To the Editor
Dogs, Canis familiaris, share more than 200 genetic disease phenotypes with humans [Patterson et al., 1982; Patterson, 2000]. Genes for specific diseases are often concentrated in purebred dogs (genetic isolates) due to founder effects, inbreeding and/or frequent use of “popular sires.” As a result, recessive, interactive, or polygenic modes of inheritance are more readily investigated, leading to identification of Quantitative Trait Loci (QTLs) [Chase et al., 2002, 2004]. The recent completion of the sequence for the canine genome has facilitated the comparison of the dog genome with human and other mammalian genomes, allowing the further investigative analysis of canine QTLs in other mammalian systems, notably the human and mouse. Here we examine changes that involve pathological remodeling of bone, altered joint conformation, and osteophyte formation, visible radiographically as osteoarthritis (OA) [Olsson, 1971; Riser, 1973]. We have related such changes in the coxofemoral (hip) joint of Portuguese Water Dogs (PWDs) to genotypes defined by SSR markers and associated them with a specific region (haplotype) of the canine genome (QTL).
Radiographs and blood for DNA were collected from 431 PWDs through the Georgie Project [http://www.georgieproject.com, Karen Miller director; Chase et al., 2002]. The dogs, ranging in age from 1.7 to 17 years (median age, 6 years), included 171 males and 260 females and represented a cross-section of the entire PWD population in the USA. They trace their ancestry to 31 founders through ca. 25 generations and consanguinities range from 0 to 0.6 with a mean of 0.2 [Chase et al., 1999]. We have associated marker alleles with a few infrequently used founders using the consanguinity between that founder and all dogs known to carry the allele. Permutation tests are used to establish the significance of each association [Alroy et al., 2000].
DNA was isolated from blood and characterized by PCR amplification and electrophoretic identification of the alleles of simple sequence repeat genetic markers [Francisco et al., 1996; Mellersh et al., 2000; Chase et al., 2002, 2004].
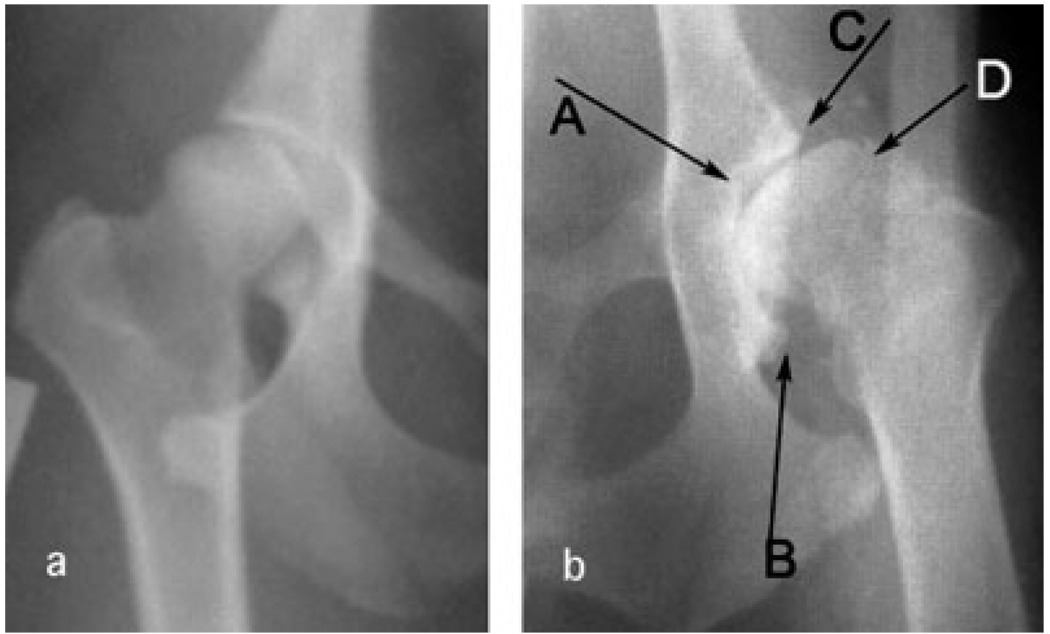
Osteoarthritis (OA) was scored from ventrodorsal radiographs of the pelvis as illustrated in Figure 2 of Chase et al. [2002]. In all, 431 dogs were scored for subchondral sclerosis of the cranial acetabular margin, osteophytes of the caudal and cranial acetabular margins, and femoral head osteophytes (illustrated in Fig. 1). The severity of each of these phenotypes was scored on an ordinal scale from 0 (none) to 3 (most severe). Left and right hips were scored independently. Scores ranged from 0 to 22 out of a possible maximum of 24 (12 left + 12 right). Fifty percent of the dogs had a score greater than 0.
