Abstract
Human prostate cancer LNCaP cells including C-33 and C-81 cells were originally derived from the lymph nodes of a patient with metastatic prostate cancer. These two cells were employed for characterization of L-selectin ligand and in vitro tumorigenicity, because they mimic the clinical conditions of early and late-stage human prostate cancer. C-81 cells exhibit higher in vitro migratory and invasive properties as compared with C-33 cells. We find that the L-selectin ligand and mucin glycan-associated MECA-79 epitope were elevated in C-81 cells. An increase of these glycotopes positively correlates with elevated tumorigenicity and expression of key glycosyl- and sulfotransferase genes. These results suggest that modulated expression of selective glycogenes correlates with altered tumorigenicity of cancer cells.
Keywords: LNCaP cells, MECA-79, L-selectin ligand, Glycosyltransferases, Sulfotransferase
Introduction
Prostate cancer is the most common solid tumor in men in the US and Western Europe, and the second most common cause of cancer-related death in men over age 55 [1]. Metastasis to the brain, bone and lymph nodes is the main cause of prostate cancer death [2]. However, the molecular mechanism by which the late-stage prostate cancer cells metastasize to the distant sites is not completely understood. One possible mechanism involves interactions of P-selectin on platelets and L-selectin on leukocytes with their respective ligands on hematogeneously disseminating tumor cells to form microemboli to facilitate their arrest in the vasculature of their target sites [3]. Such interactions are similar to the mechanisms employed by leukocytes for migration to the sites of tissue injury and lymphoid tissues [4]. These processes rely on interactions between P-selectin on the endothelial cells and sialyl Lewis x (sLex) on mucin glycan and sulfated tyrosine located at the N-terminal region of P-selectin glycoprotein ligand-1 on the leukocytes, and between L-selectin on the surface of leukocytes and the glycotopes that contain MECA-79 antigen in the high endothelial venues [5–7], respectively. Altered glycosylation, which leads to increased production of sLex, has been observed in advanced cancers [8], including prostate cancer [9]. Since sLex is an essential component of the selectin ligands, elevation of sLex coupled with expression of GlcNAc 6-sulfotransferase could enable the cancer cells to utilize the normal leukocyte migration mechanisms to metastasize [10–13].
In this communication, we provide evidence that supports the hypothesis that expression of L-selectin ligand on LNCaP cells plays a key role in leukocyte-mediated metastasis to the lymph nodes. To achieve this goal, we employed a LNCaP cell progression model, including C-33 and C-81 LNCaP cells [14, 15]. The properties exhibited by these two cell clones mimic the early and the late stages of human prostate cancer in the clinic. Thus, this is a useful in vitro cell model for studying the mechanisms of clinical progression of prostate cancer. As compared with C-33 cells, C-81 cells have higher proliferation rate, tumorigenicity, and androgen-independent secretion of prostate-specific antigen. In this study, we show that as compared to C-33 cells, C-81 cells exhibit a greater degree of in vitro migratory and invasive properties, elevated expression of L-selectin ligand, and increased expression of key glycogenes involved in the synthesis of this ligand. Thus, modulation of selective glycogenes in cancer cells could modify the selectin ligands to alter the metastatic potential of these cells. This property resembles the known tumorigenic trend of these two cell clones.
Materials and methods
Cell lines
The human prostate cancer LNCaP cell line was originally purchased from the American Type Culture Collection ATCC (Rockville, MD). The C-33 and C-81 LNCaP cell model used in the present study was previously developed [15] and characterized [14]. C-33 cells include cells between passages 25 and 35, and C-81 cells include cells between passages 81 and 125. These cells were maintained at 37°C under a 5% CO2 and water saturated environment in RPMI 1640 supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 units/ml Penicillin and 100 μg/ml Streptomycin.
