Abstract
OBJECTIVES
Acute compartment syndrome (ACS) is the result of decreased perfusion pressure (PP) and the diagnosis frequently requires invasive monitoring. Our objective was to test a new non-invasive ultrasound device for correlating PP with measurements of fascial displacement in a controlled porcine model of ACS. We hypothesized that fascial displacement in experimental compartments with impaired PP would be significantly greater than that in control compartments with normal baseline PP.
METHODS
ACS was generated in right anterior compartments of seven anesthetized pigs while contralateral compartments served as normal controls. Intramuscular pressure (IMP) in all compartments was monitored by slit catheters while IMP in experimental compartments was elevated in 10 mmHg increments by infusing 0.045% albumin in saline. A non-invasive ultrasound device continuously recorded fascial displacement corresponding to arterial pressure pulses in all compartments. Mean fascial displacement amplitude was grouped by PP and analyzed using two-way repeated measures ANOVA and contrast analysis.
RESULTS
As PP ranged from 80 to -40 mmHg, the change in fascial displacement of the infused compartments was significantly greater than that in the control compartments (ANOVA, p = 0.03). At each PP increment between 40 and –20 mmHg, fascial displacement in the infused compartments was significantly greater than that in the control compartments (contrast analysis, p < 0.014).
CONCLUSIONS
Fascial displacement is significantly greater in muscle compartments with decreased PP. Furthermore, changes in PP are associated with changes in fascial displacement over a clinically relevant range of PP, making this non-invasive technique potentially useful for monitoring in ACS.
Keywords: slit-catheter, perfusion, Volkmann’s, trauma, intracompartmental
Introduction
Acute compartment syndrome (ACS) is produced by elevated intramuscular pressure (IMP) leading to impaired perfusion of intracompartmental tissues. Historically, clinical findings associated with ACS have included the six Ps: pressure, pain, paresthesias, paralysis, pink skin, and presence of a distal pulse4, 8. Ulmer and colleagues reported a positive predictive value of 11% to 15% and a negative predictive value of 97% to 98% for diagnosing ACS based on clinical findings alone16. These results suggested that clinical findings were most useful by their absence in excluding the diagnosis of ACS. Most cases of ACS can be ruled out based on clinical findings alone. However, direct measurement of IMP is useful as an adjunct in making the diagnosis for unclear and unreliable clinical presentations as may be encountered in children and unconscious patients.
There are many criteria used for diagnosis, including an absolute IMP level greater than 30 mmHg with clinical symptoms1 or a tissue perfusion pressure (PP = mean arterial pressure — IMP) less than 30 to 40 mmHg11, 17, 18. Heppenstall and colleagues showed a correlation between tissue PP and oxygen delivery at a cellular level. They found that anaerobic metabolism occurred at a PP of approximately 30 mmHg in normal muscle and 40 mmHg in moderately traumatized muscle7. In keeping with this, Hargens and colleagues used a canine model of hemorrhagic hypotension to show that ACS occurred at a lower IMP in the setting of hypotension20. Hargens’ group also emphasized the importance of PP by applying Starling’s formula in another study, using a canine model of ACS3.
The current gold standard for detecting elevated IMP requires invasive pressure monitoring. In suspected cases of ACS, tissue pressure is obtained by the insertion of a slit catheter or similar pressure transducing device directly into the muscle compartment(s) at risk14. This technique is usually accurate and reproducible, but can at times be painful especially when measurements at multiple locations are required. Heckman and colleagues demonstrated the presence of a pressure gradient within the anterior and deep posterior muscle compartments of the leg in the setting of closed tibia fractures, suggesting that readings from multiple locations with a slit catheter or side-port needle are needed to obtain a representative set of IMPs within an affected compartment6.
Treatment of compartment syndrome relies on early recognition and prompt fasciotomy to prevent irreversible muscle death leading to contracture (such as Volkmann’s contracture) and in some cases resulting in a devascularized limb requiring amputation2, 12. The development of a fast, reliable, and non-invasive technique for measuring IMP at multiple locations within a compartment would improve the diagnostic utility of IMP in ACS, in addition to improving comfort for trauma patients with suspected ACS.
A non-invasive ultrasound device (NIUD) was used in a prior study as a means of correlating a fascial displacement waveform with IMP in patients with ACS19. The study, however, was limited by the use of an analog device, a small sample size, and a narrow range of IMPs19. In conducting the present study, our purpose was to test a new digital NIUD for measuring fascial displacement in a controlled porcine model of ACS over a broad range of IMPs and correlating the results with PP. We hypothesized that fascial displacement in experimental compartments with impaired PP would be significantly greater than fascial displacement in control compartments with normal baseline PP.
Materials and Methods
The experimental protocol was reviewed and approved by the UCSD Institutional Animal Care and Use Committee on August 16, 2006. The device is called EN-TACTTM (Emergency Non-Invasive Tissue and Compartment Testing) and is manufactured by Luna Innovations, Inc (Roanoke, VA). It is not yet FDA approved for clinical use in measuring intramuscular pressure, however it is a non-significant-risk device that is approved for experimental use.
