Abstract
The L-type Ca2+ channel (CaV1.2) is the main pathway for trans-sarcolemmal (SL) Ca2+ influx in cardiac myocytes. To maintain Ca2+ homeostasis, chronic SL Ca2+-influx must be matched by chronic SL efflux. In this study we tested the hypothesis that chronic down-regulation of SL Ca2+-entry regulates SL extrusion. We studied mRNA and Ca2+ handling responses to chronic down-regulation of Ca2+ channel current induced by over-expression of the small GTPase Rem. Rem lowered net SL diastolic Ca2+-entry, and reduced the twitch Ca2+ amplitude. Rem also significantly slowed Ca2+ transient decay kinetics (p<10-3). Rem reduced NCX1.1 protein level and function. To measure Na-Ca2+-exchange (NCX) function and sarcoplasmic reticulum (SR) store load we perfused Ca2+-free bath for 25s followed by rapid application of 50mM caffeine. In control, caffeine transient relaxations were described by a bi-exponential decay with a fast phase that was 10mM Ni2+-senstive. Rem significantly slowed caffeine-induced relaxation time course (Rem vs control, p<10-6). To test whether extrusion slowing was mediated by insufficient basal Ca2+ for allosteric NCX activation we measured the effect of increasing bath Ca2+ from 1.8 to 6mM on caffeine-induced relaxation kinetics. 6mM Ca2+ did not alter kinetics of control cells, but in Rem-over-expressed cells 6mM Ca2+ sped kinetics. We conclude that chronic block of CaV1.2 channel-mediated SL entry alters NCX expression, and coincidentally controls SR Ca loading and SL Ca2+ efflux.
Keywords: Ca-channel, Na / Ca exchanger, G-proteins, calcium (cellular), sarcolemma
1. Introduction
Sarcolemmal (SL) Ca2+ flux is crucial for beat to beat excitation-contraction coupling (ECC) in cardiac myocytes (CM). In mature CM, SL Ca2+ flux is dominated by L-type Ca2+ channel (CaV1.2) entry, this L-type Ca2+ current (ICa,L) provides the trigger for Ca2+-induced Ca2+ release (CICR) of sarcoplasmic reticular (SR) Ca2+. During the relaxation phase Ca2+ is extruded from the cytosol by activation of forward mode Na+-Ca2+ exchange current, and SR Ca2+ is returned via the SERCa-ATPase. A SL Ca-ATPase also extrudes cytosolic Ca2+, but it is slower and a relatively minor contributor in most preparations[1, 2]. To maintain Ca2+ homeostasis, CMs must balance SL Ca2+-entry to SL Ca2+-efflux. Although beat to beat imbalances may occur, a sustained mismatch of SL Ca2+ influx/efflux would result in a chronic change of internal Ca2+.
For any given beat the fraction of Ca2+ from SL/SR varies depending on species and age. In mature rodents only ∼8% of Ca2+ flux originates from the extracellular space, whereas in humans and other large mammals ∼30% of Ca2+ flux is trans-SL[3]. Similarly, an important distinction between mature and developing systems is the relative importance of trans-SL Ca2+ compared to SR as a source of cytosolic Ca2+. In the developing heart there is a greater reliance on SL Ca2+; although the SR is functional [4, 5]. In early development NCX protein levels[6] and NCX activity is elevated compared to mature heart[5].
Embryonic cardiac myocytes express L- and T-type Ca2+ channel currents ICa,L, ICa,T encoded by CaV1.2/1.3, and CaV3.1, respectively[7]. ICa,L is preferentially blocked by dihydropyridine and arylalkylamine drugs. More recently, small G-proteins of the RGK class are a novel means to selectively block ICa,L to the exclusion of effects on ICa,T [8-10].
Many studies have focused on compensatory Ca2+ handling changes induced by over-expression, knock-out, or conditional knock-out of NCX. In embryonic cardiac myocytes SL Ca2+ efflux is accomplished by NCX1.1 and to a lesser extent PM Ca2+-ATPase[5]. NCX1.1 knock-out is embryonic lethal at about embryonic day 11 (ED11;[11]-[12],) but prior to lethality transients exist due to the function of the slower efflux pathway provided by PM Ca2+-ATPase[12]. Most recently it was shown that conditional NCX knock-down resulted in compensatory reduction of ICa,L[13]. This suggests that the reciprocal may also be true. That is, we posit that PM Ca entry, primarily via CaV1 channels, ultimately determines Ca2+ regulation. This postulate takes on additional importance considering that ICa,L can be down-regulated either in disease[14]-[15], or as a consequence of therapeutic doses of Ca channel block in hypertensive patients. We now show that chronic regulation of SL Ca2+ entry is coupled with SL Ca2+ efflux via two coincident compensatory mechanisms: NCX1.1 protein level is reduced, and homeostatic regulation of diastolic cytosolic Ca2+ down regulates NCX function.
2. Materials & Methods
2.1 Cell culture & Cytosolic Calcium Imaging
E10 embryonic ventricular myocytes (EVMs) for Ca-imaging were isolated as in references [7, 8](details in expanded materials and methods). Cells were transfected with cDNA plasmids encoding either GFP-Rem or GFP (control), and cells were tested ∼48 hours post-transfection. Cardiac myocytes were loaded with 2 μM fura-2-AM for 10 minutes in a 5% CO2 incubator and then de-esterified in Tyrodes (140 NaCl, 1.8 CaCl2, 1 MgCl2, 10 Hepes (free acid), 5.4 KCl, 10 Glucose, pH = 7.4, NaOH) solution for ∼20 minutes. All recordings were performed at 37°C. The measurement of net SL diastolic calcium-entry was made by subtracting the baseline of recording (baseline defined as the mean end-diastolic Ca level for 3 successive beats) in 1.8 mM bath Ca2+ solution from lowest point between 20 and 25 seconds after 0 bath Ca2+ (140 NaCl, 1 MgCl2, 10 Hepes (free acid), 5.4 KCl, 10 Glucose, 5 EGTA, pH = 7.4, NaOH was introduced (basal calcium level). The time for 90% (or 50%) decay of Ca transients (t90 or t50) was measured by first obtaining the difference amplitude preceding the onset to the peak, and then the time for the Ca transient from the peak to reach 90% (or 50%) decay was reported.
2.2 Real-time RT-PCR and Western blots
Details and primer sequences are provided in expanded materials and methods (supplement). NCX1.1 densitometry was normalized to GAPDH. Equal protein (20 μg) was run in all lanes.