Motility and invasion assays
Motility assay of C-33 and C-81 cells was performed using 24-well plate insert chambers. Briefly, 5 × 104 cells suspended in 1 ml medium were plated on the top chamber of noncoated polyethylene terapthalate (8 μm pore size, Becton Dickinson, Bedford, MA). After incubation at 37° C for 24 h in a 5% CO2 and water saturated environment, the cells that did not migrate through the pores were removed by scraping with a cotton swab. Cells that transversed the membranes were stained with a Diff-Quick cell staining kit (Dade Behring, Inc., Newark, DE). Cells at three random fields per insert were counted with a phase contrast microscope at 40× magnification and expressed as an average number of cells per field of view. Three independent inserts were analyzed in each case. The data were expressed as mean±SD. Invasion experiment of C-33 and C-81 cells was carried out using 24-well BD BioCoat™ Matrigel™ invasion chambers (8 μm pore size; Becton Dickinson). Briefly, 5 × 104 cells suspended in 1 ml medium were seeded on top of the rehydrated Matrigel insert membrane. The cells were incubated for 22 h as described above and then the cells that did not migrate through the pores were removed by scraping with a cotton swab. Cells that transversed the membranes were stained with a Diff-Quick cell staining kit (Dade Behring, Inc., Newark, DE) and counted as described above.
Western blot analysis of MECA-79 epitope and C1β3GalT-1
Whole cell lysates of C-33 and C-81 cells were used for detection of MECA-79 epitope and C1β3GalT-1 enzyme. Aliquots of the whole cell lysates were boiled in a SDS buffer and resolved by SDS-Polyacrylamide gel electrophoresis (PAGE) on 8–10% gels, and blotted onto Immobilon-P membranes (0.45 μm pore size, Millipore, Bedford, MA). Membranes were blocked with 5% bovine serum albumin (BSA) in TBS/Tween for 1 h at RT, and treated at 4°C overnight with 3% BSA in TBS/Tween containing biotinylated rat anti-mouse MECA-79 epitope monoclonal antibody (Becton Dickinson, Biosciences; 2.5 μg/ml) or mouse polyclonal C1β3GalT-1 antibody (Abnova Corporation, Taipei, Taiwan; 0.01 μg/ml). After washing with TBS/Tween three times at 5 min each, these membranes were treated with HRP conjugated streptavidin (Zymed laboratories, San Francisco, CA; 1:1,000) and HRP conjugated goat anti-mouse antibodies (Santa Cruz Biotechnology, Inc, San Diego, CA; 1:2,000 dilution), respectively. The bound HRP was treated with the enhanced Chemiluminescent substrate, SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), and then detected according to the manufacturer's instructions.
Enzymatic characterization of the properties of MECA-79 epitope
Aliquots of cell lysates were digested with O-sialoglycoprotein endopeptidase (OSGP) (Cedarlane, Ontario, Canada) according to the manufacturer's instructions. Briefly, whole cell lysates were incubated with OSGP (50 μl/50 μg protein) at 37°C for 18 h. The cell lysates so treated were resolved on 8–10% SDS-PAGE and blotted onto Immobilon-P membranes. These membranes were treated with MECA-79 antibody and then analyzed as described above.
Flow cytometry analysis of L-selectin interactions with LNCaP cells
Flow cytometry analysis of L-selectin interactions with LNCaP cells was carried out as described previously [16]. Briefly, LNCaP Cells were detached from plates by incubation with PBS containing 2 mM EDTA for 5 min at 37°C and washed three times with Hanks' balanced salt solution (HBSS). The cells were treated with 0.5% BSA in HBSS for 30 min to block nonspecific sites before exposure to L-selectin as described next. The L-selectin used was a chimera of L-selectin fused with Fc region of human IgG purified from LSRg293 Cytel cells [16] (A kind gift from Dr. Ajit Varki, University of California at San Diego, La Jolla, CA). The L-selectin (50 μg/ml) was preincubated with a goat-anti human IgG conjugated with FITC for 1 h at room temperature before incubation with tumor cells at 4°C for 2 h. After washing with HBSS/BSA and HBSS, the cells were fixed with 2% (wt./vol.) paraformaldehyde in HBSS for 5 min. Cells were washed again with HBSS and resuspended in 1 ml of HBSS/BSA for flow cytometry analysis. Controls were stained in the presence of 5 mM EDTA (calcium chelation). Cells incubated with secondary antibody alone served as antibody control.
