Abstract
The expression of inhibitory ILTs on dendritic cells (DC) is a biomarker of tolerogenic DC. In this article we discuss current knowledge on the function of ILTs and explore the molecular mechanisms involved in modulation of DC via the inhibitory receptor and its natural ligand, rendering these cells tolerogenic. We propose a method to enhance targeting of inhibitory receptors on DC using microparticles containing a preferential ligand, HLA-G, and monoclonal antibody against the pan-DC marker CD11c. The double-coated microparticles increase the binding of ILT4 receptor and improve modulation of DC in vitro and in vivo. This targeting concept can be used for regulation of specific immune responses to antigens in transplantation, autoimmunity, allergy, and cancer.
Keywords: Inhibitory receptors, Dendritic cells, Tolerance, Transplantation, Immunotherapy
1. Introduction
Dendritic cells (DC) are a specially distributed, migratory group of bone marrow-derived leukocytes that are specialized for the uptake, transport, processing and presentation of antigens to T cells. They play a pivotal role in the induction of both immunity and tolerance. Two general mechanisms have been proposed by which DC might maintain peripheral tolerance. The first is mediated by a subtype of specialized regulatory DC [1–3]. The second is that all DC have a capacity for initiating tolerance or immunity, depending on the maturation or activation state of the DC [3,4]. A major goal in the field of transplantation and autoimmunity is to develop ideal tolerogenic DC for the application in induction of acceptance of organ/tissue allogeneic transplants or to treat and prevent autoimmune diseases. Different approaches for enhancement of the tolerogenic properties of DC have been proposed. These include pharmacologic agents that target DC to induce and promote tolerogenic properties [5–7], the use of CD8+ CD28− T suppressor cells or apoptotic cells that mediate tolerogenic effects of DC [8,9], and CpG-oligonucleotide-stimulated or HLA-G-modulated DC [10,11]. Expression profiling of tolerogenic DC using cDNA microarrays showed down-regulation of co-stimulatory molecules CD40, CD80, CD86, CD54, CD58, CD43, OX-40 ligand, and TNFR1 and up-regulation of mRNA encoding Iκβ epsilon (which inhibits NF-κB activation), the anti-apoptotic gene IAP-1, and FAS soluble protein [12]. Important biomarkers of tolerogenic DC include the expression of immunoglobulin-like transcripts (ILTs, also referred to as LILR, LIR, and CD85 molecules) [12,13].
2. Expression of ILTs on immunocompetent cells
ILTs represent Ig types of activating and inhibitory receptors that are involved in regulation of immune cell activation and control the function of immune cells [14–17]. ILTs are categorized into three groups: (i) inhibitory, those containing a cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM) and transducing an inhibitory signal (ILT2, ILT3, ILT4, ILT5, and LIR8); (ii) activating, those containing a short cytoplasmic tail and a charged amino acid residue in the transmembrane domain (ILT1, ILT7, ILT8, and LIR6α) and delivering an activating signal through the cytoplasmic immunoreceptor tyrosine-based activating motif (ITAM) of the associated common γ chain of Fc receptor; and (iii) the soluble molecule ILT6 lacking the transmembrane domain. A number of recent studies have highlighted immunoregulatory roles for ILTs on the surface of antigen presenting cells (APC). ILT2, ILT3, and ILT4 receptors, the most characterized immune inhibitory receptors, are expressed predominantly on myeloid and plasmacytoid DC. ILT3 and ILT4 are upregulated by exposing immature DC to known immunosuppressive factors, including IL-10, vitamin D3, or suppressor CD8 T cells [8,18–20]. The expression of ILTs on DC is tightly controlled by inflammatory stimuli, cytokines, and growth factors, and is down-regulated following DC activation [13]. The expression of ILT2 and ILT4 receptors is highly regulated by histone acetylation, which contributes to strictly controlled gene expression exclusively in the myeloid lineage of cells [21].