2.3 Computer simulations
To simulate calcium transients in a myocyte, we used the Shannon-Bers Model [16] which can be downloaded from: (http://www.luhs.org/depts/physio/personal_pages/bers_d/index.html). The Shannon-Bers model is a set of ordinary differential equations which simulate excitation-contraction coupling in a mature rabbit ventricular myocyte. To reproduce some features of the experimental results, we removed the reverse mode of the SERCA pump, and we adjusted the following parameters: GCaB (0.00025 -> 0.0015 mS/μF); INaCaX_Vmf(9.0 -> 18.0 A/F); and ISRCaP_Vmf(286 -> 200 μmol/l cytosol/s). To model acute LCC block, we decreased the permeability of ions through LCC by a factor of 0.01.
3. Results
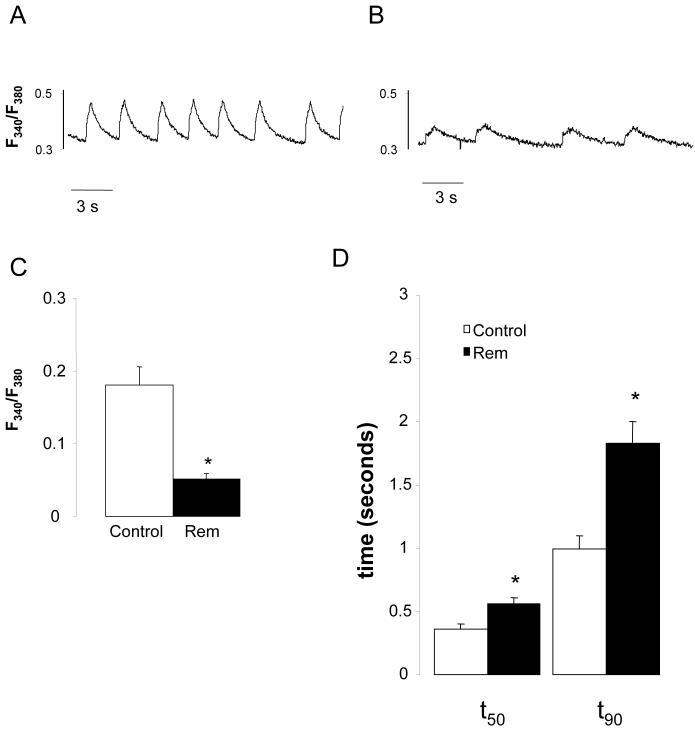
To maintain the native intracellular environment we measured Ca2+ kinetics from intact, spontaneously active embryonic day 10 ventricular myocytes (E10 EVMs). Figure 1 shows representative spontaneous, rhythmic Ca2+ transients exhibited by control cells and by cells over-expressing Rem. Three features are obvious: Rem slows the spontaneous rate (control, 0.41+/-0.07 Hz, n=14; Rem, 0.22+/-0.04 Hz, n =20; p=0.01), reduces individual twitch amplitude (control, 0.18+/-0.02 ΔF340/F380, n =14; Rem 0.05+/-0.01 ΔF340/F380, n=20; p<10-7), and significantly slows the twitch decay kinetics, t50, control 0.36 ± 0.04 (n=14) versus Rem, 0.56±0.04 (n=20; p=10-3); and t90 for control 1.0 ± 0.10 (n=14), versus Rem, 1.8 ± 0.2 (n=20; p<10-3).
Figure 1.
Rem slows spontaneous beat rate, reduces Ca2+ transient amplitude and slows Ca2+ transient kinetics. A,B) Representative Ca2+ transients from a control cell (A), and Rem-transfected cell (B). C) Rem significantly reduces twitch amplitude (p<10-7). D) Rem slows twitch Ca2+ 90% and 50% decay times (t90 and t50, p<10-3; n=14 control, n=20 Rem transfected).
3.1 Rem partially inhibits NCX1.1 expression
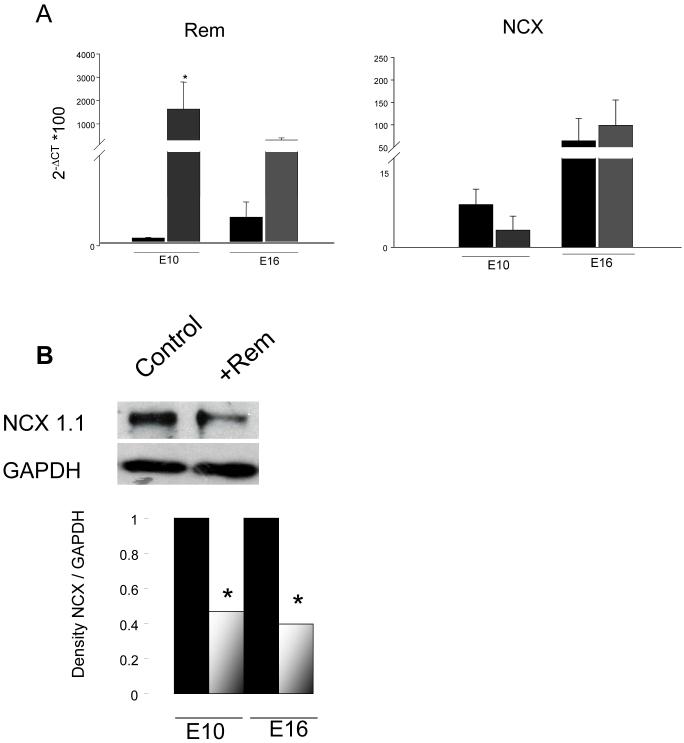
Rem and NCX1.1 mRNAs are expressed by E10 VM (Figure 2A). Rem over-expression may slow Ca2+ extrusion by down-regulating NCX. However, Rem over-expression does not significantly change NCX mRNA levels (Figure 2A). To evaluate NCX1.1 protein level a typical Western blot is shown in Figure 2B. Rem-over-expressing E10 VMs compared to control VMs show a 47% decrease of NCX1.1 protein. Early embryonic stages have more reliance on SL, but early stages pose a practical limitation - the low tissue weight creates a challenge to obtain sufficient quantity of protein for Western blot analysis. As a compromise, and secondarily to test for embryonic age-independent effects, we repeated Western blot analysis of Rem-induced changes of NCX. Rem over-expression significantly decreases NCX1 protein in E16 VMs (Figure 2B), and this decrease is similar to that in E10 EVM.
Figure 2.