RT-PCR analysis of the expression of glycosyltransferase and sulfotransferase genes
Total cellular RNAs were isolated from C-33 and C-81 cells using the TRI-REAGENT (Molecular Research Center, Inc, Cincinnati, OH) according to the manufacturer's protocol. For cDNA synthesis, 2 μg of total RNA was used as the template in a 20 μl RT reaction mixture by using Reverse-iT™ 1st strand synthesis kit (ABgene, UK) according to the manufacturer's instructions. The RT-PCR reaction was performed in an Eppendorf Mastercycler Personal using 1 μl of cDNA template in a total volume of 25 μl. RT-PCR of key Glyco- and Sulfotransferase genes involved in the synthesis of 6-sulfo-sLex and β-actin were carried out by using sense and anti-sense primers as shown below: C2GnT-1, 5′-CTCGAAA CACCTCTCTTTTCTGGC-3′ and 5′-GGTCAGTGTTT TAATGTCTCCAAAG-3′ (NM_001097633); β3GnT-3, 5′-TTCTTCA ACCTCACGCTCAAGCAG-3′ and 5′-AGCA TCTCATAAGGTAGGAAGCGG-3′ (NM_01425 6); β4GalT-1, 5′-AGTGACGTGGACCTCATTCC and 5′-CCGA TGTCCACTGTGATTTG-3′ (NM_001497); β4GalT-4, 5′-GCTGTTGACTTTGTGCCTGA-3′ and 5′-GCCT GGTGGATG ACGTAGAT-3′ (NM_003778); FUT7, 5′-CACCTCCGAGGCATCTTCAACTG-3′ and 5′-CGTT GGTATCGGCTCTCATTCATG-3′ (NM_004479); GlcNAc6ST-1, 5′-CGTGAGAGCC TACAGGTGGT-3′ and 5′-CATGGGCTGGTAGCAAAACT-3′ (NM_004267.3); ST3Gal-III, 5′-ACTCCAGTGGGAGGAGGACT-3′ and 5′-CGTGACCCCAGAGACTTGTT-3′ (NM_1749 63); ST3Gal-IV, 5′-CTAGCCATCACCAGCTCCTC-3′ and 5′-CCAT GAAGAAGGGG TTGAGA-3′ (NM_006278) and β-actin, 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCC TTA ATGTCACGGACGATTC-3′ (NM_001101). The PCR reaction conditions for all the genes were as follows: 94°C for 2 min (1 cycle); 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min (30 cycles); and then 72°C for 5 min. The expression of glycosyltransferase and sulfotransferase genes was normalized with the expression of β-actin gene, which remained constant in all samples. The PCR products were subject to electrophoresis (130 V, constant-voltage field) on 1% agarose gel equilibrated in Tris–borate electrophoresis buffer containing ethidium bromide (50 μg/ml). Gels were photographed under UV light.
Results
Elevated in vitro migration and invasion properties in C-81 LNCaP cells
The metastatic potential of human prostate cancer cells was analyzed by in vitro Matrigel Boyden chamber assay. As compared to C-33 LNCaP cells, the C-81 cells showed a significantly higher level of motility through polyethylene terapthalate (Fig. 1A) and a higher degree of invasion through matrigel (Fig. 1B).
Fig. 1.
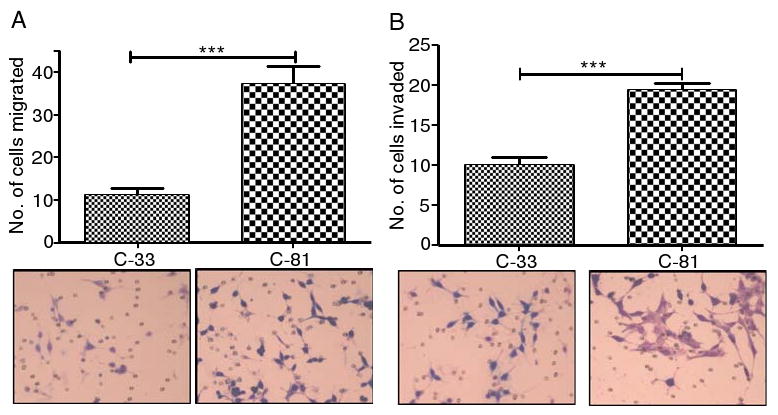
Migration and invasion analysis of LNCaP cells. C-81 and C-33 LNCaP cells of 5 × 104 cells/ml medium were inoculated in the upper chambers of 24-well migration and matrigel invasion chamber plates. After 22–24 h, the cells that transversed to bottom side of the chamber plates were fixed, stained, and then counted under a phase contrast microscope (40×) in three independent fields for each insert. C-81 cells showed highly significant level of migration (a) and invasion (b) than C-33 cells through polyethylene terapthalate membrane and matrigel invasion chambers, respectively. The data, which were expressed as mean±SD, were obtained from three independent experiments (**p<0.05 and ***p<0.001)
MECA-79 epitope is located in mucin-type glycans
The MECA-79 epitope was detected in two protein bands at 77 and 131 kDa from lysates of both C-33 and C-81 cells. The high passage LNCaP cells showed greater intensity (2.6-fold of 131 kDa and 1.8-fold of 77 kDa) than low passage LNCaP cells. The biochemical properties of MECA-79 epitopes present in C-33 and C-81 cells were characterized by digestion of cell lysates with N-glycanase, KS-II, Ch-ABC and OSGP. The MECA-79 epitope was resistant to N-glycanase, KS-II and Ch-ABC (Data not shown). However, the two protein bands carrying MECA-79 epitope were digested by OSGP, which specifically cleaves the peptide backbone of glycoproteins carrying sialylated mucin type glycans (Fig. 2) [17].
