Abstract
To examine whether escitalopram enhances net hepatic glucose uptake during a hyperinsulinemic hyperglycemic clamp, studies were performed in conscious 42-h-fasted dogs. The experimental period was divided into P1 (0–90 min) and P2 (90–270 min). During P1 and P2 somatostatin (to inhibit insulin and glucagon secretion), 4X basal intraportal insulin, basal intraportal glucagon, and peripheral glucose (2X hepatic glucose load) were infused. Saline was infused intraportally during P1 in all groups. In one group saline infusion was continued in P2 (SAL, n=11), while escitalopram was infused intraportally at 2 μg/kg/min (L-ESC, n=6) or 8 μg/kg/min (H-ESC, n=7) during P2 in two other groups. The arterial insulin concentrations rose ~four fold (to 123±8, 146±13 and 148±15 pmol/L) while glucagon concentrations remained basal (41±3, 44±9 and 40±3 ng/L) in all groups. The hepatic glucose load averaged 216±13, 223±19 and 202±12 μmol/kg/min during the entire experimental period (P1 and P2) in the SAL, L-ESC and H-ESC groups, respectively. Net hepatic glucose uptake was 11.6±1.4, 10.1±0.9 and 10.4±2.3 μmol/kg/min in P1 and averaged 16.9±1.5, 15.7±1.3 and 22.6±3.7 (P<0.05) in the SAL, L-ESC and H-ESC groups, respectively during the last hour of P2 (210–270 min). Net hepatic carbon retention (glycogen storage) was 15.4±1.3, 14.9±0.6 and 20.9±2.6 (P<0.05) μmol/kg/min in SAL, L-ESC and H-ESC respectively during the last hour of P2. Escitalopram enhanced net hepatic glucose uptake and hepatic glycogen deposition, showing that it can improve hepatic glucose clearance under hyperinsulinemic hyperglycemic conditions. Its use in individuals with diabetes may, therefore, result in improved glycemic control.
Keywords: SSRI, escitalopram, serotonin, liver, glycemia, net hepatic glucose uptake
1. Introduction
The prevalence of type 2 diabetes has increased substantially in recent years. Furthermore, evidence now suggests that neural mediators/pathways may play an important role in glucose homeostasis such that these pathways could become the site of actions for new therapeutic agents. Serotonin (5-hydroxytryptamine; 5-HT) has been shown to dose dependently decrease serum glucose levels in mice (Sugimoto et al., 1990; Yamada et al., 1989). Interestingly, type 2 diabetes is associated with reduced levels of serotonin in blood (Martin et al., 1995). Furthermore, there is a growing body of work showing that selective serotonin reuptake inhibitors (SSRI), which increase the level of endogenous serotonin, may impact glycemic control in people with type 2 diabetes (Breum et al., 1995; Maheux et al., 1997; Potter van Loon et al., 1992). Taken together, these findings suggest that serotonin may play a regulatory role in controlling glucose metabolism.
Splanchnic glucose uptake accounts for the disposal of ~1/3 of a moderately sized oral glucose load in normal individuals, with the liver being responsible for the majority of this disposal (Bajaj et al., 2002; Cherrington, 1999). Splanchnic/hepatic glucose uptake is impaired in type 2 diabetic individuals (Basu et al., 2001; Ludvik et al., 1997). Data support the possibility that hepatic serotonin levels have an impact on net splanchnic/hepatic glucose uptake. For example, intraportal infusion of 5-HT (Moore et al., 2004b) or 5-hydroxytryptophan (5-HTP) (Moore et al., 2005), the immediate precursor of 5-HT, enhanced net hepatic glucose uptake in conscious dogs during a hyperinsulinemic hyperglycemic clamp.
Further, we have demonstrated that intraportal delivery of the SSRI fluvoxamine to the liver increased net hepatic glucose uptake with concomitant stimulation of hepatic glycogen storage (Moore et al., 2004a). However, due to the limitations of drug specificity, whether these effects were caused by an inhibition of serotonin reuptake, or whether some other mechanism was involved, remains unclear.
Escitalopram (the S-enantiomer of citalopram), another commonly prescribed antidepressant, has the highest selectivity for the serotonin transporter of any SSRI currently available (Klein et al., 2006). Compared with fluvoxamine, it has little or no affinity for noradrenergic or dopaminergic transporters (Owens et al., 2001). Compared to other antidepressants, this drug has relatively modest effects on appetite and weight as well as lower potential for drug interactions (Culpepper, 2002; Hyttel, 1994). Because of these characteristics, it serves as a useful tool for determining the effects of serotonin on hepatic glucose metabolism in the absence of the previously described confounding variables. To date, the effects of escitalopram on hepatic glucose metabolism in vivo have not been elucidated. By using the arteriovenous difference technique, the effect of the drug on net hepatic glucose balance can be determined. Therefore, the purpose of this study was to determine the effect of escitalopram on net hepatic glucose uptake in conscious dogs under fixed hyperinsulinemic and hyperglycemic conditions, when the role of the liver in glucose disposition is very important to glucose homeostasis.
2. Materials and Methods
2.1. Animals and surgical procedures
Studies were carried out on healthy conscious 42-h-fasted mongrel dogs with a mean weight of 22.1± 0.4 kg. A fast of this duration was chosen because it produces a metabolic state resembling that in the overnight-fasted human and results in liver glycogen levels in the dog that are at a stable minimum (Hendrick et al., 1990). All animals were maintained on a diet of meat (Kal Kan, Vernon, CA) and chow (Purina Lab Canine Diet No. 5006; Purina Mills, St. Louis, MO) comprised of 34% protein, 14.5% fat, 46% carbohydrate, and 5.5% fiber (~ 1500 kcal/d) based on dry weight. The animals were housed in a facility that met American Association for Accreditation of Laboratory Animal Care guidelines, and the protocol was approved by the Vanderbilt University Medical Center Animal Care and Use Committee.
Approximately 16 days before study, each dog underwent a laparotomy under general anesthesia (0.01 mg/kg buprenorphine presurgery, and ~1% isoflurane inhalation anesthetic during surgery), and silicone rubber catheters for sampling were inserted in the hepatic vein, the portal vein, and a femoral artery as described in detail elsewhere (Myers et al., 1991). Catheters for intraportal infusion were also placed in a splenic and a jejunal vein, while ultrasonic flow probes (Transonic Systems, Ithaca, NY) were placed around the portal vein and the hepatic artery as described elsewhere (Adkins-Marshall et al., 1992).
Approximately 2 days before each study, blood was drawn to determine the leukocyte count and the hematocrit for each animal. The dog was studied only if it had a leukocyte count < 18,000/mm3, a hematocrit > 35%, a good appetite (as evidenced by consumption of more than 75% of daily ration), and normal stools.
On the morning of the study, catheters and the flow probe leads were exteriorized from their subcutaneous pockets using local anesthesia (2% lidocaine, Hospira, Lake Forest, IL). The contents of each catheter were aspirated, and the catheters were flushed with saline. Angiocaths (Deseret Medical, Becton Dickinson, Sandy, UT) were inserted into the cephalic veins and the saphenous veins. Each dog was allowed to stand quietly in a Pavlov harness throughout the experiment. Each animal was studied only once.
