Abstract
Primary headaches such as migraine can be aborted by systemic administration of non-steroidal anti-inflammatory drugs (NSAIDs), potentially through the non-selective inhibition of cyclooxygenase (COX) activity in the intracranial meninges. In this study we have used single and double labeling immunohistochemistry to examine the distribution of the COX-1 and COX-2 isoforms in the intracranial dura mater of the rat and identify cell types that express them. COX-1 immunoreactivity was found in medium and small dural blood vessels and was co-expressed with the endothelial cell markers vimentin and the endothelial isoform of nitric oxide synthase (ecNOS). COX-1 was also found to be present in most dural mast cells. COX-2 was mainly expressed in ED2-positive resident dural macrophages. Constitutive COX-2 expression was also found in some axonal profiles, many of which were co-labeled with the nociceptor peptide marker CGRP. The findings suggest that NSAIDs may abort headache, at least in part, by inhibiting either neuronal or non-neuronal COX activity in the dura mater.
Keywords: Cyclooxygenase, dura, headache, migraine, trigeminal, NSAID, inflammation
Introduction
Primary headaches such as migraine are believed to ensue as a result of sterile meningeal inflammation localized to the intracranial meninges and the subsequent activation and sensitization of trigeminal nociceptive fibers that innervate them [1, 21, 22]. The ability of systemic administration of non-steroidal anti-inflammatory drugs (NSAIDs) to abort these headaches [8, 15, 16, 20], potentially through the inhibition of the two cyclooxygenase isoforms COX-1 and COX-2 [26], further suggests the involvement of local meningeal inflammation and particularly the role of COX-derived prostanoids, such as prostacyclin, in promoting the activation and sensitization of meningeal nociceptors [27] and the resultant headache [24]. To better understand the potential targets of NSAIDs in the meninges, the present study utilized immunohistochemical methods to examine the localization of the COX-1 and COX-2 isoforms and identify the cell types that express them in the dura mater encephali of the rat.
Materials and Methods
Experiments were carried out on adult Sprague-Dawley male rats (250–300 g). All experimental protocols were approved by the institutional Animal Care and Use Committees of the Harvard Medical School and Beth Israel Deaconess Medical Center.
Immunohistochemistry
Rats were terminally anesthetized with sodium pentobarbital (100 mg/kg) and perfused transcardially with cold heparinized solution of 0.1M phosphate-buffered saline (PBS, pH 7.4), followed by freshly-made cold 4% paraformaldehyde in 0.1 M PBS. A large piece of the dura that included the superior sagittal sinus and transverse sinus was then removed and processed for either single or double labeling immunohistochemistry. Non-specific binding of the primary antibodies was blocked by incubating the dura for 2 h at 4°C in 0.1 M PBS containing 10% normal serum and 0.1% Triton-X. Thereafter, the dura was incubated for 24 h at 4°C with a primary antibody against a given COX isoform or a mixture of anti-COX antibody and another antibody directed against a marker specific to a given cell type. The dura was incubated with a goat polyclonal antibody direct against the C-terminus of either mouse COX-1 or COX-2 (SC-1754 or SC-1747, respectively, Santa Cruz Biochemicals, Santa Cruz, CA, both used at a 1:500 dilution). As a control for non-specific staining, dural preparations were incubated (a) without any primary antibody, (b) with control IgG isotypes, or (c) with COX-1 or COX-2 antibodies that were preadsorbed with the appropriate blocking peptides.
To examine cell types that express COX-1 and COX-2, we conducted additional double labeling of COX-1 or COX-2 in conjunction with another antibody directed against markers of: 1) endothelial cells (eNOS, 610298, 1:500 dilution, BD biosciences, San Jose, CA or vimentin SC-5565, 1:250 dilution, Santa Cruz biotechnology, Santa Cruz, CA), 2) resident macrophages (CD163, clone ED2, RDI-T3011X, 1:500, RDI, Flanders, NJ), or 3) peptidergic nociceptive sensory fibers (calcitonin gene-related peptide, CGRP, T-5027, 1:1000, Bachem, San Carlos, CA). For all double labeling immunofluorescence studies, a mixture of the appropriate secondary antibodies labeled with either Alexa Fluor 594 or Alexa Fluor 488 (each diluted 1:200, Invitrogen, Carlsbad, CA) was used. To examine potential COX expression in dural mast cells, the latter were labeled as part of the final fluorescent detection system by incubating the tissue for 1 h at room temperature with Fluorescein Isothiocyanate-conjugated egg white avidin (Avidin-FITC, 1:1000, Invitrogen, Carlsbad, CA) which binds to mast cell (MC) heparin [9]. All immunofluorescence images were acquired using an Olympus epi-illumination fluorescence microscopy system (BX50) equipped with a CoolSNAP CCD camera and RsImage software (Roper Scientific). Double labeling micrographs were created using Adobe Photoshop CS.
