Abstract
Purpose:
To validate a new method for converting MR arterial signal intensity versus time curves to arterial input functions (AIF).
Materials and Methods:
The method constrains AIF with patient's cardiac output (Q). Monte Carlo simulations of MR renography and tumor perfusion protocols were carried out for comparison with two alternative methods: direct measurement and population-averaged input function. MR renography was performed to assess the method's inter- and intra-day reproducibility for renal parameters.
Results:
In simulations of tumor perfusion, the precision of the parameters (Ktrans and ve) computed using the proposed method was improved by at least a factor of three compared to direct measurement. Similar improvements were obtained in simulations of MR renography. Volunteer study for testing inter-day reproducibility confirmed the improvement of precision in renal parameters when using the proposed method, compared to conventional methods. In another patient study (two injections within one session), the proposed method significantly increased the correlation coefficient (R) between GFR of the two exams (0.92 vs. 0.83), compared to direct measurement.
Conclusion:
A new method significantly improves the precision of DCE parameters. The method may be especially useful for analyzing repeated DCE examinations, such as monitoring tumor therapy or ACE-inhibitor renography.
Keywords: cardiac output, dynamic contrast-enhanced MRI, arterial input function
Introduction
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) measures the transit of a tracer such as a gadolinium-chelate to estimate physiologic parameters related to perfusion or permeability in vivo. Clinical applications include estimates of tumor angiogenesis (1,2) and response to therapy (3,4) as well as physiologic measurements of organ function such as kidney glomerular filtration rates (GFR) and perfusion (5-7). Ideally, these studies would be performed using a direct, well-controlled injection of the tracer bolus into the feeding vessel. Observed tissue concentration versus time curves would then reflect regional/local perfusion, permeability, or volume fraction, with minimal confounding effects due to the shape of the input function. However for practical reasons, tracers are usually injected intravenously, resulting in unpredictable dilution and widening of the bolus by the time it arrives at the feeding vessels. Therefore, accurate quantitative analysis of DCE-MRI data requires individually measured arterial input function (AIF). Reliable measurement of AIF is critical to the precision of determining the function of organ or tumor (8-10).
Several challenges limit our ability to determine AIF. First, the relationship between MR signal intensity and gadolinium concentration is nonlinear and can even be non-monotonic (11,12). Second, MR signal measurements from a blood vessel can be distorted by multiple artifacts, including inflow effect (10,13), dephasing (14), partial volume effect (15,16), and effects of flow pulsatility and turbulence. In addition, B1 inhomogeneity (17-20) affects MR signals of both blood and tissue.
Different approaches have been proposed to compute tracer concentration C(t). The simplest approach estimates concentration as proportional to normalized signal intensity (21,22). This approach is especially attractive when combined with the use of low doses of contrast, due to an approximate linearity of the relationship between low tracer concentration and its signal for commonly used gradient echo acquisition sequences (11). Alternatively, C(t) can be estimated from the longitudinal relaxation time T1(t). The estimation of T1 from signal intensity requires the knowledge of S(0) and T1(0) (11,23). This approach (termed direct measurement) is applicable to a wider range of C(t) and can yield estimates with less than 10% error in solid tissues (e.g. liver, kidneys, muscle) (11). However, it is significantly less accurate in the aorta or other major arteries, i.e. in regions used for measurement of AIF. The MR signal from these arteries is subject to artifacts listed above, and signal errors are further amplified when estimating tracer concentration by direct measurement (Figure 1).
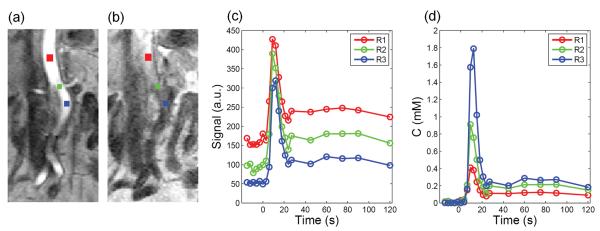
Figure 1.
Example to demonstrate the variability in aortic input function by direct measurement. (a) A cropped post-contrast image showing regions of interest (ROIs) at different levels of aorta (above renal-artery level (red), at renal-artery level (green), below renal-artery level (blue)). ROIs were placed on multiple slices and centered on aortic lumen. However, because of the tortuous vessel, some partial volume effect is unavoidable; (b) A cropped pre-contrast image showing inflow effect in upper level of aorta; (c) Signal intensity versus time curves sampled from the three regions shows different baseline levels possibly due to inflow effect, which in turn result in dramatically different (d) concentration versus time curves by direct measurement. The peak of concentration curve suffers from large variance, from 0.4 mM to 1.8 mM, while the tail varies from about 0.1 mM to 0.3 mM.
To minimize the adverse effects of AIF distortions, Parker et al. (24) and Wang et al. (9) proposed to average AIFs obtained from a group of controls and derived by direct measurement. For the analysis of patient data, rather than use actual patient's AIF, the population-averaged AIF was used. Averaging multiple AIFs is intended to reduce random, uncorrelated sources of errors, but may not correct systematic artifacts such as inflow and partial volume effect. Moreover, the magnitude and the shape of AIF depend on patient's hemodynamic status (such as cardiac output and blood volume) and on the injection protocol (25-28). By disregarding differences between patients or protocols, the use of the averaged AIF may introduce additional sources of errors.
This study presents a new method to compute AIF using a constrained conversion that takes into account the subject's cardiac output. Because the constrained method forces the area under the peak of AIF to obey the theory of indicator dilution, we hypothesize that the resulting perfusion parameters will be more robust than those by the direct measurement and averaged methods. We tested our hypothesis in simulation studies (29) of (a) tumor perfusion and (b) renal filtration, as well as in test-retest DCE-MRI studies of MR renography, and compared our method with two alternative AIF approaches.
Materials and Methods
Conversion from MR signal to tracer concentration
The quantification of tracer concentration relies on measuring the change in longitudinal relaxation time T1 due to the T1-shortening effect of the tracer. Specifically, contrast concentration C is proportional to the change in relaxation rate 1/T1,
| [1] |
where r1 is the specific relaxivity of the contrast agent.
T1(t) can be estimated from analytical relationship between signal intensity (S) and longitudinal relaxation time (T1). This relationship involves sequence parameters such as flip angle α, repetition time TR, and a scaling factor relating to spin density, system gain, coil sensitivity, and other factors (11). For a spoiled gradient recalled echo (SPGR) sequence, which is widely used for dynamic imaging,
| [2] |
where M0 reflects the equilibrium magnetization and other system gain factors. Given pre-contrast (t≤0) values S(0) and T1(0), M0 can be determined from known system parameters and substituted back into Eq. [2], allowing us to express T1(t) as
| [3] |
where and v = S(t) / S(0), u = 1− e−TR / T1(0) and w = 1− cosαe−TR / T1(0). Substituting T1(t) in Eq. [1] results in tracer concentration that corresponds to each acquired signal (29):
| [4] |
This method to obtain AIF is referred to as direct measurement.
