1-17 activity at hMC3R and hMC4R. NDNal (2’)7-ACTH1-17 has different binding sites at hMC3R and hMC4R compared to that of NDP-α-MSH. Our current study provides a better understanding that the role of the ligand Phe phenyl ring in ACTH activity is different from that of α-MSH. The results from these studies highlight the different receptor activation mechanisms of α-MSH and ACTH and how they may be valuable for future drug design.
Acknowledgments
This work has been funded by NIH Grants R03 HD047312-01A1 (Y-K, Yang) and DA 348900, DA 06248 (VJ, Hruby)
Abbreviations
- MCR
Melanocortin receptor
- GPCR
G-protein coupled receptor
- ACTH
Adrenocorticotropic Hormone
- N-ACTH
Nle4 Adrenocorticotropic Hormone
- α-MSH
α-melanocyte stimulating hormone
- ASIP
Agouti-signaling protein
- TM
transmembrane domains
- IBMX
3-Isobutyl-methylxanthine
- PCR
Polymerase chain reaction
- FACs
Flow cytometry
Reference
- 1.Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. discussion 318. [PubMed] [Google Scholar]
- 2.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 3.Gispen WH, Isaacson RL. ACTH-induced excessive grooming in the rat. Pharmacol Ther. 1981;12:209–246. doi: 10.1016/0163-7258(81)90081-4. [DOI] [PubMed] [Google Scholar]
- 4.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 5.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 6.Wessells H, Gralnek D, Dorr R, Hruby VJ, Hadley ME, Levine N. Effect of an alpha-melanocyte stimulating hormone analog on penile erection and sexual desire in men with organic erectile dysfunction. Urology. 2000;56:641–646. doi: 10.1016/s0090-4295(00)00680-4. [DOI] [PubMed] [Google Scholar]
- 7.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci. 1996;16:5182–5188. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni XP, Butler AA, Cone RD, Humphreys MH. Central receptors mediating the cardiovascular actions of melanocyte stimulating hormones. J Hypertens. 2006;24:2239–2246. doi: 10.1097/01.hjh.0000249702.49854.fa. [DOI] [PubMed] [Google Scholar]
- 9.Morgan C, Thomas RE, Cone RD. Melanocortin-5 receptor deficiency promotes defensive behavior in male mice. Horm Behav. 2004;45:58–63. doi: 10.1016/j.yhbeh.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD. Melanocortin-5 receptor deficiency reduces a pheromonal signal for aggression in male mice. Chem Senses. 2004;29:111–115. doi: 10.1093/chemse/bjh011. [DOI] [PubMed] [Google Scholar]
- 11.Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proc Natl Acad Sci U S A. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]
- 13.Lu D, Willard D, Patel IR, Kadwell S, Overton L, Kost T, Luther M, Chen W, Woychik RP, Wilkison WO, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371:799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 14.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 15.Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van der Ploeg LH, Weinberg DH. Potent and selective peptide agonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochem Biophys Res Commun. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- 16.Yang YK, Fong TM, Dickinson CJ, Mao C, Li JY, Tota MR, Mosley R, Van Der Ploeg LH, Gantz I. Molecular determinants of ligand binding to the human melanocortin-4 receptor. Biochemistry. 2000;39:14900–14911. doi: 10.1021/bi001684q. [DOI] [PubMed] [Google Scholar]
- 17.Ying J, Gu X, Cai M, Dedek M, Vagner J, Trivedi DB, Hruby VJ. Design, synthesis, and biological evaluation of new cyclic melanotropin peptide analogues selective for the human melanocortin-4 receptor. J Med Chem. 2006;49:6888–6896. doi: 10.1021/jm060768f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer JP, Hsiung HM, Flora DB, Edwards P, Smith DP, Zhang XY, Gadski RA, Heiman ML, Hertel JL, Emmerson PJ, Husain S, O'Brien TP, Kahl SD, Smiley DL, Zhang L, Dimarchi RD, Yan LZ. Discovery of a beta-MSH-derived MC-4R selective agonist. J Med Chem. 2005;48:3095–3098. doi: 10.1021/jm0501432. [DOI] [PubMed] [Google Scholar]
- 19.Richardson TI, Ornstein PL, Briner K, Fisher MJ, Backer RT, Biggers CK, Clay MP, Emmerson PJ, Hertel LW, Hsiung HM, Husain S, Kahl SD, Lee JA, Lindstrom TD, Martinelli MJ, Mayer JP, Mullaney JT, O'Brien TP, Pawlak JM, Revell KD, Shah J, Zgombick JM, Herr RJ, Melekhov A, Sampson PB, King CH. Synthesis and structure-activity relationships of novel arylpiperazines as potent and selective agonists of the melanocortin subtype-4 receptor. J Med Chem. 2004;47:744–755. doi: 10.1021/jm0304109. [DOI] [PubMed] [Google Scholar]
