Abstract
The vanilloid receptor TRPV1 is activated by ethanol and this may be important for some of the central and peripheral actions of ethanol. To determine if this receptor has a role in ethanol-mediated behaviors, we studied null mutant mice in which the Trpv1 gene was deleted. Mice lacking this gene showed significantly higher preference for ethanol and consumed more ethanol in a two-bottle choice test as compared with wild type littermates. Null mutant mice showed shorter duration of loss of righting reflex induced by low doses of ethanol (3.2 and 3.4 g/kg) and faster recovery from motor incoordination induced by ethanol (2 g/kg). However, there were no differences between null mutant and wild type mice in severity of ethanol-induced acute withdrawal (4 g/kg) or conditioned taste aversion to ethanol (2.5 g/kg). Two behavioral phenotypes (decreased sensitivity to ethanol-induced sedation and faster recovery from ethanol-induced motor incoordination) seen in null mutant mice were reproduced in wild type mice by injection of a TRPV1 antagonist, capsazepine (10 mg/kg). These two ethanol behaviors were changed in the opposite direction after injection of capsaicin, a selective TRPV1 agonist, in wild type mice. The studies provide the first evidence that TRPV1 is important for specific behavioral actions of ethanol.
Keywords: Knockout mice, Alcohol, Capsazepine, Capsaicin, Endocannabinoids, TRPV1
1. Introduction
Transient receptor potential (TRP) ion channels are receiving renewed attention in neurophysiology, sensory neuropharmacology and pain research. This cation channel superfamily represents an ancient sensory apparatus of the cell, responding to temperature, touch, sound, osmolarity, pheromones, taste, pain and other stimuli (see Clapham, 2003 for review). TRP channels are essential for humans to appreciate pungent, sweet, bitter, umami and other tastes (Zhang et al., 2003; Bandell et al., 2004) and to discriminate between different temperatures (Clapham, 2003; Patapoutian et al., 2003). The TRP channel of vanilloid type 1 (TRPV1) has been previously recognized as the receptor for capsaicin, the pungent ingredient in red pepper fruits of the genus Capsicum that include paprika, jalapeño and cayenne (Szallasi and Blumberg, 1999). TRPV1 is involved in pain responses because it is sensitive to heat, acidosis and noxious chemicals, and it becomes sensitized by inflammatory and proalgesic mediators. Thus, TRPV1 behaves as a multimodal nocisensor of afferent neurons and is hypothesized to be a key player in the hyperalgesia associated with inflammation (Caterina et al., 2000; Davis et al., 2000). Furthermore, TRPV1 has also been found in various brain areas, including dopaminergic neurons of the substantia nigra, hippocampal pyramidal neurons, hypothalamic neurons, the locus coeruleus in the brainstem and in various layers of the cortex, where it might be involved in modulation of synaptic plasticity (Mezey et al., 2000; Roberts et al., 2004). Lastly, negative reinforcers, including pain, may have a role in opiate and alcohol dependence (see Koob, 2009; Martin and Ewan, 2008 for rev.). Thus, nociceptors such as TRP channels may be important for regulation of alcohol intake because of their known role in pain and therefore potential role in negative reinforcement.
Directly the role of TRPV1 channels in mediating behavioral effects of ethanol has not been studied although the deletion of another TRP channel, Trpm5, was shown to reduce ethanol preference and intake (Blednov et al., 2008). However, for many years it has been known that ethanol produces taste responses that include a burning sensation (Hellekant, 1965; Danilova and Hellekant, 2002; Sako and Yamamoto, 1999). The presence of the TRPV1 channel in taste receptor cells (Lyall et al., 2005a-c) provides a possible mechanism for the burning effect of ethanol. Indeed, previous studies in nociceptive neurons that innervate the face and mouth, as well as TRPV1-expressing HEK293 cells, showed that TRPV1 channels responded to ethanol at concentrations of 0.3-3% (about 65-650 mM) (Trevisani et al., 2002). Specifically, ethanol potentiated the response of TRPV1 to selective TRPV1 agonist - capsaicin and protons (acidic solution of pH 6) and lowered the threshold for heat activation of TRPV1 from 42 °C to 34 °C, which is near the temperature of the tongue. This provides a likely mechanistic explanation for the ethanol-induced sensory responses of inflamed tissues (Hirota et al., 2003). Given the widespread distribution of TRPV1 in both the peripheral and the central nervous system, activation of this channel by ethanol may be important for some peripheral or central actions of ethanol.
To determine if the vanilloid receptor may participate in regulation of ethanol consumption and other ethanol-induced behaviors, we studied null mutant mice with deletion of the Trpv1 gene (Caterina et al., 2000).