Animal Preparation and Anesthesia
Seven conditioned farm pigs were selected (5 female, 2 male, weight 50 ± 2 kg) and housed in an animal facility for a minimum duration of seventy-two hours prior to experimentation. Approximately eight to twelve hours before the start of the experiment, each pig was denied food but had free access to water. A cervical intramuscular injection of Ketamine (33 mg/kg)/Xylazine (2 mg/kg)/Atropine (0.05 mg/kg) was used as a sedative prior to induction of anesthesia. The pig was then placed in a supine position on the operating table. A 21 G intravenous catheter was inserted into a dorsal ear vein and 0.9% NaCl was infused at approximately 4 ml/min for fluid replacement. Induction anesthesia was achieved with an injection of Propofol (2.4 mg/kg) into the dorsal ear vein. After induction, the pig was intubated, ventilated, and maintained in general anesthesia with isoflurane gas.
Three precordial leads were placed to monitor cardiac function. An oral temperature probe was used to monitor body temperature, which was maintained with a water heater along with blankets placed across the pig’s torso. Dissection was then performed to gain access to the carotid arteries and a single intra-arterial catheter was inserted for invasive arterial blood pressure monitoring throughout the experiment. The catheter was connected to a bag of heparinized saline in an IV compression bag through a pressure-transducing device (Transpac IV Monitoring Kit, Hospira, Lake Forest, IL) and was flushed periodically to prevent clotting. The output of the pressure transducer was displayed on a monitor (Hewlett Packard M1275A, Palo Alto, California, USA). The pig’s vital signs were continuously monitored (SurgiVet Monitor, Waukesha, WI) and manually recorded once every 10 minutes; including heart rate, respiratory rate, expired CO2, temperature, blood pressure (systolic/diastolic), mean arterial pressure, and jaw tone. Each of these parameters remained within normal limits throughout the duration of the experiments, for all animals tested. At the conclusion of each experimental trial the animals were euthanized with an IV injection of Pentobarbital (90 mg/kg) .
Experimental Set-Up
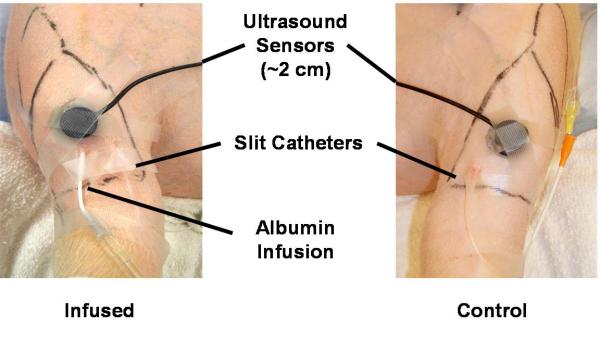
After the pig’s hind limbs were shaved, each lower leg anterior compartment was palpated in order to mark the boundaries with a pen. A 14 G angiocath needle was inserted in a caudal to rostral direction at approximately 30 degrees to the skin over the central region of the anterior compartment on each limb. The needle and catheter were advanced together approximately 4 to 5 cm, at which point the needle was retracted and a slit catheter was inserted in its place. The slit catheter was then connected to a bag of heparinized saline, through a pressure-transducing device (Transpac IV Monitoring Kit, Hospira, Lake Forest, IL), to prevent the formation of a clot around the tip of the catheter, which could result in artificially negative IMP readings. The output of the pressure transducer on the control limb was collected by the BIOPAC Systems MP150 Data Acquisition Device (Goleta, California, USA). The transducer output on the experimental limb was also collected by the BIOPAC MP150 and simultaneously displayed on the HP M1275A for viewing. A second 14 G angiocath needle was inserted into the anterior compartment of the experimental limb in the same manner as the first. The catheter was directly connected to an IV solution of bovine albumin (0.045%) in 0.9% NaCl5, 13. The albumin solution was prepared by dissolving 450 mg of bovine albumin in 1 L 0.9% NaCl. Only the experimental limb was locally infused with the albumin solution. Both slit catheters were flushed periodically in order to prevent coagulation and formation of an osmotic membrane at the tip. Care was taken to use small volumes with each flush and sufficient time was allowed between flushes to reach equilibrium in order to prevent local edema and artificially elevated IMP readings around the catheter tip. One ultrasound sensor was placed over each anterior compartment and lubricant gel was used to obtain good conduction between the sensors and the skin. Plastic tape was used to gently secure the sensor over the desired position, as shown in Figure 1.
Figure 1.
Experimental set-up. Anterior view of hind limbs with pig in supine position. Note ultrasound transducer and sensor contained in one unit.
EN-TACTTM (Emergency Non-Invasive Tissue and Compartment Testing) is a low-power ultrasound instrument with an embedded processor that employs a pulsed phase-locked loop algorithm to detect and continuously monitor very small changes in distance (on the order of microns) between the ultrasound transducer (secured on the surface of the skin with tape) and any sub-dermal tissue that is capable of reflecting the ultrasound pulse10, 19. The transducer was unfocused, with a center frequency of 1 MHz, a diameter of 0.5 inches, and an approximate bandwidth of 50%. Each arterial pulsation that courses through the muscle compartment causes a transient expansion of the compartment as the muscle fascia accommodates and rebounds from the cardiac pressure pulse, generating a periodic and characteristic fascial displacement waveform that the EN-TACTTM records in real time10, 19.