Calculation of tracer concentration using Eq. [4] requires knowledge of flip angle, TR, T1(0) and S(0). Errors in some of these values reduce accuracy of concentration estimates.
Normalization of input function using cardiac output
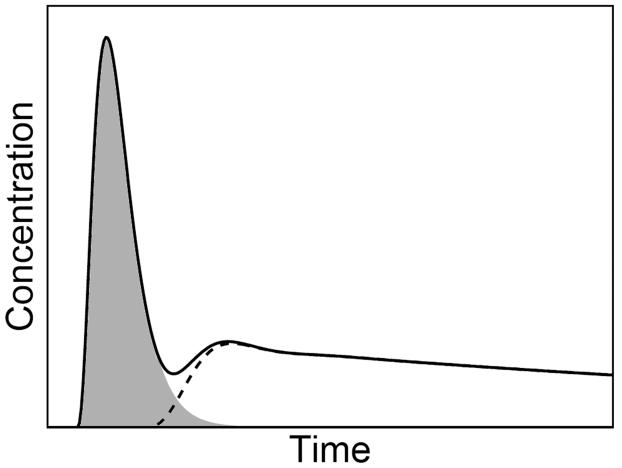
The new approach utilizes the indicator dilution (Stewart-Hamilton) principle (30) to constrain the area under AIF. After a bolus injection, arterial concentration displays an initial peak, characteristic of the “first pass” of the tracer, followed by a lower recirculation tail (Figure 2). In a system with no recirculation, AIF would consist of only the first-pass component (shaded area in Figure 2).
Figure 2.
After an intravenous bolus, the arterial input (solid line) displays an initial sharp peak, reflecting the first pass of the injected tracer (shaded area), followed by the tail due to tracer recirculation (dashed line).
The indicator dilution (or Stewart-Hamilton) principle (30) states that
| [5] |
where AUC is the area under the “first pass” concentration curve, D is the mass of the injected tracer, and Q is the cardiac output, defined as the volume of blood being pumped by the heart per minute. It should be noted that Eq. [5] is valid for AIF sampled anywhere in the body. As the sampling site moves to more distal locations, the shape of AIF becomes flatter due to tracer dispersion, without changing the area under the “first pass” component (31).
Because the precision of the AIF converted from DCE-MRI signal by direct measurement is poor, the indicator dilution method is not suitable for estimation of Q. However, if Q can be assumed or measured independently, its value can be used to improve the conversion. This is the essence of the proposed method.
The traditional “gold standard” thermodilution method for measurement of Q requires insertion of a pulmonary artery catheter. However, many noninvasive alternative techniques are becoming available and are gaining increasing acceptance (32-34). Measurement of cardiac output by MRI is routinely used as a part of clinical cardiac MRI examinations (35,36). In particular, Q can be measured rapidly using velocity-encoded phase contrast MRI with less than 10% error (32,34).
Implementation of AIF normalization
We now describe an approach for utilizing Q to derive an arterial input function. The proposed process consists of three steps S1-S3:
- S1. Fit the signal versus time curve by a gamma variate function to obtain the first-pass signal curve Sfp (i.e. eliminate recirculation) (37),
where S0 is the baseline (pre-contrast) signal level, t0 is time delay, and A, a and b are the parameters controlling the shape of gamma variate function.[6] - S2. Adjust the pre-contrast signal S0 to a new value so that the converted concentration Cfp has the expected area under curve, i.e.
In Appendix A, we show that, for fixed values A, a and b in Eq. [6], AUC of the converted Cfp is a strictly monotonic function of S0. Because of this property, a unique can always be found for any AUC predicted by Stewart-Hamilton principle.[7] S3. Shift the whole signal versus time curve S(t) to the new baseline level , and convert it to concentration using Eq. [2-4].
The above S0-shifting method was chosen for constraining AUC in this study because in our application the error in S0 seems to dominate other sources of error. However, there are other ways of constraining AUC, including simple scaling of directly-measured AIF.
Simulation studies
Monte Carlo simulations were carried out to evaluate the performance of the proposed method in dynamic MR renography and in MR tumor perfusion imaging, and to compare it with direct measurement and the previously published averaged input approach (9). Figure 3 shows the general process of simulation.
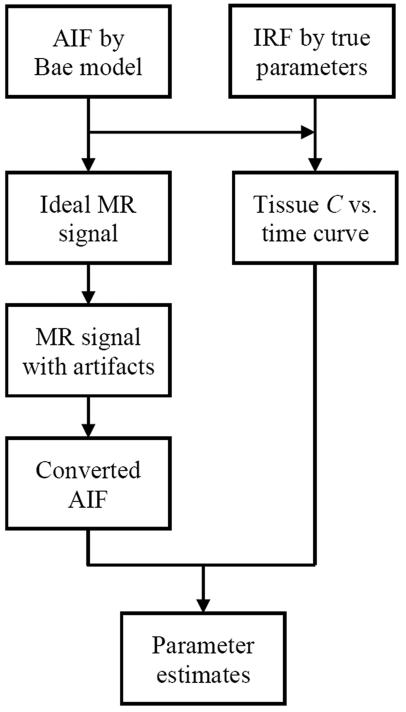
Figure 3.
Flow diagram of Monte Carlo simulation of a single individual. AIF: arterial input function; IRF: impulse retention function; C: concentration. Ideal AIF constructed by Bae's model was converted to MR signal time course. Noise was added to the signal and the parameters to simulate MR artifacts. The signals with artifact were then converted back to AIF using either direct measurement or the proposed method. The ideal AIF was also convolved with the IRF characterizing functional status of tumor or kidney, resulting in tissue concentration versus time curve. Functional parameter estimates were obtained by analyzing the converted AIF and the tissue concentration versus time curve using model-based deconvolution. Parameter estimates were then compared with true parameter values to assess the reliability of the three AIF methods.
The ideal AIF was generated by a compartmental model that was proposed by Bae et al (38,39) for describing the transit of intravascular tracer in human body (Figure 4). To simulate a random distribution of patients, flow rates and compartment volumes of the model were randomly chosen within ±30% range around their mean values (Appendix B). For a given contrast injection protocol, AIF can be derived as the output from the left-heart compartment. Realistic injection protocols (Table 1) were used to simulate tumor perfusion and kidney function applications.
