Abstract
Placental Protein 13 (PP13) is a galectin expressed by the syncytiotrophoblast. Women who subsequently develop preterm preeclampsia have low first trimester maternal serum PP13 concentrations. This study revealed that third trimester maternal serum PP13 concentration increased with gestational age in normal pregnancies (p<0.0001), and it was significantly higher in women presenting with preterm preeclampsia (p=0.02) and HELLP syndrome (p=0.01) than in preterm controls. Conversely, placental PP13 mRNA (p=0.03) and protein, as well as cytoplasmic PP13 staining of the syncytiotrophoblast (p<0.05) was decreased in these pathological pregnancies compared to controls. No differences in placental expression and serum concentrations of PP13 were found at term between patients with preeclampsia and control women. In contrast, the immunoreactivity of the syncytiotrophoblast microvillous membrane was stronger in both term and preterm preeclampsia and HELLP syndrome than in controls. Moreover, large syncytial cytoplasm protrusions, membrane blebs and shed microparticles strongly stained for PP13 in preeclampsia and HELLP syndrome. In conclusion, parallel to its decreased placental expression, an augmented membrane shedding of PP13 contributes to the increased third trimester maternal serum PP13 concentrations in women with preterm preeclampsia and HELLP syndrome.
Keywords: Brush border membrane, galectin, syncytiotrophoblast microparticle, trafficking, virtual microscopy
INTRODUCTION
Preeclampsia is a syndrome associated with shallow trophoblast invasion and abnormal spiral artery remodeling [1–4], uteroplacental ischemia and anti-angiogenic state [5–8], generalized endothelial cell dysfunction and increased maternal systemic inflammatory response [9–12]. Early-onset preeclampsia often has a severe clinical presentation that is associated with increased perinatal morbidity and mortality, and high incidence of hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome [2, 13, 14]. Moreover, the rates of uteroplacental vascular insufficiency, lesions of the villous tissues and increased syncytiotrophoblast microparticle (STBM) shedding are higher in early-onset than in late-onset preeclampsia [1, 15–19]. These differences led to the proposal that early-onset preeclampsia is a placental disease, while late-onset preeclampsia is a maternal disease [16, 20].
Placental-derived proteins, such as soluble vascular endothelial growth factor receptor-1, placental growth factor, endoglin, inhibin A and activin A have been implicated in the pathophysiology and prediction of preeclampsia [5–8, 21]. Recently, four nested case-control studies have demonstrated that patients who subsequently developed preterm preeclampsia had a significantly lower median maternal serum Placental Protein 13 (PP13) concentration in the first trimester than those women who had normal delivery at term [22–25]. The combined measurements of serum PP13 concentrations and uterine artery Doppler pulsatility indexes had even better value in the risk assessment of preterm preeclampsia [22]. However, PP13 is a less valuable marker for term preeclampsia [23, 25].
PP13 was purified and cloned from the placenta [26–28], localized to the syncytiotrophoblast brush border membrane [29], and detected in maternal and cord blood [30]. It was designated galectin-13 as it belongs to the galectin family [29, 31], whose members share similar topology, carbohydrate recognition domain and specificity for N-acetyl-lactosamine containing glycoconjugates [32]. Indeed, an in vitro study proved its lectin activity and favored binding to sugar residues widely expressed on placental microvillous surfaces [29]. Of importance, the LGALS13 gene encoding for PP13 is uniquely expressed by the placenta as shown by Northern blot [28], GNF SymAtlas (http://wombat.gnf.org/SymAtlas/) and GenBank (www.ncbi.nlm.nih.gov) gene expression data.
The unique placental expression and localization at the maternal-fetal interface might suggest that PP13 is related to the pathophysiology of preeclampsia and HELLP syndrome. To test this hypothesis, we investigated the placental expression and localization of PP13 in parallel with its maternal serum concentrations in patients with preeclampsia and HELLP syndrome.
PATIENTS, MATERIALS AND METHODS
Study design, patient groups and clinical definitions
Samples were collected at the First Department of Obstetrics and Gynecology, Semmelweis University (Budapest, Hungary), a national referral center for high-risk pregnancies, between October, 2003 and March, 2006. Pregnancies were dated according to ultrasound measurements between 8–12 weeks of gestation (GW). Patients with multiple pregnancies or with fetuses having congenital or chromosomal abnormalities were excluded. The collaborative research was approved by Regional Research Ethics Committee, and informed consent was obtained from women prior to sample collection; specimens and data were stored anonymously.
This cross-sectional study aimed 1) to determine maternal serum PP13 concentrations in normal pregnant women in the third trimester, and 2) to compare maternal serum and placental PP13 concentrations and LGALS13 gene expression in women with preeclampsia with or without HELLP syndrome as well as in gestational age matched controls at the time of delivery.
Maternal sera were collected from a first subset of control women with normal pregnancy between GW24–40. Enrollment and blood draw took place at routine laboratory sampling during prenatal care visits. Women were included in this control group (n=46) if they 1) had no medical, obstetrical or surgical complications; 2) had no labor and did not deliver at the time of blood draw; and 3) delivered at term (GW≥37) a neonate with a birth-weight appropriate for gestational age [33].
Maternal sera and placentas were also collected from women at the time of delivery. Women were enrolled at the Labor and Delivery Unit upon admission, and those who had blood draw prior to any medication were included in the analysis in the following gestational age matched groups: 1) preeclampsia with (n=12) or 2) without HELLP syndrome (n=20), and 3) a second subset of controls (n=30), which consisted of term (n=20) and preterm controls (n=10). Term controls had no medical or obstetrical complications and delivered at term (GW≥37) a newborn with birth-weight appropriate for gestational age [33]. Preterm controls included women with spontaneous preterm (GW<37) delivery without any clinical or histological signs of chorioamnionitis. Control women underwent Caesarean section secondary to previous Caesarean section or malpresentation. Preeclampsia was defined as hypertension that developed after GW20 (systolic or diastolic blood pressure ≥140 or ≥90Hgmm, respectively, measured at two different time points, 4h to 1 week apart) coupled with proteinuria (≥300mg in a 24h urine collection, or two random urine specimens obtained 4h to 1 week apart containing ≥1+ by dipstick or one dipstick of ≥2+ protein) [2, 34]. Severe preeclampsia was defined as systolic blood pressure ≥160mmHg or diastolic blood pressure ≥110mmHg and/or proteinuria greater than 5g in a 24h collection or ≥3+ protein on dipstick, and it was also diagnosed in the presence of multi-organ involvement [2, 34]. HELLP syndrome was defined as hemolysis (serum LDH >600IU/l; bilirubin >1.2mg/dl; presence of schistocytes in peripheral blood), elevated liver enzymes (serum ALT and/or AST >70IU/l) and thrombocytopenia (platelet count <100,000/mm3) [35]. In preterm preeclampsia with or without HELLP syndrome, delivery was necessitated by the symptoms before term (GW<37).