The digital NIUD is a next-generation digital implementation in comparison to the analog model used in the prior study by Wiemann and colleagues19. While the analog model provided indirect estimates of fascial displacement through post-acquisition correlation analysis, the digital model calculates displacement directly thereby providing an instantaneous output of the position of the muscle fascia10. It does so by calculating τ, which is the time it takes for an ultrasound wave to travel from the surface of the skin (where it is generated) to the muscle fascia and back to the surface of the skin (where it is detected). τ is converted to displacement using the flowing equation: , where d is displacement and c is approximate speed of sound in 2 tissue. The speed of sound in tissue is approximately 1540 m/sec and is assumed to be constant, unaffected by compartment contents, as demonstrated in a prior cadaveric study15. The digital NIUD samples τ at an adjustable rate of 300-1000 Hz (300Hz was used for this study) and converts it to a fascial displacement waveform according to the equation above, which is then displayed on the user interface and saved for post-processing.
Experimental Procedure and Data Analysis
For each pig we attempted to generate the following intracompartmental pressures in the infused limb by adjusting the infusion rate of the albumin solution: normal baseline intracompartmental pressure of 0-10 mm Hg, 10 mmHg, 20 mmHg, 30 mm Hg, 40 mm Hg, 50 mm Hg, 60 mm Hg, 70 mm Hg, 80 mm Hg, 90 mm Hg, and 100 mm Hg. It should be noted that for IMP levels greater than 80mmHg, the infusion technique became less stable in maintaining a desired pressure and actual pressure levels differed from the desired values (Table 1). The infusion technique did not allow for randomization of the order in which IMP values were generated since the infused solution could not be withdrawn. Therefore, all measurements were made in order of increasing IMP. The anterior compartment of the infused limb was gently palpated prior to elevating the intracompartmental pressure to ensure that a robust pressure response was observed on the monitor. This technique ruled out the presence of a clot at the tip of the catheter and ensured accurate pressure readings. The time required to elevate the IMP to the next incremental level ranged from 10 minutes to 1 hour with a mean of approximately 20 minutes.
Table 1.
Summary of Data Averaged Over All Animal Subjects
| Intramuscular Pressure (Mean ± SE), mm Hg |
Perfusion Pressure (Mean ± SE), mm Hg |
Fascial Displacement (Mean ± SE), μm |
Contrast Analysis, P |
||
|---|---|---|---|---|---|
| Control | Infused | Infused | Control | Infused | |
| 5.9 ± 1.3 | 10.2 ± 2.2 | 68.3 ± 0.8 | 1.6 ± 0.9 | 3.0 ± 0.9 | 0.304 |
| 8.1 ± 1.0 | 20.1 ± 0.3 | 59.4 ± 0.9 | 1.5 ± 0.8 | 2.9 ± 0.8 | 0.216 |
| 7.5 ± 1.3 | 30.8 ± 0.5 | 49.2 ± 0.8 | 2.0 ± 0.7 | 3.8 ± 0.7 | 0.095 |
| 7.8 ± 1.0 | 40.2 ± 0.6 | 39.9 ± 0.6 | 1.6 ± 0.7 | 4.4 ± 0.7 | <0.001 |
| 9.8 ± 2.1 | 51.1 ± 0.6 | 30.0 ± 1.1 | 1.7 ± 0.7 | 6.2 ± 0.7 | <0.001 |
| 7.9 ± 1.7 | 61.9 ± 0.7 | 19.7 ± 1.0 | 1.2 ± 0.7 | 6.6 ± 0.7 | <0.001 |
| 7.9 ± 0.7 | 72.2 ± 0.8 | 9.6 ± 0.9 | 1.3 ± 0.6 | 5.4 ± 0.6 | <0.001 |
| 7.0 ± 1.0 | 80.8 ± 1.0 | -11.1 ± 1.1 | 1.7 ± 0.9 | 4.7 ± 0.9 | 0.013 |
| 6.9 ± 0.8 | 90.8 ± 0.7 | -9.4 ± 1.0 | 1.1 ± 0.8 | 6.1 ± 0.8 | <0.001 |
| 7.0 ± 1.2 | 99.9 ± 0.8 | -19.6 ± 0.6 | 1.1 ± 0.8 | 4.6 ± 0.8 | 0.003 |
| 13.0 ± 1.0 | 108.5 ± 1.5 | -30.6 ± 0.8 | 1.2 ± 0.9 | 3.5 ± 0.9 | 0.056 |
| 7.1 ± 0.4 | 118.7 ± 3.3 | -41.7 ± 0.5 | 1.3 ± 1.8 | 3.3 ± 1.8 | 0.418 |
P value obtained from contrast analysis comparing fascial displacement of the control compartment against that of the infused compartments at each PP interval. Bolded values correspond to P value <0.05. MAP ranged from 60 to 99 mm Hg with a mean of 80 mm Hg.