2.2. Experimental design
Animals were divided into three experimental groups in a randomized study setting. Each experiment consisted of a 90-min equilibration period (−120 to −30 min), a 30-min basal period (−30 to 0 min), and a 270-min experimental period (0 to 270 min), which was divided into a 90 minute period (P1) followed by a 180 minute period (P2). In all experiments a constant infusion of indocyanine green dye (0.076 mg/min; Sigma Immunochemicals, St. Louis, MO) was initiated at −120 min via the left cephalic vein. At 0 min, a constant infusion of somatostatin (0.8 μg/kg/min; Bachem, Torrance, CA) was begun via the left saphenous vein to suppress endogenous insulin and glucagon secretion, and basal glucagon (0.57 ng/kg/min; Glucagen, Novo Nordisk, Bagsvaerd, Denmark) and 4-fold basal insulin (1.2 mU/kg/min; Eli Lilly & Co., Indianapolis, IN) infusions were then started through the splenic and jejunal catheters and maintained for the duration of the study. In addition, at time 0, a primed continuous infusion of 50% dextrose was started via the right cephalic vein so that the blood glucose could be quickly be clamped at the desired hyperglycemic level (~9.1 mmol/L). In P2, saline was infused intraportally in the control group (SAL, n=11), while escitalopram oxalate (Forest Research Institute, Jersey City, NJ) was infused intraportally at 2 μg/kg/min (L-ESC, n=6) or 8 μg/kg/min (H-ESC, n=7). Intraportal infusion of the drug facilitated liver targeting and reflected the route of the drug entry seen clinically. The peripheral glucose infusion rate was adjusted in P2 to maintain a similar hepatic glucose load (HGL) to that seen in P1. Femoral artery, portal vein, and hepatic vein blood samples were taken as described previously (Myers et al., 1991).
After completion of each experiment, the animal was killed with an overdose of pentobarbital and the abdomen was opened so that liver samples could be immediately freeze clamped with precooled Wallenburger tongs and stored at −70 °C. Liver samples were later pulverized under liquid nitrogen and processed for liver glycogen as described previously (Keppler and Decker, 1974). The positions of the catheter tips in their respective vessels were verified at the time of the necropsy.
2.3. Processing and analysis of samples
The collection and immediate processing of blood samples have been described previously (Galassetti et al., 1998). Four to eight 10-μl aliquots of plasma from each sample were immediately analyzed for glucose using the glucose oxidase technique in a glucose analyzer (Beckman Instruments, Inc., Fullerton, CA; Analox Instruments, Ltd., London, UK). Plasma insulin and glucagon concentrations were determined by RIA, as previously described (Morgan and Lazarow, 1962). Cortisol, lactate, glycerol, and nonesterified fatty acid concentrations were also measured as previously described (Adkins-Marshall et al., 1992). 5-HT concentrations were determined on whole blood and liver tissue by an HPLC-amperometric assay with a coefficient of variance (CV) of 4%, as described elsewhere (Moore et al., 2004a).
2.4. Calculations and data analysis
Hepatic blood flow was measured using ultrasonic flow probes and by the use of indocyanine green dye according to the method of Leevy et al. (Leevy et al., 1962). The results obtained with the flow probes and dye were not significantly different, but the data reported here were calculated using the former because their measurement did not require an assumption regarding the distribution of the arterial and portal vein contribution to hepatic blood flow.
Net hepatic glucose balance was calculated as
Net hepatic glucose balance = Loadout − Loadin.
Loadin (or hepatic glucose load) = (arterial blood glucose concentration × hepatic arterial blood flow) + (portal blood glucose concentration × hepatic portal blood flow)
The load of glucose exiting the liver was calculated as
Loadout = (hepatic vein glucose concentration × hepatic blood flow)
Where hepatic blood flow was the sum of the arterial and portal vein flows.
The average nonhepatic glucose uptake between two time points (T1 and T2) was calculated by subtracting the rate of net hepatic glucose uptake and the change in the glucose mass from the total glucose infusion rate using the equation: nonhepatic glucose uptake rate = average total glucose infusion rate between T1 and T2 − (net hepatic glucose uptakeT1 + net hepatic glucose uptakeT2)/2 − the change in the glucose mass between T1 and T2. In a previous study, we were able to determine that under hyperinsulinemic and hyperglycemic conditions virtually all of the increase in nonhepatic glucose uptake could be accounted for glucose uptake by muscle (Galassetti et al., 1998). Net hepatic carbon retention was calculated as the sum of the net balances of glucose and lactate, once the latter was converted to glucose equivalents (An et al., 2008). The calculation of net hepatic carbon retention is an established approach to estimate hepatic glycogen accretion and has been described and validated previously (Satake et al., 2002). The calculation omits the contribution of gluconeogenic substrates other than lactate and fails to account for the glucose oxidized by the liver. However, these two rates are quantitatively similar and offsetting (Satake et al., 2002). The net hepatic balances of lactate and glycerol were calculated in the same manner as glucose balance. The hepatic sinusoidal insulin and glucagon concentrations were calculated as previously described (An et al., 2008). Net fractional glucose extraction by the liver was calculated as the ratio of net hepatic glucose balance to Loadin.
For all glucose balance calculations, glucose concentrations were converted from plasma to blood values by using previously determined (Hsieh et al., 1998; Moore et al., 1999) conversion factors (CF: the mean of the ratio of the blood value to the plasma concentration). The use of whole blood glucose values ensures accurate hepatic balance measurements regardless of the characteristics of glucose entry into the erythrocyte.
2.5. Statistical analysis
All data are presented as means ± S.E.M. Time course data were analyzed with Two Way Repeated Measures ANOVA, and One Way ANOVA was used for any comparisons of other mean data. Post-hoc analysis was carried out using the Student-Newman-Keuls method. Statistical significance was accepted at P<0.05.
3. Results
3.1. 5-HT levels
The 5-HT levels in the liver were significantly increased in response to intraportal escitalopram infusion at 8 μg/kg/min (H-ESC vs. SAL, P<0.05) (Table 1). Arterial, portal and hepatic vein blood 5-HT concentrations did not change significantly in any group throughout the study (Table 1). There was a consistent, with the exception of L-ESC group during P2, albeit small net hepatic production of serotonin in each group (Table 1).
Table 1.
Serotonin concentrations and net hepatic balance in conscious 42-h-fasted dogs given saline, or a low or high dose escitalopram infusion into the hepatic portal vein
| Experimental Period |
|||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| 5-HT Levels in Liver (ng/mg protein) | |||
| SAL | N/A | N/A | 543±141 |
| L-ESC | N/A | N/A | 799±208 |
| H-ESC | N/A | N/A | 908±72a |
| Arterial Blood 5-HT (μg/L) | |||
| SAL | 1140±283 | 1054±238 | 968±250 |
| L-ESC | 835±176 | 835±165 | 702±126 |
| H-ESC | 805±203 | 766±217 | 728±172 |
| Portal Blood 5-HT (μg/L) | |||
| SAL | 1235±322 | 1139±243 | 933±200 |
| L-ESC | 873±149 | 780±126 | 733±123 |
| H-ESC | 901±240 | 834±202 | 730±173 |
| Hepatic Blood 5-HT (μg/L) | |||
| SAL | 1268±299 | 1150±220 | 1041±226 |
| L-ESC | 889±131 | 861±138 | 694±113 |
| H-ESC | 945±230 | 861±175 | 769±197 |
| Net Hepatic 5-HT Balance (μg/kg/min) | |||
| SAL | 1.5±2.0 | 1.0±0.6 | 2.6±1.5 |
| L-ESC | 0.7±1.8 | 1.8±1.1 | −0.7±1.4 |
| H-ESC | 2.0±1.8 | 0.7±1.3 | 0.9±1.3 |
Data are mean ± S.E.M.. n=6 dogs in each group
Negative values for balance data indicate net hepatic uptake
Significant statistical difference (P<0.05) from the saline group
3.2. Hormone concentrations
The arterial and hepatic sinusoidal insulin concentrations increased three- to fourfold and remained stable during P1 and P2 in all groups (Table 2). Arterial and hepatic sinusoidal plasma glucagon concentrations, on the other hand, remained near basal (Table 2). The mean plasma cortisol concentrations also remained statistically unchanged from basal in each group (Table 2).