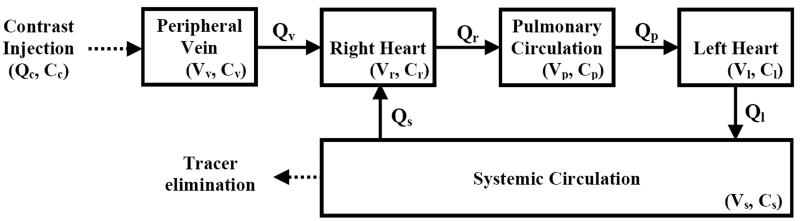
Figure 4.
A compartmental model for describing dynamic distribution of intravascular tracer in the human body modified from Bae et al. (38). Tracer with concentration Cc is injected into a peripheral vein (volume Vv, tracer concentration Cv) with injection flow rate Qc. The tracer then transits through the right heart (Vr, Cr), pulmonary circulation (Vp, Cp), the left heart (Vl, Cl), and system circulation (Vs, Cs). A fraction of tracer is eliminated from systemic circulation during every pass. Values for the parameters are in Appendix B.
Table 1.
Parameter values for simulating tracer kinetics in various tissues
| Application | Tracer Injection | Tissue type | Parameters |
|---|---|---|---|
| Tumor | 10 ml (500 mM) | High perfusion | Ktrans = 0.5 min−1, ve = 0.3 |
| at 4 ml/s | Low perfusion | Ktrans = 0.2 min−1, ve = 0.1 | |
| Kidney | 4 ml (500 mM) | Normal | GFR = 60 ml/min, RPF = 300 ml/min |
| at 2 ml/s | Impaired | GFR = 30 ml/min, RPF = 100 ml/min |
The simulated signal intensity versus time curve was constructed from each ideal AIF using Eq. [1] and [2], with pre-defined parameter values: TR = 2.3 ms, α = 9°, T1(0) = 1200 ms (40), S(0) = 50, r1 = 4.3 mM−1s−1 (41), and time interval = 3s. Zero-mean Gaussian noise with standard deviation 10% of S(0) was added to each signal point, to simulate random noise in MR signal. The signal curve was also shifted vertically with a shift randomly chosen within a conservative ±30% range of S(0), reflecting the artifacts due to inflow, dephasing and partial volume effects seen clinically.
Three methods were used to obtain AIF: (a) direct measurement, (b) the proposed method, and (c) the averaged input function that was constructed separately using Bae's model. For both direct measurement and the proposed method, the simulated signal intensity versus time curve was converted to a concentration versus time curve.
In direct measurement, the flip angle was randomly chosen within ±1° range of true flip angle, to reflect the difference between the true and the nominal flip angles due to B1 inhomogeneity (18,20). Five percent random noise was added to T1(0) (42). Pre-contrast signal S(0) was obtained by averaging 5 pre-contrast signals generated by the Monte Carlo simulation. In the proposed method, 10% random noise was added to cardiac output to reflect its measurement error (32,34).
In the averaged input function approach (9,24), one hundred AIFs simulating arterial inputs from 100 different patients were generated by the compartmental model (39,43) (Appendix B), and were subject to the same process of noise addition and direct measurement as above. These simulated AIFs were shifted in time to ensure that the peaks occurred at the same time point, and then were averaged to obtain the averaged AIF (24).
For tumor simulation, a well known model by Tofts et al (44) with transfer constant Ktrans and extracellular extravascular volume ve was employed. Representative high-perfusion and low-perfusion values (Table 1) were taken from the literature (45-48). For kidney simulation, a three-compartment renal model (5,6) was used. Normal and impaired kidneys were simulated by using different values of glomerular filtration rate, GFR and renal plasma flow, RPF (Table 1).
Random simulations were repeated Ntrial times (Ntrial = 2000), to obtain Ntrial estimates for each parameter. The standard deviation (SD) of the Ntrial estimates and the difference between their average and the true value indicates the precision and the measurement bias, respectively. Two-sampled F-test was used to compare SDs of the estimates from different methods.
To study the effect of cardiac output error on the precision of the functional parameters, conversion of the simulated AIF by the proposed method was implemented by using different cardiac-output error levels: 5%, 10%, 15% and 20%, and the parameter estimates from these different AIFs were compared.
Patient studies
Patient studies were performed to test the ability of the proposed method in improving inter- and intra-day reproducibility of DCE MRI for kidney. The studies were approved by local institutional review board, and written informed consent was obtained from all subjects.
Study A was aimed to test the ability of the proposed method in improving day-to-day or inter-day reproducibility of DCE MRI. Applications of this type include therapeutic monitoring of cancer and assessment of kidney transplantation. Four healthy volunteers (2 males and 2 females, age: 29.3±1.0 years) volunteered for this study. For each volunteer, three dimensional MR renography was repeated on three separate days with the same imaging protocol on a 1.5T Siemens Symphony: TR 2.3 ms, TE 0.8 ms, flip angle 9°, field of view 309 mm × 450 mm, slice thickness 3.0 mm, partition matrix 100 × 256 × 16 interpolated into 176 × 256 × 32, acquisition time 3.6 s. All subjects were instructed to refrain from eating and drinking for at least 8 hours prior to the exam. Subjects were given 500 ml water to drink just prior to the study. Prior to contrast administration, five 3D images were acquired during one 15 s breath-hold. Eight seconds following the start of a bolus injection of 4 ml Gd-DTPA (Magnevist, Berlex Laboratories, Wayne, NJ) at 2 ml/s (followed by 20 ml saline flush), the 3D acquisitions were repeated continuously for 30 s, during which the subject was asked to suspend respiration as long as possible. Acquisitions were repeated during separate 3 s breath-holds at multiple time points for at least 10 min thereafter.
Semi-automated image registration and segmentation was applied to the 3D renography data sets to produce aortic, renal cortical, and renal medullary signal intensity versus time curves (49). Signal curves of renal cortex and medulla were converted to concentration versus time curves using direct measurement described previously. T1 value for renal cortex and medulla without contrast were from literature, 950 and 1300 ms, respectively (50,51).
The conversion of the aortic signal intensity curves to concentration curves were implemented in three ways for comparison: direct measurement, the averaged input approach, and the proposed method. Our implementation of the averaged input approach for these test-retest data was slightly different from its original implementation. Rather than averaging AIFs across a separate group of patients, AIFs obtained by direct measurement from the three scans for the same patient were averaged, and the average AIF was then applied for analysis of each individual data of the patient. For implementing the proposed method, each AIF by direct measurement was fitted by gamma variate function for the initial 30 seconds after tracer injection to obtain the AUC of the first pass. Averaging the AUCs for the three scans of a same patient resulted in the ‘true’ AUC for the patient. The true AUC was used in the conversion of the three aortic signal curves by the proposed method.