At each increment of IMP the ultrasound operator identified the position of the muscle fascia in the echo-tracking window and obtained a 4-minute recording of the fascial displacement waveform for both limbs simultaneously. The reason for periodically and systematically determining the measurement depth at each new IMP level was to account for the shift in tissue boundaries that occurred when the compartment expanded in response to the albumin infusion. During this 4-minute interval, the BIOPAC MP150 operator recorded IMP from the two slit catheters (one in each limb) as well as the arterial blood pressure from the carotid artery catheter. Data collection was synchronized by verbal communication between the ultrasound and the BIOPAC MP150 operators.
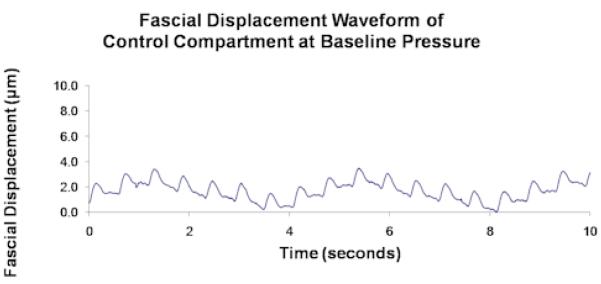
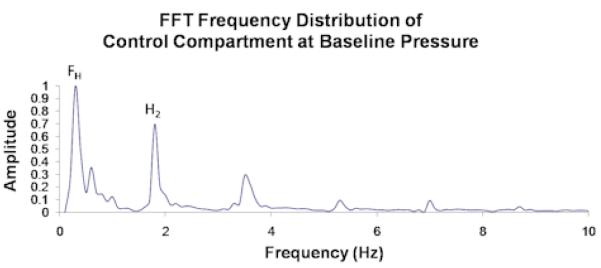
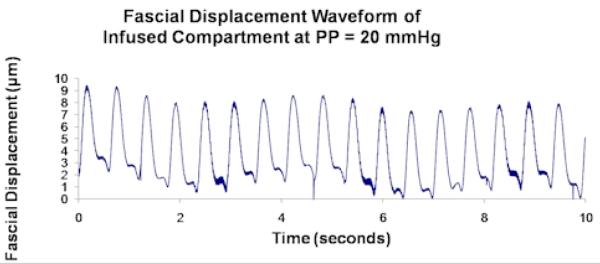
The unprocessed 4-minute recordings represent displacement of the muscle fascia from all sources including the arterial pressure pulse, respiratory pressure effect, muscle fasciculations, gross perturbation, and so forth. Each of these sources will displace the fascia at a different frequency and the collective effect is what is seen in the unprocessed data. In order to filter out the components of the overall fascial displacement that were not caused by the arterial pressure pulse, the 4-minute recordings were divided into approximately 10-second intervals and processed by fast Fourier transform (FFT) analysis. FFT converts data from a time domain to a frequency domain thereby allowing us to determine which frequencies of perturbation contribute most to the overall displacement of the fascia. The frequency of perturbation corresponding to the largest amplitude of fascial displacement is termed the fundamental harmonic, and that corresponding to the second largest amplitude is the second harmonic (Figures 2.1 and 2.2). In order for a given 10-second interval to be included in the analysis, either the fundamental or second harmonic was required to be within 5% of the cardiac pulse frequency, indicating that in that 10-second interval fascial displacement was largely caused by the arterial pressure pulse. Roughly 8% of the data consisted of 10-second intervals whose first two harmonics showed greater than 10% deviation from the cardiac pulse frequency. These data were labeled as outliers (Figure 2.3) and were visually inspected prior to rejection to ensure that they were caused by muscle fasciculations or by system noise, and not by a true pulsatile signal. The remaining acceptable data were averaged over the entire 4-minute recording to obtain an average amplitude of fascial displacement for a given IMP and a given animal.
Figure 2.1.
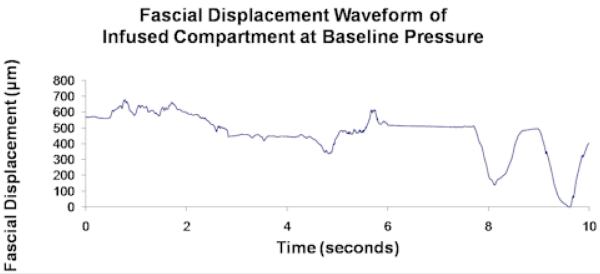
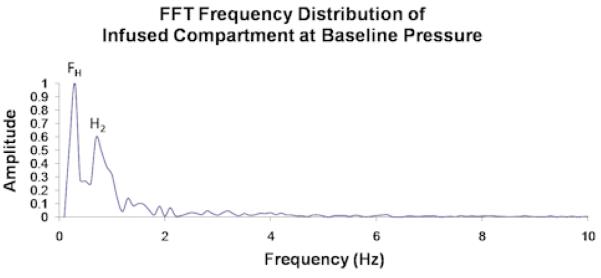
Unprocessed data from a single control compartment at baseline pressure showing (a) fascial displacement versus time and (b) the corresponding FFT frequency distribution. FH and H2 in (b) correspond to the respiratory and cardiac pulse frequencies, which respectively measure ∼ 0.25/sec and ∼ 2/sec in (a).
Figure 2.2.
