Abstract
The liver plays a central role in the transformation and degradation of endogenous and exogenous chemicals, and in the removal of unwanted cells such as damaged, genetically mutated or virus-infected cells. Because of this function, the liver is susceptible to toxicity caused by the products generated during these natural occurrences. Hepatocyte death is the major feature of liver injury. In response to liver injury specific intracellular processes are initiated to maintain the liver integrity. Inflammatory cytokines including tumour necrosis factor (TNF)α and interleukin-6 (IL-6) are key mediators of these processes as they can activate different cellular response such as proliferation, survival and death. TNFα induces specific signalling pathways in hepatocytes leading to the activation of either pro-survival mediators or effectors of cell death. While the activation of transcription factor NF-κB promotes survival, the induction of c-Jun N-terminal kinases (JNKs) and caspases represent the strategic effectors of cell death in the TNFα-mediated signalling pathway. This review summarizes recent advances in the mechanisms of TNFα-induced hepatotoxicity, suggesting that NF-κB plays a protective activity against JNK-induced hepatocyte death. The identification of the mechanisms regulating the interplay between the NF-κB and JNK pathways are required to identify novel targets for the treatment of liver disease, including hepatitis and hepatocellular carcinoma.
Keywords: Gadd45{beta}, Hepatocellular carcinoma, Liver injury, Liver regeneration, Programmed Cell Death, TNF{alpha}
Introduction
The liver is the principal “residence of metabolic clearing” for exogenous chemicals (i.e. drugs) and endogenous chemicals such as cholesterol, steroid hormones, fatty acids, and proteins that would otherwise harm the body (Parkinson, 2001; Ioannides, 2008). The liver turns both exogenous and endogenous chemicals into products that can be eliminated through the bile or urine. The inimitable job of the liver in the transformation and clearance of these substances also makes it susceptible to their toxic effects. Indeed, a number of these disposals can cause severe liver injury with consequent loss of liver function, ultimately affecting the body in many ways with important implications to the health of the entire organism (Antoine et al., 2008).
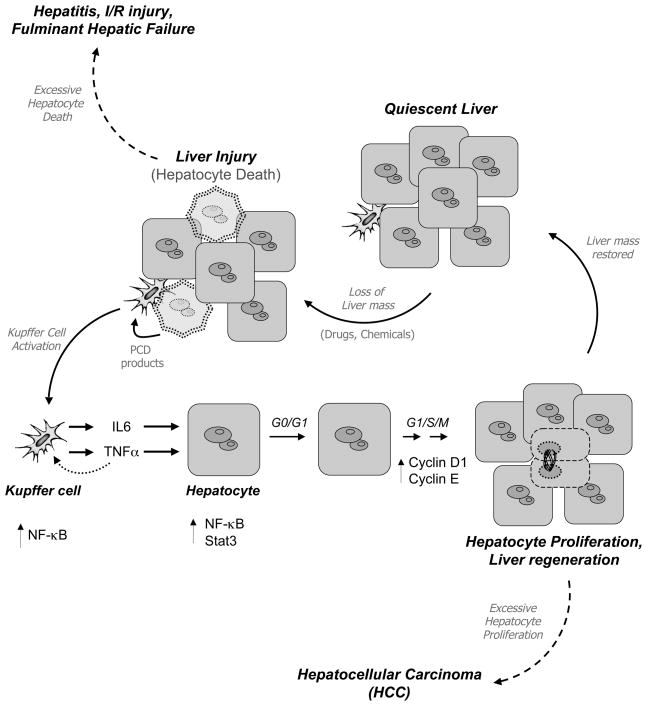
A common consequence of the livery injury is the death of hepatocytes, the major parenchyma cells that constitute this organ (Mahli and Gores, 2008). Excessive hepatocyte death has been implicated in a wide range of liver disease, including viral and alcoholic hepatitis, ischemia/reperfusion (I/R) liver injury, and fulminant hepatic failure (Figure 1) (Guicciardi and Gores, 2005). Hepatocytes, however, are well equipped to face most challenges and possess an extraordinary ability to counteract the damaging effects of cytotoxic agents. This involves the activation of specific programmes of gene expression that are capable of antagonising the programmed cell death (PCD) pathways activated by many agents, resulting in hepatocyte survival and liver integrity (Wullaert et al., 2007; Sun and Karin 2008). When these programmes fail, continued hepatocyte death can cause compensatory hepatocyte proliferation aimed at maintaining the overall liver mass, an event that promotes the development and progression of hepatocellular carcinoma (HCC) (Figure 1) (Karin, 2006). Indeed, it is becoming increasingly clear that when the balance between cell survival and cell death in the liver is tipped towards one or the other outcome, the road to liver disease is wide open (Wullaert et al., 2007). Thus, a key question is how hepatocytes regulate the balance between survival and death to ensure the tissue homeostasis.
Figure 1. Hepatocytes are well equipped to maintain liver integrity.
When the liver receives an insult, hepatocytes activate specific intracellular programmes to induce PCD and proliferation. NF-κB plays an essential role in preventing TNFα-mediated hepatocyte death. In addition, NF-κB regulates important events that induce hepatocyte proliferation. Deregulation of these signalling pathways contribute to liver disease. Excessive hepatocyte death is associated with hepatitis and liver failure. Induction of hepatocyte PCD promotes also compensatory proliferation, which can lead to the development of HCC.
An important role in the prevention of hepatocyte death is exercised by NF-κB transcription factors, which upon activation can protect hepatocytes from apoptosis induced by tumour necrosis factor (TNF)α and other death-inducing signals through the expression and activation of critical genes encoding anti-apoptotic proteins (Dutta et al., 2006; Wullaert et al., 2007). Mice deficient in components of NF-κB pathway display exacerbated hepatocyte apoptosis following TNFα stimulation both during embryonic development and in adult (mice) (Beg and Baltimore, 1996; Wullaert et al., 2007). It was shown, however, that many of these anti-apoptotic proteins can only partially protect hepatocytes from death induced by TNFα when over expressed in NF-κB-deficient cells, indicating the existence of other NF-κB-dependent protective mechanisms (Wang et al., 998; reviewed in Dutta et al., 2006; Wullaert et al., 2007). Accumulating evidences have shown now that at least some of the cytoprotective effects of NF-κB in TNFα-induced hepatotoxicity are mediated by suppression of c-Jun N-terminal kinase (JNK) activity following TNFα challenge (Figure 2) (Wullaert et al., 2006; Schwabe and Brenner, 2006; Papa et al., 2006).
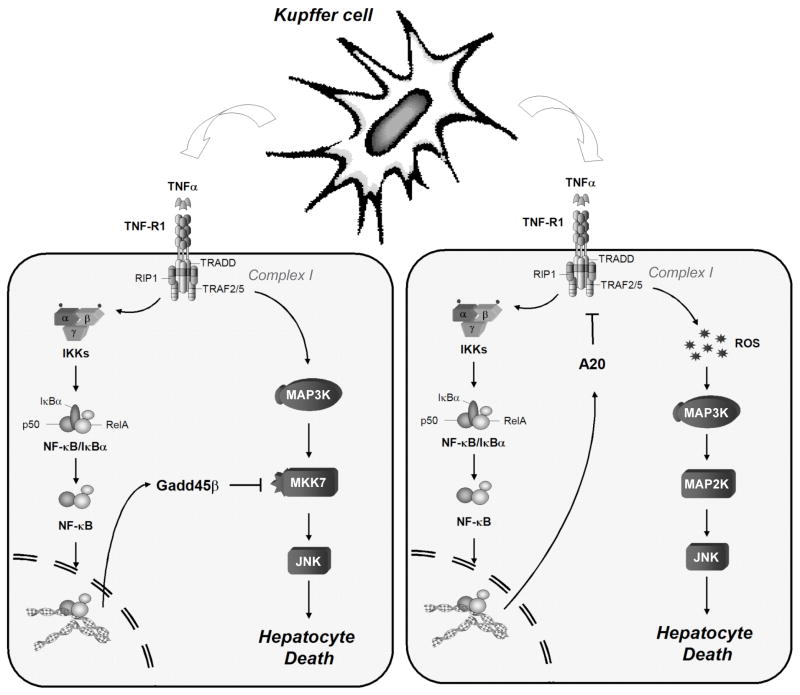
Figure 2. Mechanisms of hepatocyte protection.