Table 2.
Hormone concentrations during the basal and experimental periods in conscious 42-h-fasted dogs given saline, or a low or high dose escitalopram infusion into the hepatic portal vein
| Experimental Period |
|||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| Arterial Plasma Insulin (pmol/L) | |||
| SAL | 48±7 | 116±7a | 130±9a |
| L-ESC | 48±10 | 133±12a | 160±13a |
| H-ESC | 44±3 | 138±13a | 158±16a |
| Hepatic Sinusoidal Insulin (pmol/L) | |||
| SAL | 132±20 | 483±78a | 466±63a |
| L-ESC | 145±45 | 569±47a | 596±37a |
| H-ESC | 147±23 | 571±39a | 589±50a |
| Arterial Plasma Glucagon (ng/L) | |||
| SAL | 43±4 | 41±3 | 40±3 |
| L-ESC | 40±6 | 45±9 | 44±8 |
| H-ESC | 35±4 | 43±3 | 37±3 |
| Hepatic Sinusoidal Glucagon (ng/L) | |||
| SAL | 50±4 | 56±4 | 53±4 |
| L-ESC | 47±5 | 61±7 | 57±8 |
| H-ESC | 40±4 | 63±4a | 51±3 |
| Arterial Cortisol (nmol/L) | |||
| SAL | 109±36 | 122±21 | 78±11 |
| L-ESC | 88±27 | 87±23 | 73±16 |
| H-ESC | 100±21 | 126±16 | 75±12 |
Data are mean ± S.E.M.. n=11 dogs in the saline group (SAL), n=6 in the group that received escitalopram at 2 μg/kg/min (L-ESC) and n=7 in the group that received escitalopram at 8 μg/kg/min (H-ESC)
Significant statistical difference (P<0.05) from basal period within the group
3.3 Hepatic blood flow, blood glucose concentrations, and hepatic glucose load
Portal vein blood flow decreased ~20% in all groups during P1 in response to somatostatin infusion (Table 3). There tended to be a concomitant and somewhat offsetting increase in hepatic arterial flow. Nevertheless, total hepatic blood flow tended to be slightly reduced during P1 and P2 in all groups.
Table 3.
Average hepatic arterial, portal, and total hepatic blood flow during the basal and experimental periods in conscious 42-h-fasted dogs given saline, or a low or high dose escitalopram infusion into the hepatic portal vein
| Experimental Period |
|||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| Average Hepatic Arterial Blood Flow (ml/kg/min) | |||
| SAL | 5.1±0.4 | 5.9±0.5 | 6.8±0.9 |
| L-ESC | 5.2±0.4 | 5.9±0.8 | 6.9±1.1 |
| H-ESC | 5.0±0.2 | 5.5±0.3 | 6.0±0.6 |
| Average Hepatic Portal Blood Flow (ml/kg/min) | |||
| SAL | 22.6±1.4 | 17.9±1.2a | 17.9±1.2a |
| L-ESC | 25.6±0.7 | 18.3±0.8a | 17.3±0.6a |
| H-ESC | 21.4±1.7 | 16.3±1.2a | 17.4±1.1 |
| Average Total Hepatic Blood Flow (ml/kg/min) | |||
| SAL | 27.7±1.4 | 23.8±1.4 | 24.7±1.4 |
| L-ESC | 30.8±0.9 | 24.2±1.4a | 24.8±1.5 |
| H-ESC | 26.4±1.7 | 21.8±1.4 | 23.5±1.2 |
Data are mean ± S.E.M.. n=11 dogs in the saline group (SAL), n=6 in the group that received escitalopram at 2 μg/kg/min (L-ESC) and n=7 in the group that received escitalopram at 8 μg/kg/min (H-ESC)
Significant statistical difference (P<0.05) from basal period within the group
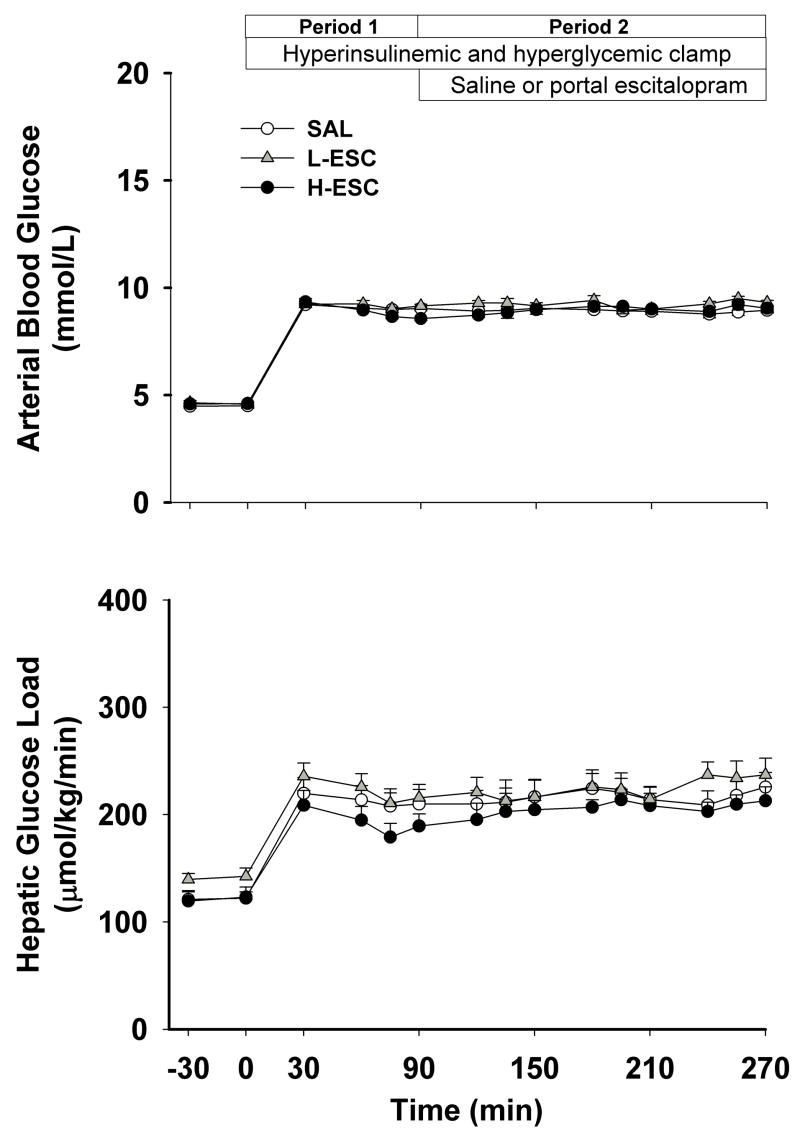
Arterial blood glucose concentrations increased in all groups from a basal value of ~4.5±0.1 to ~9.1±0.2 mmol/l during P1 and P2 (Fig. 1). The hepatic glucose loads increased proportionally and did not differ significantly among groups at any time (Fig. 1).