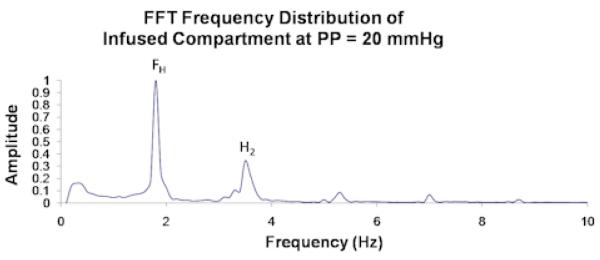
Unprocessed data from a single experimental compartment at PP = 20 mmHg showing (a) fascial displacement versus time and (b) the corresponding FFT frequency distribution. FH and H2 in (b) correspond to the cardiac pulse and respiratory frequencies, which respectively measure ∼ 2/sec and ∼ 3.5/sec.
Figure 2.3.
Unprocessed data from a single experimental compartment at baseline pressure showing (a) fascial displacement versus time and (b) the corresponding FFT frequency distribution. Note the variation in the amplitude of fascial displacement in (a) along with the numerous peaks in the FFT distribution. This segment represents an outlier.
At each increment of IMP, a corresponding PP (mean arterial pressure — IMP from slit catheter) was calculated. PP was divided into intervals of 10 mmHg ranging from −40 mmHg to 80 mmHg, with the mean arterial pressure (MAP) having an average value of 80 mmHg (Table 1). PP values between 25.0 mmHg and 34.9 mmHg were included in the 30 mmHg group, those between 35 mmHg and 44.9 mmHg were included in the 40 mmHg group, and so forth. Mean fascial displacement amplitudes were calculated for each perfusion pressure interval as described above and analyzed using two-way ANOVA, with α < 0.05. Contrast analysis was used to compare mean amplitudes of fascial displacement between the infused and control compartments for each PP interval between −40 mmHg and 80 mmHg, with α < 0.05. Finally, a receiver operator characteristic (ROC) curve was generated to determine the sensitivity and specificity of the digital NIUD for detecting PP < 30 mmHg. All statistical analyses were performed with JMP® software. The power of the study based on a sample size of 7 experimental limbs and 7 control limbs was 0.85.
Results
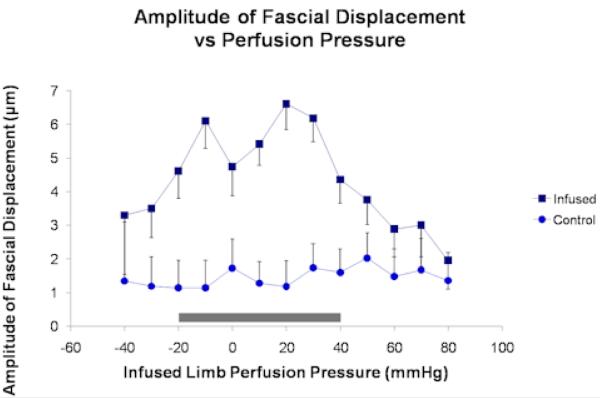
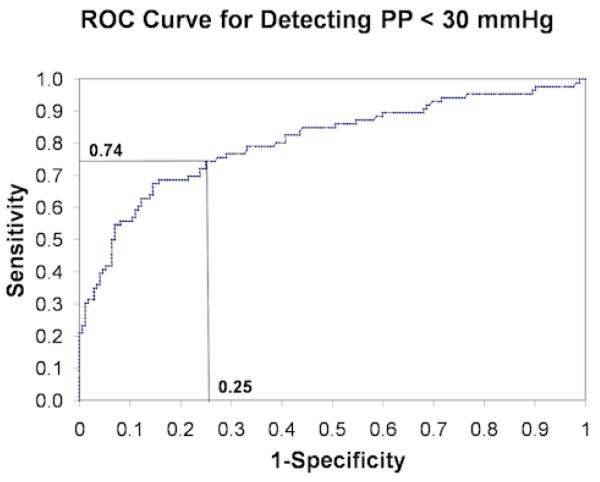
Slit catheter measurements of baseline IMP (mean ± SE) obtained in the infused and control compartments were 10.2 ± 2.2 mmHg and 5.9 ± 1.3 mmHg (t-test, p-value < 0.001), respectively. Sample unprocessed fascial displacement waveforms were plotted (Figures 2.1 and 2.2). Two-way repeated measures ANOVA analysis yielded a significant interaction between fascial displacement and PP (p = 0.03) for PP ranging between 80 and −40 mmHg (Figure 3) indicating that in this range of PP the change in amplitude of fascial displacement in the infused compartments was significantly different from that in the control compartments. Alternatively, fascial displacement in the infused compartments at each PP increment was significantly greater than that in the control compartments for PP between 40 and −20 mmHg (contrast analysis, p < 0.014, Table 1). The sensitivity and specificity of the digital NIUD for detecting PP < 30 mmHg were graphically displayed with a ROC curve (Figure 4) and determined to be 74% and 75% respectively.
Figure 3.
Fascial displacement versus infused limb perfusion pressure for infused (top curve) and control (bottom curve) compartments showing mean values along with standard error. The perfusion pressure (mean ± SE) of all the control compartments was 72 ± 1 mmHg. The shaded region corresponds to significant comparisons between the infused and control compartments (see Table 1).