Fig. 2.
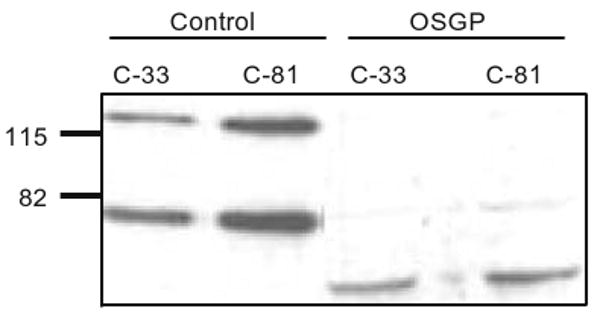
Western blot analysis and characterization of MECA-79 epitopes on C-33 and C-81 LNCaP cells. Two bands of ∼77 and 131 were recognized as MECA-79 epitopes. The high passage LNCaP cells showed more intense bands than low passage LNCaP cells. The lysates treated with OSGP showed loss of MECA-79 epitopes
L-Selectin interaction with LNCaP cells
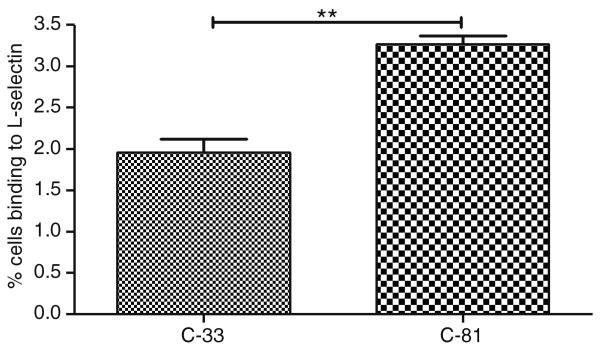
Flow cytometry analysis showed that 70% more C-81 LNCaP cells bound to L-selectin than C-33 cells (Fig. 3).
Fig. 3.
Selectin-ligand interaction on LNCaP cells. The high passage LNCaP cells showed 1.7 fold higher numbers of cells that binding to L-selectin than low passage LNCaP cells. The data represented an average of three independent experiments (**p<0.01)
Analysis of glycogenes involved in the synthesis of L-selectin ligand in LNCaP cells
To understand the difference in metastatic potential as related to L-selectin ligand expression between C-33 and C-81 LNCaP cells, a combination of western blotting and RT-PCR analysis of key glycogenes involved in the synthesis of L-selectin ligand was performed. Western blot analysis showed a 70% increase of the C1β3GalT-1 enzyme (core 1 synthase) in C-81 cells as compared to C-33 cells (Fig. 4A). RT-PCR analysis of the expression of glycosyltransferase and sulfotransferase genes involved in L-selectin ligand synthesis showed a significantly higher expression of the following glycogenes in C-81 LNCaP cells as compared to those in C-33 cells: β3GnT-3 (120%), FUT7 (20%), GlcNAc6ST-1 (50%), ST3Gal-III (30%) and ST3Gal-IV (30%) (Fig. 4B). There was no difference in the expression of C2GnT-1, β4GalT-1, β4GalT-4 and iGnT genes between these two LNCaP cells although their expression levels were relatively high in both cells.
Fig. 4.