- 20.Sebhat IK, Martin WJ, Ye Z, Barakat K, Mosley RT, Johnston DB, Bakshi R, Palucki B, Weinberg DH, MacNeil T, Kalyani RN, Tang RN, Stearns RA, Miller RR, Tamvakopoulos C, Strack AM, McGowan E, Cashen DE, Drisko JE, Hom GJ, Howard AD, MacIntyre DE, van der Ploeg LH, Patchett AA, Nargund RP. Design and pharmacology of N-[(3R)-1,2,3,4-tetrahydroisoquinolinium- 3-ylcarbonyl]-(1R)-1-(4-chlorobenzyl)- 2-[4-cyclohexyl-4-(1H-1,2,4-triazol- 1-ylmethyl)piperidin-1-yl]-2-oxoethylamine (1), a potent, selective, melanocortin subtype-4 receptor agonist. J Med Chem. 2002;45:4589–4593. doi: 10.1021/jm025539h. [DOI] [PubMed] [Google Scholar]
- 21.Vos TJ, Caracoti A, Che JL, Dai M, Farrer CA, Forsyth NE, Drabic SV, Horlick RA, Lamppu D, Yowe DL, Balani S, Li P, Zeng H, Joseph IB, Rodriguez LE, Maguire MP, Patane MA, Claiborne CF. Identification of 2-[2-[2-(5-bromo-2-methoxyphenyl)-ethyl]-3-fluorophenyl]-4,5-dihydro-1H-imidazole (ML00253764), a small molecule melanocortin 4 receptor antagonist that effectively reduces tumor-induced weight loss in a mouse model. J Med Chem. 2004;47:1602–1604. doi: 10.1021/jm034244g. [DOI] [PubMed] [Google Scholar]
- 22.Hogan K, Peluso S, Gould S, Parsons I, Ryan D, Wu L, Visiers I. Mapping the binding site of melanocortin 4 receptor agonists: a hydrophobic pocket formed by I3.28(125), I3.32(129), and I7.42(291) is critical for receptor activation. J Med Chem. 2006;49:911–922. doi: 10.1021/jm050780s. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Pontillo J, Fleck BA, Gao Y, Wen J, Tran JA, Tucci FC, Marinkovic D, Foster AC, Saunders J. 4-{(2R)-[3-Aminopropionylamido]-3-(2,4-dichlorophenyl)propionyl}-1-{2-[(2- thienyl)ethylaminomethyl]phenyl}piperazine as a potent and selective melanocortin-4 receptor antagonist--design, synthesis, and characterization. J Med Chem. 2004;47:6821–6830. doi: 10.1021/jm049278i. [DOI] [PubMed] [Google Scholar]
- 24.Hruby VJ, Cai M, Cain JP, Mayorov AV, Dedek MM, Trivedi D. Design, synthesis and biological evaluation of ligands selective for the melanocortin-3 receptor. Curr Top Med Chem. 2007;7:1085–1097. doi: 10.2174/156802607780906645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marks DL, Hruby V, Brookhart G, Cone RD. The regulation of food intake by selective stimulation of the type 3 melanocortin receptor (MC3R) Peptides. 2006;27:259–264. doi: 10.1016/j.peptides.2005.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H. Melanocortin receptors, melanotropic peptides and penile erection. Curr Top Med Chem. 2007;7:1098–1106. [PMC free article] [PubMed] [Google Scholar]
- 27.Cai M, Mayorov Av, Ying J, Stankova M, Trivedi D, Cabello C, Hruby VJ. Design of novel melanotropin agonists and antagonists with high potency and selectivity for human melanocortin receptors. Peptides. 2005;26:1481–1485. doi: 10.1016/j.peptides.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 28.Hruby VJ, Lu D, Sharma SD, Castrucci AL, Kesterson RA, al-Obeidi FA, Hadley ME, Cone RD. Cyclic lactam alpha-melanotropin analogues of Ac-Nle4-cyclo[Asp5, D-Phe7 Lys10] alpha-melanocyte-stimulating hormone-(4–10)-NH2 with bulky aromatic amino acids at position 7 show high antagonist potency and selectivity at specific melanocortin receptors. J Med Chem. 1995;38:3454–3461. doi: 10.1021/jm00018a005. [DOI] [PubMed] [Google Scholar]
- 29.Vergoni AV, Poggioli R, Marrama D, Bertolini A. Inhibition of feeding by ACTH-(1–24): behavioral and pharmacological aspects. Eur J Pharmacol. 1990;179:347–355. doi: 10.1016/0014-2999(90)90175-6. [DOI] [PubMed] [Google Scholar]
- 30.Grieco P, Balse PM, Weinberg D, MacNeil T, Hruby VJ. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- 31.Cai M, Mayorov AV, Cabello C, Stankova M, Trivedi D, Hruby VJ. Novel 3D pharmacophore of alpha-MSH/gamma-MSH hybrids leads to selective human MC1R and MC3R analogues. J Med Chem. 2005;48:1839–1848. doi: 10.1021/jm049579s. [DOI] [PubMed] [Google Scholar]
- 32.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure-activity relationships of gamma-MSH analogues at the human melanocortin MC3, MC4, and MC5 receptors. Discovery of highly selective hMC3R, hMC4R, and hMC5R analogues. J Med Chem. 2003;46:4965–4973. doi: 10.1021/jm030119t. [DOI] [PubMed] [Google Scholar]