Samples
Blood samples were immediately centrifuged (10min at 2,500rpm) after draw; sera were separated and stored at −80°C. Villous tissue samples were excised from central cotyledons after separation of the placentas from the choriodecidual layer. Native (for protein determination) and RNAlater®–ICE (Ambion Inc., St. Austin, TX) soaked (for qRT-PCR) samples were deep frozen and stored at −80°C. Whole placentas were formalin-fixed, and representative tissue blocks were paraffin-embedded. Not all specimens were available from every patient.
Placental tissue preparations for protein determination
Deep frozen placental villous tissues (500mg; n=52) were pulverized in liquid nitrogen and homogenized on ice in 500μl RIPA lysis buffer (50mM Tris-HCl pH=7.4; 150mM NaCl; 1% NP40; 0.25% Na deoxycholat, 1mM EDTA and 1mM PMSF). Homogenates were centrifuged (15min at 10,000rpm) to pellet non-soluble debris; supernatants containing soluble or solubilized cytoplasmic, membrane and nuclear proteins were collected, and their total protein contents were measured by the Bradford assay and equalized for 1mg/ml. These protein extracts were then used for ELISA, immunoprecipitation or immunoblotting.
Immunoprecipitation
Placental protein extracts (1mg; n=52) were pre-cleared on Protein A agarose (Sigma-Aldrich Co., St. Louis, MO) (1h at 4C°) and centrifuged (5min at 2,500rpm), and supernatants were transferred into fresh tubes and incubated with an excess (2μg) of anti-PP13 antibody (overnight at 4C°). 30μl of Protein A agarose was added to each tube, and mixtures were incubated (1h at 4C°). Precipitated immunocomplexes were collected by centrifugation (5min at 2,500rpm), repeatedly washed in RIPA buffer containing 0.1% detergent, and centrifuged (5min at 2,500rpm) four times. Pellets were resuspended in Laemmli buffer, boiled (5min), and centrifuged (1min at 10,000rpm), and supernatants were applied to SDS-polyacrylamide gel electrophoresis (PAGE).
SDS-PAGE and Western blot analysis
Initially, placental protein extracts (30μg) were used for Western blot analysis; however, many of the samples did not contain detectable amounts of PP13. Thus, immunoprecipitates (30μl) were subsequently run on 15% SDS-PAGE along with recombinant PP13 (rPP13) (5–50ng) as positive control (Diagnostic Technologies Ltd., Yokneam, Israel). Proteins were electroblotted to nitrocellulose membranes (Invitrogen Corporation, Carlsbad, CA) and blocked (30min at 25°C) with PBS containing 0.1% Tween-20 and 5% skim milk powder. Membranes were incubated with biotinylated anti-PP13 monoclonal antibody (Diagnostic Technologies Ltd., Yokneam, Israel) (2μg/ml; overnight at 4°C) and then with HRP-conjugated streptavidin (Jackson Immunoresearch Laboratories Inc., West Grove, PA) (1:10,000; 1h at room temperature), and developed by Super Signal West Pico ECL reagent (Pierce Biotechnology Inc., Rockford, IL). Band visualization, signal quantification and molecular weight determination were performed by a Fluorchem SP (Alpha Innotech Corporation, San Leandro, CA) or a Las3000 (Fuji Film, Tokyo, Japan) CCD imaging system. The sensitivity of both systems was 200pg PP13/lane.
ELISA
PP13 concentrations of placental protein extracts and maternal sera were determined using a solid-phase sandwich ELISA kit (Diagnostic Technologies Ltd., Yokneam, Israel). Optical density was measured at 450nm and translated into quantitative amounts using a calibration curve consisting of rPP13 standards (0–500pg/mL). The lower limit of detection was 5pg/mL. The intra- and inter-assay variations in our laboratory were 5.4% and 9.4%, respectively.
RNA isolation
Deep frozen placental tissues (n=23) were thawed and homogenized. After centrifugation at 12,000rpm, RNAlater®-ICE was removed. Total RNA was isolated with Eppendorf ® Perfect RNA Eukaryotic Kit (Eppendorf AG, Hamburg, Germany) and treated with Dnase I. Total RNA quality and integrity were determined by measuring the OD260/280 and OD260/230 ratios.
Quantitative real-time RT-PCR
cDNA was synthesized using 0.5μg Dnase-I-treated total RNA and First Strand cDNA Synthesis Kit (Roche Diagnostics Co., Indianapolis, IN). Primers (LGALS13 forward: GCAAACAATTTGAGCTGTG, reverse: CACTGAGGTCAGGGAGA; YWHAZ forward: TCCTTTGCTTGCATCCCAC, reverse: AAGGCAGACAATGACAGACCA; HPRT forward: GGCAGTATAATCCAAAGATGGTC reverse: GTCTGGCTTATATCCAACACTTG) were designed using the LightCycler Probe Design Software 1.0 (Hoffmann-La Roche Ltd, Basel Switzerland) and synthesized by Sigma-Genosys Ltd. (Haverhill, UK). PCR reactions consisted of 9μl DNA Master SYBR Green I mix (Hoffmann-La Roche Ltd., Basel, Switzerland), 1μl cDNA and 2.5pmol primers, and a negative control without cDNA template was run simultaneously with every assay. The PCRs were run in duplicate on LightCycler thermal cycler, and the average was used in the calculations. Standard curves were obtained using serial dilutions of the β-globin reference gene, and the concentration of each gene product was determined by a kinetic approach with the LightCycler software. The expression of phospholipase A2 (YWHAZ) and hypoxanthine phosphoribosyltransferase (HPRT) housekeeping genes that have great expression stability in the placenta significantly correlated in all samples, and HPRT was used for normalization.
Histopathology
Placentas were fixed and histopathologically evaluated in 38/62 cases due to temporarily limited personal and laboratory conditions. Specimens were examined according to a standard protocol by two pathologists (BH, TF) blinded to clinical details. The topography and size of all macroscopic lesions were described, and the ratio of these lesions and viable placental tissue was estimated. Seven representative histological blocks were taken from each placenta to include both ends of the umbilical cord, placental membranes, and 3 macroscopically normal central/paracentral areas of the placental disc. An en face block of the basal plate was taken for the best representation of uteroplacental arteries [36]. In addition, macroscopic lesions were also sampled. All tissue blocks were paraffin-embedded; 4μm sections were cut and mounted on SuperFrost/Plus slides (Fisherbrand, Loughborough, UK). After deparaffination and rehydration, slides were stained with haematoxylin-eosin (HE) and evaluated in 10 randomly chosen microscopic fields [37]. Macroscopic and microscopic lesions were defined according to published criteria [37–39], quantified and statistically analyzed.
Immunohistochemistry
Four μm sections were cut from full thickness blocks (n=38) of a macroscopically normal central area of each placenta. Sections were incubated with 10mM Tris (pH=9, 1mM EDTA) (30min at 100°C) to expose antigens. After inhibition of endogenous peroxidases with 3% H2O2, unspecific antibody binding was blocked with 3% BSA in PBS containing 10% goat serum (Vector Laboratories, Burlingame, CA) (30min at room temperature). Sections were incubated (90min at 37°C, then overnight at 4°C) with monoclonal anti-PP13 antibody (1:20) in PBS containing 1% BSA. After washing, the second incubation (1h at room temperature) was performed with HRP-conjugated anti-mouse IgG (1:100). Slides were developed with DAB Substrate Kit (Vector Laboratories, Burlingame, CA) and counterstained with haematoxylin (H) or HE. In negative controls, the primary antibody was omitted or preabsorbed with a ten-fold excess of rPP13.