3. Function of ILTs on DC
Engagement of the inhibitory receptors ILT2 and ILT4 alters the cytokine and chemokine secretion profile of monocytes and can inhibit Fc receptor signaling [15,22]. The role and function of ILT3 on DC have been precisely described by the Suciu-Foca group (for more details, see refs. [8,12,18,23–28]). Although the ligand for ILT3 is unknown, ILT4 is known to bind to the third domain of HLA class I molecules (HLA-A, HLA-B, HLA-C, and HLA-G), competing with CD8 for MHC class I binding [29]. The preferential ligand for several inhibitory ILT receptors is HLA-G. HLA-G plays a potential role in maternal-fetal tolerance and in the mechanisms of escape of tumor cells from immune recognition and destruction [30–32]. It is most likely that regulation of DC function by HLA-G-ILT interactions is an important pathway in the biology of DC. It has been determined that human monocyte-derived DC that highly express ILT2 and ILT4 receptors, when treated with HLA-G and stimulated with allogeneic T cells, still maintain a stable tolerogenic-like phenotype (CD80low, CD86low, HLA-DRlow) with the potential to induce T cell anergy [11]. Moreover, the HLA-G interaction with DC that highly express ILT2 and ILT4 receptors resulted in down-regulation of several genes involved in the MHC class II presentation pathway. A lysosomal thiol reductase, IFN-γ inducible lysosomal thiol reductase (GILT), abundantly expressed by professional APC, was greatly reduced in HLA-G-modified DC. The repertoire of primed CD4+ T cells can be influenced by DC expression of GILT, as in vivo T cell responses to select antigens were reduced in animals lacking GILT after targeted gene disruption [33]. The HLA-G/ILT interaction on DC interferes with the assembly and transport of MHC class II molecules to the cell surface, which might result in less efficient presentation or expression of structurally abnormal MHC class II molecules. The loading of exogenous peptides onto MHC class II molecules has been shown to be critically dependent on H2-M (HLA-DM, human). Cells from mice with a targeted mutation in the H2-M gene are unable to present intact proteins and have a markedly reduced capacity to present exogenous peptides (10-to 20 fold reduction) [34]. It was determined that HLA-G markedly decreased the transcription of invariant chain (CD74), HLA-DMA, and HLA-DMB genes on human monocyte-derived DC highly expressing ILT inhibitory receptors [11].
4. In vivo models for study of tolerogenic ILT-positive DC
A model for the study of the tolerogenic function of ILT-positive DC in vivo has been established [11]. In this ‘humanized” model, transgenic mice expressed ILT4 exclusively on DC, and the ligand, HLA-G, was delivered by injection of ligand-positive microparticles. Targeting ILT4 receptors by their natural ligand HLA-G in vivo results in significant modulation of DC, driving them to tolerogenic function. This ILT4-HLA-G-mediated tolerogenic pathway is specific for myeloid DC and does not affect lymphoid or plasmacytoid DC. This type of DC with tolerogenic function was responsible for impaired rejection of an allogeneic skin transplant, resulting from the failure of T cells to develop vigorous effector populations. The mechanisms of prolongation of graft survival in this model include development of T cell anergy and regulatory populations of T cells. T cell anergy in this model was characterized by antigen-specific T cell proliferative unresponsiveness and the inhibition of IL-2 secretion by T cells from ILT4 transgenic mice treated with HLA-G1, which resulted in prolongation of allograft survival. In contrast, T cells from untreated ILT4 transgenic mice are capable of secreting IL-2 upon activation and respond normally to proliferative signals. The mechanisms of T cell anergy induction in vivo include several sets of circumstances. It is possible that the major pathway for induction of T cell anergy by the HLA-G1-ILT4 interaction on DC is mediated by down-regulation of the expression of costimulatory molecules. In this case, DC lack costimulatory molecules, thereby causing anergy. In addition, it was determined that the HLA-G1-ILT4-mediated pathway on DC may also inhibit T cells through activation or induction of regulatory T cells, which inhibit the activation of other T cells. These IL-10-producing regulatory CD4+CD25+ T cells are characterized by high levels of expression of CTLA-4 and Foxp3 and inhibit antigen-induced proliferation of T cells. Although the mechanisms involved in regulatory T cell generation are multifactorial, it appears that lack of costimulation in combination with impaired expression of MHC class II molecules are involved in the HLA-G1-ILT4-mediated induction of regulatory T cells in vivo. It was determined that regulatory T cells isolated from ILT4 transgenic mice with prolongation of allograft survival produced a substantial amount of IL-2 and especially IFN-γ. It has been discovered that IL-2 and IFN-γ are required for the induction of tolerance to alloantigens in vivo [35]. Moreover, B. Sawitzki et al. [36] demonstrated the unique role of IFN-γ in the functional activity of alloantigen-specific regulatory T cells during the development of operational tolerance to donor alloantigens in vivo.