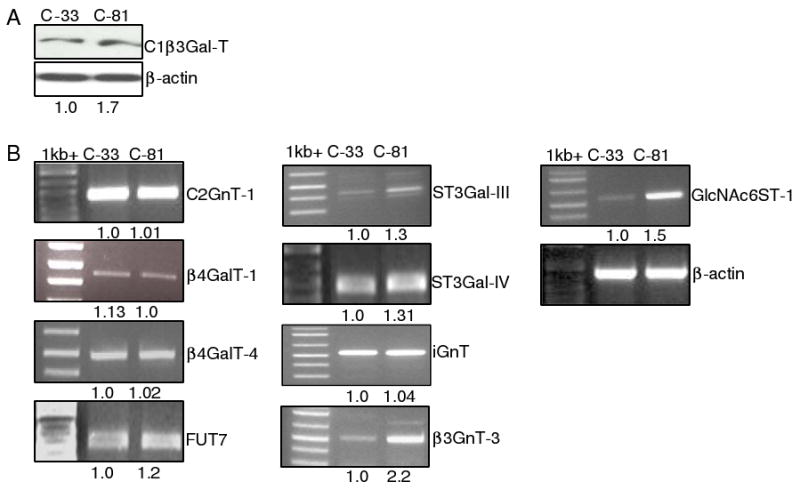
Western blotting of C1β3GalT-1 (a) and RT-PCR analysis of the expression of sulfotransfease and other glycosyltransferase genes involved in the biosynthesis of mucin-type L-selectin ligand (b). β3GnT-3, GlcNAc6ST-1, FUT7, ST3Gal-III and -IV genes were expressed higher in C-81 than C-33 LNCaP cells. There was no difference in the expression of C2GnT-1, β4galT-1, β4galT-4 and iGnT genes between these two LNCaP cells
Discussion
Cell–cell interactions involving selectins and their carbohydrate ligands present on opposite cells play a key role in a variety of biological events, such as trafficking and homing of lymphocytes to specific tissues or lymphoid organs in the body [18]. For example, the L-selectin ligand in the high endothelial venues of the lymphoid organs is responsible for the arrest of the L-selectin-carrying leukocytes that traffic through these organs. Current study demonstrates that LNCaP cells express L-selectin ligand, which may facilitate metastasis of these cells to the regional lymph nodes by hitching on the circulating leukocytes. This finding is consistent with the fact that these cells are originally derived from the lymph nodes of a patient with metastatic prostate cancer [19].
The C-33 and C-81 LNCaP cell model has been shown to mimic the clinical progression of prostate cancer metastasis [14, 15] and has been widely used for studying the mechanisms of prostate cancer progression [20–24]. We find that C-81 cells express more L-selectin ligand and exhibit higher levels of in vitro migratory and invasive properties as compared with C-33 cells. The results indicate that increased expression of L-selectin ligand correlates with elevated metastatic potential of LNCaP cells. Although elevation of L-selectin ligand can be used to explain the increased in vivo tumorigenicity, it is not clear how this property can be used to explain the enhanced in vitro metastatic potential of LNCaP cells. One possible explanation is that this property has nothing to do with the in vitro tumorigenicity, i.e. the observation is simply a co-incidental phenomenon. However, we can not exclude the possibility that this L-selectin ligand may facilitate in vitro migration of LNCaP cells because these cells express not only L-selectin ligand but also L-selectin (Radhakrishnan, unpublished observation).
6-sulfo-sLex is the L-selectin ligand, which also can be recognized by MECA-79 antibody. This antibody is specific for mucin core 1-associated 6-sulfo-N-acetyllactosamine [Galβ1–4(6-sulfo)GlcNAcβ1–3Galβ1–3GalNAcα] [25] with or without further modifications, which include α2–3 sialylation of β1-4Gal, α1–3 fucosylation of GlcNAc, and/or C6 sulfation of β1-3Gal [6, 7]. MECA-79 antibody specifically binds to luminal surface of HEV and inhibits binding of lymphocytes to HEVs in vivo and in vitro [26]. MECA-79 epitope can be found in mucin-type [17] and N-linked [27] glycans. But, they are present only in mucin-type glycans in LNCaP cells (Fig. 2).