Evaluation of immunostaining
Visual evaluation of immunostainings was performed microscopically (Carl Zeiss MicroImaging GmbH, Gottingen, Germany) by two pathologists (BH and TF). All slides (n=38) were digitally scanned by a high resolution scanner (Mirax Scan, Carl Zeiss MicroImaging GmbH, Gottingen, Germany), deposited to a virtual laboratory (www.pathonet.com), and used for virtual microscopic evaluation by a third examiner (NGT) applying Mirax Viewer 1.8.3.0 (Carl Zeiss MicroImaging GmbH, Gottingen, Germany and 3DHistech Ltd., Budapest, Hungary). All examiners were blinded to the clinical information. Immunostainings were semi-quantitatively scored by BH and NGT with a modified immunoreactive score [40]. Immunostaining intensity was graded as follows: 0=negative, 1=weak, 2=intermediate, and 3=strong. The percentage of cells staining positive was assessed as follows: 0=negative, 1=1–10%, 2=11–50%, 3=51–80%, and 4=81–100%. The final composite score for PP13 immunoreactivity was obtained by multiplying the intensity and percentage scores, giving a range of 1–12, in which a score of 1–4=weak, 5–8=moderate, 9–12=strong. Each slide was evaluated three times in 10 random fields by both examiners; the average of the scores was determined as the representative data for that sample.
Statistical analysis
Maternal serum and placental PP13 concentrations were log10 transformed to improve data normality. The association between the log transformed PP13 concentrations or LGALS13 / HPRT gene expression ratio and two explanatory variables (gestational age and disease status) were tested using a linear model strategy. In these models, the main effects and the interaction of gestational age and disease status (presence of preeclampsia) were estimated, while their significance was tested via t-tests. The models were fitted under the R statistical environment (www.r-project.org) using specialized functions. All other analyses were run by SPSS 12.0 (SPSS Inc., Chicago, IL); comparisons among the groups were performed using Chi-square test and Fisher's exact test for proportions and Kruskal-Wallis test followed by Mann-Whitney test for non-normally distributed continuous variables. A p value of <0.05 was considered statistically significant.
RESULTS
Demographic and clinical data
The 46 Caucasian women in the first subset of controls had a mean maternal age of 30±4.9 years; the mean gestational age at delivery was GW39.1±1.3. Demographic and clinical characteristics as well as placental histopathological findings of women in the second subset of controls and patients with preeclampsia with or without HELLP syndrome are displayed in Tables 1 and 2, respectively. Severe preeclampsia was diagnosed in 61.5% (8/13) of women with preterm and 57.1% (4/7) of patients with term preeclampsia. All patients with HELLP syndrome had preeclampsia. Neonatal birth-weight was lower in preeclampsia with or without HELLP syndrome than in controls. Primiparity was higher in preeclampsia than in controls at term; however, this was not the case before term. Maternal body mass index (BMI) was higher in preterm preeclampsia, and placental weight was lower in preterm preeclampsia with or without HELLP syndrome than in preterm controls (Table 1). From the twenty-two investigated macroscopic and histological placental lesion types, those consistent with maternal and fetal vascular underperfusion were more frequent in preeclampsia with or without HELLP syndrome than in controls, especially in patients who delivered preterm (Table 2).
Table 1.
| Demographic and clinical characteristics of the study groups | |||||
|---|---|---|---|---|---|
| Groups | Preterm controls | Preterm preeclampsia | Preterm HELLP | Term controls | Term preeclampsia |
| Number of cases1 | 10 | 13 | 12 | 20 | 7 |
| Maternal age (years)2 | 26.5 (5.5) | 30.9 (5.6) | 28.4 (4.5) | 32.2 (4.9) | 28.3 (3.0) |
| Gestational age at delivery (weeks)2 | 32.5 (2.8) | 30.9 (2.1) | 31.42 (2.8) | 38.7 (0.8) | 37.6 (0.8) |
| Primiparity3 | 50 | 38.5 | 58.3 | 15 | 85.7† |
| Smoking3 | 30 | 15.4 | 0 | 5 | 0 |
| Systolic blood pressure (mmHg)2,4 | 115.6 (7.2) | 162.3 (16.0)* | 162.9 (10.5)* | 120.3 (11.9) | 163.1 (15.8)* |
| Diastolic blood pressure (mmHg)2,4 | 75.0 (8.7) | 95.5 (11.7)* | 105.0 (13.8)* | 74.8 (5.8) | 103.0 (11.2)* |
| Maternal BMI (kg/m2)2 | 25.6 (4.3) | 32.4 (8.4)‡ | 26.6 (3.4) | 26.2 (4.0) | 28.3 (1.1) |
| Neonatal birth-weight (g)2 | 2246 (709.1) | 1391.5 (446.9)† | 1445.8 (421.8)† | 3317 (470) | 2714 (498)‡ |
| Placental weight (g)2 | 410.5 (65.8) | 261.5 (63.1)‡ | 262.6 (51.7)‡ | 504.0 (81.9) | 379.8 (110.9) |
| Caesarean section3 | 50 | 100 | 100 | 100 | 100 |
All women were Caucasian.
Values are presented as number
mean(±SD)
or percentage.
p<0.001
p<0.01
p<0.05 compared to gestational age matched controls.
Highest blood pressure values measured before delivery.
Table 2.
| Histopathological findings | ||||
|---|---|---|---|---|
| Preterm and term controls | Preterm preeclampsia | Preterm HELLP | Term preeclampsia | |
| Number of placentas1 (n) | 14 | 9 | 10 | 5 |
| Distal villous insufficiency (%) | 0 | 55.6† | 50† | 40‡ |
| Increased syncytial knots (%) | 57.2 | 88.9 | 70 | 100 |
| Increased perivillous fibrin (%) | 0 | 55.6† | 20 | 40‡ |
| Recent villous infarct (%) | 0 | 55.6† | 30‡ | 0 |
| Remote villous infarct (%) | 7.2 | 33.4 | 70† | 40 |
| Remote villous infarct, extensive (%) | 7.2 | 44.4‡ | 80* | 40 |
| Small placenta (%) | 0 | 66.7* | 66.7* | 60† |
| Uteroplacental vessel thrombosis (%) | 0 | 22.3 | 55.6† | 0 |
| Uteroplacental vessel adaptation problem2 (%) | 0 | 25 | 44 | 25 |
| Villous stromal fibrosis (%) | 7.2 | 66.7† | 60† | 60‡ |
| Villous dysmaturity (%) | 7.2 | 77.8† | 80* | 40 |
Three macroscopically normal central and paracentral areas of the placental discs, an en face block of the basal plates, and all blocks of the macroscopic lesions were microscopically evaluated in each placenta.
Fibrinoid necrosis with or without acute atherosis.
The Pearson's chi-square test and the Fisher's exact test were used for statistical analysis.
Values are presented as number or percentage.
p<0.001
p<0.01
p<0.05 compared to controls.