5. Mechanisms of arrest maturation/activation of DC via ILT4 receptor and ligand
Signaling events downstream of the ILT receptors and their functional impact on DC activation/maturation are incompletely understood. Evidence has been provided that engagement of ILT4 by HLA-G ligand results in recruitment of both SHP-1 and SHP-2 phosphatases and involves the IL-6-STAT3 pathway [37]. Analysis of human DC [11] and experiments with murine ILT4-positive DC suggest that one of the major targets of the HLA-G and ILT4 interaction on DC is MHC class II molecules. During maturation, DC increase their surface expression of MHC class II molecules by several fold. This increase is accompanied by a dramatic change in localization of MHC class II molecules, which are abundant in endosomal structures in immature DC but are located mostly on the plasma membrane in mature DC [38]. The control of MHC class II molecule synthesis and degradation, trafficking, and peptide loading represent key mechanisms in antigen presentation by DC. Recently, it was discovered that the IL-6-STAT3 pathway controls the intracellular MHC class II αβ dimer level through cathepsin S activity in DC [39].
Although IL-6 is involved in the development of mature T and B cell responses, it does not have only pro-inflammatory properties. It was recently demonstrated that forced activation of cytokine IL-6 in DC resulted in the development of tolerogenic DC. IL-6 knockout mice had an increased number of mature DC, indicating that IL-6 blocks DC maturation in vivo [40]. The engagement of ILT4 on DC by certain isoforms of HLA-G results in increasing the transcriptional and protein levels of IL-6 and conferring DC with tolerogenic properties [37]. The signaling pathway of IL-6 leads to the activation of STAT3 and STAT1. IL-6 activates STAT3 exclusively; when IL-6 levels were raised 10 to 100 fold, STAT1 activation was noted as well [41,42]. It has been shown that the treatment of ILT4-positive DC with HLA-G1 tetramer and HLA-G5 dimer induced phosphorylation of STAT3. Additional stimulation of DC with lipopolysaccharide (LPS) increases the levels of STAT3 protein and enhances its phosphorylation. In contrast, no activation of STAT1 was detected. Experiments with knockdown of tyrosine phosphatases determined that SHP-2 was a key molecule involved in the increase of IL-6 by the HLA-G/ILT4 interaction on DC during the maturation process that was mediated by LPS signaling [37]. Since the HLA-G/ILT4 interaction on DC especially targets MHC class II genes and does not affect the expression of MHC class I molecules, it is most likely that in ILT4-positive DC, SHP-2 modulates the NF-κB pathway in a MAP kinase-independent fashion in induction of IL-6. The exact molecular mechanisms of the induction of negative regulators of TLR4 signaling remain to be determined.
The mechanism proposed by Liang et al. [37] can be applied to control the maturation/activation of DC via HLA-G/ILT4 in the absence of a strong inflammatory response and during a moderate signal through TLR4. This situation could be similar or equivalent to a normal pregnancy or surgical procedure with tissue or organ transplantation. However, upon receiving a strong activated signal associated with a pathogen or inflammation, ILT4-positive DC will most likely force a robust rise in IL-6 levels, which will result in activation of STAT3 and STAT1, conferring DC with immune-stimulating properties.