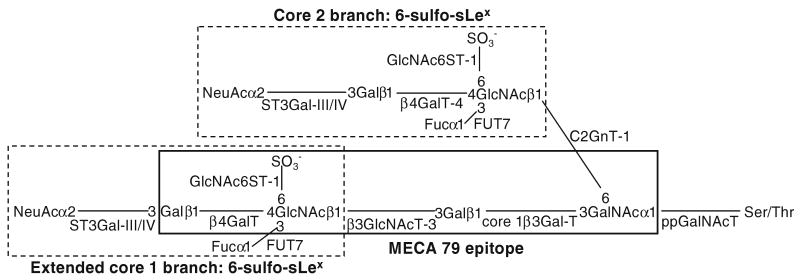
Mucin glycan-associated 6-sulfo-sLex can be found on extended core 1 and core 2 branch as shown in Fig. 5. Synthesis of this epitope involves several glycosyl- and sulfo-transferases working in a sequential ordered manner [25]. Mucin glycan synthesis is initiated by the formation of GalNAcαSer/Thr as catalyzed by peptidyl GalNAc transferases, which is followed by the formation of core 1 [Galβ1–3GalNAc] as catalyzed by core 1 synthase (C1 β3GalT-1). After the formation of core 2 [GlcNAcβ6 (Galβ1–3)GalNAcα1R] and/or extended core 1 [GlcNAcβ1–3Galβ1–3GalNAcα1R], GlcNAc 6-sulfate is generated before β1–4 galactosylation of GlcNAc. This enzymatic step is followed by sialylation and then fucosylation to complete the synthesis of the L-selectin ligand. L-selectin ligand can be located at core 1 and core 2, but only core 1-associated L-selectin ligand can be recognized by MECA-79 antibody [25]. The increase in MECA-79 epitope and L-selectin ligand in C-81 LNCaP cells as compared to C-33 cells can be explained by upregulation of the expression of several glycogenes involved in the synthesis of these glycotopes. C-81 cells express 70% more C1 β3GalT-1 than C-33 cells. Since core 1 is the obligatory precursor for core 2 [28], more core 1 may lead to the synthesis of more core 2 branch. In addition, C81 cells express 120% more core 1 extension enzyme (β3GnT-3), which may lead to the production of more extended core 1 structure. Furthermore, C-81 cells express more sulfotransferase (GlcNAc6ST-1), sialyltransferases (ST3Gal-III and -IV) and fucosyltransferase (FUT7). The expression pattern of these glycosyltransferases positively correlates with higher level expression of MECA-79 epitope and L-selectin ligand on C-81 cells than in C-33 cells. Furthermore, it is of interest to note that alteration of the expression of selective glycosyltransferase and sulfo-transferase genes is correlated with the metastatic potential of LNCaP cells, suggesting the important role played by these glycogenes in carcinogenesis. The role of each of these glycogenes plays in this process will be examined in vivo in an animal model. Further, some of these genes may serve as prognostic markers for cancer progression. Modulation of the expression of these genes may be used as a potential therapeutic approach to suppress cancer metastasis.
Fig. 5.
Schematic diagram of the biosynthetic pathway of 6-sulfo-sLex located on biantennary mucin glycans. Key glycosyltransferases and sulfotransferase involved in the biosynthesis of 6-sulfo-sLex on extended core 1 and core 2 branch structures are shown. [ppGalNAcTF, peptidyl N-acetylgalactosaminyltransferase; β3Gal-T1, β3-Galactosyltransferase-1; C2GnT-1, β1-6N-acetlyglucosaminyltransferase-1; GlcNAc6ST1, N-acetlyglucosaminyl-6-O-sulfotransferase-1; β4GalT-4, β4-Galactosyltransferase-4; FUT-7, α1–3 Fucosyltransferase-7; ST3Gal-III/IV, α2–3 sialyltransferase-III/IV; β3GlcNAcT-3, β3-N-acetlyglucosaminyltransferase-3; β4GalT, β4-galactosyltransferase]
Acknowledgments
We wish to acknowledge the research support of the State of Nebraska-NRI cancer glycobiology program, LB595, and LB506 (PWC), the Department of Defense Postdoctoral Fellowship (PR), and the NIH NCI CCSG P30CA036727-supported molecular biology core facilities. We also thank Ms. Helen Cheng for the expert technical support in cell culture, and Dr. Ajit Varki for providing 293 cells stably transfected with human L-selectin fused with Fc region of human IgG.