Following Kupffer cell activation, TNFα is released and binds to TNF-R1 exposed on hepatocytes, with consequent assembling of the Complex I. This complex is essential to signal the activation of the NF-κB and JNK cascades. NF-κB activity promotes the activation of genes such as Gadd45β and A20. These genes block the TNFα-induced hepatocyte death using two distinct mechanisms.
In addition to its beneficial function in protecting hepatocytes against TNFα-induced cytotoxicity the activation of NF-κB may promote the survival of transformed hepatocytes, thus supporting malignancy and progression in cancer (Karin, 2006). This dual role of NF-κB in hepatocytes emphasizes its intricate contribution to liver physiology and disease and also, highlights the need of studying the molecular pathways that regulate the outcome of TNFα stimulation to understand how NF-κB can exert distinct functions in the liver. It is also evident that gaining insights into the molecular pathways that regulate the hepatocyte’s decision between survival and death are of therapeutic importance. This could, in fact, lead to the identification of novel therapeutic agents capable of preventing cell death during liver injury or malignant transformation. However, the mechanisms that regulate this balance are complex, cell-type specific and remain still poorly understood.
In this review we summarize the proposed mechanisms that have been formulated currently, especially in the context of TNFα-mediated liver injury, an important aspect in the pathogenesis of several human liver conditions, including hepatitis and hepatocellular carcinoma. Precisely, we will focus on the mechanisms behind the crosstalk between NF-κB-mediated inhibition of JNK signalling in hepatocytes.
Experimental animal models of liver disease
TNFα, FasL, and other ligands of the so-called ‘death receptors’ (DRs) have been implicated in the induction of hepatocyte death but the molecular basis of the pathways controlling the death remain unclear (Wullaert et al., 2007; Malhi and Gores, 2008). Epatocyte death induced by TNFα is implicated in several human liver conditions including hepatitis, alcoholic liver disease, acute liver failure, I/R injury, liver graft rejection and HCC (Streetz et al., 2000). The distinct mechanisms regulated by TNFα have been evaluated in different animal models of liver injury.
Animal models of hepatitis
TNFα plays a prominent role in the pathogenesis of chronic hepatitis B (HBV) and C viral (HCV) infection (reviewed in Sun and Karin, 2008). Indeed, HBV-infected patients showed elevated plasma levels of TNFα and their peripheral blood mononuclear cells showed elevated TNFα production, in vitro (Gonzalez-Amaro et al., 1994). Injection of either TNFα or lypopolysaccharide (LPS) in mice recapitulates the pathogenesis of HBV and HCV infection (Kushner, 1982; Pfeffer et al., 1993). LPS has been shown to induce TNFα production from macrophages (Beutler et al., 1985). Although TNFα and LPS alone does not cause severe liver toxicity when injected in healthy mice, co-administration with GalN – a hepatotoxin that blocks transcription specifically in hepatocytes – drastically sensitizes mice to TNFα-mediated hepatotoxicity and lethality. Indeed, treatment with either TNFα/GalN or LPS/GalN induces hepatitis with manifests of elevated serum AST/ALT levels, T-cell infiltration, massive granulocyte accumulation and hepatocyte death (Kushner, 1982). Injection of LPS/GalN mediates also (directly or indirectly) many of the clinical manifestations of the alcoholic hepatitis (Hill and McClain, 2004) and fulminant liver failure (Tsuji et al., 1997).
Administration of the T-cell mitogen concanavalin A (ConA) also results in massive hepatitis associated with acute liver failure. The pathogenesis of this hepatitis involves T cells and various cytokines, including TNFα, and recapitulates several aspects of viral and autoimmune hepatitis in humans (Tiegs, 1992).
Models of liver regeneration
Wound repair activity in the liver is associated with an orchestrated cellular response leading to tissue regeneration, which involves coordinated cell activation and proliferation (Fausto et al., 2006; Michalopoulos, 2007). Liver regeneration is a process that normally does not rely on stem cells or liver cell progenitors, but rather on mitotic division of pre-existing differentiated liver cells, including hepatocytes, bile duct epithelium, and endothelial cells (Michalopoulos, 2007). The ability of the adult liver to restore its mass and function after tissue removal is a unique property of this organ. One of the most extensively characterized model systems to study liver regeneration is partial hepatectomy (PH), consisting of surgical tissue removal in rodents (Fausto et al., 2006; Michalopoulos, 2007). For instance, the left and medial lobes of the liver are excised resulting in removal of 70% of the hepatic mass. PH triggers a sequence of events that proceeds in an orderly fashion and can be observed from the first minutes up to 5–7 days following surgical removal of the liver (Fausto et al., 2006; Michalopoulos, 2007). Hepatocytes are the primary cells to enter the cell cycle. The process is highly regulated and controlled by growth factors and cytokines causing the activation of signalling pathways, such as the mitogen-activated protein kinases (MAPKs) and the nuclear transcription factors pathways (Fausto et al., 2006). TNFα seems to play a dominant role in regulating hepatocyte survival during liver regeneration, but may also contribute to promote hepatocyte proliferation (Yamada et al., 1997).
Models of hepatocellular carcinoma (HCC)
The loss of hepatic mass due to the liver injury activates the proliferation of hepatocytes that may provide a promoting environment for tumour formation. HCC rarely develops in the absence of hepatitis, fibrosis and cirrhosis (Thorgeirsson and Grisham, 2007). Indeed, it is shown that the interaction of hepatocytes with inflammatory cells results in an altered microenvironment that appears to be crucial both in the early and late stages of carcinogenesis. Hepatic carcinogenesis, however, is a complicated process and despite arduous investigation the molecular events that regulate the entire process are still largely unknown (Hussain and Harris, 2007).
The Mdr2 knockout mouse represents an interesting model of HCC. Mice lacking the Mdr2 gene fail to transport phosphatidyl choline into the bile, with consequent accumulation of phospholipids within hepatocytes. This accumulation causes TNFα-mediated hepatitis and severe inflammation, which gradually progresses in the formation of neoplastic nodules at approximately 6 months and develops tumours at approximately 18 months (Mauad et al., 1994).
Diethylnitrosamine (DEN) is a chemical commonly used to induce HCC in rodents as it causes degenerative, proliferative and neoplastic lesions in the liver (Pound et al., 1973). Administration of DEN also leads to a rapid accumulation of TNFα (Maeda et al., 2005). Although the mechanisms by which DEN induces TNFα is not clear, the release of this cytokine plays a crucial role in the carcinogenetic process (Karin, 2006). Treatments with DEN primarily affect the liver tissue where it is converted by P450-dependent mono-oxygenase into a highly reactive molecules. The consequent newly generated electrophilic DEN species bind covalently to multiple sites on DNA, proteins and other cell constituents inducing DNA damage and mutations, which cause hepatocyte death and successive compensatory proliferation (Cayama et al., 1978; Lim, 2002; Karin, 2006). In DEN-treated mice, foci of altered hepatocytes are observed at 4–5 months of age, and by 8 months malignant (macroscopic) nodules become visible. These events are considered early steps responsible for initiation and promotion of hepatocarcinogenesis as indicated by the eventual appearance of malignant tumours (Cayama et al., 1978; Karin, 2006).