- 33.Mayorov AV, Cai M, Chandler KB, Petrov RR, Van Scoy AR, Yu Z, Tanaka DK, Trivedi D, Hruby VJ. Development of cyclic gamma-MSH analogues with selective hMC3R agonist and hMC3R/hMC5R antagonist activities. J Med Chem. 2006;49:1946–1952. doi: 10.1021/jm0510326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kavarana MJ, Trivedi D, Cai M, Ying J, Hammer M, Cabello C, Grieco P, Han G, Hruby VJ. Novel cyclic templates of alpha-MSH give highly selective and potent antagonists/agonists for human melanocortin-3/4 receptors. J Med Chem. 2002;45:2644–2650. doi: 10.1021/jm020021z. [DOI] [PubMed] [Google Scholar]
- 35.Grieco P, Lavecchia A, Cai M, Trivedi D, Weinberg D, MacNeil T, Van der Ploeg LH, Hruby VJ. Structure-activity studies of the melanocortin peptides: discovery of potent and selective affinity antagonists for the hMC3 and hMC4 receptors. J Med Chem. 2002;45:5287–5294. doi: 10.1021/jm0202526. [DOI] [PubMed] [Google Scholar]
- 36.Balse-Srinivasan P, Grieco P, Cai M, Trivedi D, Hruby VJ. Structure-activity relationships of novel cyclic alpha-MSH/beta-MSH hybrid analogues that lead to potent and selective ligands for the human MC3R and human MC5R. J Med Chem. 2003;46:3728–3733. doi: 10.1021/jm030111j. [DOI] [PubMed] [Google Scholar]
- 37.Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- 38.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Aprahamian CJ, Celik A, Georgeson KE, Garvey WT, Harmon CM, Yang Y. Molecular characterization of human melanocortin-3 receptor ligand-receptor interaction. Biochemistry. 2006;45:1128–1137. doi: 10.1021/bi0521792. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Georgeson KE, Harmon CM, Haskell-Luevano C, Yang Y. Functional characterization of the modified melanocortin peptides responsible for ligand selectivity at the human melanocortin receptors. Peptides. 2006;27:2836–2845. doi: 10.1016/j.peptides.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Cai M, Aprahamian CJ, Georgeson KE, Hruby V, Harmon CM, Yang Y. Contribution of the conserved amino acids of the melanocortin-4 receptor in [corrected] [Nle4,D-Phe7]-alpha-melanocyte-stimulating [corrected] hormone binding and signaling. J Biol Chem. 2007;282:21712–21719. doi: 10.1074/jbc.M702285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Chen M, Lai Y, Gantz I, Georgeson KE, Harmon CM. Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity. J Biol Chem. 2002;277:20328–20335. doi: 10.1074/jbc.M201343200. [DOI] [PubMed] [Google Scholar]
- 43.Haskell-Luevano C, Cone RD, Monck EK, Wan YP. Structure activity studies of the melanocortin-4 receptor by in vitro mutagenesis: identification of agouti-related protein (AGRP), melanocortin agonist and synthetic peptide antagonist interaction determinants. Biochemistry. 2001;40:6164–6179. doi: 10.1021/bi010025q. [DOI] [PubMed] [Google Scholar]
- 44.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, Hadley ME. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DeBlasi A, O'Reilly K, Motulsky HJ. Calculating receptor number from binding experiments using same compound as radioligand and competitor. Trends Pharmacol Sci. 1989;10:227–229. doi: 10.1016/0165-6147(89)90266-6. [DOI] [PubMed] [Google Scholar]
- 46.Yang YK, Ollmann MM, Wilson BD, Dickinson C, Yamada T, Barsh GS, Gantz I. Effects of recombinant agouti-signaling protein on melanocortin action. Mol Endocrinol. 1997;11:274–280. doi: 10.1210/mend.11.3.9898. [DOI] [PubMed] [Google Scholar]
- 47.Mizoue LS, Bazan JF, Johnson EC, Handel TM. Solution structure and dynamics of the CX3C chemokine domain of fractalkine and its interaction with an N-terminal fragment of CX3CR1. Biochemistry. 1999;38:1402–1414. doi: 10.1021/bi9820614. [DOI] [PubMed] [Google Scholar]
- 48.István Kolossváry WCG. Low-mode conformational search elucidated: Application to C39H80 and flexible docking of 9-deazaguanine inhibitors into PNP. Journal of Computational Chemistry. 1999;20:1671–1684. [Google Scholar]
- 49.Bednarek MA, Macneil T, Tang R, Fong TM, Angeles Cabello M, Maroto M, Teran A. Analogs of alpha-melanocyte stimulating hormone with high agonist potency and selectivity at human melanocortin receptor 1b: the role of Trp(9) in molecular recognition. Biopolymers. 2008;89:401–408. doi: 10.1002/bip.20863. [DOI] [PubMed] [Google Scholar]
- 50.Gether U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr Rev. 2000;21:90–113. doi: 10.1210/edrv.21.1.0390. [DOI] [PubMed] [Google Scholar]