The exact role of ILT2 in modulation of DC remains to be determined. The generation of transgenic mice expressing ILT2 under CD11c promoter, the shRNA techniques will help to understand the mechanisms of modulation of DC by ILT2 receptor and their ligands. Experiments using transfectants and recombinant protein showed that the products of a broad range of MHC class I genes and their alleles interact with ILT2 and ILT4. However, the different isoforms of the ligands mediate distinct inhibition of immune response via ILT receptors. HLA-G is a preferential ligand for inhibitory receptors ILT2 and ILT4. HLA-G encodes at least seven isoforms as a result of alternative splicing (HLA-G1 to HLA-G7). The full-length membrane-bound isoforms, HLA-G1, is structurally similar to other class I genes, except for the truncated cytoplasmic tail. A stop sequence in intron 4 results in two soluble isoforms, HLA-G5 and HLA-G6. Our data and experiments from other groups demonstrated that not all isoforms of HLA-G delivered inhibitory signals via ILT2 or ILT4 receptors. Moreover, it has been determined that the most potent form of HLA-G is a disulfide-bonded dimer. This should be considered to design better strategies to modulate DCs function via ILT receptors.
6. Efficient targeting of ILTs on DC
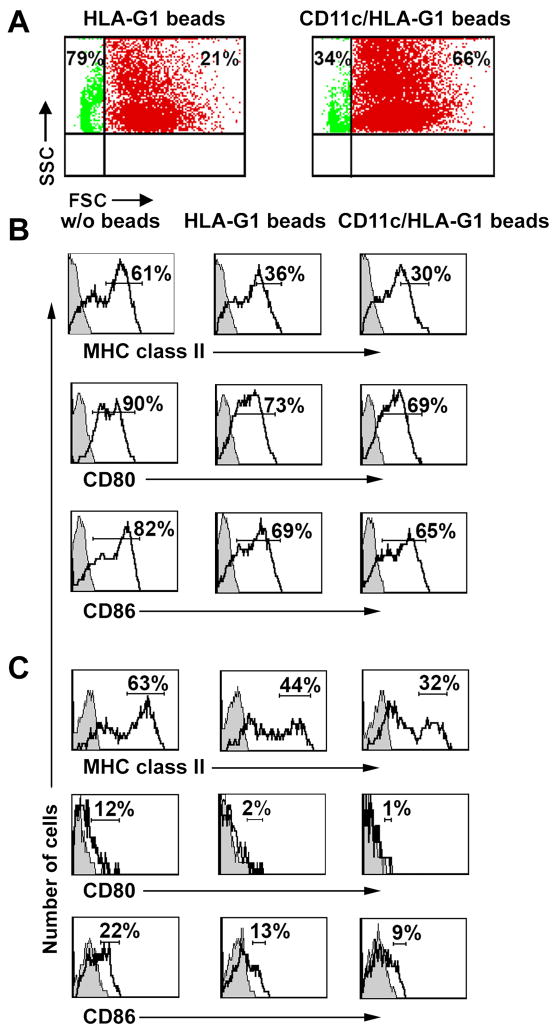
The efficient targeting of ILTs on immunocompetent cells warrants the best strategy for modulation of immune responses in vivo. For targeting ILT-positive cells, we propose to use special microparticles coated with both ligands and monoclonal antibodies recognizing the specific antigen on immunocompetent cells. For targeting ILT4-positive DC we have used microparticles coated with a ligand (HLA-G1-peptide tetrameric complexes) and with monoclonal antibody against the pan-dendritic cell marker CD11c (double-coated microparticles). The binding and modulatory effect of double-coated microparticles was determined first in vitro using bone marrow-derived DC isolated from ILT4 transgenic mice. An increasing binding to ILT4-positive DC was obtained using double-coated microparticles (increase from 21% to 66%, Figure 1A). Moreover, the analysis of stimulation of DC with LPS and treatment with HLA-G ligand demonstrated that double-coated microparticles decreased the level of expression of MHC class II and CD80 and CD86 co-stimulatory molecules to a greater extent, compared with single, ligand-containing microparticles (Figure 1B). The most striking results were obtained in vivo by injection of microparticles to the ILT4 mice. The level of expression of MHC class II and co-stimulatory molecules CD80 and CD86 was significantly reduced on DC isolated from spleen (Figure 1C) and draining lymph nodes (data not shown) of mice treated with double-coated microparticles and grafted with allogeneic skin. In addition, injection of double-coated microparticles to ILT4 mice (recipient of allogeneic skin) resulted in significant delay in rejection of skin transplant (manuscript in preparation). A major difference in the immunomodulatory effect on ILT4-positive DC in vitro and in vivo between single ligand-coated and double ligand/CD11c-coated microparticles suggested that targeting DC with ligand/DC-specific mAb microparticles is a useful and convenient approach to enhance binding of ILTs and improve immunomodulation of DC. This particular concept can be used to target specific type of cells involved in pathological pathways and to modulate their functions.