TNFα pathway
Liver homeostasis involves a balance between hepatocyte death and survival (Wullaert et al., 2007). Excessive hepatocyte death contributes to liver injury, while hepatocyte survival and protection is a crucial element for liver cancer development (Figure 1) (Karin, 2006; Wullaert et al., 2007). The proinflammatory cytokine TNFα plays a pivotal role in liver homeostasis because it has the ability to promote both hepatocyte death and survival (Wullaert et al., 2006; Schwabe and Brenner, 2006).
TNFα can exert a variety of biological effects that are mediated by its engagement of TNF-receptor 1 (TNF-R1) and TNF-R2. Whereas soluble TNFα efficiently activates TNF-R1 signalling, the activation of TNF-R2 signalling requires the binding of membrane-bound TNFα. Both receptors lack intrinsic enzymatic activity; thus, signalling by TNF-R1 and TNF-R2 is achieved by recruitment of adaptor proteins that bind to their cytosolic region (Wajant et al., 2003). For instance upon binding of the ligand, the intracellular region of TNF-R1 forms a homotrimer that interacts with TNF-R-associated death domain (TRADD). This recruitment is mediated by a direct interaction of the ‘death domain’, which is a conserved protein-protein interaction motif of 80 amino acids contained in all DRs. TNF-R1-bound TRADD is then able to engage downstream adaptors, such as ‘TNFα receptor-associated factor’ (TRAF)2 and ‘receptor-interacting protein kinase’ (RIP), which together form the so-called Complex I. The assembling of this complex is essential to signal the activation of NF-κB, as well as the JNK cascade (Figure 2) (Wajant et al., 2003; Papa et al., 2006). Numerous studies have provided evidences that NF-κB induces the activation of survival effectors in the context of TNFα-induced signalling in the liver (Streetz et al., 2000; reviewed in Schwabe and Brenner, 2006; Wullaert et al., 2007). This process is believed to involve the transcriptional expression of cytoprotective genes (Dutta et al., 2006). In contrast, the activation of JNK signalling in the liver has been associated with promotion of cell death (discussed below) (Varfolomeev and Ashkenazi, 2003; Wullaert et al., 2006; Papa et al., 2006).
Besides activating the NF-κB and JNK pathways, TNF-R1 can activate caspases proteolyses. This activation occurs when the cytosolic region of TNF-R1 is released from the membrane and recruits procaspase-8 and FADD to form Complex II, which results in the activation of caspase-8. Active caspase-8 then proteolytically cleaves several effector caspases, which are responsible for apoptosis. In addition to the activation of the downstream executioner caspases, hepatocytes require a second pathway that involves mitochondrial amplification of signals to undergo cell death. It should be noted that the caspases-dependent cell death is enhanced by the activation of JNK signalling supporting their activation (Wajant et al., 2003). Below we discuss the mechanisms and molecules involved in TNFα-mediated signalling.
TNFα-induced NF-κB Signalling
The evolutionarily conserved nuclear transcription factor NF-κB plays a crucial role in development, inflammation, immune responses, and cell survival (Hayden and Ghosh, 2004; Dutta et al., 2006). The stimulation of the TNF-R1 by TNFα represents a prototypic model of NF-κB activation. NF-κB indicates a family of dimeric transcription factors that recognize a common DNA motif called, the κB motif. The dominant form of NF-κB in most cells is a heterodimer of the p50 and p65 (alternatively named RelA) subunits. Inhibitory IκBs proteins normally sequester NF-κB complex in the cytoplasm by direct interaction. TNFα-induced signals lead to the activation of the upstream IκB kinase (IKK) complex, which consists of two catalytic subunits IKKα/IKK1 and IKKβ/IKK2 and the subunit KKγ/NEMO. Cells stimulated with TNFα rapidly activate the IKK complex. IKKβ-mediated phosphorylation of IκBα then leads to its poly-ubiquitination and subsequent proteolytic degradation, allowing NF-κB translocation into the nucleus and regulation of the transcription of target genes (Hayden and Ghosh, 2004; Dutta et al., 2006).
Numerous studies have provided evidence that NF-κB mediates pro-survival signals in the liver in the context of DRs stimulation. The NF-κB-dependent signals involve the transcriptional activation of pro-survival genes (reviewed in Hayden and Ghosh, 2004; Dutta et al., 2006). Among these genes are the caspase-8 inhibitor c-FLIP, the members of the Bcl-2 family, Bcl-xL and A1/Bfl-1, and the cellular inhibitors of apoptosis (c-IAPs) and the X-liked inhibitor of apoptosis (XIAP). While c-FLIP acts at the receptor level in the DR-induced apoptotic pathway, by competing with caspase-8 for binding to Complex II, Bcl-xL and A1/Bfl-1 act at the level of mitochondria, where they prevent tBid-induced mitochondrial depolarization and the release of cytochrome c into the cytosol. A suggested mechanism for c-IAPs-mediated suppression of apoptosis appears to be through direct inhibition of caspases. In this mechanism, c-IAPs directly bind to and inhibit effectors caspases such as caspase-3 and caspase-7, and indirectly prevent activation of caspase-6 and caspase-9. Over-expression of each of these genes in NF-κB-deficient cells, however, can only provide partial protection against TNFα-induced apoptosis, suggesting that additional NF-κB-dependent protective mechanisms exist (Dutta et al., 2006). It has recently been proposed, indeed, that the NF-κB-dependent pro-survival activity is directly linked to a suppression of the JNK pathway. Two studies in 2001 have initially described the protective crosstalk between the NF-κB and JNK pathways (De Smaele et al., 2001; Tang et al., 2001). More recent studies have shown that the liver provides a paradigm for the crosstalk between these two pathways (discussed below).
Mechanisms for TNFα-induced JNK cascade
MAPKs regulate several critical cellular functions in addition to cell death including cell proliferation and differentiation (Davis, 2000). Signal specificity is provided in part by the existence of multiple MAPKs signalling. Extra-cellular signals are orchestrated via cascades of sequential phosphorylation by their upstream activators. MAPKs are activated by upstream MAPK kinases (MAP2Ks), which, in turn, are activated by MAP2K kinases (MAP3Ks) (Davis, 2000).
JNKs are a group of serine/threonine protein MAPKs that together with p38 MAPKs and ERKs (extracellular signal-regulated kinases) mediate cellular responses through the interaction with and phosphorylation of downstream targets. Once activated, these pathways can lead to the selective activation of different types of transcription factors, ultimately regulating a myriad of biological functions, including inflammation, stress responses, cell survival and cell differentiation. For instance, the JNK and p38 cascades mostly mediate activation of the members of the transcription factor AP-1 – a heterodimeric complex composed of proteins of the Jun, Fos, Maf and ATF sub-groups. MAPKs, however, have also biological effects that are independent of their activity as transcriptional regulators (Davis, 2000; Johnson and Lapadat, 2002).
At least six protein kinases has been shown to function as MAP2Ks: MEK1 and MEK2 phosphorylating ERKs; MKK3 and MKK6 phosphorylating p38s; MKK4 and MKK7 phosphorylating JNKs. It is worth noting that activation of each protein kinase strictly depends on the stimulus and the cell type in which it is activated. In the context of JNK pathway, MKK4 is primary activated by stress, whereas MKK7 is activated by both inflammatory cytokines, such as TNFα, and stress. Interestingly, MKK4 may also phosphorylates p38, suggesting that it can also regulates the p38 pathway in the same tissue (Davis, 2000; Johnson and Lapadat, 2002).
More complex is the regulation of molecules in the MAP3K family. For instance, in the context of TNF-R1, previous studies have shown that TNFα-induced activation of JNK (and p38) is mediated by the recruitment to TNF-R1 of the adaptor TRAF-2 and subsequent activation of four major MAP3K groups: the MEKK group (MEKK1/2/3/4), the MLK group (MLK1/2/3, DLK, and LZK), the ASK group (ASK1 and ASK2) and TAK1. Many of these MAP3Ks, however, can activate more than one MAPK pathway and can also trigger activation of the NF-κB pathway, underscoring the complexity of this signalling (Davis 2000).