Fig. 1.
Examples of (a) no osteoarthritis (score = 0) and (b) severe osteoarthritis (score = 11). A: Subchondral sclerosis of the cranial acetabular margin; (B and C) osteophytes of the caudal and cranial acetabular margins respectively; (D) femoral head osteophytes.
Methods for estimating heritability (h2), identifying the QTL and estimating its effect on the variation of specific phenotypes (R2) have been described previously [Chase et al., 2004]. Estimation of QTL significance used permutations, as described in Chase et al. [2002].
In the PWD population OA is heritable (h2 = 30%). About half of the population show some degree of OA. OA is significantly correlated with the Norberg Angle (an indicator of joint laxity). However, whereas the Norberg Angle is significantly greater for the right than the left hip, there is no significant difference in the OA scores between the right and left joints. OA also is significantly correlated with the 4th Principal Component (PC) defined by variation in the skeletal metrics of the pelvis and limb bones. There is no significant correlation with other PCs. We had identified two QTLs on autosome 1 (CFA 1) associated with the Norberg Angle [Chase et al., 2004] and several QTLs associated with PC4 (unpublished data). In addition, we have identified two QTLs related to autoimmune Addison’s disease (unpublished data). We analyzed these QTLs (11 in all) for association with OA. One QTL identified by the SSR marker, FH2320 on CFA 3 and associated with PC4, also was significantly associated with OA (P ≤ 0.002, corrected for pedigree effects and number of QTLs tested [Chase et al., 2002]). Table I presents the relevant region of the canine genome and its syntenic counterparts on the human and mouse genomes.
TABLE 1.
Map Location and Synteny of the Canine Genome Region Associated With Osteoarthritis
| Marker FH2320 on CFA 3: 44,880,174–44,880,416 | |
|---|---|
| 4 Mb Haplotype synteny: | |
| Canine chr 3 | 42,880,174–46,880,416 |
| Mouse chr 7 | 51,790,661–55,790,661 |
| Human chr 15 | 76,830,782–80,830,782 |
We have characterized the effects of this QTL in greater detail. It accounts for about 16% of the OA variation (R2 = 16.4%), and involves primarily cranial and caudal acetabular marginal osteophytes. This same QTL affects PC4 through its effect on the ischial tuberosity, the width of which is segregating in the PWD population (R2 = 7.5% for the trait residual after removing the effects of PCs 1, 2, and 3). This QTL also affects several other bone metric residuals including the length of the 2nd vertebrae above the sacrum (R2 = 9.6%), the inner diameter of the radius (R2 = 6.7%), lever arms on the limb bones, e.g., the calcaneus (R2 = 6.7%) and inlever (R2 = 5.3%) two metrics of the heel, as well as the pisiform or wrist (R2 = 5.6%).
In the Water Dog, OA is associated with a rare haplotype of this QTL. This haplotype is defined by a FH2320 allele that is a relatively small contributor to the existing gene pool. Importantly, this haplotype is associated with average values of the ischial tuberosity and not with either extreme. Moreover, the association of the QTL with metrics of other pelvic bones is not driven by the OA haplotype. Thus, the regulation of OA by this QTL does not segregate with pelvic shape and the OA phenotype cannot be due to differences in the shape of the pelvis.
A more acceptable working hypothesis may be that the QTL regulates normal skeletal growth of certain bones (among others the ischial tuberosity) and that this particular OA haplotype contains a gene that has a high propensity toward promoting abnormal growth in joints. A survey of syntenic regions of the Mouse, Rat, and Human genomes, suggests two possible candidate genes close to the FH2320 marker sequence: IGF1R, an IGF1 receptor [Abbott et al., 1992]; and DMN, a cytoskeletal protein conferring resistance to mechanical stress [Mizuno et al., 2001].