Figure 4.
Receiver operator characteristic (ROC) curve for PP < 30 mmHg. Sensitivity is defined as with specificity calculated by (TP = true positive rate, TN = true negative rate, FP = false positive rate, FN = false negative rate).
Discussion
The results of our study provided evidence for the hypothesis that fascial displacement in experimental compartments with impaired PP would be significantly greater than fascial displacement in control compartments with normal baseline PP. We demonstrated a well-controlled porcine model of acute compartment syndrome with the use of an albumin infusion and achieved intramuscular pressures of up to 120 mmHg. We obtained measurements of fascial displacement with the next generation digital NIUD, yielding a significant two-way ANOVA interaction term between albumin infusion and control. For values of PP between 40 and −20 mmHg fascial displacement in the infused compartments was significantly greater than that in the control compartments, by contrast analysis.
The relationship between IMP, muscle compliance, and arterial pressure-pulse induced fascial displacement is one that is complex and not fully understood. Each arterial pressure-pulse within a muscle compartment is transmitted to the enveloping fascia and results in a fascial displacement that can be measured with ultrasound technology. Generally, the transmission of a pressure wave through a medium is dependent on the density or tightness of that medium. Local edema within a muscle compartment results in elevated IMP, decreased PP, and a shift along the pressure-volume curve to a position of greater slope, representing increased compartment tightness. With progression of compartment edema, the increasing compartment tightness is theorized to alter the transmission of arterial pressure-pulses to the muscle fascia resulting in varying amplitudes of fascial displacement.
Wiemann and colleagues used the previous generation analog NIUD to measure a voltage potential associated with fascial displacement. They then performed an FFT analysis of the fascial displacement waveform to obtain the ratio of the fundamental harmonic to the second harmonic. This allowed for the shape of the waveform to be quantified by a ratio of harmonics, which was found to be linearly correlated with IMP, both in a thigh-tourniquet model of ACS and in trauma patients with ACS19. Their study was paramount in demonstrating that measurements of fascial displacement with a NIUD could potentially be used as a diagnostic indication of elevated IMP. However, being a preliminary report it was limited by the small number of patients with ACS, and additionally the use of a thigh-tourniquet model of ACS generated a narrow range of IMPs (from 10 to 40 mmHg)19 making its application to trauma patients limited.
The next generation digital NIUD used in our study improved on the previous analog model by providing a direct measurement of fascial displacement rather than a correlation with voltage output. Additionally, in our study we measured fascial displacement over a wide range of IMPs and analyzed our data with PP. We quantified the diagnostic utility of the digital NIUD for detecting PP < 30 mmHg by calculating a sensitivity of 74% and a specificity of 75% (Figure 4). However, we were unable to corroborate the results of the harmonic ratio data in the prior study, as we found no correlation between the ratio of the fundamental to the second harmonic and IMP. We speculate that this may be due to a more compliant osseofascial membrane in our porcine model of ACS compared to the osseofascial membrane of humans, however we do not have evidence in support of this theory. Given that the harmonic ratio did not yield a significant interaction with IMP, it was excluded from our analysis.
One advantage of using the digital NIUD over the more invasive slit catheter technique was the prospect of simultaneously monitoring all four muscle compartments. Another advantage was that the sensors could be used continuously to monitor fascial displacement and compartment diameter over time allowing the physician to objectively assess progression of the syndrome. Lastly, the small ultrasound transducer could be placed anywhere along the skin over the compartment of interest, at various distances from the fracture site without subjecting the patient to multiple needle punctures.
One limitation of our study included the use of an animal model of acute compartment syndrome. The porcine hind-limb anterior compartment physiology may have been limited in its relevance to a larger and presumably less compliant human muscle compartment. Also, porcine skin was significantly thicker and more difficult to penetrate ultrasonically when compared to human skin. Therefore, we used higher power settings that yielded a more complex echo analysis and made it more difficult for an operator to accurately distinguish the fascial membrane from other tissues. Values of MAP and IMP in our animal model, however, were similar to values observed in humans. In addition, the infusion technique for generating ACS had been well established in both canine and porcine animal models by prior studies3, 5, 13.
The mean baseline values of IMP in the experimental and control compartments were noted to be significantly different (t-test, p-value < 0.0001), and may have been the result of localized tissue trauma from the insertion of two catheters in each of the experimental compartments as compared to one catheter in each of the control compartments. If this is the case then the discordance of these initial values is unlikely to have impacted the results of our study since we do not normalize subsequent measurements of fascial displacement to the initial baseline values. Conversely, the initial difference in IMP could have been due to an infusion related focal fluid-collection within the experimental compartment as a result of initial flushing. This would represent a constant error that would impact the results of the study. Future studies could address this issue by varying the relative insertion-site distance between the infusion catheter and the slit catheter in order to observe the effect on baseline IMP measurements. Also, a second catheter could be inserted into each control compartment and serve as a sham infusion to normalize the effect of tissue trauma from the presence of a second catheter.