Figure 1.
Arterial blood glucose and hepatic glucose loads in 42-h-fasted conscious dogs during the basal (−30–0 min) and experimental periods (period [P] 1, 0–90 min; P2, 90–270 min). Somatostatin was infused peripherally and insulin (4-fold basal) and glucagon (basal) were given intraportally, while glucose was delivered peripherally at a variable rate to increase the hepatic glucose load 2-fold basal during P1 and P2. SAL group (n=11), received intraportal normal saline during P2; L-ESC group (n=6), received intraportal escitalopram (2 μg/kg/min) during P2; H-ESC group (n=7), received intraportal escitalopram (8 μg/kg/min) during P2. Data are mean ± S.E.M.
3.4. Net hepatic glucose balance and net hepatic fractional glucose extraction
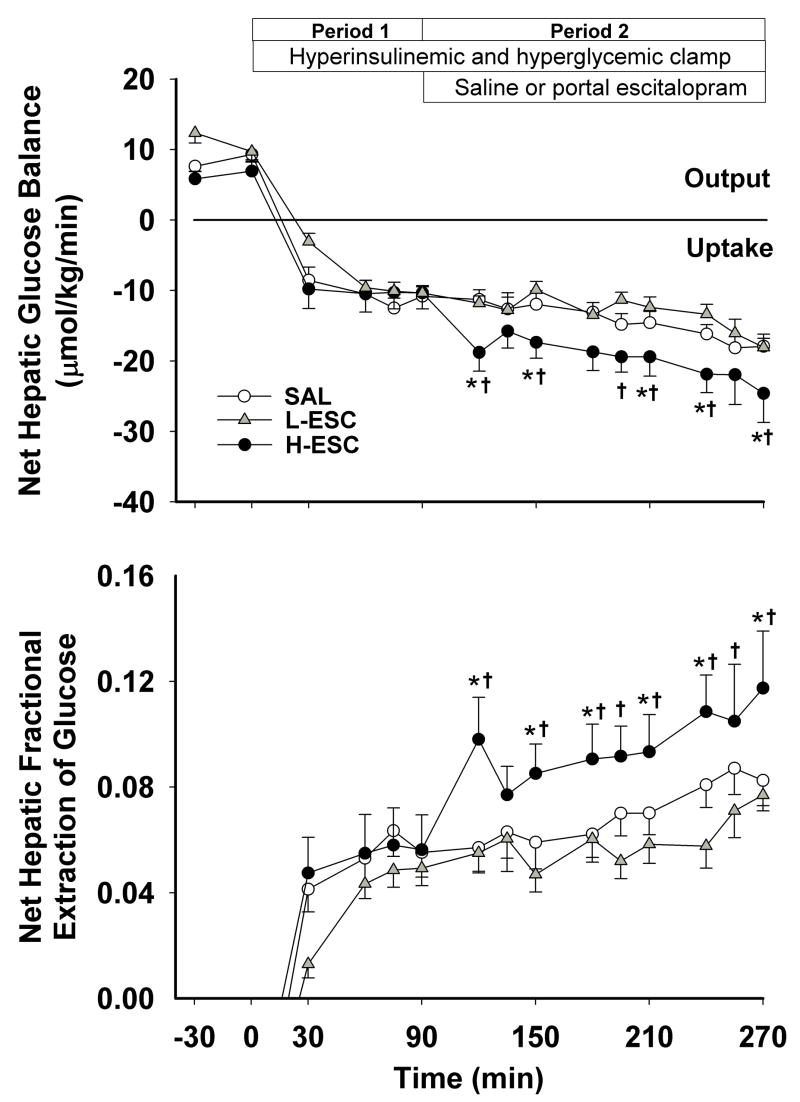
All groups exhibited a similar rate of net hepatic glucose output during the basal period. Coincident with the start of the experimental period (4X basal insulin, basal glucagon and hyperglycemia), all groups switched from net output to net uptake of glucose, with the rates not being significantly different among groups during P1 (Fig. 2). Subsequently, the rate of net hepatic glucose uptake remained relatively stable in SAL and L-ESC (13.3±1.6 and 13.3±1.3 μmol/kg/min), whereas it increased in H-ESC and averaged 22.6±3.7 μmol/kg/min during the last hour of P2 (P<0.05 vs. SAL). The net hepatic fractional glucose extraction followed a similar pattern, increasing significantly in response to intraportal escitalopram infusion at 8 μg/kg/min (Fig. 2).
Figure 2.
Net hepatic glucose uptake and net hepatic fractional extraction of glucose in 42-h-fasted conscious dogs during the basal and experimental periods. See Fig. 1 for description of study conditions. Data are mean ± S.E.M. P<0.05 compared to SAL group (*), and compared with the L-ESC group (†).
3.5. Glucose infusion rates, nonhepatic glucose uptake
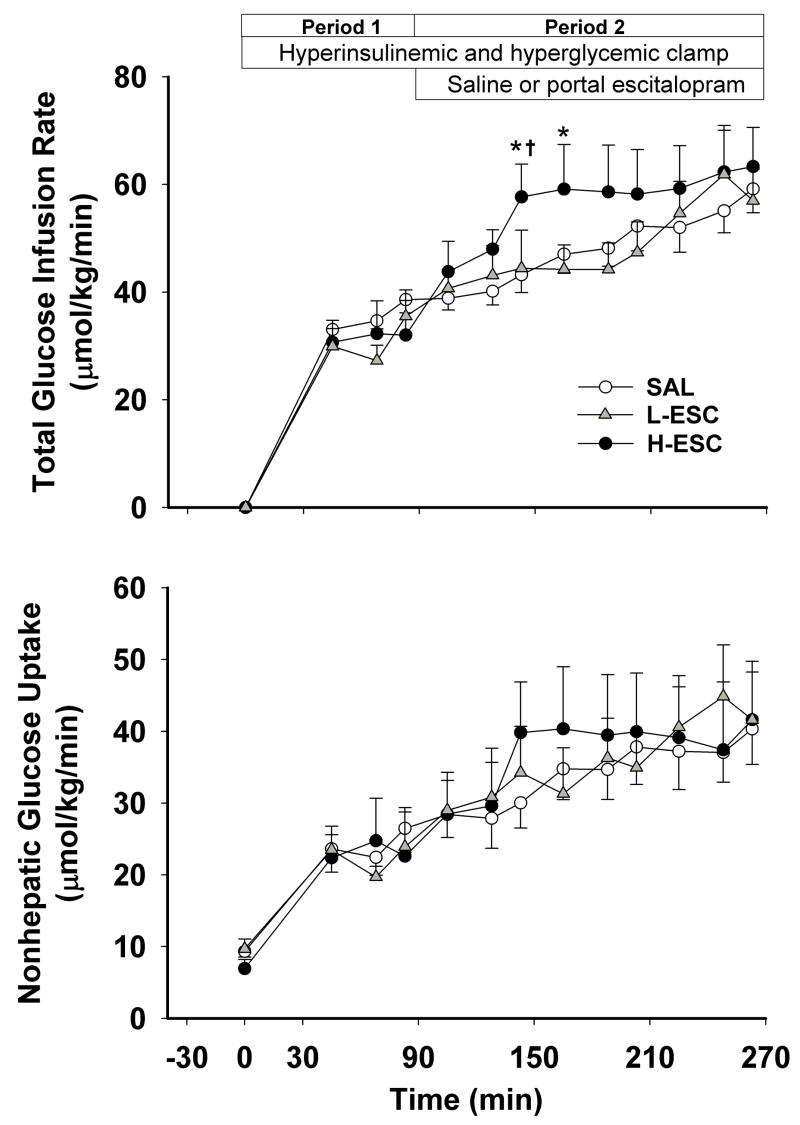
The glucose infusion and nonhepatic glucose uptake rates increased over time in all groups (Fig. 3). In response to intraportal escitalopram infusion at 8 μg/kg/min, the area under the curve (AUC) for the change from baseline in glucose infusion rate was significantly greater than in the other two groups (709±149 in H-ESC vs. 272±58 in SAL and 368±82 μmol/kg/min X180 min in L-ESC group, P<0.05) during P2 (90–270 min). Nonhepatic glucose uptake did not differ significantly among groups at any time.