Results
Using specific antibodies raised against COX-1 or COX-2 we detected constitutive expression of both COX isoforms in the intracranial dura mater of the rat. Control studies, including omission of the primary antibody or using control IgG isotypes resulted in no labeling. Preadsorption of the primary antibodies with the appropriate peptide immunogen eliminated both COX-1 and COX-2 labeling indicating the specificity of the antibodies used.
COX-1 immunoreactivity was mainly found within medium to small (second and third order) blood vessels (Fig 1a). Some of the labeled vessels could be identified as branches of the middle meningeal artery or tributaries of the adjacent middle meningeal vein. Within a labeled blood vessels, COX-1 was co-localized with both vimentin (Fig 1c) and endothelial nitric oxide synthase (eNOS) (Fig 1f) thus indicating the bulk of COX-1 expression to be within the vascular endothelium. In addition to vascular labeling, COX-1 was also observed in most dural MCs (Fig 1i). COX-1 was not detected in dural nerve fibers.
Figure 1.
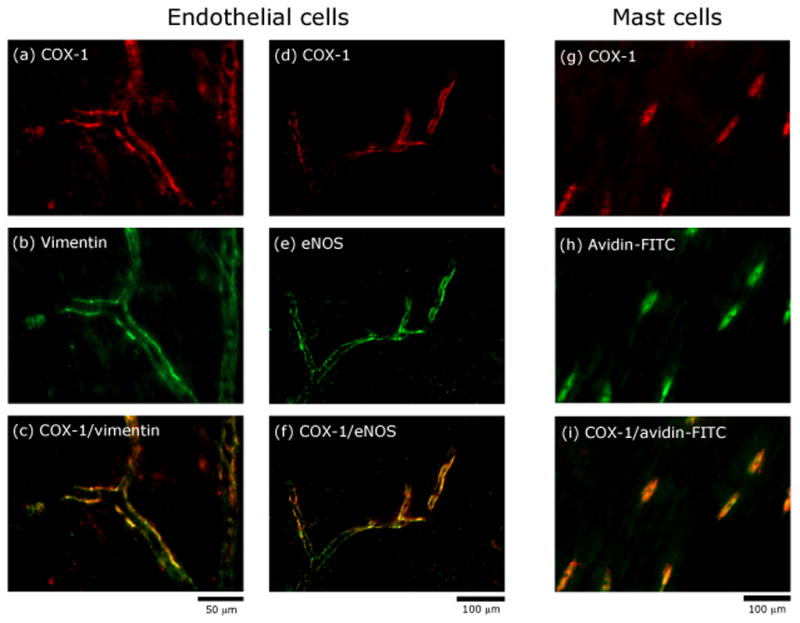
Localization of COX-1 immunoreactivity in endothelial and mast cells in a whole-mount preparation of the rat dura. Co-localization of COX-1 and vimentin immunoreactivity (ac) indicating presence of COX-1 in endothelial cells. Co-localization of COX-1 and eNOS immunoreactivity (d–f) confirming the presence of COX-1 in endothelial cells. Co-localization of COX-1 immunoreactivity and avidin (g–i) suggesting presence of COX-1 in mast cells.