The concentration curve of tissues and aorta were analyzed by a three-compartment model (6) to estimate GFR and RPF for each of the three studies from the same patient. We expected that, using the proposed method for normalizing AIF, the standard deviation (SD) of the three estimates of one parameter would be reduced, compared with the other two methods.
Study B was performed to evaluate the proposed method's ability in improving intra-day reproducibility of DCE MRI, which is important for detecting short-term physiologic changes induced by an exogenous stimulus. The additional challenge for reproducible analysis in such a study is the gadolinium circulatory residual from the first scan at the start of the second scan (52). As an example of such a study, intra-day repeated DCE MRI with angiotensin converting enzyme inhibitor (ACEi) was performed for 6 patients (69.8 ± 16.3 years, range 39 – 87) without significant renal artery stenosis (RAS). Without RAS, ACEi should not induce any change in GFR, and GFR estimates by pre- and post-ACEi scans can thus be used to test the reproducibility of DCE MRI.
In the first DCE exam, serial coronal 3D spoiled gradient recalled echo (GRE) images were acquired at 1.5 T (Avanto, Siemens Medical Solutions, Erlangen, Germany) using the following parameters: TR/TE/flip angle = 2.84 ms/1.05 ms/12°, partition matrix 161 × 256 × 20 interpolated to 256 × 256 × 40, field of view 400 × 400 × 100 mm, voxel size 1.6 × 1.6 × 2.5 mm, parallel imaging acceleration factor of 3, acquisition time 3 s. Protocols for tracer injection and timing of breath hold were same as in study A. About 3 min before the end of the first DCE acquisition, an ACEi enalaprilat (0.04 mg/kg, up to 2.5 mg) was administered intravenously over 3 min. Typically 7 - 10 min after the baseline scan ended, the second DCE scan started, with 8 ml bolus of Gd-DTPA injected and the same MRI imaging protocol as in the first scan.
The techniques used for image segmentation, conversion of tissue signal to concentration, and model fitting in study B were the same as those in study A, except the following two differences. First, T1 values for renal cortex and medulla were measured (rather than assumed) (23). Second, in analyzing data of the second scan with 3-compartment model, gadolinium circulatory residual from the first scan was accounted for by the model parameters (52).
For converting aortic signals to AIF, two methods were used: direct measurement and the proposed method. The implementation of the proposed method for each patient in study B utilized cardiac output measured by phase-contrast MRI described in next paragraph. For each method of AIF conversion, we compared GFR values of the first exam (GFR1) and those from second exam (GFR2) for the cases in study B, using correlation coefficient (R). We hypothesized that the use of the proposed method would increase R, compared with direct measurement.
For cardiac output measurement, 2D prospective ECG-gated phase-contrast velocity images were acquired before DCE scans using the following protocol: imaging plane perpendicular to the ascending aorta approximately 20 mm above the aortic valve; TR/TE/FA 48.9 ms/3.09 ms/30°, slice thickness 6 mm, field of view 270 × 320 mm, matrix 216 × 256, 20 frames per cardiac cycle, acquisition time ~20 sec. Patients were asked to hold their breath during data acquisition. The stroke volume was computed as the area under the flow versus time curve. Heart rate was recorded continuously. The cardiac output was obtained by multiplying the stroke volume by the heart rate.
Results
Simulation
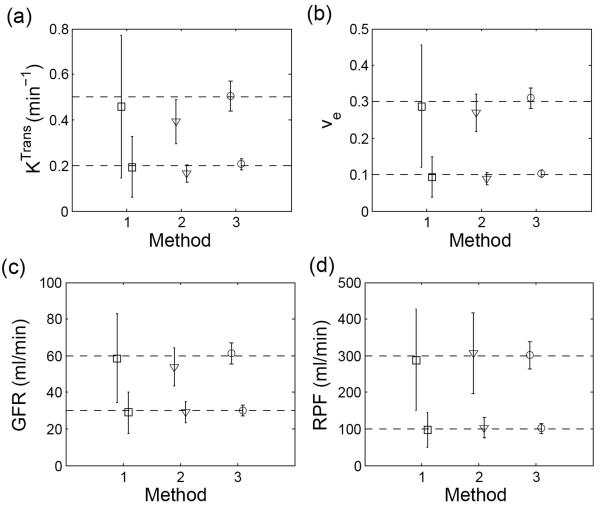
The distribution of tumor perfusion parameters (Ktrans and ve) estimated using the different AIF methods is shown in Figure 5 (a) and (b). The proposed method improved the precisions of both parameters by at least a factor of three compared with the direct method. For example, in the high-perfusion tumor simulation (nominal value Ktrans = 0.5 min−1), the SD of Ktrans was 0.08 min−1 using the constrained AIF versus 0.31 min−1 using the direct measurement method. Precision improved by a factor of 4 for ve. Use of averaged AIF also improved the precision, but not as much as the proposed cardiac output method. In addition, the averaged AIF was associated with a systematic deviation of computed parameters. For example, the systematic bias for high-perfusion Ktrans was −41% and for ve was −8%, whereas, the estimates by the proposed method showed minimal deviation from their true values: −4.6% for Ktrans, −0.8% for ve.
Figure 5.
Comparison of parameter estimates by the different input methods in simulation. (a) Ktrans and (b) ve from simulated MR tumor imaging; (c) GFR (d) RPF from simulated MR renography. Method ‘1’: direct measurement; ‘2’: averaged input approach; ‘3’: the proposed method. The error bars denote SD of the parameters.
Figure 5 (c) and (d) show the estimates for the renal parameters (GFR and RPF) using Monte Carlo simulations to test different AIF methods. Similar to the results in tumor simulation, the precisions of all parameters for both normal and impaired kidneys were significantly improved (about three-fold for GFR and three-fold for RPF) when using the proposed method and the deviation of these estimates from their true values was small (less than 1 ml/min for GFR, less than 2 ml/min for RPF).