Figure 1. Double-coated microparticles increase binding to ILT4-positive DC in vitro and in vivo and enhance their inhibitory effect.
(A) Binding of single (HLA-G1-coated), double (HLA-G1- and anti-CD11c mAb-coated) microparticles to ILT4-positive DC. Bone marrow-derived DC (BMDC) were prepared from ILT4 transgenic mice as described previously [11]. 5 × 106 microparticles (Invitrogen, Carlsbad, CA) were coated with HLA-G1 tetrameric complexes [37] or with both a purified anti-CD11c mAb (N418, hamster IgG, eBioscience, San Diego, CA) and HLA-G1 tetramer, and added to DC for 3 h. Cells were analyzed by flow cytometry. Numbers indicate the percentage of microparticles bound to ILT4-positive DC (red) and unbound (green) microparticles. Data shown are from one of four independent experiments.
(B) Effect of single (HLA-G1-coated) and double (HLA-G1 and anti-CD11c mAb-coated) microparticles on activation/maturation of ILT4-positive DC in vitro. Immature BMDC from ILT4 mice (1 × 106) were treated for 3 h with the same amount of the indicated microparticles, and cells were stimulated an additional for 18 h with a titrated concentration of LPS (Sigma-Aldrich, St. Louis, MO; 100 ng/ml of LPS as shown). Cells were stained with anti-MHC class II mAb (M5/114.15.2, rat IgG2b, BD Pharmingen, San Diego, A), anti-CD80 mAb (16-10A1, hamster IgG), and anti-CD86 mAb (GL1, rat IgG2a, both mAbs from e-Bioscience). Histograms shown were gated on the CD11c+ population. Numbers indicate percentage of total gated cells falling into selected quadrants. Numbers for MHC class II represent by population of cells, which highly express these molecules. Filled histograms represent isotype controls. Data are representative of four independent experiments.
(C) Effect of single (HLA-G1-coated) and double (HLA-G1and anti-CD11c mAb-coated) microparticles on activation/maturation of ILT4-positive DC in vivo induced by allogeneic skin transplant. Draining lymph node cells isolated from ILT4 transgenic mice (H-2b) grafted with allogeneic skin transplant from a MHC class II-mismatch donor (B6. C-H-2bm12, H-2b) were analyzed on day 3 for the expression of MHC class II and costimulatory molecules CD80 and CD86. One day before grafting, mice received single- or doubled-coated microparticles as indicated. Histograms shown were gated on the CD11c+ population. Numbers indicate percentage of total gated cells falling into selected quadrants. Numbers for MHC class II represent by population of cells, which highly express these molecules. Filled histograms represent isotype controls. Data are representative of three independent experiments.