2. Materials and methods
2.1. Animals
Null mutant Trpv1 (-/-) allele mice were created using homologous recombination as previously described (Caterina et al., 2000). Commercially available breeding pairs of Trpv1 (-/-) mice on 129X1/SvJ × C57Bl/6J genetic background were purchased from Jackson Laboratories, Bar Harbor, ME. In preliminary experiments wild type as well as knockout mice from this colony showed very low level of ethanol intake. To increase ethanol consumption, mice were backcrossed two times to C57Bl/6J, and heterozygous mice from the F2 generation were used to establish the experimental colony. All behavioral analyses were performed on homozygous knockout (-/-) and wild type (+/+) littermates generated from crosses between heterozygous animals. Mice were group-housed three-five per cage based on sex. Food and water were available ad libitum. The vivarium was maintained on a 12:12 h light:dark cycle with lights on at 7:00 a.m. The temperature (22 °C) and humidity (40-60%) of the room were controlled. All experiments were performed during the light phase of the light/dark cycle. Male mice were used for all studies and were 10-16 wk old at the time of analysis; within each experimental paradigm, mice of similar ages were used. All experiments were conducted in the isolated behavioral testing rooms in the animal facility to avoid external distractions. All experiments were approved by the Institutional Animal Care and Use Committee.
2.2. Rationale for the used behavioral tests
Two-bottle choice allows measurement of ethanol preference and intake under conditions of voluntary consumption. Because the ethanol produces taste responses (sweet and bitter) it is critical to analyze the sensitivity of the genotypes to bitter (quinine solutions) and sweet (saccharin solutions) tastes. Conditioned taste aversion is used as the index of aversive properties to ethanol and the response in this test is negatively correlated with voluntary ethanol intake. Loss of righting reflex shows the anesthetic or sedative activities of ethanol and may negatively correlate with voluntary ethanol consumption. Acute ethanol withdrawal shows the sensitivity to the development of ethanol physical dependence and also negatively correlates with ethanol intake in two-bottle choice paradigm. The rotarod test shows the motor incoordination as well as recovery from mild acute ethanol intoxication.
2.3. Ethanol preference
The two-bottle choice protocol was carried out as previously described (Blednov et al., 2001). Briefly, mice were allowed to acclimate for 1 wk to individual housing. Two drinking tubes were available continuously to each mouse, and tubes were weighed daily. One tube always contained water. Food was available ad libitum, and mice were weighed every 4 d. After 4 d of water consumption (both tubes), mice were offered 3% ethanol (v/v) vs. water for 4 d. Tube positions were changed every day to control for position preferences. Quantity of ethanol consumed (g/kg body weight/24 h) was calculated for each mouse and these values were averaged for every concentration of ethanol. Immediately following 3% ethanol, a choice between 6% (v/v) ethanol and water was offered for 4 d, then 9% (v/v) ethanol vs. water for 4 d, then 12% (v/v) ethanol vs. water for 4 d and finally 15% (v/v) ethanol vs. water for 4 d. Throughout the experiment, evaporation/spillage estimates were calculated every day from two bottles placed in an empty cage, one containing water and the other containing the appropriate ethanol solution.
2.4. Preference for non-ethanol tastants
Wild type or knockout mice were also tested for saccharin and quinine consumption. One tube always contained water and the other contained the tastant solution. Mice were serially offered saccharin (0.033% and 0.066%) and quinine hemisulfate (0.03 mM and 0.06 mM) and intakes were calculated. Each concentration was offered for 4 d, with bottle position changed every day. For each tastant, the low concentration was always presented first, followed by the higher concentration. Between tastants, mice had two bottles both containing water for two weeks.
2.5. Ethanol-induced acute withdrawal
Mice were scored for handling-induced convulsions (HICs) severity 30 min before and immediately before i.p. ethanol administration. The two pre-drug baseline scores (PRE) were averaged. A dose of 4 g/kg of ethanol in saline was injected i.p., and the HIC score was tested every hour until the HIC level reached baseline. Acute withdrawal was quantified as the area under the curve but above PRE level and handling-induced convulsion scoring was performed according to Crabbe et al. (1991). Briefly, each mouse was picked up gently by the tail and, if necessary, gently rotated 180°, and HICs scored as follows: 5, tonic-clonic convulsion when lifted; 4, tonic convulsion when lifted; 3, tonic-clonic convulsion after a gentle spin; 2, no convulsion when lifted, but tonic convulsion elicited by a gentle spin; 1, facial grimace only after a gentle spin; 0, no convulsion.