Figure 3.
Glucose infusion rate and nonhepatic glucose uptake in 42-h-fasted conscious dogs during the basal and experimental periods. See Fig. 1 for description of study conditions. Data are mean ± S.E.M. P<0.05 compared to SAL group (*), and compared with the L-ESC group (†).
3.6. Lactate metabolism, net hepatic carbon retention and hepatic glycogen content
The arterial blood lactate concentrations rose in all groups during P1 and P2. After the experimental period began, net hepatic lactate balance changed from uptake to output, and the output rates remained elevated in all groups during P2 (Table 4). Net hepatic carbon retention (μmol/kg/min) did not differ between SAL, L-ESC and H-ESC at any time during P1 but was increased in H-ESC relative to the other two groups during P2 (20.9±2.6 in H-ESC vs. 15.4±1.3 in SAL and 14.9±0.6 L-ESC group, respectively, P<0.05). In agreement with this, the hepatic glycogen content at the end of study was greater in H-ESC than in L-ESC and SAL (5.6 ± 0.4 vs. 3.3± 0.2 and 4.0 ± 0.4 g/100 g liver, respectively, P<0.05).
Table 4.
Average lactate, glycerol and non-esterified fatty acid concentrations and net hepatic balances during the basal and experimental periods in conscious 42-h-fasted dogs given saline, or a low or high dose escitalopram infusion into the hepatic portal vein
| Experimental Period |
|||
|---|---|---|---|
| Group | Basal Period | Period 1 | Period 2 |
| Arterial Blood Lactate (μmol/L) | |||
| SAL | 327±58 | 673±84 | 783±81 |
| L-ESC | 395±32 | 934±98 | 855±73 |
| H-ESC | 551±90 | 1025±192 | 986±103 |
| Net Hepatic Lactate Balance (μmol/kg/min) | |||
| SAL | −7.3±1.1 | 3.5±4.3 | 3.0±2.8 |
| L-ESC | −6.6±0.9 | 6.8±1.6 | 3.6±1.4 |
| H-ESC | −5.2±1.5 | 4.1±1.5 | 3.5±1.8 |
| Arterial Blood Glycerol (μmol/L) | |||
| SAL | 82±14 | 43±11 | 34±15 |
| L-ESC | 97±9 | 34±5 | 24±4 |
| H-ESC | 98±7 | 46±10 | 31±7 |
| Net Hepatic Glycerol Uptake (μmol/kg/min) | |||
| SAL | 1.8±0.4 | 1.0±0.4 | 0.6±0.3 |
| L-ESC | 2.1±0.3 | 0.5±0.1 | 0.5±0.1 |
| H-ESC | 1.4±0.2 | 0.7±0.1 | 0.6±0.2 |
| Arterial Plasma Non-esterified Fatty Acid (μmol/L) | |||
| SAL | 803±114 | 108±11 | 90±18 |
| L-ESC | 909±75 | 133±17 | 96±14 |
| H-ESC | 1027±57 | 138±20 | 100±14 |
| Net Hepatic Non-esterified Fatty Acid Uptake (μmol/kg/min) | |||
| SAL | 4.4±1.5 | 0.2±0.1 | 0.1±0.1 |
| L-ESC | 3.2±0.4 | 0.4±0.1 | 0.2±0.1 |
| H-ESC | 3.0±0.5 | 0.4±0.1 | 0.2±0.1 |
Data are mean ± S.E.M.. n=11 dogs in the saline group (SAL), n=6 in the group that received escitalopram at 2 μg/kg/min (L-ESC) and n=7 in the group that received escitalopram at 8 μg/kg/min (H-ESC)
All values in each group during the experimental period were significantly different (P<0.05) from the basal period. Negative values for balance data indicate net hepatic uptake
3.7. Glycerol and nonesterified fatty acid metabolism
Arterial blood glycerol concentrations and net hepatic glycerol uptakes were reduced by 40–65% in response to hyperglycemia and hyperinsulinemia and remained suppressed in all groups throughout P1 and P2 (Table 4). Arterial plasma nonesterified fatty acid concentrations and net hepatic nonesterified fatty acid uptakes changed in a pattern similar to glycerol, decreasing 85–95% during P1 and P2 in all groups (Table 4).
4. Discussion
To our knowledge, this study is the first in vivo investigation to explore the effects of the SSRI escitalopram on the ability of the liver to take up and store glucose. In the presence of fourfold basal insulin and basal glucagon, hyperglycemia caused net hepatic glucose uptake of ~11 μmol/kg/min during P1 in all three groups. Portal infusion of the SSRI escitalopram at 2 μg/kg/min (L-ESC) did not significantly enhance net hepatic glucose uptake during P2 compared with the control group (SAL). However, when the infusion rate was increased to 8 μg/kg/min (H-ESC), net hepatic glucose uptake was ~60% greater than the corresponding rate in SAL. Similar to net hepatic glucose uptake, net hepatic carbon retention did not differ among groups during P1. The enhancement of net hepatic glucose uptake during P2 in H-ESC was accompanied by a ~60% increase in net hepatic carbon retention, and the hepatic glycogen content at the end of study was greater in H-ESC than in SAL and L-ESC. At the same time, glucose uptake by nonhepatic tissues was not significantly altered by escitalopram infusion. There was a tendency (not statistically significant) for arterial and sinusoidal insulin levels to be higher in the L-ESC and H-ESC groups than in SAL group in period 2 when escitalopram was given. Since somatostatin inhibited insulin secretion, as confirmed by reduced arterial c-peptide levels (data not shown), this could have resulted from a drug induced decrease in insulin clearance or more than likely a random difference in insulin clearance in the L-ESC and H-ESC groups. In an earlier study, it was demonstrated that SSRI Sertraline increases plasma insulin levels in rats without changing peripheral insulin sensitivity (Gomez et al., 2001a). Nevertheless, it seems unlikely that the tendency of insulin to be higher in the H-ESC group was responsible for the enhancement of net hepatic glucose uptake seen in that group since it was a small change and the same tendency for insulin to be higher in the L-ESC group did not result in increased net hepatic glucose uptake.
