TO THE EDITOR: Glioblastoma is a lethal malignant brain tumor with overall survival rates of less than 3.3% at 5 years1. Very few effective treatment options are currently available for patients. Even for those who show temporary radiographic responses to treatments, the durability of such responses is limited and median survival is less than two years.
At UCLA, we have an ongoing IRB-approved Phase I clinical trial investigating the use of autologous dendritic cell (DC) vaccination as adjunctive therapy in malignant glioma. All patients receive surgery, standard radiation and Temozolomide chemotherapy, and subsequent vaccination using DC pulsed with autologous tumor lysate. We have enrolled 14 newly diagnosed glioblastoma (WHO grade IV) patients to date, with some interesting immunological “responses”.
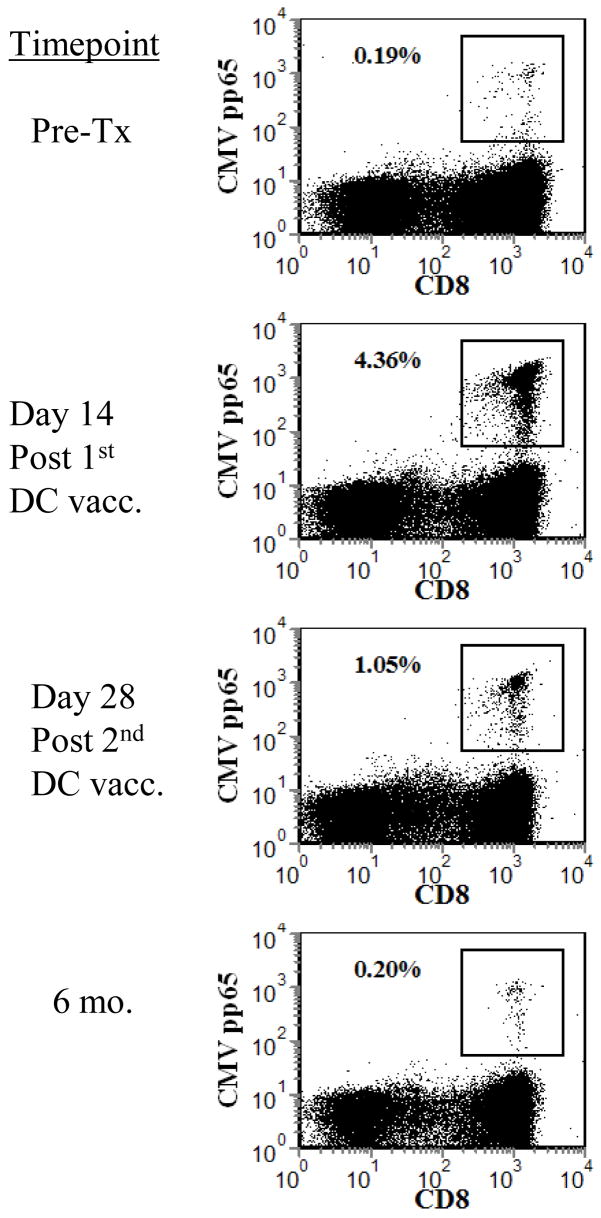
Herein, we report on a patient with glioblastoma who developed an extremely robust human cytomegalovirus (HCMV)-specific CD8+ T-cell response to the pp65 CMV immunodominant epitope that began immediately after one injection of autologous tumor lysate-pulsed DC (figure 1A,B). This rapid expansion of an anti-CMV immune response was compelling (4.39% of CD8+ T cells became specific for pp65 after vaccination) and was not associated with any systemic symptoms of viral illness. Local lymphadenopathy was observed at the same time as the HCMV-specific T-cell expansion. Such focal axillary and cervical lymph node swelling on the side of DC injection has been occasionally observed in our other clinical trial patients following vaccination 2.
Figure 1. Induction of HCMV-specific immune responses following autologous tumor-lysate-pulsed DC vaccination.