2.6. Conditioned taste aversion
Subjects were adapted to a water-restriction schedule (2 h of water per day) over a 7-d period. At 48-h intervals over the next 10 d (days 1, 3, 5, 7, 9 and 11), all mice received 1-h access to a solution of saccharin (0.15% w/v sodium saccharin in tap water). Immediately after 1-h access to saccharin, mice received injections of saline or ethanol (2.5 g/kg) (days 1, 3, 5, 7 and 9). Mice also received 30-min access to tap water 5 h after each saccharin access period to prevent possible dehydration followed by injection of ethanol (2.5 g/kg, i.p.) (days 1, 3, 5, 7 and 9). On intervening days, mice had 2 h continuous access to water at standard times in the morning (days 2, 4, 6, 8 and 10).
2.7. Loss of righting reflex (LORR)
Sensitivity to ethanol was determined using the standard duration of LORR assay. Ethanol was diluted in 0.9% saline (20% v/v) and administered in doses (3.2 g/kg, 3.4 g/kg and 3.8 g/kg, i.p.) adjusted by injection volumes. Mice were injected with ethanol and when they became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 30 s. LORR was defined as the time from being placed in the supine position until they regained their righting reflex. During all LORR assays, room temperature was 22 °C. Mice that failed to lose the righting reflex (misplaced injections) or had duration of LORR greater than two standard deviations from the group mean were excluded from the analysis.
2.8. Rotarod
Mice were trained on a fixed speed rotarod (Economex; Columbus Instruments; speed of rod, 5.0 rpm), and training was complete when mice were able to remain on the rotarod for 60 s. Every 10 min after injection of ethanol (2 g/kg i.p.), each mouse was placed back on the rotarod and latency to fall was measured until the mouse was able to stay on the rotarod for 60 s.
2.9. Drug injection
All ethanol (Aaper Alcohol and Chemical, Shelbyville, KY) solutions were made in saline (20%, v/v) and injected i.p. in a volume of 0.1 ml/10 g of body weight. Capsazepine (Tocris, Ellisville, MO; 10 mg/kg i.p.), an antagonist of TRPV1, was prepared as a suspension of drug in saline with 4-5 drops of Tween-80 and injected in wild type Trpv1 (+/+) mice in a volume of 0.1 ml/10 g of body weight 15 min before administration of ethanol in LORR and rotarod experiments. Capsaicin (Tocris, Ellisville, MO; 1 mg/kg i.p.), an agonist of TRPV1, was prepared as a suspension of drug in saline with 4-5 drops of Tween-80.
2.10. Ethanol metabolism
Animals were given a single dose of ethanol (3.8 g/kg i.p.), and blood samples were taken from the retro-orbital sinus 30, 60, 120, 180 and 240 min after injection. Blood ethanol concentration (BEC) values, expressed as mg ethanol per ml blood were determined spectrophotometrically using an enzyme assay (Lundquist, 1959).
2.11. Statistical analysis
Data are reported as the mean ± S.E.M. The statistics software program GraphPad Prizm (Jandel Scientific, Costa Madre, CA) was used throughout. To evaluate differences between groups, analysis of variance (two-way ANOVA with Bonferroni post hoc analysis) and Student’s t-test were applied.
3. Results
3.1. Ethanol consumption
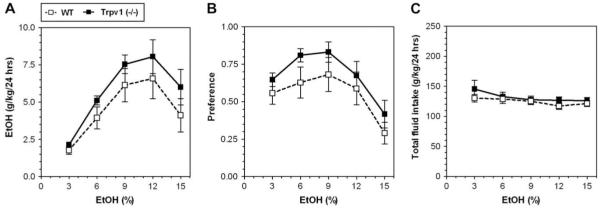
In a two-bottle test in which mice could drink either water or an ascending series of ethanol concentrations (3, 6, 9, 12 and 15%), mice lacking Trpv1 displayed increased ethanol consumption (F(1,95) = 5.0; p < 0.05, main effect of genotype; F(4,95) = 11.3; p < 0.001, main effect of concentration; 2-way AVOVA with repeated measures, factors were - genotype and ethanol concentration) (Fig. 1A) as well as increased preference for ethanol (F(1,95) = 5.7; p < 0.05, main effect of genotype and F(4,95) = 7.0; p < 0.001, main effect of concentration) (Fig. 1B). No genotype × concentration interaction was found. There were no differences in total intake of fluid (water + ethanol solution) between wild type and null mutant mice (Fig. 1C).
Fig. 1.
Increased ethanol (EtOH) consumption and preference in Trpv1 knockout (-/-) mice. A. Ethanol consumption was significantly greater in mutant Trpv1 (-/-) compared to wild type (WT) mice. B. Preference for ethanol was greater in null mutant compared to wild type mice. C. The total amount of fluid (water + ethanol) intake was stable across ethanol concentrations for mutant and wild type mice. Values are mean ± SEM, n = 10-11 for both genotypes.