Contributor Information
Prakash Radhakrishnan, Department of Biochemistry and Molecular Biology, College of Medicine, 985870, Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198, USA.
Ming-Fong Lin, Department of Biochemistry and Molecular Biology, College of Medicine, 985870, Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198, USA, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE, USA.
Pi-Wan Cheng, Department of Biochemistry and Molecular Biology, College of Medicine, 985870, Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198, USA, pcheng@unmc.edu, Department of Pharmaceutical Sciences, College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, USA, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE, USA.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 3.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: Mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA. 2001;98:3352–3357. doi: 10.1073/pnas.061615598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pratt MR, Bertozzi CR. Syntheses of 6-sulfo sialyl lewis X glycans corresponding to the L-selectin ligand “sulfoadhesin”. Org Lett. 2004;6:2345–2348. doi: 10.1021/ol0493195. [DOI] [PubMed] [Google Scholar]
- 7.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohyama C, Tsuboi S, Fukuda M. Dual roles of sialyl lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999;18:1516–1525. doi: 10.1093/emboj/18.6.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martensson S, Bigler SA, Brown M, Lange PH, Brawer MK, Hakomori S. Sialyl-lewis(x) and related carbohydrate antigens in the prostate. Hum Pathol. 1995;26:735–739. doi: 10.1016/0046-8177(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 10.Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997;14:577–584. doi: 10.1023/A:1018532409041. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi A, Ding K, Maehara M, Goi T, Nakagawara G. Expression of nm23-H1 gene and sialyl lewis X antigen in breast cancer. Oncology. 1998;55:357–362. doi: 10.1159/000011878. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Nakayama J, Ohyama C, Suzuki M, Suzuki A, Fukuda M, et al. Sialyl lewis X-dependent lung colonization of B16 melanoma cells through a selectin-like endothelial receptor distinct from E- or P-selectin. Cancer Res. 2002;62:4194–4198. [PubMed] [Google Scholar]
- 13.McEver RP. Selectin–carbohydrate interactions during inflammation and metastasis. Glycoconj J. 1997;14:585–591. doi: 10.1023/A:1018584425879. [DOI] [PubMed] [Google Scholar]
- 14.Igawa T, Lin FF, Lee MS, Karan D, Batra SK, Lin MF. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- 15.Lin MF, Lee MS, Zhou XW, Andressen JC, Meng TC, Johansson SL, et al. Decreased expression of cellular prostatic acid phosphatase increases tumorigenicity of human prostate cancer cells. J Urol. 2001;166:1943–1950. doi: 10.1016/S0022-5347(05)65725-4. [DOI] [PubMed] [Google Scholar]
- 16.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA. 2002;99:2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puri KD, Finger EB, Gaudernack G, Springer TA, Sialomucin CD. 34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, et al. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 20.Li Y, Koeneman KS. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Urol Oncol. 2008;26:106–107. doi: 10.1016/j.urolonc.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun A, Tang J, Hong Y, Song J, Terranova PF, Thrasher JB, et al. Androgen receptor-dependent regulation of bcl-xL expression: Implication in prostate cancer progression. Prostate. 2008;68:453–461. doi: 10.1002/pros.20723. [DOI] [PubMed] [Google Scholar]
- 22.Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- 23.Veeramani S, Yuan TC, Chen SJ, Lin FF, Petersen JE, Shaheduzzaman S, et al. Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen-independent proliferation of prostate cancer. Endocr Relat Cancer. 2005;12:805–822. doi: 10.1677/erc.1.00950. [DOI] [PubMed] [Google Scholar]
- 24.Stewart LV, Lyles B, Lin MF, Weigel NL. Vitamin D receptor agonists induce prostatic acid phosphatase to reduce cell growth and HER-2 signaling in LNCaP-derived human prostate cancer cells. J Steroid Biochem Mol Biol. 2005;97:37–46. doi: 10.1016/j.jsbmb.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 26.Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, Yu SY, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- 28.Williams D, Longmore G, Matta KL, Schachter H. Mucin synthesis. II. Substrate specificity and product identification studies on canine submaxillary gland UDP-GlcNAc:Galβ1–3GalNAc (GlcNAc-GalNAc)β6-N-acetylglucosaminyltransferase. J Biol Chem. 1980;255:11253–11261. [PubMed] [Google Scholar]