Evidence shows that serotonin has a range of biological functions including acting as a neurotransmitter and as a regulator of vascular tone and glucose metabolism. Treatment with serotonin or its precursor tryptophan induces hypoglycemia in rodents (Smith and Pogson, 1977; Yamada et al., 1989), whereas 5-HT receptor antagonists cause hyperglycemia (Wozniak and Linnoila, 1991). In addition, SSRIs improve glucose tolerance and insulin sensitivity in obese and diabetic rats (Gomez et al., 2001b; Picarel-Blanchot et al., 1994) and humans (Breum et al., 1995; Maheux et al., 1997; Potter van Loon et al., 1992). However, the mechanisms that account for hypoglycemia or improved glucose tolerance in individuals with diabetes after treatment with SSRIs are not fully understood.
In our previous study, infusion of the SSRI fluvoxamine into the liver through the hepatic portal vein increased net hepatic glucose uptake and hepatic carbon storage under hyperglycemic hyperinsulinemic conditions in conscious dogs (Moore et al., 2004a). Fluvoxamine inhibits reuptake of serotonin into the presynaptic nerve terminals, but whether this is the mechanism by which it brings about an increase in net hepatic glucose uptake remains unknown. In the present study, escitalopram was chosen because it is the most selective SSRI available, having a very low affinity for serotonergic (5-HT1–7), alpha- and beta-adrenergic, dopamine (D1–5), histamine (H1–3), muscarinic (M1–5), and benzodiazepine receptors (Waugh and Goa, 2003). Since some of these receptors could influence glucose metabolism, using escitalopram allows us to more definitively study the effect of serotonin on hepatic glucose metabolism. The patterns of enhancement in net hepatic glucose uptake and net hepatic carbon retention in response to intraportal escitalopram infusion at 8 μg/kg/min were similar to those seen during intraportal infusion of fluvoxamine, confirming the role of hepatic serotonin in the regulation of hepatic glucose uptake. Fluvoxamine may also have inhibited peripheral glucose uptake since it was shown to significantly reduced nonhepatic glucose uptake albeit at a single time point at the end of our earlier study (Moore et al., 2004a). There was no such effect of escitolapram on nonhepatic glucose metabolism in the present study. It should be noted, however, that there was a tendency for an elevation in the plasma epinephrine level to occur when fluvoxamine was infused but not when escitolapram was given (data not shown), thereby potentially explaining the reduction in nonhepatic glucose uptake.
5-HT receptor subtypes have been grouped into seven families, 5-HT receptors 1 to 7, based on the second messenger system they employ as well as their sequence homologies. Each family comprises several variant subtypes. With the exception of the 5-HT3 receptor, 5-HT receptors are G-protein-coupled receptors. 5-HT1, 5-HT2 and 5-HT4 receptors are found in relative abundance in the liver (genecards.org). 5-HT4 receptor is coupled to Gs, which activates adenylyl cyclase (AC), and increases synthesis of cAMP. cAMP in turn activates protein kinase A (PKA), and PKA phosphorylates downstream protein kinases. PKA also causes activation of glycogen phosphorylase in hepatocytes thereby triggering the degradation of hepatic glycogen. 5-HT2 receptor is coupled to Gq, which activates phospholipase C (PLC), yielding diacylglycerol (DAG). DAG activates protein kinase C (PKC), which then activates downstream protein kinases. By contrast, 5-HT1 receptor is coupled to Gi, which inhibits AC and PKA. It is possible that the activation of hepatic 5-HT1 receptor will cause inactivation of PKA, and in turn result in an increase in net hepatic glucose uptake and hepatic glycogen deposition in response to escitalopram. The latter possibility is supported by a recent study which showed that a 5-HT1 receptor agonist could enhance glycogen synthesis in cultured hepatocytes (Hampson et al., 2007).
Twenty mg escitalopram by mouth OQD is commonly prescribed to treat depression. It has been shown in human subjects that i.v. infusion of escitalopram at a rate of 10 mg/h (~2 μg/kg/min) brings about a similar pharmacokinetic profile to 20 mg of the drug p.o. (Sogaard et al., 2005). In the present study, when escitalopram was infused intraportally of at 2 μg/kg/min, a dose which was believed to mimic the pharmacokinetic profile in humans taking 20 mg escitalopram daily, no change of net hepatic glucose uptake was observed. However net hepatic glucose uptake was significantly enhanced in response to the portal infusion of escitalopram at 8 μg/kg/min. Little is known about how the pharmacokinetics of escitalopram in dogs and humans compare, but an early citalopram (escitalopram racemate) study showed that its half life is much shorter and its systemic plasma clearance rate is much higher in dogs than in humans (Fredricson Overo, 1982). This suggests that in order to mimic the pharmacokinetic profile in humans, higher dose of escitalopram are needed in the dog model. Therefore, the enhancement of net hepatic glucose uptake seen in the present study with a high dose of escitalopram may reflect that seen in humans with a typical oral dose of the drug. The enhancement of net hepatic glucose uptake was accompanied by an increase in net hepatic carbon retention, which reflects hepatic glycogen deposition. In a net sense, ~85% of the glucose taken up by the liver in response to the drug was deposited in liver glycogen, while most of the remaining glucose left the liver as lactate. It appears that portal escitalopram infusion had no effects on nonhepatic glucose uptake, although it is hard to conclusively rule out such a possibility because of the variability associated with the measurement of this parameter. This means that the increase in whole body glucose disposal seen in response to intraportal escitalopram infusion at 8 μg/kg/min was most likely the result of augmented net hepatic glucose uptake.