Oxidative stress can also partly activates MAPKs pathway, in particular the p38 and JNK pathway. This condition occurs when the levels of reactive oxygen species (ROS) in the cells exceed the capacity of antioxidant factors to limit their accumulation. In response to certain stress stimuli and cytokines (including TNFα), ROS formation and accumulation above a certain threshold can induce cell damage. This is, in fact, a common mechanism in liver injury. The precise mechanism(s) by which ROS trigger cell damage, however, is unclear. It has been proposed that, in some circumstances, ROS-inflicted cytotoxicity involves an inhibition of NF-κB activity (reviewed in Bubici et al., 2006). In addition, it is becoming increasingly clear that in other systems, this cytotoxicity is mediated in part by the activation of JNK. Recent studies from Karin and colleagues, for instance, reported that TNFα-induced ROS oxidize and inactivate the MAPK phosphatases (MKPs) group such as MKP-1, MKP-3, MKP-5 and MKP-7 – known to dephosphorylate MAPKs (Karin and Gallagher, 2005; Kamata et al., 2005). Another study reported that ROS also prompt the induction of ASK1 (reviewed in Matsuzawa and Ichijo, 2008). It was shown that Ask1−/− MEFs exhibit a defect in the induction of JNK signalling and cell death following stimulation of TNF-R1 (Matsuzawa and Ichijo, 2008). Hence, ROS may influence the activation of the JNK pathway downstream of TNF-Rs both by inducing MAP3Ks and inhibiting MKPs. The relative importance of each of these mechanisms to the sustained induction of the TNF-Rs signalling is likely to depend on the tissue and biological context.
Role of NF-κB in health and liver disease
NF-κB in embryonic liver development
NF-κB signalling plays a key role in liverphysiology and development (Hayden and Ghosh, 2004; Dutta et al., 2006). RelA-deficient mice provide the first evidence for NF-κB in regulating embryonic liver homeostasis. Disruption of the RelA gene locus leads to embryonic lethality at mid-gestation and this lethality is associated with a massive induction of PCD in the liver (Beg and Baltimore, 1995). Further studies showed that RelA is not critical for liver development but may serve as a vital protective function against TNF-R1-induced PCD. Indeed, double mutants mice harbouring compound mutation of RelA and TNF-R1 (RelA−/−/TnfR1−/−) survived during the embryonic development. At birth RelA−/−/TnfR1−/− mice have normal liver morphology, but soon after most animals develop acute hepatitis and die around 10 days of age (Rosenfeld et al., 2000). The lethality phenotype showed in embryos of RelA−/− mice has been also detected in mice lacking either IKKβ or IKKγ, two subunits of the IKK complex (Li et al., 1999; Makris et al., 2000; Rosenfeld et al., 2000).
NF-κB protects against liver injury
Numerous reports have now confirmed the pivotal role that NF-κB plays in preventing TNFα-mediated PCD also in adult hepatocytes. Karin and collaborators showed that hepatocyte-specific deletion of IKKβ (IKKβΔHep) sensitizes mice to liver failure and apoptosis following ConA administration (Maeda et al., 2003). IKKβΔHep mice, however, are apparently otherwise healthy and fertile with normal postnatal liver development and function. Indeed, some reports shown that administration of LPS in IKKβΔHep mice induces similar degree of liver damage than in the wild-type strain (Maeda et al., 2003). Consistent with these findings, Trautwein and colleagues recently found that injectable TNFα or challenge with LPS does not sensitize livers of either IKKβΔHep or another mouse model IKKβf/f-Mx-Cre, which after Cre-mediated recombination showed an efficient knockout of IKKβ in Kupffer, non-parenchymal and lymphoid cells (Luedde et al., 2005). A possible explanation for the selective role of IKKβ and NF-κB in liver protection against ConA-induced apoptosis manifests because this agent induces membrane-bound TNFα expression from cells, which can then activates both TNF-R1 and TNF-R2 in hepatocytes. In contrast, LPS/TNFα-mediated apoptosis is dependent on soluble TNFα, which can only activate TNF-R1. As a consequence, ConA-challenged IKKβΔHep mice exhibit a more robust and prolonged JNK activation than mutants animals treated with LPS. Indeed, inhibition of JNK results in a reduction of ConA-induced liver damage, demonstrating that the primary mechanisms by which IKKβ-dependent NF-κB activation prevents TNFα-induced apoptosis in vivo is through attenuation of JNK activation (discussed below) (Maeda et al., 2003; Wullaert et al., 2007). Another study showed that hepatocyte-specific deletion of IKKβdoes not lead to increased apoptosis upon challenge with ConA, which is in sharp contrast with previous results (Luedde et al., 2005). Yet, activation of NF-κB signalling after pharmacological inhibition of IKKβ was not impaired after TNFα stimulation. This study concluded that in the adult liver activation of NF-κB induced by TNFα could occur independently of IKKβ. However, it has been shown that IKKβΔHep mice are resistant to hepatic post-ischemic reperfusion (I/R) injury, mimicking events that can occurs during organ transplantation, tissue resection and hemorrhagic shock in humans. Conversely, pharmacological inhibition of IKKβ was shown to prevent hepatic I/R injury (Luedde et al., 2005). Together these results supports that different IKK subunits can mediate differential functions in the adult liver.
Hepatocyte-specific deletion of the IKKγ gene (IKKγΔHep) renders mice highly susceptible to LPS-induced injury, carrying massive hepatocyte apoptosis (Luedde et al., 2005). Yet, in a mouse model of IKKγf/f-Mx-Cre, which after Cre-mediated recombination showed an efficient knockout of IKKγ in both myeloid and non-parenchymal cells, challenge with TNFα caused a higher degree of liver injury, promoting both apoptotic and necrotic PCD. These findings are consistent with the notion that deletion of IKKγ completely abolishes activation of NF-κB; in contrast, IKKβ deletion only partially inhibits this activation due to the redundant function of IKKα activity (Luedde et al., 2005; Wullaert et al., 2007).
Whether or not NF-κB activation depends on IKKβ, all together the data presented here agree that NF-κB activity plays a protective role required to prevent TNFα-induced cell death in the adult liver. These conclusions are in agreement also with a recent study, which shows that the genetic inactivation of RelA in hepatocytes sensitizes mice to cytotoxicity induced by treatment with either LPS or TNFα (Geisler et al., 2007). Clinical evidences also support this view. NF-κB is activated in the livers of subjects with chronic hepatitis C, and expression of RelA in these livers is associated with lower degree of apoptosis, and slower progression to fibrosis-associated chirrosis (Boya et al., 2001). Likewise, RelA expression is suppressed in pregnant women with chronic hepatitis E (HEV) infection and correlates with the severe liver damage and high mortality seen in these patients (Prusty et al., 2007).