Maternal serum PP13 concentrations
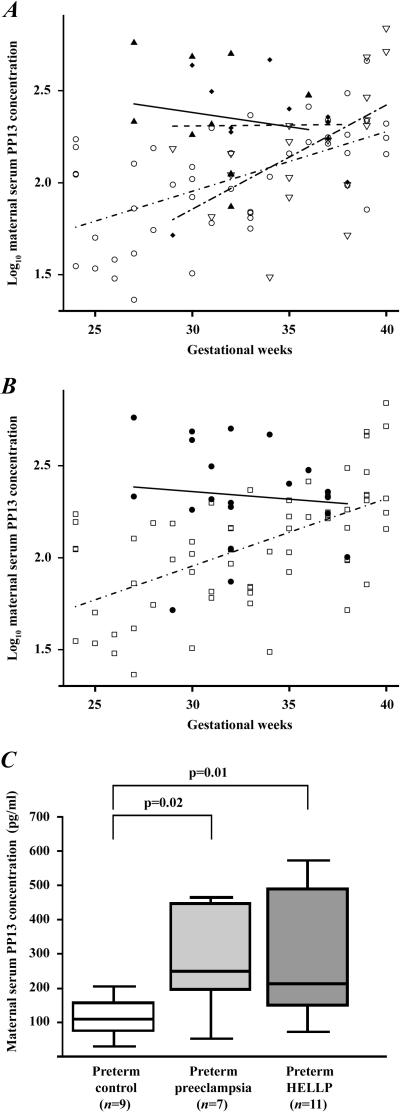
The analysis of the first subset of control women revealed that log10 PP13 concentration increased as a function of gestational age in their sera taken at routine prenatal care visits (p<0.0001) (Figure 1A). When analyzing the second subset of control women, log10 PP13 concentrations in maternal serum taken at the time of delivery increased similarly as a function of gestational age (p=0.02). In contrast, maternal serum PP13 concentrations did not correlate with gestational age in patients with preeclampsia with or without HELLP syndrome (Figure 1A).
Figure 1. Maternal serum PP13 concentrations.
(A) Log10 PP13 concentration increased as a function of gestational age in sera of normal pregnant women taken at routine prenatal care visits (n=46; ○ -·-) and in preterm and term controls taken at the time of delivery (n=18; ▿ –-–); however, did not correlate with gestational age in patients with preeclampsia with (n=11; ▲—) or without HELLP syndrome (n=12; ◆- -) at the time of delivery. (B) The regression line for log10 serum PP13 concentrations was different in the pooled group of controls (n=64; ◻- · -) from the pooled group of preeclampsia (●—). (C) Median maternal serum PP13 concentrations were significantly higher in women with preterm preeclampsia with or without HELLP syndrome than in preterm controls. Boxes represent the median±25th percentiles, whiskers the extreme values.
Since neither the mean log10 maternal serum PP13 concentrations at any given GW nor their rate of increase with gestational age were different between the two subsets of controls, these groups were pooled together to increase the statistical power. For the same reasons, the groups of preeclampsia with or without HELLP syndrome were also combined. Then, log10 PP13 concentrations () were calculated and a linear regression model was fitted to the results as a function of gestational age (expressed in GW), disease status (DS; control=0; preeclampsia=1) and their interaction. The following equation gives the estimated log10 PP13 concentrations:
| (1) |
The slopes of the regression lines for log10 PP13 concentrations were significantly different between controls and patients with preeclampsia (p=0.02), and the difference between the two groups decreased with advancing gestational age (Figure 1B and Table 3).
Table 3.
| Maternal serum PP13 concentrations adjusted to the linear regression model (pg/ml)1 | ||
|---|---|---|
| Gestational weeks | Control2 | Preeclampsia2 |
| 24 | 53.8 | NA |
| 25 | 58.5 | NA |
| 26 | 63.7 | NA |
| 27 | 69.3 | 239.9 |
| 28 | 75.4 | 235.4 |
| 29 | 82.0 | 230.9 |
| 30 | 89.2 | 226.6 |
| 31 | 97.1 | 222.3 |
| 32 | 105.6 | 218.2 |
| 33 | 114.9 | 214.1 |
| 34 | 125.0 | 210.0 |
| 35 | 136.0 | 206.1 |
| 36 | 148.0 | 202.2 |
| 37 | 161.0 | 198.4 |
| 38 | 175.1 | 194.7 |
| 39 | 190.6 | 191.0 |
| 40 | 207.3 | NA |
The equation of the linear model was used for the intrapolation of gestational age-specific maternal serum PP13 concentrations in control women and in patients with preeclampsia.
Each value represents an intersection on the regression lines for the given gestational week.
Subsequently, women were sub-divided into term and preterm subgroups to reveal the differences in maternal serum PP13 concentrations that may exist between the second subset of control women and patients with distinct subforms of preeclampsia at the time of onset of disease. Median maternal serum PP13 concentrations in preterm preeclampsia without [250.0pg/ml (range: 52–462), p=0.02] and with HELLP syndrome [213.0 (74–571), p=0.01] were significantly higher than in preterm controls [109.6 (31–203)] (Figure 1C). There was no difference between the term preeclampsia [211.1 (100–226)] and term control [218.0 (52–685)] groups.
Placental PP13 concentrations
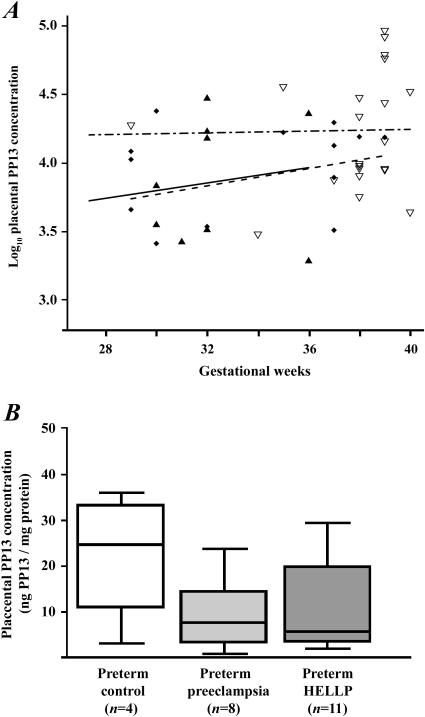
Placental PP13 concentrations did not correlate with gestational age in any of the groups (Figure 2A). Median placental PP13 concentrations in preterm preeclampsia without [7.5ng/mg total protein (range: 0.7–23.8, p=0.13)] and with HELLP syndrome [5.6 (1.9–29.3, p=0.15)] were lower than in preterm controls [24.7 (3.0–35.8)] (Figure 2B). There was no difference between the preeclampsia [14.3 (3.2–19.6)] and control [12.1 (4.3–92.1)] groups at term.
Figure 2. Placental PP13 concentrations.
(A) Log10 placental PP13 concentrations did not correlate with gestational age either in control women (n=22; ▿– - –) or in patients with preeclampsia with (n=11; ▲ —) or without HELLP syndrome (n=14; ◆- -). (B) Median placental PP13 concentrations were lower in women with preeclampsia without (3.3-fold) or with (4.4-fold) HELLP syndrome than in controls. Boxes represent the median±25th percentiles, whiskers the extreme values.