The reliance on an arterial pressure pulse to generate the fascial displacement waveform may have been another limitation as the magnitude of the pressure pulse was likely to vary from subject to subject. In addition, we propose that the proportion of the pressure pulse that reaches the muscle compartment fascia is a function of the IMP. As stated earlier, when IMP rises the compliance of the muscle compartment falls and a greater proportion of the arterial pressure pulse is transmitted to the muscle compartment fascia9. Furthermore, as IMP approaches MAP, blood-flow in pre-capillary arterioles becomes severely restricted and the system behaves like a column of stagnant fluid3. Such a system is thought to deliver more energy to the vessel wall with each arterial pressure pulse, given that virtually none of the energy is consumed in generating flow. This phenomenon could have explained the observed increase in amplitude of fascial displacement when IMP approached MAP or when PP approached 0 mmHg (Figure 3). It is also important to note that as IMP rose above MAP, the amplitude of fascial displacement trended back down, seemingly taking on a maximum value at IMP ≈ MAP (Figure 3). One possible explanation for this observation is the idea that as IMP rises above MAP, the area of arterial compression where flow becomes severely restricted begins to migrate proximally from pre-capillary arterioles to larger arterioles, ultimately reaching the main artery supplying the compartment. When only a small portion of the main compartmental artery is patent enough to receive a pressure pulse and transmit that pulse to the muscle fascia, the effective source of pressure waves to induce fascial displacement is decreased resulting in a diminished amplitude of displacement. The exact mechanism has not been demonstrated experimentally and further work is needed to determine the cause of the fascial displacement variation with IMP. The clinical significance of this variation, though, is that the amplitude of fascial displacement takes on similar values at both high and low IMP. Therefore, the measurements made by the NIUD should be supplemented with additional data in order to distinguish between these two important extremes in the progression of ACS. The use of an external energy source to induce fascial displacement may address the problem. However, this approach has not yet been validated.
It should be noted that although we were able to generate values of IMP reaching 120 mmHg with our infusion model, the self limiting nature of compartment swelling makes such high levels physiologically possible only in the setting of direct arterial injury3. Patients who have direct arterial injury or patients with severe hypotension, though, may lack a significant arterial pressure pulse to generate a detectable amplitude of fascial displacement, precluding the use of this technique. In such cases, an external pressure source could be applied to the skin surface in order to generate fascial displacements, however as stated previously this technique has not yet been validated.
One additional consideration is the fact that our experimental model of ACS does not account for changes in fascial compliance that may occur with prolonged exposure to elevated IMP. In the clinical setting, patients at risk for ACS with worsening physical exam findings may be subjected to invasive IMP monitoring for up to 72 hours post injury. We have yet to determine whether exposure to elevated levels of IMP could results in plastic deformation of the muscle fascia over a clinically relevant period of 72 hours. If future studies provide evidence for this hypothesis, then the amplitude of fascial displacement, which is a function of fascial compliance, would be significantly different for compartments with the same PP, but at varying times after injury. Along the same lines, the extent of direct fascial trauma from the original injury may have a significant impact on the fascial compliance. If so, then the etiology of ACS (fracture, crush injury, ischemia/reperfusion, etc.) could have an impact on the amplitude of fascial displacement, irrespective of PP.
In this study, we demonstrated the utility of the next generation digital NIUD in monitoring the anterior compartment of the porcine hind limb. Currently, advanced software for data processing is available and may allow for monitoring of all four leg compartments simultaneously, such that this technique could be further investigated in future studies. With future changes made in the user interface, the NIUD will be more applicable in a clinical setting and will theoretically provide amplitudes of fascial displacement in less than two minutes. In addition to this, the interface will be simple to use and will not require expertise in echo analysis.
For future studies, improved technology will enable the use of the digital NIUD to automatically find the measurement depth of the muscle fascia in the echo-tracking window, more accurately than would a human operator. A digital NIUD would also automate the process of filtering out non-pulsatile displacements, thereby intrinsically reducing the number of outliers obtained. However, the technique may still generate false positive readings if local muscle fasciculations result in a pulsatile waveform that mimics the waveform associated with an arterial pulse. It is important to point out that the amplitude of fascial displacement provided by the NIUD will ultimately have to be converted to a value of IMP for each human subject and additional studies will have to be performed to investigate this conversion. The most important point to be addressed in future studies, though, is whether the relationship between PP and amplitude of fascial displacement is consistent among different limbs, different compartments, different injuries, and different animal models including humans. If so, then the technique could be utilized clinically with confidence, allowing the orthopedist to relate a measured degree of fascial displacement to a reliable corresponding PP, aiding in the diagnosis and prompt treatment of ACS.