NF-κB in liver regeneration
Hepatocytes have a remarkable proliferative capacity, but are quiescent in healthy liver. Liver regeneration after PH provides a fundamental paradigm for the liver response to injury (Fausto et al., 2006; Michalopoulos, 2007). NF-κB is one of the transcription factors that protect cells from undergoing apoptosis in a wide range of cell types, including hepatocytes (Fausto et al., 2006). After PH, NF-κB is rapidly activated and has been shown both to protect hepatocytes from apoptosis and to promote hepatocyte proliferation. Although the factors responsible for the induction of NF-κB during liver regeneration are still under investigation, a likely candidate is TNFα itself (Schwabe and Brenner, 2006). Expression of this cytokine is induced during liver regeneration (Evan et al., 1985) and neutralizing anti-TNFα antibodies can inhibit hepatocyte DNA synthesis during this process (Akerman et al., 1992). Furthermore, NF-κB activity is markedly impaired in TNF-R1 knockout mice after PH challenge (Yamada et al., 1997). Moreover, the infection with adenovirus expressing the super-repressor IκBα (IκBα-S), which blocks NF-κB activity, results in the induction of massive apoptosis (Iimuro et al., 1998). Similar effects were obtained using pharmacological inhibitors of NF-κB activity (Cressman et al., 1994; Plumpe et al., 2000). Whereas initial studies using adenoviral IκBα-S delivery have reported that NF-κB is implicated in hepatocyte survival and proliferation after PH (Iimuro Y et al., 1998), subsequent studies demonstrated that hepatocyte-specific IκBα-S expression has no adverse effect on these processes (Chaisson et al., 2002). There is still a controversy as to which liver cell types NF-κB primarily carries out its function during regeneration. It has been reported that PH induces NF-κB activation primarily in Kupffer cells where it promotes the synthesis of factors that stimulate hepatocyte growth (Chaisson et al., 2002; Abshagen et al., 2007). Other studies suggested a role for NF-κB activation in hepatocytes during regeneration (reviewed in Wullaert et al., 2007). Support for these roles comes from other models of compensatory hepatocyte growth, which showed that activation of NF-κB in hepatocytes induces protective genes that antagonize TNFα-mediated PCD (discussed below) (Maeda et al., 2005; Luedde et al., 2007). Additional studies contribute to shed further light on the basis for the discrepancies between earlier conclusions. Trautwein and collaborators reported that IKKβ deletion in hepatocytes accelerates hepatocyte proliferation after PH (Malato et al., 2008). Altogether studies in the literature, therefore, support the notion that NF-κB plays important roles in liver regeneration both within hepatocytes and non-parenchymal cells.
NF-κB in liver carcinogenesis
Tumour development involves an imbalance between cell proliferation and cell death (Karin, 2006; Wullaert et al., 2007). Given the prominent role of NF-κB in the control of liver cell proliferation and cell death, it would be logical to expect that NF-κB is involved in both initiation and development of liver cancer (Karin, 2006). The evidence or the participation of NF-κB in carcinogenesis, indeed, has recently become more apparent. Studies in animal models underscore the fundamental role of the NF-κB pathway in the regulation of PCD and proliferation in hepatocytes that when deregulated may contribute to the onset of HCC.
The first evidence of NF-κB in hepatocarcinogenesis using animal models comes from analyses of Mdr2-knockout mice, a model of inflammation-driven HCC. A study by Pikarsky et al. demonstrates that hepatocytes adjacent to regions of inflammation in this model express high levels of nuclear NF-κB. It was also shown that NF-κB protects transformed hepatocytes from PCD through the upregulation of pro-survival genes. This and other data suggested that NF-κB is a tumour-promoting factor in hepatocytes (Pikarsky et al., 2004). Another recent study, however, using the DEN-induced HCC model showed that hepatocyte-specific deletion of IKKβ markedly increases hepatocarcinogenesis, whereas deletion of IKKβ in both hepatocytes and hematopoietic-derived cells inhibits this process. These results support a model whereby lower activation of NF-κB augments hepatocyte death in the form of necrosis releasing products that induce the secretion of pro-inflammatory cytokines (i.e. TNFα) from myeloid cells stimulating compensatory proliferation of surviving, DNA-damaged hepatocytes (Figure 1) (Maeda et al., 2005; Sakuray et al., 2008). The compensatory proliferation of NF-κB-deficient hepatocytes in response to DEN treatment plays a key role in the development of liver cancer. Thus, whereas in the hepatocytes NF-κB plays an inhibitory role, in Kupffer cells it promotes hepatocarcinogenesis. A growth-suppressing role for IKKβ in hepatocytes during liver regeneration was also suggested by studies showing that extracellular matrix, basement membrane, and expression of integrins were increased in IKKβΔHep mice (Koch et al., 2009). These findings were recently supported by other studies which showed that conditional IKKγ deletion in hepatocytes causes steatohepatitis and HCC, suggesting a tumour-suppressor role for NF-κB activity (Luedde et al., 2007). On the other hand, transgenic mice in which a constitutively active form of IKKβ was expressed in hepatocytes showed that NF-κB activation in these cells did not significantly affect formation of DEN-induced HCC (Yau et al., 2009).
Like NF-κB, the p38α MAPK is also suggested to suppress DEN-induced cancer development in the liver. It was shown that by using distinct mechanisms NF-κB and p38α cooperate to prevent ROS accumulation, which promotes the necrotic cell death that drives compensatory proliferation during this process (Sakurai et al., 2008).
Because of these seemingly conflicting results in different models of HCC, whether NF-κB activity in hepatocytes is important for carcinogenesis remains unclear. It is worth mentioning in this regard that this might depend on differences in the specific mouse model used (Vainer et al., 2008). Given that HCC develops in the settings of inflammation-driven cancer associated to challenge with hepatitis virus and chronic alcoholism, it would be appropriate in the future to obtain further data in these and probably other models of animals or in humans. For instance, rather than using chemical hepatocarcinogenetic compounds, more emphasis should be placed in developing mouse model that resembles the infection with viral hepatitis and alcohol consumption, which are the major agents causing HCC in humans. Another important challenge will be to delineate the mechanisms and the critical checkpoints that control hepatocyte death and proliferation in HCC.
NF-κB inhibits JNK-mediated cell injury and death
In perhaps no other tissues the crosstalk between NF-κB and JNK in antagonism of TNF-R1-induced PCD has been more thoroughly documented than in the liver. Indeed much work has been done so far to elucidate the mechanisms behind the protective activity of NF-κB against TNFα-mediated injury in the liver. It is now accepted that a crosstalk between NF-κB and JNK activity is a major determinant in the fate of hepatocytes during TNFα challenge (reviewed in Schwabe and Brenner, 2006). One of the mechanisms through which NF-κB provides its survival signals in TNFα-induced cell death is the inhibition of the prolonged activation of JNK. Blocking NF-κB by either ablation of RelA or IKKβ or by the expression of IκBα-S impairs the shutdown of TNFα-induced JNK signalling. Interestingly, it is the persistent rather than the transient activation of JNK that is ultimately responsible for triggering death. Thus, the NF-κB-mediated suppression of this prolonged activation is crucial for inhibition of TNFα-induced cell death (De Smaele et al., 2001; Tang et al., 2001; Papa et al., 2004a).
Indeed the analyses of IKKβ/JNK1 double mutants showed that the absence of JNK1 – one of the two major JNK isoforms expressed in the liver – delays embryonic mice lethality caused by compromised NF-κB activation, suggesting that JNK signalling is an important mediator of apoptosis in the liver during embryogenesis and the suppression of this signalling is a key mechanism by which NF-κB promotes survival during liver development (Chang et al., 2006). Evidence of the protective crosstalk between NF-κB and JNK also comes from the analyses of adult livers with defects in NF-κB activation. The proapoptotic effects of ConA-induced hepatitis are increased in IKKβ deficient hepatocytes, and this increase correlates with a persistent activation of JNK. Treatment of IKKβ-Δhep primary cells with a small molecule inhibitor of JNK (SP600125) results in the inhibition of TNFα-induced cell death (Maeda et al., 2003). Likewise, ConA-challenged (but not LPS-challenged) IKKβ-Δhep mice exhibit more robust and prolonged JNK activation than similarly treated control mice demonstrating that prolonged JNK activation is necessary for both TNF-R1- and TNF-R2-mediated liver injury (Maeda et al., 2003). Similar results were obtained with RelA-deficient mice, where the intravenous injection of either murine or human TNFα was shown to promote prolonged JNK activation in hepatocytes. Primary cultures of RelA-deficient hepatocytes treated with SP600125 reduce the ability of TNFα to induce hepatocyte death. The authors of these studies, however, questioned whether prolonged JNK activation is mediated only in systems where TNFα-inflicted cytotoxicity is dependent on both TNF-R1 and TNF-R2, as seen only in ConA-induced liver injury (Geisler et al., 2007).