As noted above, the augmentation of net hepatic glucose uptake and hepatic glucose storage could have resulted from a direct interaction of endogenous serotonin with its receptor on hepatocytes. Recently, it was reported that physiological concentrations of serotonin or a 5-HT1 receptor agonist have direct stimulatory effects on glycogen synthesis in primary cultures of rat hepatocytes (Hampson et al., 2007). It is also possible, however, that the increase in net hepatic glucose uptake seen in the present study could have been brought about by a neural signal initiated in the liver. A rise in hepatic serotonin levels resulting from intraportal serotonin injection can decrease the afferent firing rate in the hepatic branch of the vagus nerve, similar to the effect seen in response to intraportal glucose injection (Niijima, 1981) (Niijima, 1996). The vagus nerve communicates with the hypothalamus via the hypothalamic projections to the medulla oblongata and the liver could receive autonomic input as a result of hypothalamic nuclei receiving serotonergic input (Buijs et al., 2003; Hosoya, 1985). Furthermore, stimulation of hypothalamic autonomic efferents can enhance hepatic glycogen synthase activity (Shimazu, 1967). These investigations suggest a mechanism by which a change of serotonin levels in the liver could elicit a neural signal which may be coordinated by the hypothalamus to enhance net hepatic glucose uptake. In line with this theory, we observed an increase in 5-HT levels in the liver in response to intraportal escitalopram infusion at 8 μg/kg/min. Consistent with our previous observations with fluvoxamine, however, we did not detect a significant increase in 5-HT levels in the blood. Such measurements may not be very sensitive readouts for alterations in neurotransmitter levels in the synapse (Ahren et al., 1988), given the variance of the analytical method and the limited power to detect a change of 5-HT levels (the power calculation was set based on detecting differences in net hepatic glucose balance). We also cannot rule out the involvement of central serotonergic sensing since the infused SSRI escitalopram can penetrate the blood-brain barrier and may thus activate the brain serotonergic system directly. The central serotonergic system has recently been shown to regulate peripheral glucose metabolism without changing energy expenditure, locomotor activity or body weight (Zhou et al., 2007). These authors showed that 5-HT2C receptor agonists significantly improved glucose tolerance and reduced the plasma insulin level in mouse models of obesity and type 2 diabetes. Furthermore, they showed that the mechanism required downstream activation of melanocortin-4 receptors in the CNS. The precise workings of the serotonin receptors/pathways regulating hepatic glucose metabolism require further elucidation. Highly selective receptor agonists/antagonists will undoubtedly provide valuable mechanistic insights into the role of serotonin receptors in modulating glucose homeostasis.
In conclusion, our data indicate that intraportal infusion of a SSRI, escitalopram, enhanced net hepatic glucose uptake and hepatic glycogen deposition under hyperglycemic and hyperinsulinemic conditions in conscious 42-h-fasted dogs. These findings raise the possibility that serotonin may play a role in directing the disposition of an oral glucose load. Hepatic-targeted SSRIs might therefore help to control postprandial hyperglycemia in individuals with diabetes.
Acknowledgments
The authors appreciate the assistance and support of Jon Hastings, Patsy Raymer, the Diabetes Research and Training Center Hormone Core, and Vanderbilt Neurochemistry Core in these studies. We would also like to thank Phil Williams for his technical assistance. The drug escitalopram oxalate used in this work was generously provided by Forest Research Institute (Jersey City, NJ). This research was supported by the National Institutes of Health grant RO1 DK43706 and Vanderbilt University Diabetes Research and Training Center grant P60 DK020593. Part of this work was presented at the 67th Annual Meeting of the American Diabetes Association, Chicago, June, 2007. Dr. Cherrington is the Jacquelyn A. Turner and Dr. Dorothy J. Turner Chair in Diabetes Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins-Marshall B, Pagliassotti MJ, Asher JR, Connolly CC, Neal DW, Williams PE, Myers SR, Hendrick GK, Adkins RB, Jr, Cherrington AD. Role of hepatic nerves in response of liver to intraportal glucose delivery in dogs. Am J Physiol. 1992;262:E679–686. doi: 10.1152/ajpendo.1992.262.5.E679. [DOI] [PubMed] [Google Scholar]
- Ahren B, Dunning BE, Havel PJ, Veith RC, Taborsky GJ., Jr Extraction of epinephrine and norepinephrine by the dog pancreas in vivo. Metabolism. 1988;37:68–73. doi: 10.1016/0026-0495(88)90031-5. [DOI] [PubMed] [Google Scholar]
- An Z, Dicostanzo CA, Moore MC, Edgerton DS, Dardevet DP, Neal DW, Cherrington AD. Effects of the nitric oxide donor SIN-1 on net hepatic glucose uptake in the conscious dog. Am J Physiol Endocrinol Metab. 2008;294:E300–306. doi: 10.1152/ajpendo.00380.2007. [DOI] [PubMed] [Google Scholar]
- Bajaj M, Berria R, Pratipanawatr T, Kashyap S, Pratipanawatr W, Belfort R, Cusi K, Mandarino L, DeFronzo RA. Free fatty acid-induced peripheral insulin resistance augments splanchnic glucose uptake in healthy humans. Am J Physiol Endocrinol Metab. 2002;283:E346–352. doi: 10.1152/ajpendo.00329.2001. [DOI] [PubMed] [Google Scholar]
- Basu A, Basu R, Shah P, Vella A, Johnson CM, Jensen M, Nair KS, Schwenk WF, Rizza RA. Type 2 diabetes impairs splanchnic uptake of glucose but does not alter intestinal glucose absorption during enteral glucose feeding: additional evidence for a defect in hepatic glucokinase activity. Diabetes. 2001;50:1351–1362. doi: 10.2337/diabetes.50.6.1351. [DOI] [PubMed] [Google Scholar]
- Breum L, Bjerre U, Bak JF, Jacobsen S, Astrup A. Long-term effects of fluoxetine on glycemic control in obese patients with non-insulin-dependent diabetes mellitus or glucose intolerance: influence on muscle glycogen synthase and insulin receptor kinase activity. Metabolism. 1995;44:1570–1576. doi: 10.1016/0026-0495(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Cherrington AD. Banting Lecture 1997. Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–1214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- Culpepper L. Escitalopram: A New SSRI for the Treatment of Depression in Primary Care. Prim Care Companion J Clin Psychiatry. 2002;4:209–214. doi: 10.4088/pcc.v04n0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredricson Overo K. Kinetics of citalopram in test animals; drug exposure in safety studies. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:297–309. doi: 10.1016/s0278-5846(82)80180-2. [DOI] [PubMed] [Google Scholar]
- Galassetti P, Shiota M, Zinker BA, Wasserman DH, Cherrington AD. A negative arterial-portal venous glucose gradient decreases skeletal muscle glucose uptake. Am J Physiol. 1998;275:E101–111. doi: 10.1152/ajpendo.1998.275.1.E101. [DOI] [PubMed] [Google Scholar]
- Gomez R, Huber J, Lhullier F, Barros HM. Plasma insulin levels are increased by sertraline in rats under oral glucose overload. Braz J Med Biol Res. 2001a;34:1569–1572. doi: 10.1590/s0100-879x2001001200009. [DOI] [PubMed] [Google Scholar]