Placental LGALS13 gene expression
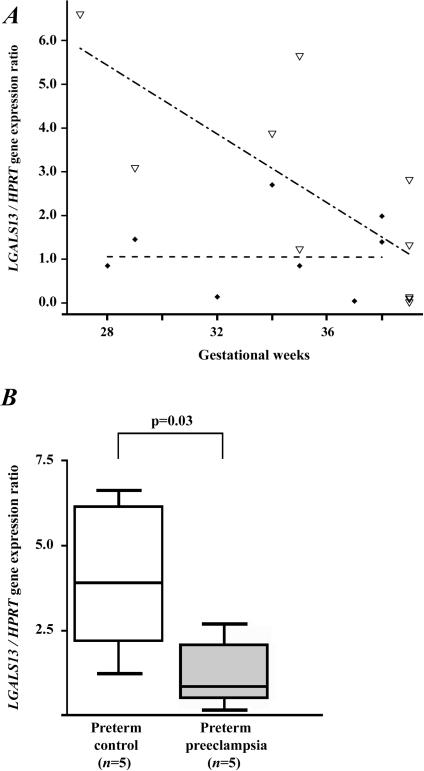
The mean LGALS13 / HPRT gene expression ratio decreased with advancing gestational age in the second subset of controls (p=0.01); however, this did not change in patients with preeclampsia (Figure 3A). In addition, the mean LGALS13 / HPRT gene expression ratio was higher in controls than in the preeclampsia group (p=0.02). The LGALS13 / HPRT gene expression ratios were fitted to a linear regression model:
| (2) |
Not surprisingly, the median LGALS13 / HPRT gene expression ratio was significantly lower in patients with preterm preeclampsia than in preterm controls (3.45-fold; p=0.03) (Figure 3B); however, no difference was detected between the term preeclampsia and term control groups (1.02-fold).
Figure 3. Placental LGALS13 gene expression.
(A) The regression line for the LGALS13 / HPRT gene expression ratio decreased with advancing gestational age in control women (n=10; ▿ – - –), while it did not change in patients with preeclampsia (n=9; ◆ - -). (B) The LGALS13 / HPRT gene expression ratio was 3.5-fold lower in women with preterm preeclampsia than in preterm controls. Boxes represent the median±25th percentiles, whiskers the extreme values.
Molecular size of PP13
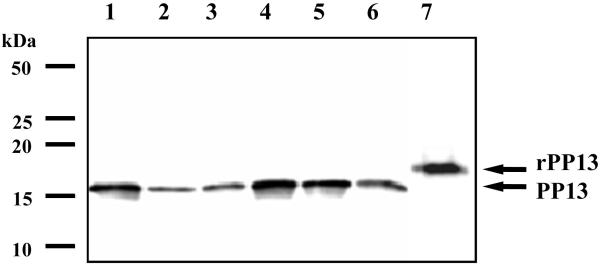
To verify the specificity of the antibody, we performed Western blots on immunoprecipitated placental protein extracts. Recombinant, His-tagged PP13 was recognized as a 18kDa band, while placental-derived PP13 migrated as a 16kDa band in all investigated samples (Figure 4). These results provided the biochemical evidence for the nature of the immunostained protein and revealed that the same molecular size PP13 was detectable in all control and pathological placentas.
Figure 4. Identification of recombinant and placental expressed PP13 by Western blotting.
The His-tagged rPP13 (7) migrated as a 18 kDa band, while immunoprecipitated placental PP13 was recognized as an immunologically identical 16 kDa band in all samples (1–2: term controls; 3: term preeclampsia; 4: preterm control; 5: preterm preeclampsia; 6: preterm HELLP syndrome). The positions of molecular mass markers are signed in the left.
Placental PP13 immunostaining
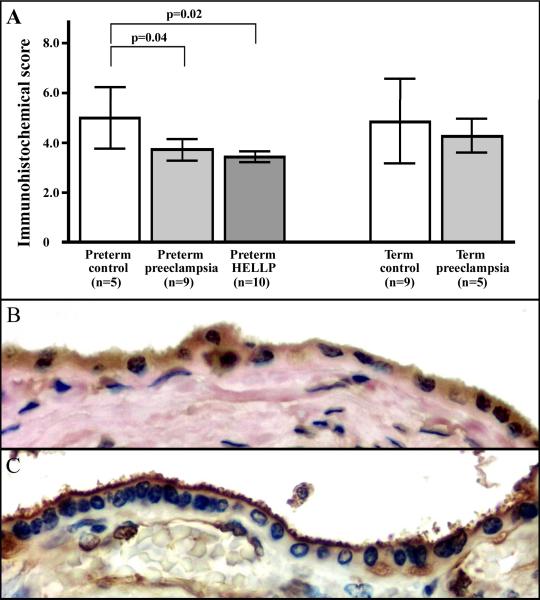
The intensity of cytoplasmic staining of the syncytiotrophoblast was significantly weaker in central cotyledons in preterm preeclampsia without (mean composite score±SD: 3.7±0.4, p=0.04) and with HELLP syndrome (3.5±0.7, p=0.02) compared to preterm controls (5.0±1.2). However, there was no significant difference between preeclampsia (4.3±0.7) and controls (4.9±1.7) at term (Figure 5A–E, Figure 6A). The coefficients of variation (CVs) between the scores provided by the two examiners for cytoplasmatic and membrane staining were 10.53% (p=0.13) and 8.68% (p=0.1), respectively.
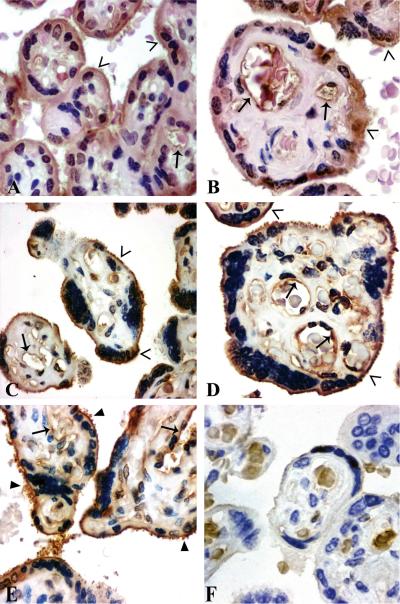
Figure 5. Placental immunolocalization of PP13.
Syncytiotrophoblastic immunostaining was moderate in preterm controls (A: GW 35), and weak in term controls (B: GW 38), preterm (C: GW 29) and term preeclampsia (D: GW 37) and HELLP syndrome (E: GW 36). The brush border membrane (open arrowheads) stained moderately in controls (A,B), and strongly in preeclampsia without (C,D) or with HELLP syndrome (E). Cytoplasm protrusions, membrane blebs and shed membrane particles were PP13 positive (filled arrowheads) (C–E). The villous endothelium (arrows) was immunopositive in all sections (A–E). Negative control: isotype-matched IgG staining (F). HE (A,B) or hematoxilin (C–F) counterstain. 500x (A,C,E) or 700x (B,D,F) magnifications.