Other than in hepatitis models, the interplay between NF-κB and JNK has been extensively investigated in mouse models where tissue injury triggers compensatory proliferation. Ben-Neriah and colleagues suggested that the specific inhibition of NF-κB in hepatocytes of Mdr2−/− mice augments JNK activation at 7 months of age, the time at which premalignant changes began to occur. The elevation of JNK activation in this model was dependent on continuous release of TNFα, because Mdr2−/− mice challenged with anti-TNFα antibodies shown suppressed activation of JNK and expression of c-Jun in the liver (Pikarsky et al., 2004). Similar results were obtained with the model of DEN-induced HCC. Hepatocyte deletion of IKKβ augments DEN-induced JNK activation and hepatocarcinogenesis. Generation of double mutants mice harbouring specific inhibition of both IKKβ and JNK1 (IKKβ-Δhep/Jnk1−/−) provided further evidences for the dependence of hepatocyte death on JNKs. Indeed, JNK1 deletion reversed increased hepatocyte death and the susceptibility of mice to progress in HCC in the absence of IKKβ (Sakuray et al., 2006). Consistent with these findings recently studies in Jnk1−/− and Jnk2−/− mice challenged with DEN reported that the JNK1 pathway has a dual, stage-dependent function in HCC: one in the early stages of carcinogenesis in mediating DEN-induced PCD; another at late stages where it promotes the compensatory proliferation of hepatocytes, which supports the formation of liver cancer (Hui et al., 2008).
It was suggested that ROS, whose production is induced by TNFα, are responsible for the prolonged JNK activation (Sakon et al., 2003). Whereas NF-κB inhibits JNK-mediated CD, ROS accumulation induces JNK-mediated PCD. Indeed, pre-treatment with the antioxidant butylated hydroxyanisole (BHA) agent protects mice from ConA-induced liver failure, a response that requires sustained JNK activation (Kamata et al., 2005). Like NF-κB, p38 suppresses JNK activation (Sakurai et al., 2008; Heinrichhsdorff et al., 2008). Whether this suppression of JNK by p38 is also involved in inhibition of TNFα-mediated liver injury, however, remain unknown.
Mechanisms of JNK-mediated liver injury
The exact mechanisms through which JNK activation promotes TNFα-induced apoptosis are unknown. It was initially suggested that JNK act at a level upstream of caspase-8, through generation of a novel Bid cleavage product, jBid (Deng et al., 2003). These analyses, however, await confirmation in animal models, including models of liver injury. Analyses of Jnk1−/− and Jnk2−/− mice challenged with either LPS/GalN or ConA/GalN, however, have recently suggested that activation of Bid is independent of JNK activity, suggesting a parallel role for Bid and JNK signalling in mediating TNFα-induced liver injury (Ni et al., 2009).
Other studies suggested that JNK activation in IKKβ-ΔHep livers after challenge with either ConA or LPS sequentially phosphorylates and activates the ubiquitin ligase, Itch. Once activated, Itch promotes the degradation of c-FLIP and TNFα-induced PCD (Chang et al., 2006). It remains unclear, however, whether the enhanced proteasomal turnover of c-FLIP represents a general mechanism that is required for TNFα-induced PCD in hepatocytes. For instance, the abrogation of the NF-κB response in RelA-deficient hepatocytes leads to a prolonged JNK activation after TNFα challenge in vivo. Yet, the degradation of c-FLIP was detected in this system before the onset of morphologic signs of JNK-mediated apoptosis, raising doubt on the interpretation that c-FLIP degradation is reduced as consequence of JNK-mediated phosphorylation of Itch (Geisler et al, 2007). Indeed, the authors suggested that the prolonged JNK activation in NF-κB-deficient hepatocytes might not mediate cell death only through an Itch/c-FLIP pathway, but also through another target upstream of the mitochondria, more likely at the level of caspase-8 activation (Geisler et al, 2007). In support of this view, it was recently shown that JNK contributes to TNF-R1-induced PCD signalling in hepatocytes by activating another proapoptotic BH3-only proteins, Bim rather than Bid. Combined loss of Bid and Bim rendered mice more resistant to induction of hepatitis after challenge with LPS/GalN. The injection of the JNK inhibitor, D-JNKI1 reduces LPS/GalN-induced hepatitis in Bid−/− but not in Bim−/− mice (Kaufmann T et al., 2009).
The activation of JNK signalling is also required for the expression of TNFα from hematopoietic cells, which it is necessary for promoting ConA-induced hepatitis (Das et al., 2009). These studies, however, also shown that JNK is not required for TNFα-stimulated PCD during the development of hepatitis, which appears to be in conflict with what was shown in other recent reports (Maeda et al., 2003; Chang et al., 2006; Wang et al., 2006; Kodama et al., 2009). Indeed, while there is a controversy over whether the two hepatic JNK isoforms, JNK1 and JNK2, have specialized functions in cell survival, growth and PCD, knockout studies have shown that JNK activation contribute to TNF-R-mediated hepatic death (Maeda et al., 2003; Chang et al., 2006; Wang et al., 2006; Kodama et al., 2009).
JNK-mediated apoptosis has been also documented in activation of Fas and TNF-associated apoptosis-inducing ligand (TRAIL) death-receptor in hepatocytes. Elevated hepatic bile acid concentrations facilitate Fas and TRAIL activation (Higuchi and Gores 2003). Häussinger and collegues investigated that activation of NADPH oxidase is responsible for bile acid-induced ROS production, which accounts for the activation of JNK signalling (Reinehr et al., 2005). However the molecular mechanisms leading to activation of such signalling and its contribution to the final apoptotic event are not fully understood.
Mechanisms of NF-κB-mediated suppression of JNK signalling
The interplay between the NF-κB and JNK signalling can also occur at the transcriptional level (Papa et al., 2006; Papa et al., 2004a). To date, at least three NF-κB-dependent genes have been identified as potential candidates of NF-κB-mediated control of TNFα-induced JNK activation: Growth arrest DNA damage-inducible gene 45β (Gadd45β), X-liked inhibitor of apoptosis (XIAP), and the zinc finger protein A20. As discussed below, while Gadd45β appears to have a crucial role in the protection against JNK-induced hepatocytes death in the PH model, the role of XIAP and A20 in vivo in the context of TNFα signalling remain to be delineated. Indeed, whereas A20 was shown to protect mice against LPS/GalN-induced hepatocytes apoptosis, whether this protective function is mediated by an inhibition of JNK remains unknown (Figure 2) (reviewed in Wullaert et al., 2007).
With the regard to the in vivo role of Gadd45β in cytoprotection, the Gadd45β-mediated inhibition of JNK activity appears to involve a blockade of MKK7 (Papa et al., 2008). Gadd45β directly associates with MKK7 blocking its catalytic activity and precluding access of ATP to its catalytic pocket (Papa et al., 2007; Papa et al., 2004b). Early studies have suggested that Gadd45β is not involved in the NF-κB-mediated control of TNFα-induced PCD, as Gadd45β −/− MEFs cells were found to have no defect in the suppression of JNK induction or PCD downstream of TNF-Rs (Amanullah et al., 2003). Thus, the role of Gadd45β in cytoprotection against TNFα-mediated JNK activation might be tissue- and context-dependent.