Rem has no effect on NCX1.1 mRNA level, but decreases NCX1.1 protein similarly in E10 and E16 embryonic ventricular myocytes. A) qRT-PCR for Rem (left) and NCX1.1 (right) in control versus Rem over-expressing cells. (E10, n=3; E16 n=4; triplicates for each n=1). B) Western blot of NCX1.1 and GAPDH in control and Rem over-expressing cells. *p<0.001. (E10, n=3; E16, n=6).
3.2 Rem inhibits Ca2+ efflux
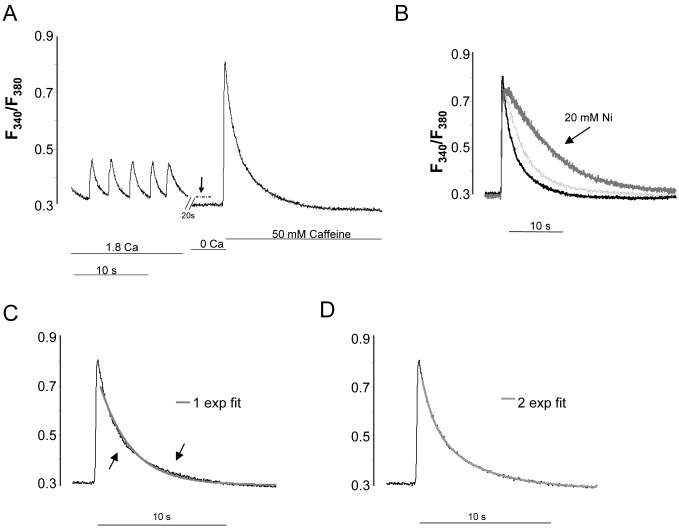
We previously established that Rem blocks cardiac ICa,L in E10 VM [8]. The prolonged Ca2+ extrusion kinetics shown in Figure 1, and reduced protein level (Figure 2) suggest a Rem effect on NCX as well. To assess SL Ca2+ efflux, we switched the bath solution to 0 Ca2+ bath (=0-added Ca2+, 5mM EGTA) for 25s, and then abruptly added 50 mM caffeine. Removal of bath Ca2+ causes an instantaneous cessation of spontaneous activity and a drop of cytosolic Ca2+ (Figure 3A, dashed line). Subsequent addition of caffeine induces a large transient (CaffTr) with relaxation kinetics (Figure 3B) that is well-defined by bi-exponential function in control cells, but not adequately fitted by a single exponential function (Figure 3C-D). In the presence of Ni2+ relaxation kinetics required only a single, slow exponential function (supplemental data). 10 mM Ni2+ eliminated the fast component of relaxation, but had no effect on the slow component. 20 mM Ni2+ slowed relaxation further (Figure 3B).
Figure 3.
Representative control cell demonstrating SL contribution, SR load, and SL efflux. A) Ca2+ dynamics in response to sequential change of solutions from normal tyrodes, to Ca2+-free, to Ca2+-free+50 mM caffeine. The vertical arrow and dashed line highlights the difference between diastolic Ca of spontaneously beating cells, and Ca2+ in the absence of SL Ca2+ influx. For all experiments, 25 s lapsed between onset of Ca2+-free and addition of caffeine to Ca2+-free bath solution. B) Caffeine-induced Ca2+ transient in Ca2+-free bath solution superimposed over that elicited with the addition of 10 (light grey) or 20 mM Ni2+ (dark grey). Note that increasing Ni2+ progressively slows decay kinetics. C,D) The caffeine-induced Ca2+ transient decay (CaffTr) is not well described by a single exponential function (C), but is well-described by a bi-exponential function (D). CaffTr + Ni2+ exhibited only the slow exponential component (not shown).
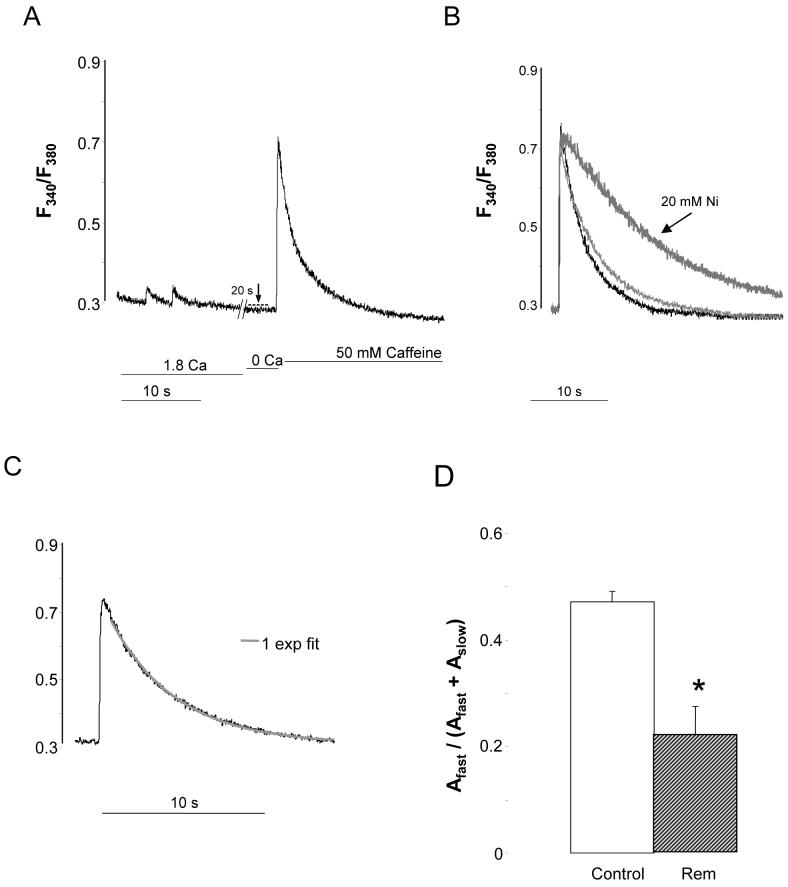
Rem over-expression significantly reduces net SL diastolic Ca2+-entry (control, 0.049+/-0.005 ΔF340/F380, n=14; Rem, 0.030+/-0.003 ΔF340/F380, n=20; p=0.003) (Figure 4A-dashed line), and significantly slows the caffeine relaxation time (Figure 4A; Table 1). This Reminduced slowing is progressively more pronounced for t50 than t75 or t90 (Table 1). In 10 of 20 cells no fast component of relaxation was observed (Figure 4C), and in the remaining 10 cells the fast component was significantly slower than that in control cells. 10 mM and 20 mM Ni2+ progressively slowed relaxation rates (Figure 4B; Supplemental Table). In control ionic conditions, chronic Rem over-expression significantly reduced the fast relaxation of the caffeine transient (47.3+/-2.0%, n=14, versus 22.0+/-5.5%, n=20; control versus Rem, p<10-3); Figure 4D). These results suggest that Rem over-expression inhibits NCX function.