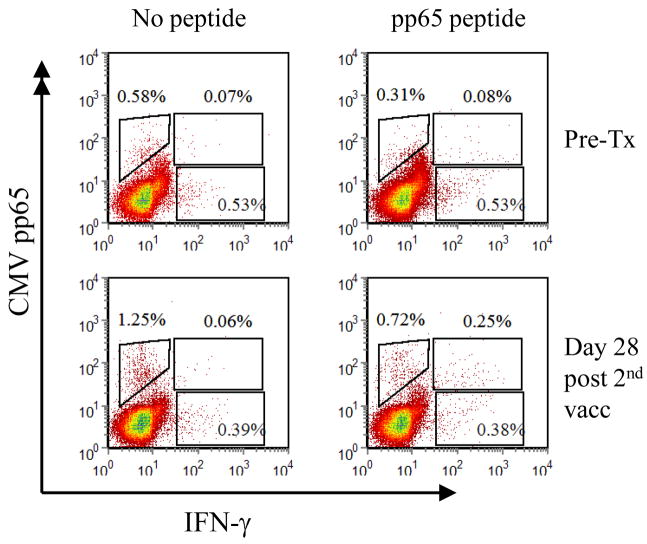
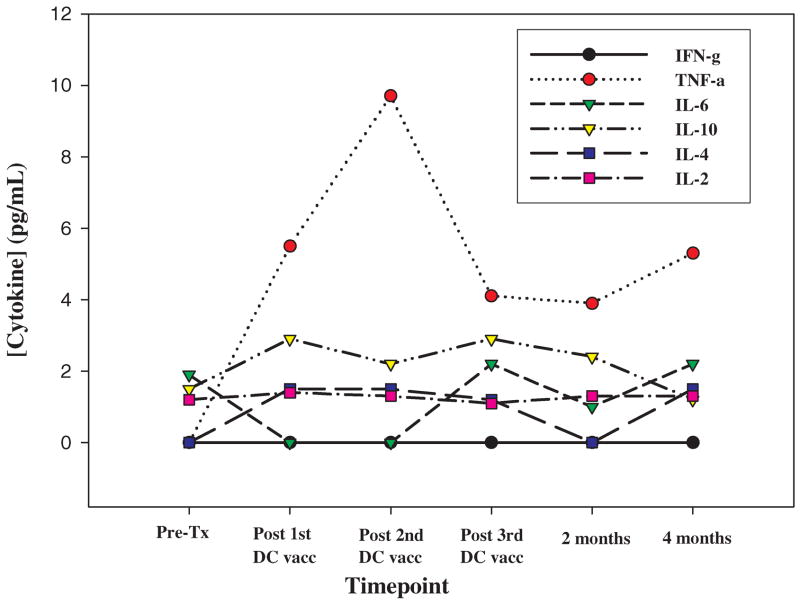
(A) Histograms showing HCMV-specific CD8+ T cells (CD4/13/19−CD3+CD8+CMVpp65 tetramer+) prior to treatment, after one and two DC vaccinations, and at 6 months after DC vaccination. Annotated numbers reflect the percentage of all CD3+CD8+ T cells. (B) Combined tetramer and intracellular cytokine staining of patient #4-908 PBMC from pre-treatment (Top plots) and after 2 DC vaccinations (bottom plots). PBMC were restimulated for six hours with (right plots) or without (left plots) the immunodominant HCMV peptide in the presence of Brefeldin A, followed by surface antibody labeling, fixation/permeabilization and labeling of intracellular IFN-γ. Annotated boxed numbers reflect the frequency of CMV+IFN-γ−, CMV+IFN-γ+ and CMV−IFN-γ+ T cells gated from the CD3+CD8+ population.~ (C) Immunohistochemical localization of HCMV pp65 in glioblastoma. 6 μm sections were deparaffinized in xylene, subjected to an antigen retrieval solution (Dako) and incubated with the pp65-specific mAb (Novocastra) overnight in a humidified chamber at 4°C. Expression was revealed after the incubation with a biotinylated secondary mAb (Vector Labs) and DAB color development (Vector Labs). (D) Serum cytokine levels during the initial bi-weekly DC vaccination regimen. 50 microliters of serum was tested for the presence of TH1 (IL-2, TNF–α, IFN-γ) and TH2 (IL-4, IL-6, IL-10)-type cytokines by cytometric bead array at the indicated timepoints.
Interestingly, the patient’s tumor appeared to be infected with CMV. Immunohistochemical staining on tumor tissue demonstrated the focal expression of the HCMV pp65 within this patient’s tumor (#4-908), but not in a similar HLA-A0201+ glioblastoma patient (#2-261) who did not show increased CMV-specific anti-tumor immune responses after vaccination (figure 1C). Serum cytokine measurements revealed detectable increases in TNF-α following one DC vaccination, which peaked two weeks after the second DC vaccination (figure 1D).
Recently, an association has been discovered between human cytomegalovirus (HCMV) and malignant gliomas in vivo3–5. To our knowledge, this is the first report describing the induction of a CMV-specific T-cell immune response in a patient with glioblastoma following therapeutic vaccination. Our results suggest that the selective tropism of HCMV for glial cells may represent an attractive immunotherapeutic target in glioblastoma patients. The immune recognition of such foreign, viral antigens is promising in light of the comparative ease for which cellular immune responses can be generated against viral epitopes compared with “self” tumor antigens. This case report highlights the potential for targeted DC-based vaccination strategies as an adjunct to standard treatments for glioblastoma, although further multi-center, randomized studies will be necessary to demonstrate true clinical efficacy.
References
- 1.Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20:E1. doi: 10.3171/foc.2006.20.4.E1. [DOI] [PubMed] [Google Scholar]
- 2.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 3.Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50. [PubMed] [Google Scholar]
- 4.Ho KL, Gottlieb C, Zarbo RJ. Cytomegalovirus infection of cerebral astrocytoma in an AIDS patient. Clin Neuropathol. 1991;10:127–33. [PubMed] [Google Scholar]
- 5.Mitchell DA, Xie W, Schmittling R, et al. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro Oncol. 2008;10:10–8. doi: 10.1215/15228517-2007-035. [DOI] [PMC free article] [PubMed] [Google Scholar]