Figure 6. IHC score analysis and immunolocalization of PP13 in the syncytiotrophoblast.
The composite IHC score of the syncytiotrophoblast was higher in preterm controls than in preterm preeclampsia with or without HELLP syndrome (A). High magnification (800x) images show uniformly moderate cytoplasmic and brush border membrane staining in a preterm control (B), while weak cytoplasmic and strong membrane staining in a preterm HELLP syndrome placenta (C). Cytoplasm protrusions, membrane blebs and shed membrane particles stained intensely for PP13 (C). HE (B) or hematoxilin (C) counterstain.
Because of the extensive loss of the brush border membrane in preeclamptic placentas, it was only possible to evaluate the membrane intensity score. Staining of the microvillous membrane was uniform in term (membrane intensity score±SD: 1.8±0.4) and preterm (1.6±0.4) controls (Figure 5A,B, Figure 6B), while it was stronger in term preeclampsia (2.2±0.3, p=0.04) and preterm preeclampsia without (2.2±0.6) and with HELLP syndrome (2.3±0.4, p=0.01). Syncytial cytoplasm protrusions, membrane blebs and shed membrane particles stained intensely; however, there was no PP13 staining at the sites of membrane loss (Figure 5C–E, Figure 6C). The villous capillary endothelium was weakly immunoreactive in all sections (Figure 5A–E, Figure 6C).
DISCUSSION
PP13 localizes to the syncytiotrophoblast microvillous membrane and villous endothelium
Our previous [29] and present studies localized PP13 to the cytoplasm and brush border membrane of the syncytiotrophoblast, which is a highly polarized cell-layer with distinct proteomic and glycomic profiles on its apical and basement membranes [41, 42]. The brush border membrane contains an abundance of N-acetyl-galactosamine [42], the strongest carbohydrate ligand for PP13 [29], which may suggest a causal link between the binding specificity and sublocalization of PP13. As other galectins, PP13 lacks a secretion signal peptide but may accumulate directly below the plasma membrane and can be secreted to the cell surface via non-classical pathways, such as through the extrusion of membrane blebs or exovesicle shedding [43]. Indeed, syncytiotrophoblast membrane blebs were immunopositive for PP13 in this study, and exovesicles generally contain annexin-II [44],a ligand for PP13 at the syncytiotrophoblast apical membrane [29]. Of note, annexin-II is a major component of lipid rafts in microvillous surfaces, and it functions as an interface between lipid rafts and the actin cytoskeleton [44]. As a high similarity of the syncytiotrophoblast microvillous membrane to lipid rafts has recently been identified [41], and galectins bear an important role in lipid rafts on microvillous surfaces [44], it would be intriguing to verify whether PP13 has a placenta-specific role in membrane trafficking and organization of the microvillous membrane through its interaction with annexin-II and β/γ-actin [29].
As a novel finding of this study, the villous endothelium was immunopositive for PP13 in all investigated placentas, which is in accord with a previous report that showed comparable amounts of PP13 in maternal and cord blood [30]. Thus, it would be important to test whether differences exist in cord blood PP13 concentrations in healthy and pathological pregnancies.
The localization and expression of PP13 is altered in preeclampsia and HELLP syndrome
This study has shown first that PP13 staining of the syncytiotrophoblast microvillous membrane is increased in preeclampsia with or without HELLP syndrome, irrespective of gestational age, suggesting a shift in the intracellular trafficking of PP13 in these syndromes. In contrast, cytoplasmic PP13 staining of the syncytiotrophoblast is decreased in preterm preeclampsia with or without HELLP syndrome when compared to gestational age matched controls. In accordance with these, placental LGALS13 gene expression and PP13 protein content were lower in preterm preeclampsia than in gestational age-matched controls, and a similar decrease in placental PP13 protein content was found in preterm HELLP syndrome. The impairment of LGALS13 gene expression in preterm preeclampsia and HELLP syndrome is underlined by the following: 1) spatial and temporal gene expression differences within the placental disks might have had only minimal impact on our expression data as only central cotyledons were investigated, and patients with preeclampsia and control women were matched for gestational age; 2) the immunostaining of the syncytiotrophoblast was also significantly weaker in these central cotyledons in preterm preeclampsia with or without HELLP syndrome when compared to controls.
Our previous [29] and present studies localized PP13 to the syncytiotrophoblast but not to cytotrophoblasts, suggesting that the regulation of the LGALS13 gene expression is related to syncytialization [49–51]. Of note, recent studies have provided evidence that syncytin-1, a major regulator of syncytialization [45], was abnormally localized to the apical microvillous membrane in preeclampsia [46]; moreover, its placental expression was significantly decreased in preterm but not in term preeclampsia and HELLP syndrome [46–48]. The altered Syncytin gene expression and protein localization was suggested to impair cell-fusion and syncytiotrophoblast formation [46–48], which may lead to decreased LGALS13 gene expression in preterm preeclampsia. Further functional studies are warranted to elucidate the regulation of LGALS13 gene expression and its relation to syncytin-1 and villous trophoblast differentiation.
Shedding of PP13 immunopositive membrane particles is enhanced in preterm preeclampsia and HELLP syndrome
Syncytiotrophoblast cytoplasm protrusions, microvillous membrane blebs and shed membrane particles were strongly PP13 immunopositive, which is a novel finding. Previous studies revealed that early- rather than late-onset preeclampsia is characterized by frequent placental lesions and increased STBM shedding [15–19]. An intensive loss of the brush border membrane, cytoplasm protrusions and microvillous blebs [52–54] were implicated as sources of the large quantities of STBMs in preterm preeclampsia [18, 55]. These morphological changes were suggested to be the consequence of placental vascular underperfusion and ischaemia [48, 52, 54]. We observed that 1) the frequency of characteristic changes in placental histopathological findings consistent with placental vascular underperfusion, 2) the magnitude of syncytiotrophoblast brush border distortions and 3) the extent of microvillous membrane shedding were highest in preterm preeclampsia with or without HELLP syndrome. Moreover, shed membrane particles were strongly immunopositive for PP13, which may lead to an increase in maternal serum PP13 concentrations in these cases.
Maternal serum PP13 concentrations are elevated in preterm preeclampsia and HELLP syndrome in the third trimester
Maternal serum PP13 concentrations increased as a function of gestational age in the control groups, similar to the increase in maternal serum concentrations of syncytiotrophoblastic proteins localized to the brush border membrane, such as PP5/TFPI-2, heat-stable alkaline phosphatase (PLAP) and pregnancy-specific beta1-glycoprotein (SP1) [27]. This rise in serum PP13 concentrations with advancing gestation was also similar to the increase in trophoblast cell volumes as assessed by stereological methods [56]. Thus, concentrations of PP13 in maternal serum are dependent on the volume of its origin, the trophoblast, in normal pregnancies.