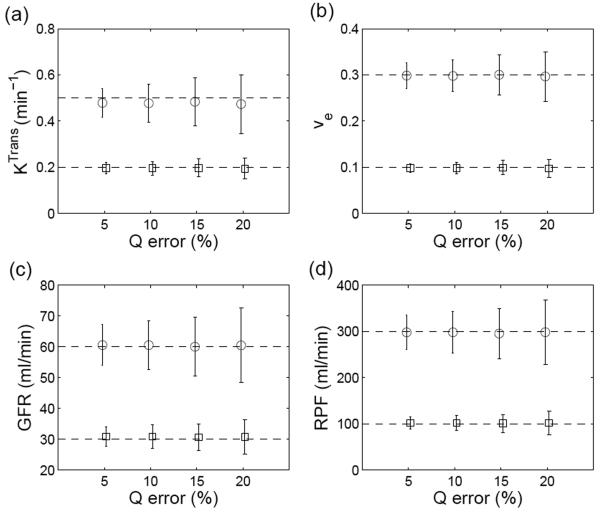
The simulations described above were obtained assuming the relative error of cardiac output (Q) measurement of 10%. Figure 6 demonstrates a nearly linear relationship between the precision of Q and the precision of functional parameters computed using the proposed method. For a relative error of Q = 5%, the coefficients of variation (CV) for the parameters were 9%~13%, while for relative error of 20%, CV for the parameters were 17%~23%.
Figure 6.
Effect of measurement error in cardiac output (Q) on the estimation of (a) Ktrans, (b) ve, (c) GFR and (d) RPF when using the proposed method. The circles are estimates for high perfusion or normal renal function, and the squares are for low perfusion or impaired renal function. The error bars denote SD of the parameters.
Patient studies
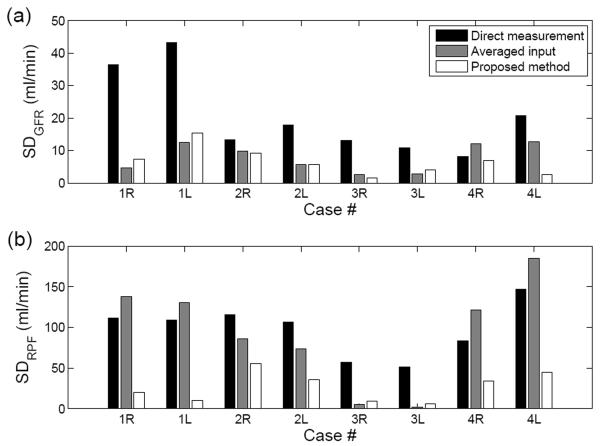
In study A, GFR estimates in healthy volunteers on three separate days showed greater consistency using the proposed cardiac output-corrected method than the other two methods. SD of the three GFR estimates by the proposed method, 6.4±4.4 ml/min, was significantly lower than that by direct measurement, 20.5±12.7 ml/min, and also significantly lower than that by the averaged input approach, 7.8±4.4 ml/min (Figure 7 (a)). As shown in Figure 7 (b), the SD of RPF estimates by the proposed method, 27.4±17.8 ml/min, was lower than that by direct measurement, 97.8±32.1 ml/min, or by averaged input approach, 92.7±64.5 ml/min.
Figure 7.
Standard deviations (SD) of three estimates for (a) GFR and (b) RPF from three independent scans in four patients (R, right kidney, and L, left kidney) in study A. The proposed method consistently resulted in lower SD for both GFR and RPF, as compared to the direct measurement or averaged AIF methods.
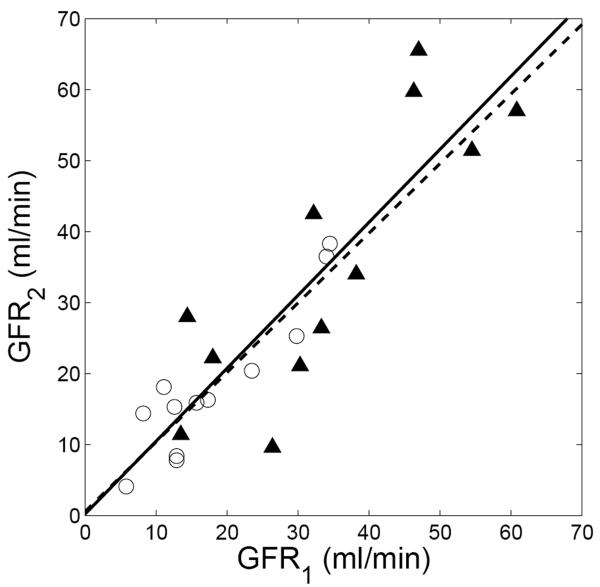
Study B tested the reproducibility of GFR measured within a single session. The cardiac output measurements for the patients by phase-contrast MRI were 4.7 ± 1.2 L/min (range 3.3 – 6.6 L/min). Using direct measurement, the correlation coefficient (R) between GFR1 and GFR2 was 0.83, with regression line: GFR2 = 1.03 GFR1 + 0.21 (Figure 8). The proposed method increased R to 0.92 (regression line: GFR2 = 0.98 GFR1 + 0.57).
Figure 8.
Comparison of baseline GFR (GFR1) and second-injection GFR (GFR2) for patients in study B. For direct measurement (displayed as triangle), correlation coefficient (R) is 0.83, and regression line is GFR2 = 1.03 GFR1 + 0.21 (solid line); for the proposed method (circles), R is 0.92, and regression line is GFR2 = 0.98 GFR1 + 0.57 (dashed line).
Discussion
In this study, a novel method is described for converting MR signal intensity of aortic blood into tracer concentration. According to the indicator dilution principle, knowledge of the subject's cardiac output allows us to constrain the area under the first pass of arterial input function (AIF). In our experience, the main source of error in deriving AIF is the inaccurate baseline signal intensity, S(0), mostly resulting from inflow artifacts and which become especially pronounced when the acquisition plane is not aligned with the direction of the flowing blood in-plane. By applying the proposed method, the baseline, unenhanced arterial signal intensity level can be corrected and ensure that the first-pass component of the corrected AIF has the correct area under curve and thus the correct magnitude.
The performance of the proposed method was evaluated using Monte Carlo simulations and with patient data. In the Monte Carlo simulations, the physiologic parameters of kidney filtration and tumor perfusion were estimated with three-fold higher precision than the estimates by direct measurement. The coefficients of variation of the parameters using the proposed method were reduced from ~30% to approximately 10%. These values can be further improved with more precise measurement of cardiac output. Patient studies measuring renal function confirmed the ability of the method to reduce variability in intra- and inter-day DCE MRI measurements.
The averaged input approach was originally proposed to reduce the overall effect of the various artifacts. However, averaging does not cancel the systematic artifacts such as inflow and partial volume effects. In addition, assuming a same AIF for different patients is only valid when the patient population is homogeneous. Clearly, this assumption does not apply in most clinical settings and when dealing with patients of unknown hemodynamic status. In our simulations, the averaged input approach resulted in a low accuracy of the perfusion parameters, with 15%~28% deviation in RPF and 35%~40% deviation in Ktrans. This observation confirms that perfusion parameters are highly sensitive to errors in the first-pass peak (6).