Figure 4.
Rem decreases SL Ca2+-entry and slows CaffTr kinetics. A) Representative Ca2+ dynamics in Rem-transfected CM using same protocol as Figure 2A. B) CaffTr in Ca2+-free bath (black trace) and in response to 10 mM Ni2+ (light grey) and 20 mM Ni2+. C) Single-exponential fit describes CaffTr in representative Rem-transfected cells. D) Pooled data of the fraction of fast amplitude of CaffTr. Control ampfast was significantly greater than control cells with 10 mM Ni2+, or control cells versus Rem-transfected cells (p<10-3; n=20 Rem, n=14 control).
Table 1.
Caffeine transient decay times
| Condition | Caffeine Decays (n) | t50 (s) | t75 (s) | t90 (s) |
|---|---|---|---|---|
| Control | 14 | 1.15 ± 0.05 | 2.59 ± 0.13 | 4.82 ± 0.25 |
| Rem | 20 | 3.11 ± 0.28* | 6.37 ± 0.50* | 10.41 ± 0.63* |
| Fold-slowing | 2.7 | 2.5 | 2.2 |
p<10-7
3.3 Cytosolic Ca2+ Homeostasis as a Mechanism of Rem-induced reduction of trans-PM Ca2+ efflux
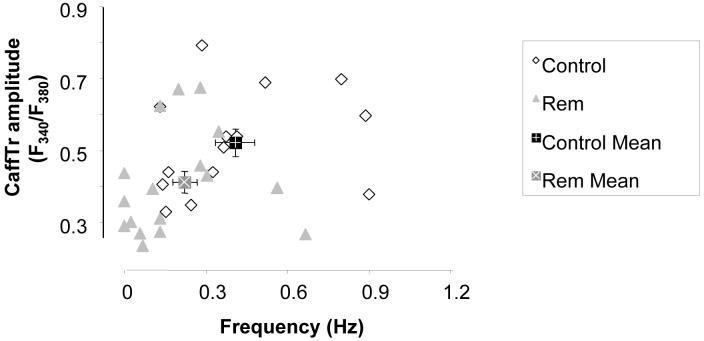
In this study, the beating frequency ranged from 0 (quiescent) to ∼0.9 Hz. As shown above (Figure 1), Rem slows the natural frequency. Thus an important caveat is that frequency may regulate SR Ca2+ load secondary to Rem effects. To address this issue we plotted the amplitude of caffeine-releasable Ca2+ transient (CaffTr) as a function of beat frequency. Figure 5 shows that there is no dependence of SR Ca2+ load (defined as CaffTr amplitude) on frequency within the Rem or control groups. However, both SR Ca2+ load (control, 0.522+/-0.038 ΔF340/F380, n=14; Rem, 0.411+/-0.030 ΔF340/F380, n=20; p=0.01), and frequency (Figure 1) are reduced by Rem. Thus, we conclude that ICa,L-mediated Ca2+ entry regulates SR Ca2+ load and beat rate independently.
Figure 5.
SR Ca2+ load does not significantly vary as a function of natural beating frequency. Caffeine-induced Ca2+ transient amplitude plotted versus prior spontaneous beat frequency for control cells (open diamonds) and Rem-transfected cells (closed triangles). However, mean frequency and mean SR load are significantly different between control (closed square), and Rem-transfected cells (grey).
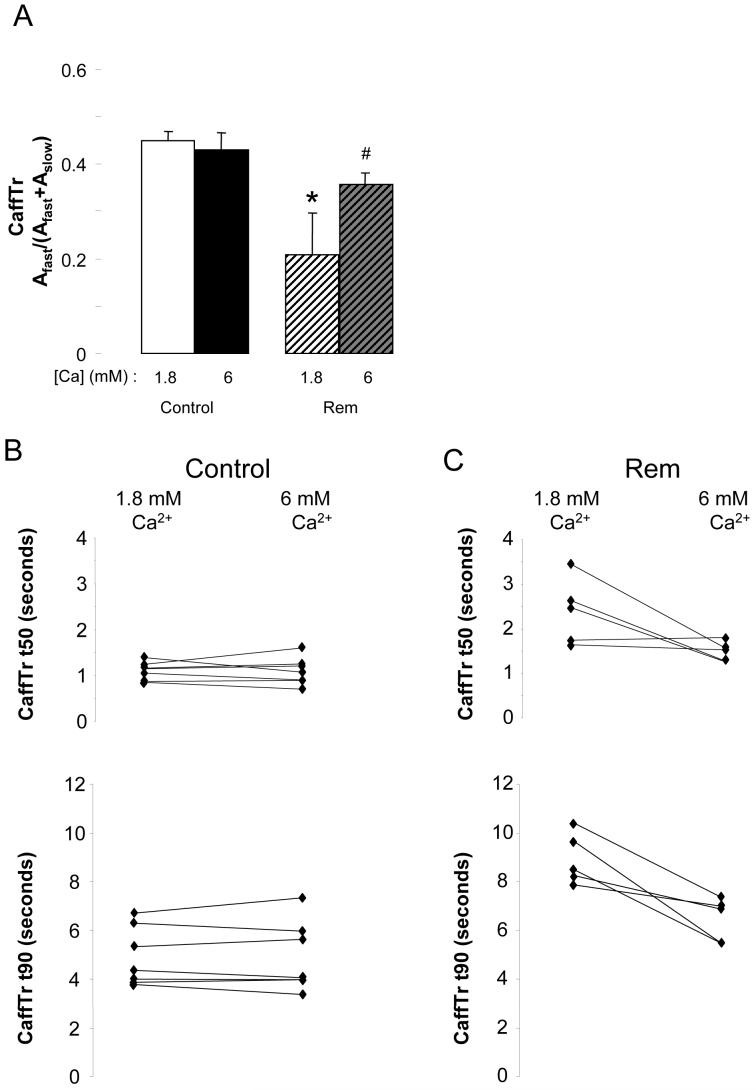
We next tested the hypothesis that the kinetics of the CaffTr relaxation was slower in Rem because low diastolic Ca2+ caused a memory-dependent inactivation of NCX[17]. To address this we tested the effect of raising bath Ca from 1.8 to 6mM on the decay kinetics of Ca2+ transients induced by caffeine in the absence of bath Ca2+. Figure 6A shows that raising bath Ca2+ had no effect on the fast phase of CaffTr in control (1.8 mM Ca2+, 44.8+/-2.1%, n=6; 6 mM Ca2+, 43.0+/-3.7%, n=6). In contrast, elevated bath Ca2+ significantly increased the fast component in cells over-expressing Rem (1.8 mM Ca2+, 20.9+/-8.6%, n=5; 6 mM Ca2+, 35.8+/-2.4%, n=5; p=0.04). This fast component of relaxation is inhibited by 10mM Ni2+ as expected for NCX. Similarly, elevated bath Ca2+ speeded kinetics of the caffeine transient relaxation in Rem over-expressing cells (Figure 6C), but kinetics was unchanged in control cells (Figure 6B).