Of importance, no difference was detected in serum PP13 concentrations in preterm controls presenting with labor that led to preterm delivery when compared to normal pregnant women who delivered at term. In contrast, maternal serum PP13 concentrations were higher in women presenting with preterm preeclampsia with or without HELLP syndrome than in preterm controls; however, no such difference was found between patients and controls at term. Furthermore, characteristic changes were also detected in placental histopathology, PP13 content and immunostaining in preterm preeclampsia, but no similar differences were found between patients and controls at term. These findings may underline the different mechanisms of disease in term and preterm preeclampsia [14, 16, 20]. Moreover, as placentas in preterm preeclampsia with or without HELLP syndrome showed a PP13 pattern more typical of term than preterm controls, this phenomenon might be considered as a biochemical correlate of the accelerated maturation of the villous tree, represented by our frequent findings of distal villous insufficiency in cases of preterm preeclampsia.
Our study did not show differences in maternal serum and placental PP13 concentrations and immunostainings between preterm preeclampsia alone or coupled with HELLP syndrome, suggesting a common pathway for the dysregulation of PP13 production and placental shedding in the two syndromes. This finding is in accord with the current view that although preeclampsia and HELLP syndrome might have distinct familial segregation and genetic background [57–61], independent genes and mechanisms in various subsets of these syndromes may initiate shared pathophysiological pathway(s), common clinical symptoms and placental dysfunction [61, 62].
What is the cause of the high maternal serum PP13 concentrations in preterm preeclampsia? 1) The LGALS13 gene is predominantly expressed by the placenta [28]; therefore, this organ is the probable source for the elevated amounts of PP13 in the maternal circulation. 2) There is a lower placental LGALS13 gene expression in preterm preeclampsia, which obviously cannot account for an increased placental PP13 production. 3) Thus, our data suggest that the excess syncytiotrophoblastic shedding of PP13 is probably the major source of its increased third trimester maternal serum concentrations in preterm preeclampsia and HELLP syndrome, which is similar to that observed with other brush border localized proteins, such as PLAP and PP5/TFPI-2 [27, 40].
We suggest that a constitutionally decreased LGALS13 gene expression throughout pregnancy might account for the low first trimester maternal serum PP13 concentrations in patients destined to develop early-onset/preterm preeclampsia [22–25]; however, PP13 concentrations increase parallel with the augmented shedding of STBMs in these subset of patients in the third trimester [18].
CONCLUSIONS
Our combined approach has shed new light on the behavior of PP13 in preeclampsia and HELLP syndrome at the onset of clinical symptoms. Essential differences in placental histopathology, PP13 expression, and apical membrane shedding of PP13 in preterm preeclampsia and HELLP syndrome have been demonstrated, and the latter might contribute to the increased maternal serum PP13 concentrations in these cases. Our findings may reflect a different mechanism of disease between term and preterm preeclampsia.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Zsolt Csapo and Dr. Katalin Hertelendy for their support in the Histopathological and Chemistry Laboratories; to Dr. Timea Kovats, Dr. Julia Dienes, Dr. Maria Lengyel, Katalin Karaszi, Krisztina Mekli and Istvan Szabo for their helpful technical assistance; to Julia Olah, Katalin Lang, Katalin Raum and all the colleagues in the laboratories, operating theatres and Labor and Delivery Unit of the 1st Department of Obstetrics and Gynecology for their help with the specimens. The authors thank Dr. Offer Erez, Dr. Chong Jai Kim, Dr. Derek Wildman and Sara Tipton for their critical reading of the manuscript and valuable advices.
The Fluorchem SP CCD imaging system was a generous donation of the Hungarian Terry Fox Foundation to the First Department of Obstetrics and Gynecology. N.G.T. is grateful to the Hungarian Academy of Sciences for the János Bolyai Scholarship.
Funding This research was funded by the Hungarian Országos Tudományos Kutatási Alapprogramok (T/046473 to N.G.T.) and by grants from the European Union (FP6, “Pregenesys - 037244” to H.M. and N.G.T.) and the Israel Chief Scientist (31851, 37324, 14128 to H.M.).
Footnotes
Conflict of interest statement We declare that we have no conflict of interest.
Reference List
- 1.Myatt L. Role of placenta in preeclampsia. Endocrine. 2002;19:103–111. doi: 10.1385/ENDO:19:1:103. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy--a review. Placenta. 2005;26(Suppl A):S31–S36. doi: 10.1016/j.placenta.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Espinoza J, Romero R, Mee KY, et al. Normal and abnormal transformation of the spiral arteries during pregnancy. J Perinat Med. 2006;34:447–458. doi: 10.1515/JPM.2006.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torry DS, Wang HS, Wang TH, et al. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Taylor RN, Musci TJ, et al. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1989;161:1200–1204. doi: 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- 10.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 11.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185:792–797. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 12.Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myatt L, Miodovnik M. Prediction of preeclampsia. Semin Perinatol. 1999;23:45–57. doi: 10.1016/s0146-0005(99)80059-7. [DOI] [PubMed] [Google Scholar]
- 14.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 15.Moldenhauer JS, Stanek J, Warshak C, et al. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 16.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 17.Sebire NJ, Goldin RD, Regan L. Term preeclampsia is associated with minimal histopathological placental features regardless of clinical severity. J Obstet Gynaecol. 2005;25:117–118. doi: 10.1080/014436105400041396. [DOI] [PubMed] [Google Scholar]
- 18.Goswami D, Tannetta DS, Magee LA, et al. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Egbor M, Ansari T, Morris N, et al. Morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG. 2006;113:580–589. doi: 10.1111/j.1471-0528.2006.00882.x. [DOI] [PubMed] [Google Scholar]
- 20.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996;175:1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 21.Zwahlen M, Gerber S, Bersinger NA. First trimester markers for pre-eclampsia: placental vs. non-placental protein serum levels. Gynecol Obstet Invest. 2007;63:15–21. doi: 10.1159/000094672. [DOI] [PubMed] [Google Scholar]
- 22.Nicolaides KH, Bindra R, Turan OM, et al. A novel approach to first-trimester screening for early pre-eclampsia combining serum PP-13 and Doppler ultrasound. Ultrasound Obstet Gynecol. 2006;27:13–17. doi: 10.1002/uog.2686. [DOI] [PubMed] [Google Scholar]
- 23.Spencer K, Cowans NJ, Chefetz I, et al. First-trimester maternal serum PP-13, PAPP-A and second-trimester uterine artery Doppler pulsatility index as markers of pre-eclampsia. Ultrasound Obstet Gynecol. 2007;29:128–134. doi: 10.1002/uog.3876. [DOI] [PubMed] [Google Scholar]