With regard to liver injury, we recently reported that Gadd45β is important for protection of hepatocytes during liver regeneration. Whereas wild-type mice fully recovered from surgery, the survival rate of Gadd45β −/− mice was dramatically reduced. The analyses of livers from these mice showed that loss of Gadd45β is associated with liver injury. In contrast, wild-type mice showed no signs of this injury. Gadd45β −/− livers also displayed reduced hepatocytes proliferation after PH, but it is likely that this reduction is owed in part by a secondary consequence of an elevation of PCD in Gadd45β −/− mice. Significantly, the induction of PCD in Gadd45β −/− mice during liver regeneration is associated with augmented JNK activation, which correlates with increased caspases activation in Gadd45β −/− livers (Papa et al., 2008). Given a crucial role of TNFα in liver regeneration, these findings are consistent with a model in which TNFα stimulates NF-κB activity in hepatocytes following PH (Figure 2). Transcriptional activity of NF-κB up-regulate the expression of Gadd45β, which in turn binds to and specifically blocks MKK7-JNK activity. Indeed, double mutants mice harbouring the compound deletion of Jnk2 and Gadd45β (Gadd45β −/−/Jnk2−/−) exhibit reduced hepatocyte death and increased proliferation. Although the data strengthen that Gadd45β promotes hepatocyte survival during liver regeneration by modulating JNK-mediated PCD, its role in liver protection against TNFα-mediated injury in model of TNF-R-mediated liver challenge is uncertain. It is likely in fact that Gadd45β protects the liver during challenges in which activation of JNK is independent of ROS production. Indeed, using a model of ConA-induced hepatitis – a model where JNK activation is dependent on ROS – Gadd45β does not appear to be required for inhibition of JNK activation. However, in model like PH, in which activation of JNK does not rely on induction of ROS, Gadd45β is able to mediate the protective activity of NF-κB in blocking TNFα-induced, JNK-mediated PCD (Figure 2) (Papa et al., 2008).
Concluding remarks
Growing evidences indicate that programmed cell death regulates different physiological and pathological conditions (Guiccairdi and Gores, 2005). As outlined in this review, the liver offers a paradigmatic example on how cell death can be detrimental for the entire organism as it has been associated with the onset of liver disease (Karin, 2006; Wullaert et al., 2007; Su and Karin, 2008). While there are no effective treatments for many liver disorders, our recent findings support possible step forwards (Papa et al., 2008). Much work has been done in the way of understanding the mechanisms of liver disease. Among these, NF-κB and JNK signalling have been found to represent important elements in the liver pathology (Wullaert et al., 2007; Su and Karin, 2008). Taken together, the studies reported here demonstrate that NF-κB activation is important for sustaining hepatocyte survival during TNFα-mediated chronic liver injury, through the inhibition of the JNK pathway. However, the pro-survival activity of NF-κB could also play a detrimental role because it may enhance the progression of hepatocellular carcinoma (especially during late stage of carcinogenesis), in part by enhancing the survival of ‘transformed’ hepatocytes, indicating that NF-κB signalling is a problematic therapeutic target.
Because the NF-κB pathway has a pivotal role in inflammatory response, blockers of this pathway are currently used for treatment of inflammatory liver conditions. Drugs that target NF-κB activity, however, can alleviate the inflammatory response, but at the same time might cause elevated hepatocyte death, because of its pro-survival activity (Su and Karin, 2008). Therefore additional efforts are necessary in the future to unravel the proteins under transcriptional regulation of NF-κB that mediate the biological function of this transcription factor in specific pathological settings, including liver cancer. Indeed, inhibition of NF-κB activity may be achieved by targeting individual downstream effectors of NF-κB. Specifically, there is the need for developing agents that enable the selective blockade of the pro-survival action of NF-κB, without significantly compromising its capacity to serve in immunity and development. Such selective blockade could help limit the side effects associated to global inhibitors of the NF-κB pathway.
Gadd45β might represent a promising target. Given that Gadd45β exerts its specificity and accuracy to control the JNK signalling during TNF-R-mediated challenge, the Gadd45β-MKK7 interaction may in fact provide a suitable molecular target for devising tools that would not directly impact on NF-κB functions in immunity and development.
Acknowledgments
Work is supported in part by grants from NIH CA84040 and CA98583 and Cancer Research UK C26587-A8839 (GF), American Italian Cancer Foundation (CB), and Italian Association for Cancer Research (FZ). S. Papa is supported in part by the PhD Program in Experimental Medicine and Endocrinology, University of L'Aquila. This work is dedicated to the students of University of L'Aquila who lost their lives during the earthquake on the 6th April 2009.
References
- Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- Akerman PA, Cote P, Yang SQ, McClain C, Nelson S, Bagby GJ, Diehl AM. Antibodies to tumor necrosis factor-a inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585. doi: 10.1152/ajpgi.1992.263.4.G579. [DOI] [PubMed] [Google Scholar]
- Amanullah A, Azam N, Balliet A, Hollander C, Hoffman B, Fornace A, Liebermann D. Cell signalling: cell survival and a Gadd45-factor deficiency. Nature. 2003;424:741. doi: 10.1038/424741b. discussion 742. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Williams DP, Park BK. Understanding the role of reactive metabolites in drug-induced hepatotoxicity: state of the science. Expert Opin Drug Metab Toxicol. 2008;4:1415–1427. doi: 10.1517/17425255.4.11.1415. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Beutler B, Mahoney J, Le Trang N, Pekala P, Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985;161:984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P, Larrea E, Sola I, Majano PL, Jiménez C, Civeira MP, Prieto J. Nuclear factor-kB in the liver of patients with chronic hepatitis C: decreased RelA expression is associated with enhanced fibrosis progression. Hepatology. 2001;34:1041–1048. doi: 10.1053/jhep.2001.29002. [DOI] [PubMed] [Google Scholar]
- Bubici C, Papa S, Dean K, Franzoso G. Mutual crosstalk between reactive oxygen species and nuclear factor-kB: molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- Cayama E, Tsuda H, Sarma DS, Farber E. Initiation of chemical carcinogenesis requires cell proliferation. Nature. 1978;275:60–62. doi: 10.1038/275060a0. [DOI] [PubMed] [Google Scholar]
- Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kB leads to apoptosis after TNFa–treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor κB in hepatocytes, a primary response in the regenerating liver. J Biol Chem. 1994;269:30429–30435. [PubMed] [Google Scholar]
- Das M, Sabio G, Jiang F, Rincón M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Dutta J, Fan Y, Gupta N, Fan G, Gélinas C. Current insights into the regulation of programmed cell death by NF-kB. Oncogene. 2006;25:6800–6816. doi: 10.1038/sj.onc.1209938. [DOI] [PubMed] [Google Scholar]
- Evan GI, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr Opin Cell Biol. 1995;7:825–834. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Geisler F, Algül H, Paxian S, Schmid RM. Genetic inactivation of elA/p65 sensitizes adult mouse hepatocytes to TNF-induced apoptosis in vivo and in vitro. Gastroenterology. 2007;132:2489–2503. doi: 10.1053/j.gastro.2007.03.033. [DOI] [PubMed] [Google Scholar]
- González-Amaro R, García-Monzón C, García-Buey L, Moreno-Otero R, Alonso JL, Yagüe E, Pivel JP, López-Cabrera M, Fernández-Ruiz E, Sánchez-Madrid F. Induction of tumor necrosis factor alpha production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179:841–848. doi: 10.1084/jem.179.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Heinrichsdorff J, Luedde T, Perdiguero E, Nebreda AR, Pasparakis M. p38alpha MAPK inhibits JNK activation and collaborates with IκB kinase 2 to prevent endotoxin-induced liver failure. EMBO Rep. 2008;9:1048–1054. doi: 10.1038/embor.2008.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Gores GJ. Bile Acid Regulation of Hepatic Physiology. IV. Bile acids and death receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G734–G738. doi: 10.1152/ajpgi.00491.2002. [DOI] [PubMed] [Google Scholar]