Concluding remarks
In recent years the molecular pathways involved in the ligand and ILT interaction on DC have begun to be resolved. We are also beginning to gain a better understand of how cytokines, chemokines, and growth factors may contribute to the development of tolerogenic DC. In the future it will be important to understand the specific role of and the molecular pathways mediated by other inhibitory receptors, especially ILT2, and their interaction with ligands on DC. Future studies on tolerogenic DC should determine the influence of the various receptors on one another. In addition, a better understanding of the importance of different forms of ILT ligands (monomer, dimer) is required. Results from several groups, including ours, demonstrated a novel HLA-G-based strategy used by different isoforms of HLA-G to down-regulate DC activation and function to render them tolerogenic. The identification of powerful ligands that specifically target ILTs in DC might be useful for certain clinical applications for the development of tolerogenic vaccines. In the future, it is hoped that these pathways can be manipulated not only for prevention of allograft rejection, but also for the development of tolerogenic vaccines.
Acknowledgments
We are grateful to Dr. Rhea-Beth Markowitz for critical reading of the manuscript. This work is supported in parts by grants from the National Institutes of Health and the Roche Organ Transplantation Foundations.
Abbreviations used
- APC
antigen-presenting cell
- DC
dendritic cells
- IFN
interferon
- IgG
immunoglobulin G
- ILT
immunoglobulin-like transcript
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κB
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- SHP
Src homology protein
- STAT
signal transducer and activator of transcription
Footnotes
Disclosure statement A.H. has patents pending on HLA-G.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, et al. IL-6 inhibits the tolerogenic function of CD8 alpha+ dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol. 2001;167:708–714. doi: 10.4049/jimmunol.167.2.708. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 5.Coates PT, Colvin BL, Kaneko K, Taner T, Thomson AW. Pharmacologic, biologic, and genetic engineering approaches to potentiation of donor-derived dendritic cell tolerogenicity. Transplantation. 2003;75:32S–36S. doi: 10.1097/01.TP.0000067949.90241.CB. [DOI] [PubMed] [Google Scholar]
- 6.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 7.Xiao BG, Huang YM, Link H. Tolerogenic dendritic cells: the ins and outs of outcome. J Immunother. 2006;29:465–471. doi: 10.1097/01.cji.0000210387.55951.8b. [DOI] [PubMed] [Google Scholar]
- 8.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 9.Skoberne M, Beignon AS, Larsson M, Bhardwaj N. Apoptotic cells at the crossroads of tolerance and immunity. Curr Top Microbiol Immunol. 2005;289:259–292. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 10.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu YJ, et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 11.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 12.Suciu-Foca N, Manavalan JS, Scotto L, Kim-Schulze S, Galluzzo S, Naiyer AJ, Fan J, Vlad G, Cortesini R. Molecular characterization of allospecific T suppressor and tolerogenic dendritic cells: review. Int Immunopharmacol. 2005;5:7–11. doi: 10.1016/j.intimp.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Ju XS, Hacker C, Scherer B, Redecke V, Berger T, Schuler G, Wagner H, et al. Immunoglobulin-like transcripts ILT2, ILT3 and ILT7 are expressed by human dendritic cells and down-regulated following activation. Gene. 2004;331:159–164. doi: 10.1016/j.gene.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Borges L, Fanger N, Cosman D. Interactions of LIRs, a family of immunoreceptors expressed in myeloid and lymphoid cells, with viral and cellular MHC class I antigens. Curr Top Microbiol Immunol. 1999;244:123–136. doi: 10.1007/978-3-642-58537-1_11. [DOI] [PubMed] [Google Scholar]
- 15.Colonna M, Nakajima H, Navarro F, Lopez-Botet M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J Leukoc Biol. 1999;66:375–381. doi: 10.1002/jlb.66.3.375. [DOI] [PubMed] [Google Scholar]