Acknowledgments
Support: This research was supported by the following grants: 1. Grant Number 1 R43 AR053750-01 by NIH 2. Grant Number 5 T32 R007484-23 by NIH 3. LUNA Innovations Incorporated (Roanoke, Virginia)
Reference
- 1.Bernot M, Gupta R, Dobrasz J, Chance B, Heppenstall RB, Sapega A. The effect of antecedent ischemia on the tolerance of skeletal muscle to increased interstitial pressure. J Orthop Trauma. 1996;10(8):555–559. doi: 10.1097/00005131-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Feliciano DV, Cruse PA, Spjut-Patrinely V, Burch JM, Mattox KL. Fasciotomy after trauma to the extremities. Am J Surg. 1988;156(6):533–536. doi: 10.1016/s0002-9610(88)80547-6. [DOI] [PubMed] [Google Scholar]
- 3.Hargens AR, Akeson WH, Mubarak SJ, Owen CA, Gershuni DH, Garfin SR, Lieber RL, Danzig LA, Botte MJ, Gelberman RH. Kappa Delta Award paper. Tissue fluid pressures: from basic research tools to clinical applications. J Orthop Res. 1989;7(6):902–909. doi: 10.1002/jor.1100070617. [DOI] [PubMed] [Google Scholar]
- 4.Hargens AR, Mubarak SJ. Current concepts in the pathophysiology, evaluation, and diagnosis of compartment syndrome. Hand Clin. 1998;14(3):371–383. [PubMed] [Google Scholar]
- 5.Hargens AR, Schmidt DA, Evans KL, Gonsalves MR, Cologne JB, Garfin SR, Mubarak SJ, Hagan PL, Akeson WH. Quantitation of skeletal-muscle necrosis in a model compartment syndrome. J Bone Joint Surg Am. 1981;63(4):631–636. [PubMed] [Google Scholar]
- 6.Heckman M, Whitesides TJ, Grewe S, Rooks M. The relationship between tissue pressure, compartment, and the distance from teh site of the fracture. J Bone Joint Surg Am. 1994;76(9):1285–1292. doi: 10.2106/00004623-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Heppenstall RB, Sapega AA, Scott R, Shenton D, Park YS, Maris J, Chance B. The compartment syndrome. An experimental and clinical study of muscular energy metabolism using phosphorus nuclear magnetic resonance spectroscopy. Clin Orthop Relat Res. 1988;(226):138–155. [PubMed] [Google Scholar]
- 8.Kam JL, Hu M, Peiler LL, Yamamoto LG. Acute compartment syndrome signs and symptoms described in medical textbooks. Hawaii Med J. 2003;62(7):142–144. [PubMed] [Google Scholar]
- 9.Kim K, Weitzel W, Rubin J, Xie H, Chen X, O’Donnell M. Vascular Intramural Strain Imaging Using Arterial Pressure Equilization. Ultrasound in Med. & Biol. 2004;30(6):761–771. doi: 10.1016/j.ultrasmedbio.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JE, Heyman JS, Hargens AR. Ultrasonic device for the noninvasive diagnosis of compartment syndrome. Physiol Meas. 2004;25(1):N1–9. doi: 10.1088/0967-3334/25/1/n01. [DOI] [PubMed] [Google Scholar]
- 11.McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures. The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78(1):99–104. [PubMed] [Google Scholar]
- 12.Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36–40. doi: 10.1016/0268-0890(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 13.Odland R, Schmidt AH, Hunter B, Kidder L, Bechtold JE, Linzie BM, Pedowitz RA, Hargens AR. Use of tissue ultrafiltration for treatment of compartment syndrome: a pilot study using porcine hindlimbs. J Orthop Trauma. 2005;19(4):267–275. doi: 10.1097/01.bot.0000155308.20133.71. [DOI] [PubMed] [Google Scholar]
- 14.Perron AD, Brady WJ, Keats TE. Orthopedic pitfalls in the ED: acute compartment syndrome. Am J Emerg Med. 2001;19(5):413–416. doi: 10.1053/ajem.2001.24464. [DOI] [PubMed] [Google Scholar]
- 15.Ueno T, Ballard RE, Shuer LM, Cantrell JH, Yost WT, Hargens AR. Noninvasive measurement of pulsatile intracranial pressure using ultrasound. Acta Neurochir Suppl. 1998;71:66–69. doi: 10.1007/978-3-7091-6475-4_21. [DOI] [PubMed] [Google Scholar]
- 16.Ulmer T. The clinical diagnosis of compartment syndrome of the lower leg: are clinical findings predictive of the disorder? J Orthop Trauma. 2002;16(8):572–577. doi: 10.1097/00005131-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 17.White TO, Howell GE, Will EM, Court-Brown CM, McQueen MM. Elevated intramuscular compartment pressures do not influence outcome after tibial fracture. J Trauma. 2003;55(6):1133–1138. doi: 10.1097/01.TA.0000100822.13119.AD. [DOI] [PubMed] [Google Scholar]
- 18.Whitesides TE, Haney TC, Morimoto K, Harada H. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975;(113):43–51. doi: 10.1097/00003086-197511000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Wiemann JM, Ueno T, Leek BT, Yost WT, Schwartz AK, Hargens AR. Noninvasive measurements of intramuscular pressure using pulsed phase-locked loop ultrasound for detecting compartment syndromes: a preliminary report. J Orthop Trauma. 2006;20(7):458–463. doi: 10.1097/00005131-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Zweifach SS, Hargens AR, Evans KL, Smith RK, Mubarak SJ, Akeson WH. Skeletal muscle necrosis in pressurized compartments associated with hemorrhagic hypotension. J Trauma. 1980;20(11):941–947. doi: 10.1097/00005373-198011000-00006. [DOI] [PubMed] [Google Scholar]