3.2. Preference for non-ethanol tastants
Mice lacking Trpv1 did not differ from wild type mice in preference for saccharin (F(1,36) = 0.5, p > 0.05 and F(1,36)7.0,= p < 0.05, main effect of genotype and concentration, respectively; 2-way AVOVA with repeated measures, factors were - genotype and ethanol concentration) or for quinine (F(1,38) = 0.3, p > 0.05 and F(1,38) = 8.8, p < 0.01, main effect of genotype and concentration, respectively; 2-way AVOVA with repeated measures, factors were - genotype and ethanol concentration) (Fig. 2A and C). There were no significant differences in total fluid intake (g/kg/day) between wild type and null mutant mice for saccharin (F(1,38) = 0.1, p > 0.05 and F(1,38) = 33.5, p < 0.001, main effect of genotype and concentration, respectively) or quinine (F(1,38) = 0.3, p > 0.05 and F(1,38) = 0.2, p > 0.05, main effect of genotype and concentration, respectively)(Fig. 2B and D).
Fig. 2.
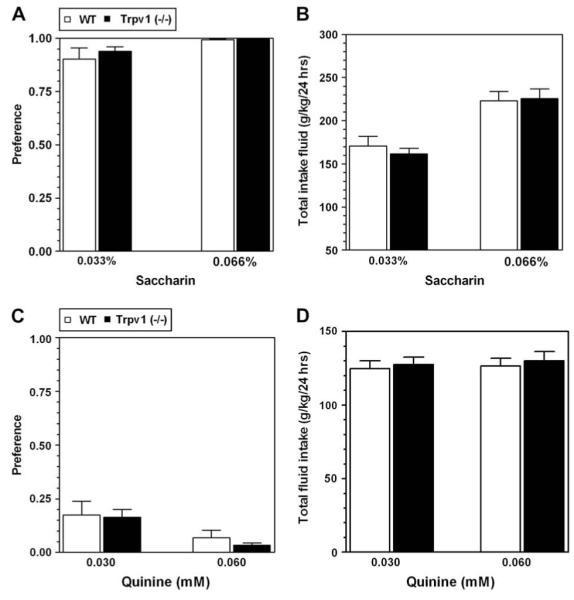
No difference in preference for sweet (saccharin) or bitter (quinine) solutions between wild type (WT) and null mutant Trpv1 (-/-) mice. A. Preference for saccharin solutions was similar in wild type and mutant mice. B. Total fluid (saccharin + water) intake was similar in wild type and mutant mice. C. Preference for quinine was similar in wild type and mutant mice. D. Total fluid (quinine + water) intake was similar in wild type and mutant mice. Values are mean ± SEM, n = 10-11 for both genotypes.
3.3. Depressant effect of ethanol
Because the experiments for each dose of ethanol in both wild type and knockout mice were done on different days and with different mice they can be considered as independent experiments and a Student t-test was used to analyze these data. The duration of loss of righting reflex (LORR) produced by ethanol was decreased in null mutants compared with wild type at 3.2 g/kg (p < 0.05) and 3.4 g/kg (p < 0.01) (Fig. 3A). No differences in duration of LORR between wild type and knockout mice were found at dose of 3.8 g/kg.
Fig. 3.
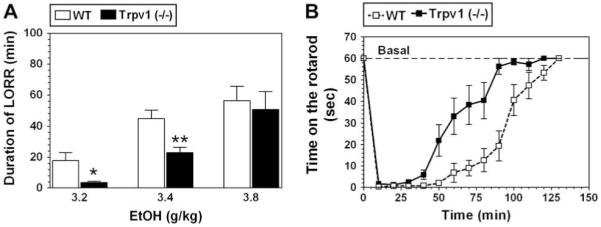
Reduced depressant effect and faster recovery from motor incoordinating effect of ethanol in Trpv1 knockout (-/-) mice. A. Duration of LORR produced by 3.2 and 3.4 g/kg ethanol (EtOH) was significantly reduced in mutant mice Trpv1 (-/-) compared to wild type (WT). Values are mean ± SEM, n = 10-14 for each dose and genotype; *p < 0.05, **p < 0.01, significant difference compared to wild type for same dose of ethanol (Student’s t-test). B. Mutant mice showed faster recovery from the motor impairing effect of ethanol (2 g/kg) compared to wild type mice. n = 10 per genotype.
3.4. Ethanol-induced motor incoordination
Acute administration of ethanol (2 g/kg) produced motor incoordination in both genotypes, but null mutant mice recovered from this impairment faster than wild type mice (F(1,252) = 65; p < 0.0001, dependence on genotype; F(13,252) = 59; p < 0.0001, dependence on time; 2-way AVOVA with repeated measures, factors were - genotype and time) (Fig. 3B). There was a significant genotype × time interaction (F(13,252) = 4.5; p < 0.0001).