Several features of the proposed method make it advantageous over the previous methods. First, the AIF is calibrated using the area under the first-pass aortic curve, which is calculated for each individual patient. Second (as demonstrated in Figure 1), the estimate of high concentration in the first-pass peak is more sensitive to MR artifacts than low concentration in the tail. As a consequence, calibration of AUC for the first-pass peak corrects most of the AIF error. Third, although not correct all errors in AIF perfectly, the proposed method constrains AUC of AIF to its correct value. In appendix C, we give a theoretical derivation that the precision of ve is directly determined by the precision of AUC.
Because of its ability to significantly improve reproducibility, the proposed method should be of value in applications where repeated measurements are compared, for example in the monitoring of tumor response to therapy, or when using a pharmacologic challenge such as angiotensin-converting enzyme inhibitor-enhanced renography. These interventions typically have a subtle effect on physiologic parameters. For example, ACE inhibitor induces approximately 10% decrease in GFR in patients with renovascular hypertension (53). Detection of subtle changes requires high reproducibility of the measurements. In our volunteer study, the proposed method improved the precision of GFR from 20.5±12.7 ml/min to 6.4±4.4 ml/min. We note that in these applications, the measurement of cardiac output is not absolutely necessary, provided that cardiac output change across serial DCE-MRI exams can be neglected.
Our study has several limitations. First, our current patient study, without reference values for the DCE-derived parameters, can not be used to test the ability of the proposed method to improve the accuracy of these parameters. Future work will include reference measurements of parameters that can be validated with other methods, such as renal GFR. Second, measurement of cardiac output may be cumbersome whenever the tissue of interest is outside of the torso, i.e. when the aortic root is not in the field of view. For such situations, the MRI table may need to be adjusted for measuring cardiac output. Third, the method requires that the vascular curve contains a well-defined first-pass peak. However, in our experience, using an intravenous injection rate of 2 ml/sec is sufficient in all subjects (CO ranging from 3.3 – 6.6 L/min) to visualize first-pass peak.
Future work is clearly needed to validate our approach in other clinical DCE MRI applications and with larger groups of patients. Further investigations are also needed to facilitate online, real-time DCE image processing with the approach.
In conclusion, this study presents a novel method for reducing the effect of artifacts when estimating AIF from DCE MRI data. Simulations and patient studies showed that the method significantly improves our ability to measure functional parameters in MR renography and in tumor perfusion measurements. Improved reproducibility may be especially useful for the applications where repeated measurements are to be compared.
Acknowledgments
GRANTS: National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-063183 and DK-061599.
Appendix A: AUC of the first-pass component (Cfp) as a strictly monotonic function of S0
The definition of AUC and its partial derivative with respect to S0 are expressed as
| [A1] |
The partial derivative of tracer concentration with respect toS0 is
| [A2] |
where v = S(t)/S0, u = 1 − e−TR/T1(0) and w = 1−cosαe−TR/T1(0). Since ∂C/∂S0 is negative, ∂AUC/∂S0 is also negative. As a consequence AUC is a strictly monotonic function of S0. With this property, we can always find a unique S0 for every predicted AUC.
Appendix B: A whole-body tracer kinetic model
Monte Carlo simulation in this study required the construction of an ideal AIF, and this was achieved by using a whole body compartmental model of intravascular tracer (38,39). This model includes peripheral venous, the right heart, pulmonary system, the left heart, and peripheral arterial compartments. The model is described by a set of differential equations based on mass conservation for each compartment:
| [B1] |
where Vv, Vr, Vp, Vl, Vs are respectively the volume of venous, right heart, pulmonary, left heart and systemic compartments. Cv, Cr, Cp, Cl, Cs are the respective concentration of venous, right heart, pulmonary, left heart and systemic compartments, and Qv, Qr, Qp, Ql, Qs represent the flows out of each compartment. Qc and Cc represent the injection flow rate and concentration of the tracer. Parameter f denotes the rate of tracer elimination from human body, mainly due to renal excretion. Typical values for the parameters were: Vv = 40 mL, Vr = 250 mL, Vp = 450 mL, Vl = 250 mL, Vs = 5000 mL; Qv = 250 mL/min, Qr = Qp = Ql = Qs = 5000 mL/min; f = 0.2 (38,39). A system-circulation delay, 24s, was included for recirculation (54). For simulating AIF for different patients, the flow rates and compartment volumes were randomly chosen from ±30% range around their typical values.
Appendix C: Constraint of AUC eliminates error in v
In general there is no simple analytic relationship between the error in AIF and the corresponding error in perfusion parameters. In case of a general kinetic model of Tofts and Kermode (44), there is one parameter, the volume of extracellular and extravascular compartment, ve, for which we can demonstrate an exact relationship. We do not need to limit the proof to a specific impulse residue function R(t) of the model. The concentration of tracer within tissue ROI, CT(t), can be expressed as the convolution,
| [C1] |
where VT is the volume of tissue ROI, F is the blood flow, and CA is the arterial input function.
It is known that the area under a convolution is the product of the areas under the two factors:
| [C2] |
The arterial concentration CA can be expressed as the sum of all its passes (blood pumped out of heart comes back to heart, and then is pumped again and again),
| [C3] |
Integrating both sides of [C3], we get
| [C4] |
The area under the first pass CA1 on the right side of Eq. [C4] is denoted as AUC. The area under the second pass can be expressed as f·AUC, where the factor f is less than 1 because of tracer elimination. If we assume that the rate of tracer elimination is constant (time invariant), we can express area under the third pass as f2·AUC, and that of the fourth pass as f3·AUC etc. Equation [C4] can thus be rewritten as
| [C5] |
Substituting [C5] into [C2], we get
| [C6] |
According to central volume theorem, the transit volume (v) equals flow rate multiplied by mean transit time (area under IRF), i.e.
| [C7] |
Substituting [C7] into [C6], we obtain
| [C8] |
According to [C8], if we ignore errors from other sources, constraining arterial input so as to eliminate AUC error eliminates the error in v. In general kinetic model of Toft and Kermode v corresponds to ve, the volume of EES.