- 16.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 17.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue antigens. 2004;64:215–225. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 18.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, et al. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–258. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 19.Beinhauer BG, McBride JM, Graf P, Pursch E, Bongers M, Rogy M, et al. Interleukin 10 regulates cell surface and soluble LIR-2 (CD85d) expression on dendritic cells resulting in T cell hyporesponsiveness in vitro. Eur J Immunol. 2004;34:74–80. doi: 10.1002/eji.200324550. [DOI] [PubMed] [Google Scholar]
- 20.Vlad G, Piazza F, Colovai A, Cortesini R, Della Pietra F, Suciu-Foca N, et al. Interleukin-10 induces the upregulation of the inhibitory receptor ILT4 in monocytes from HIV positive individuals. Hum Immunol. 2003;64:483–489. doi: 10.1016/s0198-8859(03)00040-5. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima H, Asai A, Okada A, Ping L, Hamajima F, Sata T, et al. Transcriptional regulation of ILT family receptors. J Immunol. 2003;171:6611–6620. doi: 10.4049/jimmunol.171.12.6611. [DOI] [PubMed] [Google Scholar]
- 22.Colonna M, Samaridis J, Cella M, Angman L, Allen RL, O’Callaghan CA, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol. 1998;160:3096–3100. [PubMed] [Google Scholar]
- 23.Manavalan JS, Kim-Schulze S, Scotto L, Naiyer AJ, Vlad G, Colombo PC, et al. Alloantigen specific CD8+CD28− FOXP3+ T suppressor cells induce ILT3+ ILT4+ tolerogenic endothelial cells, inhibiting alloreactivity. Int Immunol. 2004;16:1055–1068. doi: 10.1093/intimm/dxh107. [DOI] [PubMed] [Google Scholar]
- 24.Kim-Schulze S, Seki T, Vlad G, Scotto L, Fan J, Colombo PC, et al. Regulation of ILT3 gene expression by processing of precursor transcripts in human endothelial cells. Am J Transplant. 2006;6:76–82. doi: 10.1111/j.1600-6143.2005.01162.x. [DOI] [PubMed] [Google Scholar]
- 25.Cortesini R, Suciu-Foca N. ILT3+ ILT4+ tolerogenic endothelial cells in transplantation. Transplantation. 2006;82:S30–32. doi: 10.1097/01.tp.0000231437.12890.64. [DOI] [PubMed] [Google Scholar]
- 26.Vlad G, Liu Z, Zhang QY, Cortesini R, Suciu-Foca N. Immunosuppressive activity of recombinant ILT3. Int Immunopharmacol. 2006;6:1889–1894. doi: 10.1016/j.intimp.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Suciu-Foca N, Cortesini R. Central role of ILT3 in the T suppressor cell cascade. Cell Immunol. 2007;248:59–67. doi: 10.1016/j.cellimm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Vlad G, Cortesini R, Suciu-Foca N. CD8(+) T suppressor cells and the ILT3 master switch. Hum Immunol. 2008;69:681–686. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 29.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003;100:8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunt JS, Petroff MG, McIntire RH, Ober C. HLA-G and immune tolerance in pregnancy. Faseb J. 2005;19:681–693. doi: 10.1096/fj.04-2078rev. [DOI] [PubMed] [Google Scholar]
- 31.Rouas-Freiss N, Goncalves RM, Menier C, Dausset J, Carosella ED. Direct evidence to support the role of HLA-G in protecting the fetus from maternal uterine natural killer cytolysis. Proc Natl Acad Sci U S A. 1997;94:11520–11525. doi: 10.1073/pnas.94.21.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65:10139–10144. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 33.Maric M, Arunachalam B, Phan UT, Dong C, Garrett WS, Cannon KS, et al. Defective antigen processing in GILT-free mice. Science. 2001;294:1361–1365. doi: 10.1126/science.1065500. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, et al. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 35.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 36.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6--STAT3 signaling pathway. Proc Natl Acad Sci U S A. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villadangos JA, Schnorrer P, Wilson NS. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol Rev. 2005;207:191–205. doi: 10.1111/j.0105-2896.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 39.Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 41.Barton BE. STAT3: a potential therapeutic target in dendritic cells for the induction of transplant tolerance. Expert Opin Ther Targets. 2006;10:459–470. doi: 10.1517/14728222.10.3.459. [DOI] [PubMed] [Google Scholar]
- 42.Darnell JE., Jr Reflections on STAT3, STAT5, and STAT6 as fat STATs. Proc Natl Acad Sci U S A. 1996;93:6221–6224. doi: 10.1073/pnas.93.13.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]