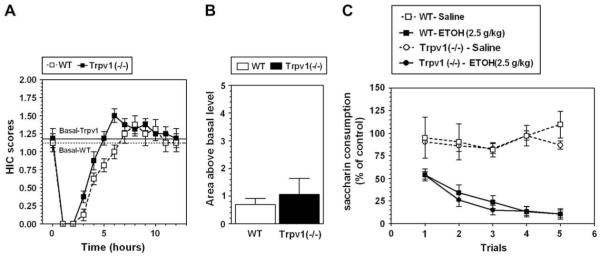
3.5. Acute ethanol withdrawal severity
Trpv1 null mutant and wild type mice did not differ in levels of basal handling-induced convulsions (HICs). A single 4 g/kg ethanol dose suppressed basal HIC in both the knockout and the wild type mice for about 5 h, followed by increased HIC (Fig. 4A). Consistent with their genetic background animals of both genotypes demonstrated weak signs of withdrawal (HIC scores higher than the basal level). However, there were no differences in area under the curve for HIC or above the basal level during withdrawal (0.69 ± 0.23 and 1.06 ± 0.58 for wild type and knockout, respectively; Student t-test) (Fig. 4B).
Fig. 4.
No differences in ethanol-induced conditioned taste aversion (CTA) and severity of acute ethanol withdrawal between wild type (WT) and Trpv1 knockout (-/-) mice. A. Ethanol (4 g/kg) affected HIC in a similar manner in wild type and mutant mice. B. There were no differences between wild type and mutant mice in area under the HIC curve or above the basal level (n = 8 for both genotypes). C. Saccharin intake decreased in both wild type and mutant mice when ethanol (EtOH) was paired with saccharin compared with saline/saccharin pairings (n = 6 for saline injection in both genotypes; n = 8 for ethanol injection in both genotypes). Values are mean ± SEM.
3.6. Conditioned taste aversion
There was no difference in consumption of saccharin on trial 0 (before conditioning) between wild type and Trpv1 null mutant mice (58.6 ± 3.9 g/kg body weight and 56.9 ± 2.9 g/kg body weight, respectively). To correct for any initial fluctuations in intake of tastant, intake was calculated as a percentage of the trial 0 consumption for each subject by dividing the amount of saccharin solution consumed on subsequent conditioning trials by the amount of saccharin solution consumed on trial 0 (before conditioning). Ethanol-saccharin pairings produced reduction in saccharin intake across trials compared with saline-saccharin pairings, indicating the development of conditioned taste aversion (CTA) in wild type mice (F(1,62) = 91; p < 0.001, effect of treatment; 2-way AVOVA with repeated measures, factors were - treatment and trial) as well as for knockout mice (F(1,62) = 366; p < 0.001, effect of treatment; F(4,62) = 6.3; p < 0.001 - dependence on trial; F(4,62) = 5.7; p < 0.001, genotype × trial interaction; 2-way AVOVA with repeated measures, factors were - treatment and trial) (Fig. 4C). However, there were no differences between wild type and Trpv1 null mice for comparison of saline-treated groups as well as for comparison of ethanol-treated groups (2-way AVOVA with repeated measures, factors were - genotype and trial).
3.7. Ethanol metabolism
There were no differences in metabolism of ethanol between wild type and mutant mice. Ethanol clearance expressed in mg of ethanol per dl of blood in 1 h was 59 ± 3 and 63 ± 4 for wild type and knockout mice, respectively (n = 5 per genotype; Student t-test).
3.8. Capsazepine administration in vivo
To determine whether the differences in ethanol behavioral phenotypes of the null mutant mice are the result of deletion of Trpv1 or compensatory changes resulting from loss of this channel, we studied the effects of an antagonist of TRPV1, capsazepine, on several ethanol behaviors.
Capsazepine (10 mg/kg) significantly attenuated the sedative effect of ethanol only at 3.4 g/kg (p < 0.001, Student t-test) but had no effect on ethanol-induced LORR at dose 3.8 g/kg (Fig. 5A). Wild type mice injected with capsazepine recovered from the motor impairing effect of ethanol faster than the saline-treated group (F(1,126) = 65; p < 0.001, main effect of treatment; F(13,126) = 75; p < 0.001, main effect of time; F(13,126) = 5.6; p < 0.001, treatment × time interaction; 2-way AVOVA with repeated measures, factors were - treatment and time) (Fig. 5B).
Fig. 5.