Figure 6.
Increasing bath Ca2+ from 1.8 to 6 mM restores fast component of CaffTr relaxation. A) Pooled fractional amplitudes of bi-exponential decay fit of CaffTr relaxation. In control cells elevated Ca2+ has no effect; in Rem transfected cells elevated Ca2+ significantly increases fast component. * indicates p<0.005, compared to 1.8 mM control cell; # indicates p<0.05 compared to 1.8 mM Rem cell. B, C) Scatter plot of t50 and t90 of CaffTr in (B) control and (C) Rem transfected cells in response to elevation of bath Ca2+ from 1.8 to 6 mM. Note that bath Ca2+ elevation had no effect in control cells, but decreased CaffTr total time in all Rem-transfected cells (p<0.02).
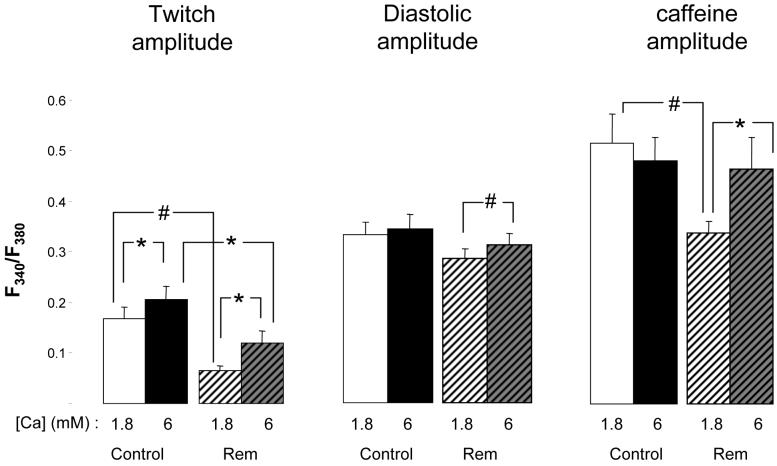
Given that NCX is deactivated by low internal Ca2+, and that Rem blocks Ca2+ entry we posited that an explanation for Rem inhibition of NCX function is attributable to lowered cytosolic Ca2+. Raising bath Ca2+ to 6 mM significantly increased NCX function in Rem-expressed cells (Figure 6). Elevated bath Ca2+ also caused an increase of twitch amplitude (1.8 mM Ca2+, 0.066+/-0.008 ΔF340/F380 (n=5); 6 mM Ca2+, 0.120+/-0.023 ΔF340/F380 (n=5; p=0.02; Figure 7A), and diastolic amplitude (1.8 mM Ca2+, 0.280+/-0.043 F340/F380 (n=5); 6 mM Ca2+, 0.315+/-0.024 F340/F380; n=5; p=0.002; Figure 7B). Similar trends occurred in control cells consistent with bath Ca2+ increasing Ca2+ entry. SR Ca2+ load was not altered by elevated bath Ca2+ (1.8 mM Ca2+, 0.51+/-0.06 ΔF340/F380, (n=6); 6 mM Ca2+, 0.48+/-0.5 ΔF340/F380; n=6; Figure 7C), but in Rem-over-expressed cells SR Ca2+ load was significantly increased (1.8 mM Ca2+, 0.34+/-0.02 ΔF340/F380, n=5; 6 mM Ca2+, 0.50+/-0.05 ΔF340/F380, n=5; p=0.01; Figure 7C). We conclude that partial SL Ca2+-entry inhibition by Rem can be overcome by elevated bath Ca2+ leading to normalized SR load and increased diastolic Ca2+. As a result of increased cytosolic Ca2+, NCX function is normalized despite chronic ICa,L partial blockade.
Figure 7.
Ca responses to elevation of bath Ca from 1 to 6 mM in control and Rem over-expressing cells. Left) Twitch amplitude is significantly increased regardless of Rem expression. Also, Rem cells have significantly lower amplitude than that of control controls. Middle) Net SL diastolic Ca2+-entry is only increased in Rem over-expressing cells. Right) CaffTr amplitude, a measure of SR Ca load, is only increased in Rem over-expressing cells. Note that in 1.8mM bath Ca, SR Ca is significantly less in Rem over-expressing cells than control, but elevation of bath Ca normalizes SR Ca load. *p<0.02; #p<0.002; n=14 and 20 for control and Rem-transfected, respectively.