We have used simulations to set limits on the extent to which our database can be used to refine the haplotype region surrounding any marker. This region is large, 4 Mb. However, an advantage of the canine system is that OA, regulated by this QTL, may be segregating in other breeds. If that is the case, it will be possible to reduce the haplotype by comparing linkage disequilibrium among breeds, an approach used successfully for shrinking the region of the canine genome containing the gene for Collie Eye Anomaly [Ostrander, personal communication].
ACKNOWLEDGMENTS
We thank Makiko Uemura, Kerry Matz, and Norma Bartlett for technical assistance. Radiographs and blood for DNA were collected from owners contacted by Deborah Broughton and identified through the Georgie Project, Karen Miller, Director. This research was supported by gifts from the Judith Chiara Family Trust and from more than 100 PWD owners.
Grant sponsor: NIH; Grant number: GM 63056; Grant sponsor: NSF; Grant number: IBN-0212141.
Contributor Information
Kevin Chase, Department of Biology, University of Utah, Utah.
David R. Carrier, Department of Biology, University of Utah, Utah
Karl G. Lark, Department of Biology, University of Utah, Utah
Dennis F. Lawler, Pet Products Research and Development, Nestle Purina Co., Missouri
REFERENCES
- Abbott AM, Bueno R, Pedrini MT, Murray JM, Smith RJ. Insulin-like growth factor I receptor gene structure. J Biol Chem. 1992;267:10759–10763. [PubMed] [Google Scholar]
- Alroy J, Rush JE, Freeman L, Amarendhra Kumar MS, Karuri A, Chase K, Sarkar S. Inherited infantile dilated cardiomyopathy in dogs: Genetic, clinical, biochemical, and morphologic findings. Am J Med Genet. 2000;95:57–66. [PubMed] [Google Scholar]
- Chase K, Adler FR, Miller-Stebbings K, Lark KG. Teaching a new dog old tricks: Identifying quantitative trait loci [in dogs] using lessons from plants. J Hered. 1999;90:43–51. doi: 10.1093/jhered/90.1.43. [DOI] [PubMed] [Google Scholar]
- Chase K, Carrier D, Adler FR, Jarvik T, Ostrander EA, Lorentzen TD, Lark KG. Genetic basis for systems of skeletal quantitative traits: Principal component analysis of the canid skeleton. Proc Natl Acad Sci USA. 2002;99:9930–9935. doi: 10.1073/pnas.152333099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase K, Lawler DF, Adler FR, Ostrander EA, Lark KG. Bilaterally asymmetric effects of Quantitative Trait Loci (QTLs): QTLs that affect laxity in the right vs. left coxofemoral (hip) joints of the dog (Canis familiaris) Am J Med Genet. 2004;124A:239–247. doi: 10.1002/ajmg.a.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LV, Langston AA, Mellersh CS, Neal CL, Ostrander EA. A class of highly polymorphic tetranucleotide repeats for canine genetic mapping. Mamm Genome. 1996;7:359–362. doi: 10.1007/s003359900104. [DOI] [PubMed] [Google Scholar]
- Mellersh CS, Hitte C, Richman M, Vignaux F, Priat C, Jouquand S, Werner P, Andre C, DeRose S, Patterson DF, Ostrander EA, Galibert F. An integrated linkage-radiation hybrid map of the canine genome. Mamm Genome. 2000;11:120–130. doi: 10.1007/s003350010024. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Puca AA, O’Brien KF, Beggs AH, Kunkel LM. Genomic organization and single-nucleotide polymorphism map of desmuslin, a novel intermediate filament protein on chromosome 15q26.3. BMC Genet. 2001;2:8. doi: 10.1186/1471-2156-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson S-E. DJD (osteoarthrosis): A review with special reference to the dog. J Small Animal Practice. 1971;12:333–342. doi: 10.1111/j.1748-5827.1971.tb06238.x. [DOI] [PubMed] [Google Scholar]
- Patterson DF. Canine Genetic Disease Information System: A Computerized Knowledgebase of Genetic Diseases in Dogs. St. Louis, MO: Mosby-Harcourt; 2000. [Google Scholar]
- Patterson DF, Haskins ME, Jezyk PF. Models of human genetic disease in domestic animals. Adv. Hum. Genet. 1982;12:263–339. doi: 10.1007/978-1-4615-8315-8_4. [DOI] [PubMed] [Google Scholar]
- Riser WH. The dysplastic hip joint: Its radiographic and histologic development. J Am Vet Radiol Soc. 1973;14:35–50. [Google Scholar]