- Hill DB, McClain CJ. Anti-TNF therapy in alcoholic hepatitis. Am J Gastroenterol. 2004;99:261–263. doi: 10.1111/j.1572-0241.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- Hui L, Zatloukal K, Scheuch H, Stepniak E, Wagner EF. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. J Clin Invest. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–280. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- Iimuro Y, Nishiura T, Hellerbrand C, Behrns KE, Schoonhoven R, Grisham JW, Brenner DA. NF-κB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest. 1998;101:802–811. doi: 10.1172/JCI483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannides C. In: Cytochromes P450: Role in the Metabolism and Toxicity of Drugs and other Xenobiotics. Ioannides C, editor. Cambridge, England: RSC Publishing; 2008. [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Karin M. Nuclear factor-kB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- Karin M, Gallagher E. From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance. IUBMB Life. 2005;57:283–295. doi: 10.1080/15216540500097111. [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Jost PJ, Pellegrini M, Puthalakath H, Gugasyan R, Gerondakis S, Cretney E, Smyth MJ, Silke J, Hakem R, et al. Fatal hepatitis mediated by tumor necrosis factor TNFalpha requires caspase-8 and involves the BH3-only proteins Bid and Bim. Immunity. 2009;30:56–66. doi: 10.1016/j.immuni.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KS, Maeda S, He G, Karin M, Leffert HL. Targeted deletion of hepatocyte Ikkbeta confers growth advantages. Biochem Biophys Res Commun. 2009;380:349–354. doi: 10.1016/j.bbrc.2009.01.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Taura K, Miura K, Schnabl B, Osawa Y, Brenner DA. Anti-apoptotic effect of c-Jun N-terminal Kinase-1 through Mcl-1 stabilization in TNF-induced hepatocyte apoptosis. Gastroenterology. 2009;136:1423–1434. doi: 10.1053/j.gastro.2008.12.064. [DOI] [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann NY Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Van Antwerp D, Mercurio F, Lee KF, Verma IM. Severe liver degeneration in mice lacking the IkappaB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim IK. Spectrum of molecular changes during hepatocarcinogenesis induced by DEN and other chemicals in Fischer 344 male rats. Mech Ageing Dev. 2002;123:1665–1680. doi: 10.1016/s0047-6374(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Luedde T, Assmus U, Wüstefeld T, Meyer zu Vilsendorf A, Roskams T, Schmidt-Supprian M, Rajewsky K, Brenner DA, Manns MP, Pasparakis M, Trautwein C. Deletion of IKK2 in hepatocytes does not sensitize these cells to TNF-induced apoptosis but protects from ischemia/reperfusion injury. J Clin Invest. 2005;115:849–859. doi: 10.1172/JCI23493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Maeda S, Chang L, Li ZW, Luo JL, Leffert H, Karin M. IKKbeta is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFalpha. Immunity. 2003;19:725–737. doi: 10.1016/s1074-7613(03)00301-7. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krähn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M. Female mice heterozygous for IKKgamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- Malato Y, Sander LE, Liedtke C, Al-Masaoudi M, Tacke F, Trautwein C, Beraza N. Hepatocyte-specific inhibitor-of-κB-kinase deletion triggers the innate immune response and promotes earlier cell proliferation during liver regeneration. Hepatology. 2008;47:2036–2050. doi: 10.1002/hep.22264. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJM, Schinkel AH, Notenboom RGE, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP, van der Valk MA, Borst P, Offerhaus GJA. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Chen X, Shi YH, Liao Y, Beg AA, Fan J, Yin XM. Genetic delineation of the pathways mediated by bid and JNK in tumor necrosis factor-alpha-induced liver injury in adult and embryonic mice. J Biol Chem. 2009;284:4373–4382. doi: 10.1074/jbc.M807259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa S, Bubici C, Zazzeroni F, Pham CG, Kuntzen C, Knabb JR, Dean K, Franzoso G. The NF-kB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Differ. 2006;13:712–729. doi: 10.1038/sj.cdd.4401865. [DOI] [PubMed] [Google Scholar]
- Papa S, Monti SM, Vitale RM, Bubici C, Jayawardena S, Alvarez K, De Smaele E, Dathan N, Pedone C, Ruvo M, Franzoso G. Insights into the structural basis of the GADD45β-mediated inactivation of the JNK kinase, MKK7/JNKK2. J Biol Chem. 2007;282:19029–19041. doi: 10.1074/jbc.M703112200. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-kappaB: a key to survival. J Cell Sci. 2004a;117:5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, et al. Gadd45β mediates the NF-κB suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004b;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- Papa S, Zazzeroni F, Fu YX, Bubici C, Alvarez K, Dean K, Christiansen PA, Anders RA, Franzoso G. Gadd45β promotes hepatocyte survival during liver regeneration in mice by modulating JNK signaling. J Clin Invest. 2008;118:1911–1923. doi: 10.1172/JCI33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull’s Toxicology: The Basic Science of Poisons. 6. New York: McGraw Hill; 2001. pp. 133–224. [Google Scholar]
- Pfeffer K, Matsuyama T, Kündig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Krönke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Plumpe J, Malek NP, Bock CT, Rakemann T, Manns MP, Trautwein C. NF-kB determines between apoptosis and proliferation in hepatocytes during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2000;278:G173–G183. doi: 10.1152/ajpgi.2000.278.1.G173. [DOI] [PubMed] [Google Scholar]
- Pound AW, Lawson TA, Horn L. Increased carcinogenic action of dimethylnitrosamine after prior administration of carbon tetrachloride. Br J Cancer. 1973;27:451–459. doi: 10.1038/bjc.1973.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty BK, Hedau S, Singh A, Kar P, Das BC. Selective suppression of NF-kB/p65 in hepatitis virus-infected pregnant women manifesting severe liver damage and high mortality. Mol Med. 2007;13:518–526. doi: 10.2119/2007-00055.Prusty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr R, Becker S, Keitel V, Eberle A, Grether-Beck S, Haussinger D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology. 2005;129:2009–2031. doi: 10.1053/j.gastro.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Prichard L, Shiojiri N, Fausto N. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am J Pathol. 2000;156:997–1007. doi: 10.1016/S0002-9440(10)64967-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M. Loss of hepatic NF-κB activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–5899. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- Streetz K, Leifeld L, Grundmann D, Ramakers J, Eckert K, Spengler U, Brenner D, Manns M, Trautwein C. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology. 2000;119:446–460. doi: 10.1053/gast.2000.9364. [DOI] [PubMed] [Google Scholar]
- Sun B, Karin M. NF-kB signaling, liver disease and hepatoprotective agents. Oncogene. 2008;27:6228–6244. doi: 10.1038/onc.2008.300. [DOI] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nature Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J Clin Invest. 1992;90:196–203. doi: 10.1172/JCI115836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H, Harada A, Mukaida N, Nakanuma Y, Bluethmann H, Kaneko S, Yamakawa K, Nakamura SI, Kobayashi KI, Matsushima K. Tumor necrosis factor receptor p55 is essential for intrahepatic granuloma formation and hepatocellular apoptosis in a murine model of bacterium-induced fulminant hepatitis. Infect Immun. 1997;65:1892–1898. doi: 10.1128/iai.65.5.1892-1898.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainer GW, Pikarsky E, Ben-Neriah Y. Contradictory functions of NF-kappaB in liver physiology and cancer. Cancer Lett. 2008;267:182–188. doi: 10.1016/j.canlet.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullaert A, Heyninck K, Beyaert R. Mechanisms of crosstalk between TNF-induced NF-kB and JNK activation in hepatocytes. Biochem Pharmacol. 2006;72:1090–1101. doi: 10.1016/j.bcp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Wullaert A, van Loo G, Heyninck K, Beyaert R. Hepatic tumor necrosis factor signaling and nuclear factor-kB: effects on liver homeostasis and beyond. Endocr Rev. 2007;28:365–386. doi: 10.1210/er.2006-0031. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau TO, Chan CF, Gee-San Lam S, Cheung OF, Ching YP, Jin DY, Sham MH, Ng IO. Hepatocyte-specific activation of NF-κB does not aggravate chemical hepatocarcinogenesis in transgenic mice. J Pathol. 2009;217:353–361. doi: 10.1002/path.2451. [DOI] [PubMed] [Google Scholar]