3.4 Effect of short term block of ICa,L block on trans-SL Ca2+ efflux
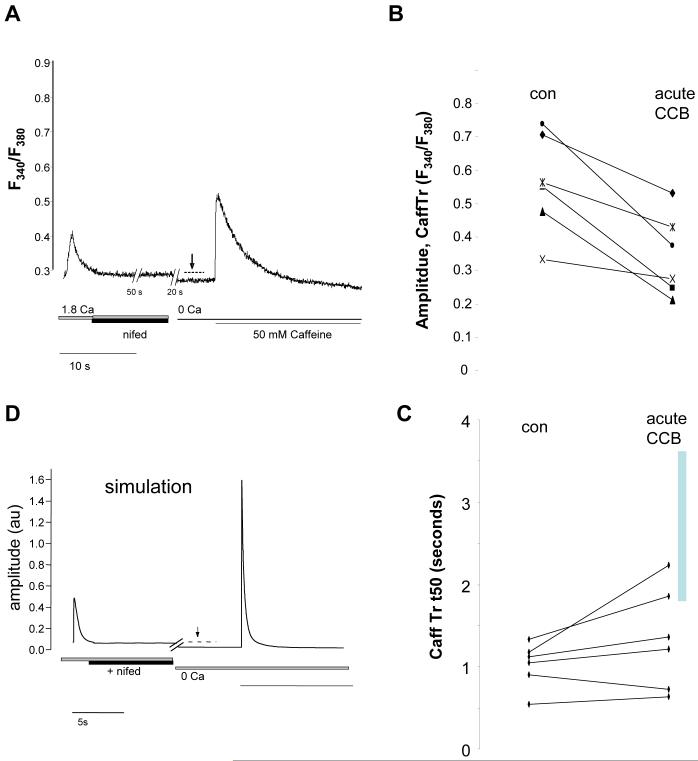
We next determined whether acute Ca2+ channel block (CCB) yields similar effects as chronic ICa,L inhibition. Acute CCB by 10 μM n ifedipine ceased all spontaneous activity. After 1 minute of 10 μM nifedipine in physiological salt solution, the cells were bathed in 0 Ca2+ bath solution with 10 μM nifedipine for 25 seconds and then 50 mM caffeine (also containing10 μM nifedipine) was applied abruptly (Figure 8). We found no significant difference in net SL diastolic Ca2+-entry in controls in response to acute CCB for 1 minute (Figure 8, dashed line). CaffTr amplitude (SR load) was significantly reduced, (Figure 8B; control, 0.56+/-0.06 ΔF340/F380, n=6; 10 μM Nifedipine, 0.34+/-0.05 ΔF340/F380, n=6; p=0.003). In contrast to chronic ICa,L blockade, the acute blocked cells showed a slight slowing of CaffTr kinetics, but the Rem-effect led to significantly slower CaffTr decay kinetics (Figure 8C). These results lead us to the conclusion that short term ICa,L blockade alters loading of the SR, but chronic Rem-mediated ICa,L blockade leads to further homeostatic regulation mechanisms governed by reduced diastolic calcium levels thereby inhibiting NCX forward mode.
Figure 8.
Acute blockade of ICa,L by 10 μM nifedipine does not block CaffTr relaxation time. A) Representative Ca2+ dynamics; sequential bath solutions were: normal tyrodes, normal tyrodes+10 μM nifedipine for 60s, 0 Ca/ EGTA bath solution for 25s, 0Ca / EGTA + 50 mM caffeine. Note that nifedipine rapidly blocks spontaneous activity, but in distinction to Rem-transfected cells the diastolic Ca2+ level is not lower than control cells in normal tyrodes. B) Acute nifedipine reduces CaffTr amplitude. C) Acute nifedipine has no significant effect on CaffTr kinetics. The vertical gray bar shows the range of t50 for Rem treated cells. Acute block is CaffTr decay is significantly faster than Rem over-expressing cells (p<10-3). D) Computer simulation of the effect of ICa,L blockade. Nifedipine had no effect on CaffTr. Nifedipine and control traces are superimposed from 0 Ca onward and are indistinguishable.
To verify these conclusions we simulated Ca2+ transients with an established model for mature cardiac myocytes[16]. The model reproduced the effect of acute Ca2+ channel blockade by nifedipine, but could not reproduce the Rem-effect of slowing CaffTr relaxation. Thus, we conclude that long-term block of ICa,L homeostatically regulates Ca2+ entry and commensurately regulates NCX-based Ca2+ efflux across the SL.
4. Discussion
The major finding of this study is that a chronic partial block of ICa,L mediated by Rem over-expression results in a reduction of NCX-mediated extrusion. We show direct evidence for two non-competing mechanisms that may contribute to this result. First, Rem reduces NCX1.1 protein post-translationally as manifested by a decrease of protein, but without a change in mRNA. Second, Rem over-expression results in reduced cytosolic Ca2+, but this reduction of Ca2+ can be restored by elevating bath Ca2+, and this partially restores NCX function. The end-point of these findings - reduced ICa,L leads to reduced NCX is reciprocal to finding in NCX knockout mice[13], but the compensatory mechanisms differ. Primary alteration of NCX leads to no change in CaV1.2 protein[18], whereas primary alteration of CaV1.2 leads to reduced NCX1 protein level (present study).
We suggest that a Ca2+ channel-centric perspective for control of Ca2+ handling is a critically important consideration. For example, in the CaV1.2 transgenic mouse model an ICa,L increase precedes hypertrophy[19], supporting the notion that ICa,L level is a cause rather than a consequence of Ca2+ dynamics remodeling in pathological conditions. In line with our findings, a modest increase of SL Ca2+ entry in this transgenic model induced a compensatory up-regulation of NCX activity[19]. Although ICa,L up-regulation is the opposite experimental manipulation of our study, it invokes the same homeostatic balance mechanisms of SL Ca2+. Importantly, pathological conditions naturally may reduce ICa,L [20, 21]. More work will be needed to determine whether reduction of ICa,L is a potentially protective mechanism to prevent Ca2+Ca,L overload by preventing Ca2+ entry, or exacerbates Ca2+ overload by triggering compensatory down-regulation of NCX based efflux.
NCX over-expression in mature rabbit ventricular myocytes yields a decrease of SR load[22, 23]. In the same vein, Rem over-expression in the present study decreases SR load. The commonality is that these manipulations decrease cytosolic Ca2+, either by increasing exit or by decreasing entry, respectively. Rem selectively blocks L-type Ca2+ channels in muscle [10] reinforcing the notion that ICa,L is a long-term regulator of SR Ca2+ loading, though increased Ca2+ entry may increase systolic Ca2+ to normalize SR load [24].
We find that CaffTr decay is well described by a biexponential function. A biexponential decay suggests a sequential process, yet under conditions of sustained caffeine exposure the relevant major cytosolic extrusion pathways - NCX and sarcolemmal Ca-ATPase are of course arranged in parallel. A plausible explanation is that NCX is allosterically deactivated by declining cytosolic Ca. As the caffeine-induced Ca transient decays -that is as cytosolic Ca lowers, NCX deactivates[25],[26]. This well established feature of NCX [25, 27-31]is captured in the Shannon-Bers model[16], and here we find that a simulation of a caffeine-induced transient in 0 bath Ca results in a Ca decay that is biexponential (not shown). The time course of allosteric activation of NCX upon increasing cytosolic Ca2+ is controversial. In some studies activation occurs in <200 ms[25],[27], though in others a time constant of ∼0.6s was noted[32], and a lag was detected[17]. Regardless these times are well within the seconds-long CaffTr decay. With NCX deactivated, the remaining extrusion is dominated by the relatively slow sarcolemmal Ca-ATPase. Thus, two molecular mechanisms arranged in parallel may lead to an apparent biexponential process as long as NCX deactivation is accounted for.