References
- 1.Barrett T, Brechbiel M, Bernardo M, Choyke PL. MRI of tumor angiogenesis. J Magn Reson Imaging. 2007;26(2):235–249. doi: 10.1002/jmri.20991. [DOI] [PubMed] [Google Scholar]
- 2.Kiessling F, Morgenstern B, Zhang C. Contrast agents and applications to assess tumor angiogenesis in vivo by magnetic resonance imaging. Current medicinal chemistry. 2007;14(1):77–91. doi: 10.2174/092986707779313516. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull LW. Dynamic contrast-enhanced MRI in the diagnosis and management of breast cancer. NMR in biomedicine. 2008 doi: 10.1002/nbm.1273. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CD, Ladam-Marcus V, Cucu C, Bouche O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: Current standards and perspectives. Critical reviews in oncology/hematology. 2008 doi: 10.1016/j.critrevonc.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Lee VS, Rusinek H, Bokacheva L, et al. Renal Function Measurements from MR Renography and a Simplified Multicompartmental Model. American journal of physiology. 2007;292:F1548–1559. doi: 10.1152/ajprenal.00347.2006. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JL, Rusinek H, Bokacheva L, et al. Functional assessment of the kidney from magnetic resonance and computed tomography renography: impulse retention approach to a multicompartment model. Magn Reson Med. 2008;59(2):278–288. doi: 10.1002/mrm.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackstein N, Kooijman H, Tomaselli S, Rau WS. Glomerular filtration rate measured using the Patlak plot technique and contrast-enhanced dynamic MRI with different amounts of gadolinium-DTPA. J Magn Reson Imaging. 2005;22(3):406–414. doi: 10.1002/jmri.20401. [DOI] [PubMed] [Google Scholar]
- 8.Roberts C, Buckley DL, Parker GJ. Comparison of errors associated with single- and multi-bolus injection protocols in low-temporal-resolution dynamic contrast-enhanced tracer kinetic analysis. Magn Reson Med. 2006;56(3):611–619. doi: 10.1002/mrm.20971. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Huang W, Panicek DM, Schwartz LH, Koutcher JA. Feasibility of using limited-population-based arterial input function for pharmacokinetic modeling of osteosarcoma dynamic contrast-enhanced MRI data. Magn Reson Med. 2008;59(5):1183–1189. doi: 10.1002/mrm.21432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peeters F, Annet L, Hermoye L, Van Beers BE. Inflow correction of hepatic perfusion measurements using T1-weighted, fast gradient-echo, contrast-enhanced MRI. Magn Reson Med. 2004;51(4):710–717. doi: 10.1002/mrm.20032. [DOI] [PubMed] [Google Scholar]
- 11.Bokacheva L, Rusinek H, Chen Q, et al. Quantitative determination of Gd-DTPA concentration in T(1)-weighted MR renography studies. Magn Reson Med. 2007;57(6):1012–1018. doi: 10.1002/mrm.21169. [DOI] [PubMed] [Google Scholar]
- 12.Materne R, Smith AM, Peeters F, et al. Assessment of hepatic perfusion parameters with dynamic MRI. Magn Reson Med. 2002;47(1):135–142. doi: 10.1002/mrm.10045. [DOI] [PubMed] [Google Scholar]
- 13.Ivancevic MK, Zimine I, Montet X, et al. Inflow effect correction in fast gradient-echo perfusion imaging. Magn Reson Med. 2003;50(5):885–891. doi: 10.1002/mrm.10633. [DOI] [PubMed] [Google Scholar]
- 14.Heilmann M, Walczak C, Vautier J, et al. Magma. 4. Vol. 20. New York, NY: 2007. Simultaneous dynamic T1 and T2* measurement for AIF assessment combined with DCE MRI in a mouse tumor model; pp. 193–203. [DOI] [PubMed] [Google Scholar]
- 15.van der Schaaf I, Vonken EJ, Waaijer A, Velthuis B, Quist M, van Osch T. Influence of partial volume on venous output and arterial input function. Ajnr. 2006;27(1):46–50. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JJ, Smith MR, Frayne R. Partial volume effect in quantitative magnetic resonance perfusion imaging. Conf Proc IEEE Eng Med Biol Soc. 2004;2:1132–1135. doi: 10.1109/IEMBS.2004.1403364. [DOI] [PubMed] [Google Scholar]
- 17.Wang J, Mao W, Qiu M, Smith MB, Constable RT. Factors influencing flip angle mapping in MRI: RF pulse shape, slice-select gradients, off-resonance excitation, and B0 inhomogeneities. Magn Reson Med. 2006;56(2):463–468. doi: 10.1002/mrm.20947. [DOI] [PubMed] [Google Scholar]
- 18.Warntjes JB, Dahlqvist O, Lundberg P. Novel method for rapid, simultaneous T1, T*2, and proton density quantification. Magn Reson Med. 2007;57(3):528–537. doi: 10.1002/mrm.21165. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Qiu M, Kim H, Constable RT. T1 measurements incorporating flip angle calibration and correction in vivo. J Magn Reson. 2006;182(2):283–292. doi: 10.1016/j.jmr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Cheng HL, Wright GA. Rapid high-resolution T(1) mapping by variable flip angles: accurate and precise measurements in the presence of radiofrequency field inhomogeneity. Magn Reson Med. 2006;55(3):566–574. doi: 10.1002/mrm.20791. [DOI] [PubMed] [Google Scholar]
- 21.Wen JG, Chen Y, Ringgaard S, et al. Evaluation of renal function in normal and hydronephrotic kidneys in rats using gadolinium diethylenetetramine-pentaacetic acid enhanced dynamic magnetic resonance imaging. The Journal of urology. 2000;163(4):1264–1270. [PubMed] [Google Scholar]
- 22.Jones RA, Easley K, Little SB, Scherz H, Kirsch AJ, Grattan-Smith JD. Dynamic contrast-enhanced MR urography in the evaluation of pediatric hydronephrosis: Part 1, functional assessment. AJR Am J Roentgenol. 2005;185(6):1598–1607. doi: 10.2214/AJR.04.1540. [DOI] [PubMed] [Google Scholar]
- 23.Bokacheva L, Huang AJ, Chen Q, et al. Single breath-hold T1 measurement using low flip angle TrueFISP. Magn Reson Med. 2006;55(5):1186–1190. doi: 10.1002/mrm.20845. [DOI] [PubMed] [Google Scholar]
- 24.Parker GJ, Roberts C, Macdonald A, et al. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med. 2006;56(5):993–1000. doi: 10.1002/mrm.21066. [DOI] [PubMed] [Google Scholar]
- 25.Le Sech C, Capderou A. Determination of pulmonary mean transit time and cardiac output using a one-dimensional model. Bulletin of mathematical biology. 1996;58(6):1155–1170. doi: 10.1007/BF02458387. [DOI] [PubMed] [Google Scholar]