- Gomez R, Huber J, Tombini G, Barros HM. Acute effect of different antidepressants on glycemia in diabetic and non-diabetic rats. Braz J Med Biol Res. 2001b;34:57–64. doi: 10.1590/s0100-879x2001000100007. [DOI] [PubMed] [Google Scholar]
- Hampson LJ, Mackin P, Agius L. Stimulation of glycogen synthesis and inactivation of phosphorylase in hepatocytes by serotonergic mechanisms, and counter-regulation by atypical antipsychotic drugs. Diabetologia. 2007;50:1743–1751. doi: 10.1007/s00125-007-0696-y. [DOI] [PubMed] [Google Scholar]
- Hendrick GK, Frizzell RT, Williams PE, Cherrington AD. Effect of hyperglucagonemia on hepatic glycogenolysis and gluconeogenesis after a prolonged fast. Am J Physiol. 1990;258:E841–849. doi: 10.1152/ajpendo.1990.258.5.E841. [DOI] [PubMed] [Google Scholar]
- Hosoya Y. Hypothalamic projections to the ventral medulla oblongata in the rat, with special reference to the nucleus raphe pallidus: a study using autoradiographic and HRP techniques. Brain Res. 1985;344:338–350. doi: 10.1016/0006-8993(85)90812-1. [DOI] [PubMed] [Google Scholar]
- Hsieh PS, Moore MC, Neal DW, Cherrington AD. Hepatic glucose uptake rapidly decreases after removal of the portal signal in conscious dogs. Am J Physiol. 1998;275:E987–992. doi: 10.1152/ajpendo.1998.275.6.E987. [DOI] [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs) Int Clin Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Keppler D, Decker K. Glycogen: determination with amyloglycosidase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Verlag Chemie Weinheim, Academic Press; New York: 1974. pp. 1127–1131. [Google Scholar]
- Klein N, Sacher J, Geiss-Granadia T, Attarbaschi T, Mossaheb N, Lanzenberger R, Potzi C, Holik A, Spindelegger C, Asenbaum S, Dudczak R, Tauscher J, Kasper S. In vivo imaging of serotonin transporter occupancy by means of SPECT and [123I]ADAM in healthy subjects administered different doses of escitalopram or citalopram. Psychopharmacology (Berl) 2006;188:263–272. doi: 10.1007/s00213-006-0486-0. [DOI] [PubMed] [Google Scholar]
- Leevy CM, Mendenhall CL, Lesko W, Howard MM. Estimation of hepatic blood flow with indocyanine green. J Clin Invest. 1962;41:1169–1179. doi: 10.1172/JCI104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvik B, Nolan JJ, Roberts A, Baloga J, Joyce M, Bell JM, Olefsky JM. Evidence for decreased splanchnic glucose uptake after oral glucose administration in non-insulin-dependent diabetes mellitus. J Clin Invest. 1997;100:2354–2361. doi: 10.1172/JCI119775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheux P, Ducros F, Bourque J, Garon J, Chiasson JL. Fluoxetine improves insulin sensitivity in obese patients with non-insulin-dependent diabetes mellitus independently of weight loss. Int J Obes Relat Metab Disord. 1997;21:97–102. doi: 10.1038/sj.ijo.0800372. [DOI] [PubMed] [Google Scholar]
- Martin FJ, Miguez JM, Aldegunde M, Atienza G. Effect of streptozotocin-induced diabetes mellitus on serotonin measures of peripheral tissues in rats. Life Sci. 1995;56:51–59. doi: 10.1016/0024-3205(94)00407-j. [DOI] [PubMed] [Google Scholar]
- Moore MC, DiCostanzo CA, Dardevet D, Lautz M, Farmer B, Neal DW, Cherrington AD. Portal infusion of a selective serotonin reuptake inhibitor enhances hepatic glucose disposal in conscious dogs. Am J Physiol Endocrinol Metab. 2004a;287:E1057–1063. doi: 10.1152/ajpendo.00313.2004. [DOI] [PubMed] [Google Scholar]
- Moore MC, Geho WB, Lautz M, Farmer B, Neal DW, Cherrington AD. Portal serotonin infusion and glucose disposal in conscious dogs. Diabetes. 2004b;53:14–20. doi: 10.2337/diabetes.53.1.14. [DOI] [PubMed] [Google Scholar]
- Moore MC, Hsieh PS, Flakoll PJ, Neal DW, Cherrington AD. Differential effect of amino acid infusion route on net hepatic glucose uptake in the dog. Am J Physiol. 1999;276:E295–302. doi: 10.1152/ajpendo.1999.276.2.E295. [DOI] [PubMed] [Google Scholar]
- Moore MC, Kimura K, Shibata H, Honjoh T, Saito M, Everett CA, Smith MS, Cherrington AD. Portal 5-hydroxytryptophan infusion enhances glucose disposal in conscious dogs. Am J Physiol Endocrinol Metab. 2005;289:E225–231. doi: 10.1152/ajpendo.00614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CR, Lazarow A. Immunoassay of insulin using a two-antibody system. Proc Soc Exp Biol Med. 1962;110:29–32. doi: 10.3181/00379727-110-27411. [DOI] [PubMed] [Google Scholar]
- Myers SR, McGuinness OP, Neal DW, Cherrington AD. Intraportal glucose delivery alters the relationship between net hepatic glucose uptake and the insulin concentration. J Clin Invest. 1991;87:930–939. doi: 10.1172/JCI115100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niijima A. Visceral afferents and metabolic function. Diabetologia. 1981;20(Suppl):325–330. [PubMed] [Google Scholar]
- Niijima A. Afferent impulse discharges from glucoreceptors in the liver of the guinea pig. Nutrition. 1996;12:390, 392–393. [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Picarel-Blanchot F, Bailbe D, Portha B. d-Fenfluramine improves hepatic insulin action in streptozotocin-diabetic rats. Eur J Pharmacol. 1994;264:227–232. doi: 10.1016/0014-2999(94)00547-8. [DOI] [PubMed] [Google Scholar]
- Potter van Loon BJ, Radder JK, Frolich M, Krans HM, Zwinderman AH, Meinders AE. Fluoxetine increases insulin action in obese nondiabetic and in obese non-insulin-dependent diabetic individuals. Int J Obes Relat Metab Disord. 1992;16:79–85. [PubMed] [Google Scholar]
- Satake S, Moore MC, Igawa K, Converse M, Farmer B, Neal DW, Cherrington AD. Direct and indirect effects of insulin on glucose uptake and storage by the liver. Diabetes. 2002;51:1663–1671. doi: 10.2337/diabetes.51.6.1663. [DOI] [PubMed] [Google Scholar]
- Shimazu T. Glycogen synthetase activity in liver: regulation by the autonomic nerves. Science. 1967;156:1256–1257. doi: 10.1126/science.156.3779.1256. [DOI] [PubMed] [Google Scholar]
- Smith SA, Pogson CL. Tryptophan and the control of plasma glucose concentrations in the rat. Biochem J. 1977;168:495–506. doi: 10.1042/bj1680495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard B, Mengel H, Rao N, Larsen F. The pharmacokinetics of escitalopram after oral and intravenous administration of single and multiple doses to healthy subjects. J Clin Pharmacol. 2005;45:1400–1406. doi: 10.1177/0091270005280860. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Kimura I, Yamada J, Watanabe Y, Takeuchi N, Horisaka K. Effects of serotonin on blood glucose and insulin levels of glucose- and streptozotocin-treated mice. Jpn J Pharmacol. 1990;54:93–96. doi: 10.1254/jjp.54.93. [DOI] [PubMed] [Google Scholar]
- Waugh J, Goa KL. Escitalopram : a review of its use in the management of major depressive and anxiety disorders. CNS Drugs. 2003;17:343–362. doi: 10.2165/00023210-200317050-00004. [DOI] [PubMed] [Google Scholar]
- Wozniak KM, Linnoila M. Hyperglycemic properties of serotonin receptor antagonists. Life Sci. 1991;49:101–109. doi: 10.1016/0024-3205(91)90023-5. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Kimura I, Takeuchi N, Horisaka K. Serotonin-induced hypoglycemia and increased serum insulin levels in mice. Life Sci. 1989;45:1931–1936. doi: 10.1016/0024-3205(89)90547-x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK. Serotonin 2C Receptor Agonists Improve Type 2 Diabetes via Melanocortin-4 Receptor Signaling Pathways. Cell Metab. 2007;6:398–405. doi: 10.1016/j.cmet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]