In our work, the fast component of CaffTr decay is completely eliminated by 10mM Ni2+. Several past works, including that by Trafford, Diaz, & Eisner document that the 10 mM Ni2+-sensitive component of the caffeine transient is largely ascribable to NCX mediated Ca-extrusion (reviewed by [2]). Diaz et al. [33]) found a biexponential decay of the caffeine transient - the fast component was attributed to Ca buffers. Diaz et al. (2001) was, in part, built on earlier work that showed a slower decay as a function of peak Ca released[34]. Thus, it follows that one possible explanation could be that our observation of a slower decay in Rem-treated cells is secondary to the decrease of caffeine-releasable Ca in these cells. However, two experiments argue against a buffering-only explanation for the slower decay in Rem-treated cells. First, when we applied acute nifedipine we measured decreased CaffTr amplitude without any effect on the time-course of CaffTr decay. Fortuitously, CaffTr amplitude following acute nifedipine in control cells was similar to CaffTr amplitude for Rem-treated cells without acute addition of drug. Second, Ni2+ blocked the fast component completely, but did not alter the amplitude of the caffeine-releasable Ca transient. This does not negate buffering as a consideration for interpreting Ca transient kinetics in intact cells. However, the reproducible attenuation of Ni2+-sensitivity is consistent with our new hypothesis that Rem decreases NCX function.
Given the complex nature of Ca2+ dynamics, computational models contain many unconstrained parameters. Nevertheless, the application of such models can be informative. We used the Shannon-Bers model[16] which was developed for a mature myocyte. Although the time scale of the calcium dynamics is an order of magnitude faster than the experimental results, features captured by the modeling are revealing. First, the decay of the CaffTr depends on the operation of NCX and the SL calcium pump. Second, acute CCB effects on Ca2+ dynamics are reproduced: a switch to 0 bath Ca2+ decreases diastolic Ca2+ and CaffTr kinetics is unaffected. Features not captured by modeling are also revealing. Following the removal of bath calcium, the experimental results showed a sudden drop in cytosol calcium, which was not reproduced by the computational model [16]. We found that increasing the conductance of the background calcium current helped the model mimic this experimental result. Assuming that Rem block is specific for ICa,L [8, 10], a possible implication is that leak ICa,L is a contributor to steady-state, diastolic Ca2+ entry. Interestingly, the model simulation of the decay of the caffeine induced calcium transient does not depend on the length of time of the ICa,L block. Yet, the experimental results indicate that the sudden drop in [Ca]i disappears with chronic ICa,L block. Therefore, some component of the background calcium current through the SL is associated with the long term operation of the L-type Ca2+ channel.
In conclusion, we suggest that long-term regulation of trans-SL Ca2+-pathways is an essential regulator of the Ca2+ handling. This study uncovers the importance of long-term homeostatic regulation of Ca2+ dynamics initiated by long-term partial blockade of ICa,L.
Supplementary Material
6. Acknowledgments
We acknowledge Paul Cornelius and Ken Campbell for statistical and programming consultation, and Miranda Byse for constructive comments. We also are grateful to Zachary Fulkerson and Kristen Edenfield for support. Supported by NIH (HL074091 & HL072936). J.S. is an Established Investigator of the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- 1.Bers DM, Despa S. Cardiac Myocytes Ca(2+) and Na(+) Regulation in Normal and Failing Hearts. J Pharmacol Sci. 2006 doi: 10.1254/jphs.cpj06001x. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers; Dordrecht: 2001. [Google Scholar]
- 3.Negretti N, O’Neill SC, Eisner DA. The relative contributions of different intracellular and sarcolemmal systems to relaxation in rat ventricular myocytes. Cardiovasc Res. 1993;27:1826–30. doi: 10.1093/cvr/27.10.1826. [DOI] [PubMed] [Google Scholar]
- 4.Haddock PS, Coetzee WA, Cho E, Porter L, Katoh H, Bers DM, Jafri MS, Artman M. Subcellular [Ca2+]i Gradients During Excitation-Contraction Coupling in Newborn Rabbit Ventricular Myocytes. Circ Res. 1999;85:415–427. doi: 10.1161/01.res.85.5.415. [DOI] [PubMed] [Google Scholar]
- 5.Artman M, Henry G, Coetzee WA. Cellular basis for age-related differences in cardiac excitation-contraction coupling. Prog Pediatr Cardiol. 2000;11:185–194. doi: 10.1016/s1058-9813(00)00049-7. [DOI] [PubMed] [Google Scholar]
- 6.Linask KK, Han MD, Artman M, Ludwig CA. Sodium-calcium exchanger (NCX-1) and calcium modulation: NCX protein expression patterns and regulation of early heart development. Dev Dyn. 2001;221:249–64. doi: 10.1002/dvdy.1131. [DOI] [PubMed] [Google Scholar]
- 7.Cribbs LL, Martin BL, Schroder EA, Keller BB, Delisle BP, Satin J. Identification of the T-Type Calcium Channel (CaV3.1d) in Developing Mouse Heart. Circ Res. 2001;88:403–407. doi: 10.1161/01.res.88.4.403. [DOI] [PubMed] [Google Scholar]
- 8.Crump SM, Correll RN, Schroder EA, Lester WC, Finlin BS, Andres DA, Satin J. L-type calcium channel alpha-subunit and protein kinase inhibitors modulate Rem-mediated regulation of current. Am J Physiol Heart Circ Physiol. 2006;291:H1959–71. doi: 10.1152/ajpheart.00956.2005. [DOI] [PubMed] [Google Scholar]
- 9.Finlin BS, Mosley AL, Crump SM, Correll RN, Ozcan S, Satin J, Andres DA. Regulation of L-type Ca2+ channel activity and insulin secretion by the Rem2 GTPase. J Biol Chem. 2005 doi: 10.1074/jbc.M414261200. [DOI] [PubMed] [Google Scholar]