- 26.Reiser UJ. Study of bolus geometry after intravenous contrast medium injection: dynamic and quantitative measurements (Chronogram) using an X-ray CT device. Journal of computer assisted tomography. 1984;8(2):251–262. [PubMed] [Google Scholar]
- 27.Hany TF, McKinnon GC, Leung DA, Pfammatter T, Debatin JF. Optimization of contrast timing for breath-hold three-dimensional MR angiography. J Magn Reson Imaging. 1997;7(3):551–556. doi: 10.1002/jmri.1880070316. [DOI] [PubMed] [Google Scholar]
- 28.Boos M, Scheffler K, Haselhorst R, Reese E, Frohlich J, Bongartz GM. Arterial first pass gadolinium-CM dynamics as a function of several intravenous saline flush and Gd volumes. J Magn Reson Imaging. 2001;13(4):568–576. doi: 10.1002/jmri.1080. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Quon H, Loevner LA, et al. Transcytolemmal water exchange in pharmacokinetic analysis of dynamic contrast-enhanced MRI data in squamous cell carcinoma of the head and neck. J Magn Reson Imaging. 2007;26(6):1607–1617. doi: 10.1002/jmri.21207. [DOI] [PubMed] [Google Scholar]
- 30.Zierler KL. Equations for Measuring Blood Flow by External Monitoring of Radioisotopes. Circ Res. 1965;16:309–321. doi: 10.1161/01.res.16.4.309. [DOI] [PubMed] [Google Scholar]
- 31.Millard RK. Indicator-dilution dispersion models and cardiac output computing methods. The American journal of physiology. 1997;272(4 Pt 2):H2004–2012. doi: 10.1152/ajpheart.1997.272.4.H2004. [DOI] [PubMed] [Google Scholar]
- 32.Rebergen SA, van der Wall EE, Doornbos J, de Roos A. Magnetic resonance measurement of velocity and flow: technique, validation, and cardiovascular applications. American heart journal. 1993;126(6):1439–1456. doi: 10.1016/0002-8703(93)90544-j. [DOI] [PubMed] [Google Scholar]
- 33.Berton C, Cholley B. Critical care. 3. Vol. 6. London, England: 2002. Equipment review: new techniques for cardiac output measurement--oesophageal Doppler, Fick principle using carbon dioxide, and pulse contour analysis; pp. 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szolar DH, Sakuma H, Higgins CB. Cardiovascular applications of magnetic resonance flow and velocity measurements. J Magn Reson Imaging. 1996;6(1):78–89. doi: 10.1002/jmri.1880060117. [DOI] [PubMed] [Google Scholar]
- 35.Kuehne T, Yilmaz S, Schulze-Neick I, et al. Magnetic resonance imaging guided catheterisation for assessment of pulmonary vascular resistance: in vivo validation and clinical application in patients with pulmonary hypertension. Heart (British Cardiac Society) 2005;91(8):1064–1069. doi: 10.1136/hrt.2004.038265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. Eur Heart J. 2004;25(21):1940–1965. doi: 10.1016/j.ehj.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 37.Davenport R. The derivation of the gamma-variate relationship for tracer dilution curves. J Nucl Med. 1983;24(10):945–948. [PubMed] [Google Scholar]
- 38.Bae KT, Heiken JP, Brink JA. Aortic and hepatic contrast medium enhancement at CT. Part II. Effect of reduced cardiac output in a porcine model. Radiology. 1998;207(3):657–662. doi: 10.1148/radiology.207.3.9609887. [DOI] [PubMed] [Google Scholar]
- 39.Bae KT. Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology. 2003;227(3):809–816. doi: 10.1148/radiol.2273020102. [DOI] [PubMed] [Google Scholar]
- 40.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54(3):507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40(11):715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 42.Cheng HL. T1 measurement of flowing blood and arterial input function determination for quantitative 3D T1-weighted DCE-MRI. J Magn Reson Imaging. 2007;25(5):1073–1078. doi: 10.1002/jmri.20898. [DOI] [PubMed] [Google Scholar]
- 43.Robert P, Violas X, Santus R, Le Bihan D, Corot C. Optimization of a blood pool contrast agent injection protocol for MR angiography. J Magn Reson Imaging. 2005;21(5):611–619. doi: 10.1002/jmri.20324. [DOI] [PubMed] [Google Scholar]
- 44.Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7(1):91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- 45.Padhani AR, Hayes C, Landau S, Leach MO. Reproducibility of quantitative dynamic MRI of normal human tissues. NMR in biomedicine. 2002;15(2):143–153. doi: 10.1002/nbm.732. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson JP, Rosen M, Sun W, et al. Phase I trial of the antivascular agent combretastatin A4 phosphate on a 5-day schedule to patients with cancer: magnetic resonance imaging evidence for altered tumor blood flow. J Clin Oncol. 2003;21(23):4428–4438. doi: 10.1200/JCO.2003.12.986. [DOI] [PubMed] [Google Scholar]
- 47.Galbraith SM, Maxwell RJ, Lodge MA, et al. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J Clin Oncol. 2003;21(15):2831–2842. doi: 10.1200/JCO.2003.05.187. [DOI] [PubMed] [Google Scholar]
- 48.Hayes C, Padhani AR, Leach MO. Assessing changes in tumour vascular function using dynamic contrast-enhanced magnetic resonance imaging. NMR in biomedicine. 2002;15(2):154–163. doi: 10.1002/nbm.756. [DOI] [PubMed] [Google Scholar]
- 49.Rusinek H, Boykov Y, Kaur M, et al. Performance of an automated segmentation algorithm for 3D MR renography. Magn Reson Med. 2007;57(6):1159–1167. doi: 10.1002/mrm.21240. [DOI] [PubMed] [Google Scholar]
- 50.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230(3):652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 51.Bluml S, Schad LR, Stepanow B, Lorenz WJ. Spin-lattice relaxation time measurement by means of a TurboFLASH technique. Magn Reson Med. 1993;30(3):289–295. doi: 10.1002/mrm.1910300304. [DOI] [PubMed] [Google Scholar]
- 52.Zhang JL, Rusinek H, Bokacheva L, et al. Angiotensin-converting enzyme inhibitor-enhanced MR renography: repeated measures of GFR and RPF in hypertensive patients. American journal of physiology. 2009;296(4):F884–891. doi: 10.1152/ajprenal.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor AT, Jr., Fletcher JW, Nally JV, Jr., et al. Procedure guideline for diagnosis of renovascular hypertension. Society of Nuclear Medicine. J Nucl Med. 1998;39(7):1297–1302. [PubMed] [Google Scholar]
- 54.Brandfonbrener M, Landowne M, Shock NW. Changes in cardiac output with age. Circulation. 1955;12(4):557–566. doi: 10.1161/01.cir.12.4.557. [DOI] [PubMed] [Google Scholar]