- 24.Chafetz I, Kuhnreich I, Sammar M, et al. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35–37. doi: 10.1016/j.ajog.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Kusanovic JP, Than NG, et al. First trimester maternal serum PP13 in the risk assessment for preeclampsia. Am J Obstet Gynecol. 2008;199(2):122.e1–122.e11. doi: 10.1016/j.ajog.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohn H, Kraus W, Winckler W. Purification and characterization of two new soluble placental tissue proteins (PP13 and PP17) Oncodev Biol Med. 1983;4:343–350. [PubMed] [Google Scholar]
- 27.Than GN, Bohn H, Szabo DG. Advances in pregnancy-related protein research. CRC Press; Boca Raton: 1993. [Google Scholar]
- 28.Than NG, Sumegi B, Than GN, et al. Isolation and sequence analysis of a cDNA encoding human placental tissue protein 13 (PP13), a new lysophospholipase, homologue of human eosinophil Charcot-Leyden Crystal protein. Placenta. 1999;20:703–710. doi: 10.1053/plac.1999.0436. [DOI] [PubMed] [Google Scholar]
- 29.Than NG, Pick E, Bellyei S, et al. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271:1065–1078. doi: 10.1111/j.1432-1033.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 30.Burger O, Pick E, Zwickel J, et al. Placental protein 13 (PP-13): effects on cultured trophoblasts, and its detection in human body fluids in normal and pathological pregnancies. Placenta. 2004;25:608–622. doi: 10.1016/j.placenta.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Visegrady B, Than NG, Kilar F, et al. Homology modelling and molecular dynamics studies of human placental tissue protein 13 (galectin-13) Protein Eng. 2001;14:875–880. doi: 10.1093/protein/14.11.875. [DOI] [PubMed] [Google Scholar]
- 32.Barondes SH, Castronovo V, Cooper DN, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- 33.Papp Cs, Szabo G, Toth-Pal E, et al. Fetal growth rate and its variations 1988/89. Orv Hetil. 1991;132:1865–1870. [PubMed] [Google Scholar]
- 34.ACOG ACOG practice bulletin: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. Number 33, January 2002. [DOI] [PubMed] [Google Scholar]
- 35.Barton JR, Sibai BM. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin Perinatol. 2004;31:807–33. vii. doi: 10.1016/j.clp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Khong TY. A topographical and clinical approach to examination of the placenta. Pathology. 2001;33:174–186. doi: 10.1080/00313020120038700. [DOI] [PubMed] [Google Scholar]
- 37.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 38.Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:449–476. [PubMed] [Google Scholar]
- 39.Hargitai B, Marton T, Cox PM. Best practice no 178. Examination of the human placenta. J Clin Pathol. 2004;57:785–792. doi: 10.1136/jcp.2003.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogawa M, Yanoma S, Nagashima Y, et al. Paradoxical discrepancy between the serum level and the placental intensity of PP5/TFPI-2 in preeclampsia and/or intrauterine growth restriction: possible interaction and correlation with glypican-3 hold the key. Placenta. 2007;28:224–232. doi: 10.1016/j.placenta.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 41.Paradela A, Bravo SB, Henriquez M, et al. Proteomic analysis of apical microvillous membranes of syncytiotrophoblast cells reveals a high degree of similarity with lipid rafts. J Proteome Res. 2005;4:2435–2441. doi: 10.1021/pr050308v. [DOI] [PubMed] [Google Scholar]
- 42.Jones CJ, Carter AM, Aplin JD, et al. Glycosylation at the fetomaternal interface in hemomonochorial placentae from five widely separated species of mammal: is there evidence for convergent evolution? Cells Tissues Organs. 2007;185:269–284. doi: 10.1159/000102175. [DOI] [PubMed] [Google Scholar]
- 43.Nickel W. Unconventional secretory routes: direct protein export across the plasma membrane of mammalian cells. Traffic. 2005;6:607–614. doi: 10.1111/j.1600-0854.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 44.Danielsen EM, Hansen GH. Lipid raft organization and function in brush borders of epithelial cells. Mol Membr Biol. 2006;23:71–79. doi: 10.1080/09687860500445604. [DOI] [PubMed] [Google Scholar]
- 45.Aplin JD, Straszewski-Chavez SL, Kalionis B, et al. Trophoblast differentiation: progenitor cells, fusion and migration -- a workshop report. Placenta. 2006;27(Suppl A):S141–S143. doi: 10.1016/j.placenta.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 46.Knerr I, Beinder E, Rascher W. Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am J Obstet Gynecol. 2002;186:210–213. doi: 10.1067/mob.2002.119636. [DOI] [PubMed] [Google Scholar]
- 47.Lee X, Keith JC, Jr., Stumm N, et al. Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta. 2001;22:808–812. doi: 10.1053/plac.2001.0722. [DOI] [PubMed] [Google Scholar]
- 48.Langbein M, Strick R, Strissel PL, et al. Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol Reprod Dev. 2008;75:175–183. doi: 10.1002/mrd.20729. [DOI] [PubMed] [Google Scholar]
- 49.Benirschke K, Kaufmann P. Pathology of the human placenta. 4th Edn. Springer-Verlag; New York: 2000. [Google Scholar]
- 50.Gude NM, Roberts CT, Kalionis B, et al. Growth and function of the normal human placenta. Thromb Res. 2004;114:397–407. doi: 10.1016/j.thromres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 51.Bischof P, Irminger-Finger I. The human cytotrophoblastic cell, a mononuclear chameleon. Int J Biochem Cell Biol. 2005;37:1–16. doi: 10.1016/j.biocel.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 52.Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1:61–76. doi: 10.1016/s0143-4004(80)80016-6. [DOI] [PubMed] [Google Scholar]
- 53.de Luca Brunori I, Battini L, Brunori E, et al. Placental barrier breakage in preeclampsia: ultrastructural evidence. Eur J Obstet Gynecol Reprod Biol. 2005;118:182–189. doi: 10.1016/j.ejogrb.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 54.Crocker I. Pre-eclampsia and villous trophoblast turnover: perspectives and possibilities. Placenta. 2007;28(Suppl A):S4–13. doi: 10.1016/j.placenta.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 55.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007 doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 56.Mayhew TM, Wadrop E, Simpson RA. Proliferative versus hypertrophic growth in tissue subcompartments of human placental villi during gestation. J Anat. 1994;184(Pt 3):535–543. [PMC free article] [PubMed] [Google Scholar]
- 57.Rigo J, Jr., Nagy B, Fintor L, et al. Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutation and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy. 2000;19:163–172. doi: 10.1081/prg-100100132. [DOI] [PubMed] [Google Scholar]
- 58.Lachmeijer AM, Arngrimsson R, Bastiaans EJ, et al. A genome-wide scan for preeclampsia in the Netherlands. Eur J Hum Genet. 2001;9:758–764. doi: 10.1038/sj.ejhg.5200706. [DOI] [PubMed] [Google Scholar]
- 59.van Dijk M, Mulders J, Poutsma A, Könst AA, et al. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37:514–519. doi: 10.1038/ng1541. [DOI] [PubMed] [Google Scholar]
- 60.Sziller I, Babula O, Hupuczi P, et al. Mannose-binding lectin (MBL) codon 54 gene polymorphism protects against development of pre-eclampsia, HELLP syndrome and pre-eclampsia-associated intrauterine growth restriction. Mol Hum Reprod. 2007;13:281–285. doi: 10.1093/molehr/gam003. [DOI] [PubMed] [Google Scholar]
- 61.Oudejans CB, van DM, Oosterkamp M, et al. Genetics of preeclampsia: paradigm shifts. Hum Genet. 2007;120:607–612. doi: 10.1007/s00439-006-0259-1. [DOI] [PubMed] [Google Scholar]
- 62.Cross JC. The genetics of pre-eclampsia: a feto-placental or maternal problem? Clin Genet. 2003;64:96–103. doi: 10.1034/j.1399-0004.2003.00127.x. [DOI] [PubMed] [Google Scholar]