- 10.Finlin BS, Crump SM, Satin J, Andres DA. Regulation of voltage-gated calcium channel activity by the Rem and Rad GTPases. PNAS. 2003 doi: 10.1073/pnas.2437756100. 2437756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wakimoto K, Kobayashi K, Kuro OM, Yao A, Iwamoto T, Yanaka N, Kita S, Nishida A, Azuma S, Toyoda Y, Omori K, Imahie H, Oka T, Kudoh S, Kohmoto O, Yazaki Y, Shigekawa M, Imai Y, Nabeshima Y, Komuro I. Targeted disruption of Na+/Ca2+ exchanger gene leads to cardiomyocyte apoptosis and defects in heartbeat. J Biol Chem. 2000;275:36991–8. doi: 10.1074/jbc.M004035200. [DOI] [PubMed] [Google Scholar]
- 12.Reuter H, Henderson SA, Han T, Mottino GA, Frank JS, Ross RS, Goldhaber JI, Philipson KD. Cardiac excitation-contraction coupling in the absence of Na(+) - Ca2+ exchange. Cell Calcium. 2003;34:19–26. doi: 10.1016/s0143-4160(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 13.Pott C, Philipson KD, Goldhaber JI. Excitation-contraction coupling in Na+-Ca2+ exchanger knockout mice: reduced transsarcolemmal Ca2+ flux. Circ Res. 2005;97:1288–95. doi: 10.1161/01.RES.0000196563.84231.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 15.Yatani A, Shen YT, Yan L, Chen W, Kim SJ, Sano K, Irie K, Vatner SF, Vatner DE. Down regulation of the L-type Ca2+ channel, GRK2, and phosphorylated phospholamban: protective mechanisms for the denervated failing heart. J Mol Cell Cardiol. 2006;40:619–28. doi: 10.1016/j.yjmcc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Shannon TR, Wang F, Puglisi J, Weber C, Bers DM. A mathematical treatment of integrated Ca dynamics within the ventricular myocyte. Biophys J. 2004;87:3351–71. doi: 10.1529/biophysj.104.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves JP, Condrescu M. Allosteric activation of sodium-calcium exchange activity by calcium: persistence at low calcium concentrations. J Gen Physiol. 2003;122:621–39. doi: 10.1085/jgp.200308915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pott C, Yip M, Goldhaber JI, Philipson KD. Regulation of cardiac L-type Ca2+ current in Na+-Ca2+ exchanger knockout mice: functional coupling of the Ca2+ channel and the Na+-Ca2+ exchanger. Biophys J. 2007;92:1431–7. doi: 10.1529/biophysj.106.091538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song LS, Guia A, Muth JN, Rubio M, Wang SQ, Xiao RP, Josephson IR, Lakatta EG, Schwartz A, Cheng H. Ca(2+) signaling in cardiac myocytes overexpressing the alpha(1) subunit of L-type Ca(2+) channel. Circ Res. 2002;90:174–81. doi: 10.1161/hh0202.103230. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Piacentino V, III, Furukawa S, Goldman B, Margulies KB, Houser SR. L-Type Ca2+ Channel Density and Regulation Are Altered in Failing Human Ventricular Myocytes and Recover After Support With Mechanical Assist Devices. Circ Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- 21.He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- 22.Schillinger W, Fiolet JW, Schlotthauer K, Hasenfuss G. Relevance of Na+-Ca2+ exchange in heart failure. Cardiovasc Res. 2003;57:921–33. doi: 10.1016/s0008-6363(02)00826-x. [DOI] [PubMed] [Google Scholar]
- 23.Ranu HK, Terracciano CM, Davia K, Bernobich E, Chaudhri B, Robinson SE, Bin Kang Z, Hajjar RJ, MacLeod KT, Harding SE. Effects of Na(+)/Ca(2+)-exchanger overexpression on excitation-contraction coupling in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2002;34:389–400. doi: 10.1006/jmcc.2001.1521. [DOI] [PubMed] [Google Scholar]
- 24.Trafford AW, Diaz ME, Eisner DA. Coordinated control of cell Ca(2+) loading and triggered release from the sarcoplasmic reticulum underlies the rapid inotropic response to increased L-type Ca(2+) current. Circ Res. 2001;88:195–201. doi: 10.1161/01.res.88.2.195. [DOI] [PubMed] [Google Scholar]
- 25.Weber CR, Ginsburg KS, Philipson KD, Shannon TR, Bers DM. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J Gen Physiol. 2001;117:119–31. doi: 10.1085/jgp.117.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chernysh O, Condrescu M, Reeves JP. Calcium-dependent regulation of calcium efflux by the cardiac sodium/calcium exchanger. Am J Physiol Cell Physiol. 2004;287:C797–806. doi: 10.1152/ajpcell.00176.2004. [DOI] [PubMed] [Google Scholar]
- 27.Hilgemann DW, Collins A, Matsuoka S. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol. 1992;100:933–61. doi: 10.1085/jgp.100.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuoka S, Nicoll DA, Hryshko LV, Levitsky DO, Weiss JN, Philipson KD. Regulation of the cardiac Na(+)-Ca2+ exchanger by Ca2+. Mutational analysis of the Ca(2+)-binding domain. J Gen Physiol. 1995;105:403–20. doi: 10.1085/jgp.105.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trafford AW, Diaz ME, O’Neill SC, Eisner DA. Comparison of subsarcolemmal and bulk calcium concentration during spontaneous calcium release in rat ventricular myocytes. J Physiol. 1995;488(Pt 3):577–86. doi: 10.1113/jphysiol.1995.sp020991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujioka Y, Hiroe K, Matsuoka S. Regulation kinetics of Na+-Ca2+ exchange current in guinea-pig ventricular myocytes. J Physiol. 2000;529(Pt 3):611–23. [PubMed] [Google Scholar]
- 31.Weber CR, Piacentino V, 3rd, Ginsburg KS, Houser SR, Bers DM. Na(+)-Ca(2+) exchange current and submembrane [Ca(2+)] during the cardiac action potential. Circ Res. 2002;90:182–9. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- 32.Kappl M, Hartung K. Rapid charge translocation by the cardiac Na(+)-Ca2+ exchanger after a Ca2+ concentration jump. Biophys J. 1996;71:2473–85. doi: 10.1016/S0006-3495(96)79441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diaz ME, Trafford AW, Eisner DA. The role of intracellular Ca buffers in determining the shape of the systolic Ca transient in cardiac ventricular myocytes. Pflugers Arch. 2001;442:96–100. doi: 10.1007/s004240000509. [DOI] [PubMed] [Google Scholar]
- 34.Bers DM, Berlin JR. Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol. 1995;268:C271–7. doi: 10.1152/ajpcell.1995.268.1.C271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.