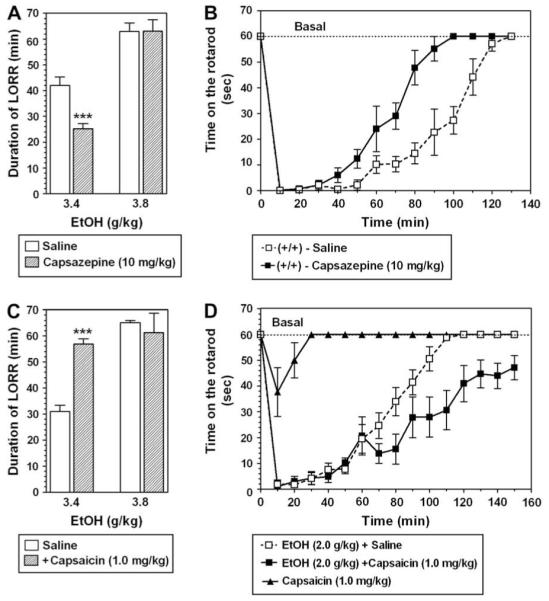
Capsazepine (10 mg/kg) and capsaicin (1.0 mg/kg) have opposite effects on ethanol-induced sedation and tolerance in wild type mice. A. Capsazepine significantly decreased the sedative effects of 3.4 g/kg ethanol but had no effect on a higher dose of ethanol in wild type mice (n = 7 for each group, ***p < 0.01 significant difference between saline and capsazepine for same dose of ethanol, Student t-test). B. Capsazepine decreased the recovery time for the motor impairing effect of ethanol (2 g/kg) in wild type mice (n = 6 for each group). C. Capsaicin significantly increased the sedative effects of the lower dose of ethanol (n = 6 for each group). D. Capsaicin increased the recovery time of the motor impairing effect of (EtOH) ethanol (2 g/kg) (n = 6-9 for each group, ***p < 0.001 significant difference between saline and capsaicin for same dose of ethanol, Student t-test). Values are mean ± SEM.
3.9. Capsaicin administration in vivo
We next used the inverse of the null approach, asking whether the activation of TRPV1 could produce changes opposite to deletion of Trpv1. We studied the effects of an agonist of TRPV1, capsaicin, on ethanol-induced LORR and recovery from motor incoordination produced by ethanol in wild type mice.
Capsaicin potentiated the sedative effect of ethanol at 3.4 g/kg (p < 0.001; Student t-test) and had no effect on ethanol-induced LORR produced by 3.8 g/kg (Fig. 5C).
Wild type mice injected with capsaicin recovered from the motor impairing effect of ethanol slower than the saline-treated group (F(1,256) = 41.5; p < 0.001, main effect of treatment; F(15,256) = 49.3.2; p < 0.001, main effect of time; F(15,256) = 2.7; p < 0.01, treatment × time interaction; 2-way AVOVA with repeated measures, factors were - treatment and time) (Fig. 5D). Capsaicin alone produced a very short loss of motor coordination (F(1,208) = 377; p < 0.001, main effect of treatment; F(15,208) = 10.2; p < 0.001, main effect of time; F(15,208) = 5.9;p < 0.001 treatment × time interaction); however, this cannot explain the long recovery in mice treated with capsaicin and ethanol together (Fig. 5D).
4. Discussion
Deletion of Trpv1 significantly increases ethanol preference and consumption, reduces the sedative effect of ethanol and shortens the recovery from acute ethanol intoxication. One potential problem in interpretation of results obtained with mutant mice is whether compensatory changes in expression of other genes occur as a result of deletion of the target gene (Ponomarev et al., 2006). In this context, it is important to note that two of three behavioral differences (sedative effect of ethanol and recovery from acute ethanol intoxication) between wild type and mutant mice were reproduced in wild type mice after administration of a TRPV1 antagonist, capsazepine. The effects of a selective TRPV1 agonist, capsaicin, were opposite to those of capsazepine, and further support a direct role of TRPV1 in these ethanol behaviors.
Increased ethanol consumption in mice lacking Trpv1 could be due to reduced ethanol-induced burning sensations since high concentrations of ethanol (3%, equivalent to 510 mM) activate TRPV1 (Trevisani et al., 2002). Indeed, Trpv1 null mutant mice trained to drink a sweet solution (saccharin), showed no aversive response after addition of capsaicin (100 μM), whereas wild type mice trained to drink saccharin, vigorously avoided capsaicin solutions (Caterina et al., 2000). However, one piece of data that is not entirely consistent with the idea that ethanol consumption is promoted by removal of the burning sensation in the null mutants is that the increase in alcohol consumption was greatest for 9% ethanol solutions. One might expect the more concentrated solutions (e.g.,15%) to show the largest effect of the mutation, and this was not the case.
An alternative explanation emerges from studies showing a negative correlation between hypnotic (LORR) effects of ethanol and voluntary ethanol consumption (Thiele et al., 1998, 2000), and the reduced LORR and increased ethanol consumption resulting from deletion of TRPV1. Therefore, reduction of ethanol-induced sedation together with increased recovery from acute ethanol-induced intoxication could lead to increased ethanol consumption found in Trpv1 knockout mice. In Trpv1 null mutants, we did not see changes in two other ethanol behaviors that negatively correlate with ethanol consumption and potentially could change ethanol consumption: ethanol-induced conditioned taste aversion (for review see Chester and Cunningham, 2002) and acute ethanol withdrawal (Metten et al., 1998). One approach that might distinguish between the two possibilities (peripheral burning or central actions) is injection of capsazepine or capsaicin and asks if they change drinking (as was done for ataxia and LORR). However, this is not feasible because drinking in wild type and knockout mice was measured over a 24 h period and the duration of action of these drugs is too short to change drinking over this time frame. We attempted to employ shorter drinking schedules (e.g., measurement of drinking during 2 h at the beginning of the dark phase for either continuous access or limited access to alcohol), but drinking in these tests was not affected by deletion of Trpv1, thus they could not be used to test the effects of TRPV1 agonists or antagonists (data not shown).
There is interest in endogenous ligands that may use TRPV1 for inter- or intracellular signaling (Di Marzo et al., 2001; van der Stelt and Di Marzo, 2004). Although somewhat controversial, anandamide (AEA) was proposed as a candidate because of its structural similarity to capsaicin (Suh and Oh, 2005). AEA activates TRPV1 in recombinant and endogenous systems, which reinforces the possibility that it functions as an endovanilloid (Di Marzo et al., 2002). Lipids are also thought to regulate TRP channel function (Hofmann et al., 1999). The extensive co-localization of fatty acid amide hydrolase (FAAH) with TRPV1 in the mouse brain is consistent with the hypothesis that AEA may act as a physiological ligand of brain TRPV1 (see Starowicz et al., 2007 for review). The role of endocannabinoids in neural circuitry regulating motivation for alcohol and other drugs of abuse is well established (see Colombo et al., 2005; Maldonado et al., 2006; Gardner, 2005; Hungund and Basavarajappa, 2004). AEA is inactivated through cellular reuptake and enzymatic degradation by FAAH, which hydrolyzes AEA to arachidonic acid (Cravatt et al., 1996), and it is notable that the changes in ethanol behaviors produced by deletion of TRPV1 are identical to those produced by deletion of FAAH (Blednov et al., 2007). These data raise the possibility that endocannabinoid signaling through TRPV1 receptors opposes the effects of endocannabinoids mediated by brain cannabinoid (CB1) receptors. Consistent with this proposal, AEA is known to act simultaneously on CB1 and TRPV1 receptors to induce opposite effects. Ahluwalia et al. (2003) found that AEA had dual inhibitory and excitatory effects on calcitonin gene-related peptide release from cultured primary sensory neurons. The inhibitory effect of AEA was completely blocked by the CB1 receptor antagonist, SR141716A, while the excitatory effect of AEA was abolished by capsazepine, indicating that the inhibitory and excitatory effects of AEA were mediated exclusively by CB1 and TRPV1, respectively. Dual AEA effects on neuropeptide release were also mediated via TRPV1 and CB1 receptors as reported in a tracheal preparation in vitro (Nemeth et al., 2003). Thus, exogenous AEA can affect TRPV1 function, but it is not clear that the levels of endocannabinoids produced within neurons are sufficient to act as endogenous ligands for TRPV1.
Ethanol changes the levels of brain endocannabinoids, decreasing AEA with acute or short term treatments (Rubio et al., 2007; Caillé et al., 2007; Ferrer et al., 2007) and increasing the level of endocannabinoids but reducing the number of CB1 receptors during chronic ethanol-induced dependence and withdrawal (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999; Mitrirattanakul et al., 2007). It is possible that fluctuations in endocannabinoids can alter the behavioral actions of ethanol through actions on both CB1 and TRPV1 receptors. Our genetic and pharmacological dissections indicate that these two pathways will have opposite consequences on ethanol sedation, tolerance and consumption, thus providing homeostatic regulation. Chronic, excessive alcohol consumption, which has more pronounced effects on endocannabinoids and cannabinoid receptors compared to short term, moderate consumption (Mitrirattanakul et al., 2007), may disrupt this homeostatic balance (Koob and Le Moal, 2001).
Acknowledgments
This study or research was supported by grants from the National Institute of Alcohol Abuse and Alcoholism (AA U01 13520 - INIA Project) and NIH A06399. The authors would like to thank Virginia Bleck and Danielle Walker for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Ahluwalia J, Urban L, Bevan S, Nagy I. Anandamide regulates neuropeptide release from capsaicin-sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. Eur. J. Neurosci. 2003;17:2611–2618. doi: 10.1046/j.1460-9568.2003.02703.x. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J. Neurochem. 1999;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J. Pharmacol. Exp. Ther. 2001;298:521–530. [PubMed] [Google Scholar]
- Blednov YA, Cravatt BF, Boehm SL, 2nd, Walker D, Harris RA. Role of endocannabinoids in alcohol consumption and intoxication: studies of mice lacking fatty acid amide hydrolase. Neuropsychopharmacology. 2007;32:1570–1582. doi: 10.1038/sj.npp.1301274. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J. Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptor modulation of the rewarding and aversive effects of ethanol. Alcohol. 2002;26:131–143. doi: 10.1016/s0741-8329(02)00199-4. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Colombo G, Serra S, Vacca G, Carai MA, Gessa GL. Endocannabinoid system and alcohol addiction: pharmacological studies. Pharmacol. Biochem. Behav. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill C, Belknap JK. Acute dependence on depressant drugs is determined by common genes in mice. J. Pharmacol. Exp. Ther. 1991;257:663–667. [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Danilova V, Hellekant G. Oral sensation of ethanol in a primate model III: responses in the lingual branch of the trigeminal nerve of Macaca mulatta. Alcohol. 2002;26:3–16. doi: 10.1016/s0741-8329(01)00178-1. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Brandi I, Jefferson RG, Winckler RL, Davis JB, Dasse O, Mahadevan A, Razdan RK, Martin BR. Highly selective CB(1) cannabinoid receptor ligands and novel CB(1)/VR(1) vanilloid receptor “hybrid” ligands. Biochem. Biophys. Res. Commun. 2001;281:444–451. doi: 10.1006/bbrc.2001.4354. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Blumberg PM, Szallasi A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- Ferrer B, Bermúdez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodríguez de Fonseca F. Regulation of brain anandamide by acute administration of ethanol. Biochem. J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol. Biochem. Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Hellekant G. The effect of ethyl alcohol on non-gustatory receptors of the tongue of the cat. Acta Physiol. Scand. 1965;65:243–250. doi: 10.1111/j.1748-1716.1965.tb04267.x. [DOI] [PubMed] [Google Scholar]
- Hirota K, Smart D, Lambert DG. The effects of local and intravenous anesthetics on recombinant rat VR1 vanilloid receptors. Anesth. Analg. 2003;96:1656–1660. doi: 10.1213/01.ANE.0000061580.89627.91. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Basavarajappa BS. Role of endocannabinoids and cannabinoid CB1 receptors in alcohol-related behaviors. Ann. N.Y. Acad. Sci. 2004;1025:515–527. doi: 10.1196/annals.1316.064. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissue. Methods Biochem. Anal. 1959;7:217–251. [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem. Senses. 2005a;30:i42–i43. doi: 10.1093/chemse/bjh104. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, Desimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J. Gen. Physiol. 2005b;125:587–600. doi: 10.1085/jgp.200509264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, DeSimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. I. Effect on TRC volume and Na+ flux. J. Gen. Physiol. 2005c;125:569–585. doi: 10.1085/jgp.200409213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Ewan E. Chronic pain alters drug self-administration: implications for addiction and pain mechanisms. Exp. Clin. Psychopharmacol. 2008;16:357–366. doi: 10.1037/a0013597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm. Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like inmmunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin. Exp. Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Nemeth J, Helyes Z, Than M, Jakab B, Pinter E, Szolcsanyi J. Concentration-dependent dual effect of anandamide on sensory neuropeptide release from isolated rat tracheae. Neurosci. Lett. 2003;336:89–92. doi: 10.1016/s0304-3940(02)01221-1. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Maiya R, Harnett MT, Schafer GL, Ryabinin AE, Blednov YA, Morikawa H, Boehm SL, 2nd, Homanics GE, Berman AE, Lodowski KH, Bergeson SE, Harris RA. Transcriptional signatures of cellular plasticity in mice lacking the α1 subunit of GABAA receptors. J. Neurosci. 2006;26:5673–5683. doi: 10.1523/JNEUROSCI.0860-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC, Davis JB, Benham CD. [3H]-Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004;995:176–183. doi: 10.1016/j.brainres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Rubio M, McHugh D, Fernández-Ruiz J, Bradshaw H, Walker JM. Shortterm exposure to alcohol in rats affects brain levels of anandamide, other N-acylethanolamines and 2-arachidonoyl-glycerol. Neurosci. Lett. 2007;421:270–274. doi: 10.1016/j.neulet.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Electrophysiological and behavioral studies on taste effectiveness of alcohols in rats. Am. J. Physiol. 1999;276:R388–R396. doi: 10.1152/ajpregu.1999.276.2.R388. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- van der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Suh YG, Oh U. Activation and activators of TRPV1 and their pharmaceutical implication. Curr. Pharm. Des. 2005;11:2687–2698. doi: 10.2174/1381612054546789. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J. Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Tognetto M, Gunthorpe M, Campi B, Amadesi S, Gray J, Jerman J, Owen D, Smith G, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]