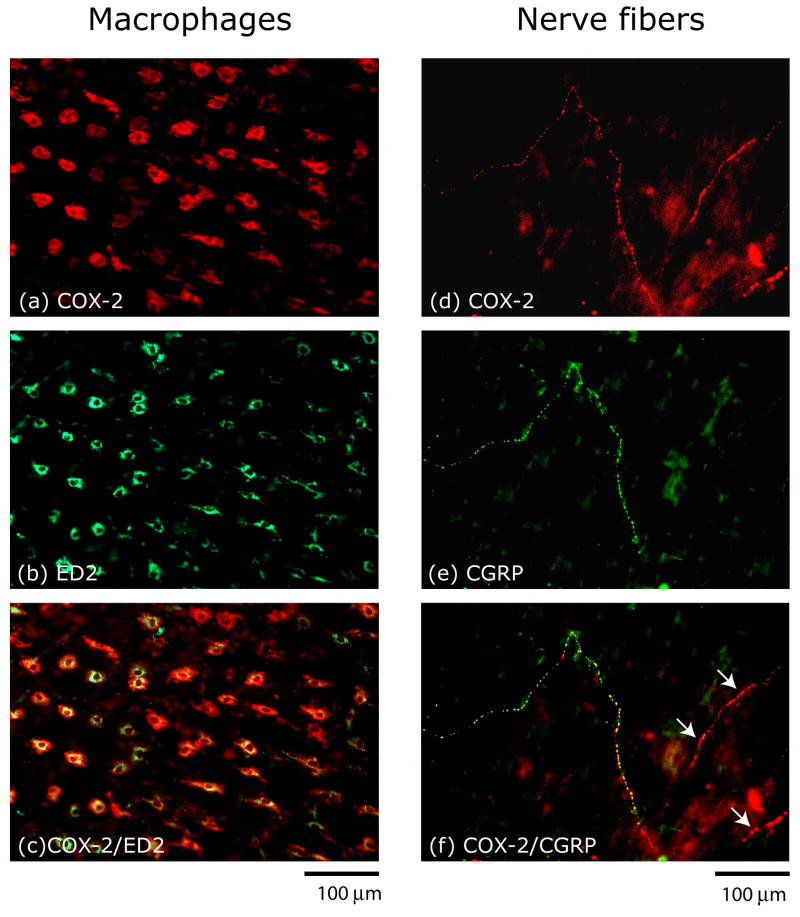
COX-2 immunoreactivity was also found throughout the dura mater, but unlike COX-1 it was not found in blood vessels or mast cells. The bulk of COX-2 labeling was localized to resident macrophages as indicated by the double labeling with the macrophage marker ED2 (Fig. 2c). COX-2 expression was also found in some axonal profiles, many of which were co-labeled with the nociceptor peptide marker CGRP (Fig. 2f).
Figure 2.
Localization of COX-2 immunoreactivity in macrophages and nerve fibers in a whole-mount preparation of the rat dura. Co-localization of COX-2 and ED2 immunoreactivity (a–c) indicating the presence of COX-2 in resident macrophages. Co-localization of COX-2 and CGRP immunoreactivity (d–f) indicating expression of COX-2 in meningeal sensory fibers. Note that COX-2 is also present in CGRP-negative fibers (arrows in f).
Discussion
Our findings suggest that intracranial meningeal COX-1 is mainly expressed in the vascular endothelium and within dural mast cells. Constitutive expression of COX-2 is mostly localized to dural macrophages and some peptidergic dural afferents fibers. Previous studies have identified basal COX-1 expression in cell bodies of primary afferent neurons in dorsal root ganglia of both rats [2] and mice [3]. We, however, failed to detect axon profiles showing COX-1-immunoreactivity throughout the trigeminal-innervated dura mater. Such negative findings suggest that COX-1 expression in trigeminal primary afferent nociceptive neurons, if present, may be limited to the cell body and not transported to the nociceptor’s peripheral endings in the dura. Our findings are supported by other studies which also failed to detect COX-1 expression in peripheral nerves [10, 12, 19], but are in contrast to a recent study showing COX-1 immunoreactivity in large cutaneous nerve branches, as well as small intradermal nerve bundles and nerve endings [13]. Such discrepancies may be due to differences between the innervation of the skin and the dura or more generally between trigeminal and non-trigeminal sensory innervations.
Although dural axons lacked COX-1, there was ample presence of COX-1 immunoreactivity in the dural vascular endothelium and adjacent mast cells. Such constitutive endothelial expression suggests that prostanoids produced by COX-1 are likely involved in controlling the circulatory homeostasis of the dura, potentially in conjunction with endothelial NOS-derived nitric oxide [4, 14].
The constitutive expression of COX-2 in perivascular macrophages and some nerve fibers in the dura (present study) and perivascular macrophages in the choroid plexus [18], as well as the brain [25] and spinal cord [5], suggest that COX-2 is not strictly an inducible isoform, as was believed for many years [23]. The co-localization of COX-2 and CGRP immunoreactivity in some dural nerve fibers suggest that COX-2 is expressed in small myelinated and non myelinated meningeal sensory fibers, likely nociceptors. Such findings are consistent with evidence for COX-2 expression in sciatic and saphenous axons [12, 13] and in cell bodies of primary afferent neurons in dorsal root ganglia [7] and the trigeminal ganglion [6].
The cellular localization of the two COX isoforms in the dura mater is in agreement with previous studies documenting the release of COX-1 and COX-2-derived prostanoids by mast cells [17] and macrophages [11] respectively. The constitutive expression of COX in meningeal blood vessels, mast cells, macrophages and nociceptive axons supports the notion that local release of prostanoids, such as PGI2 [27], could initiate as well as maintain the activation and sensitization of meningeal nociceptors thus contributing to the pathophysiology of intracranial headaches, such as migraine. Of note is a recent study demonstrating the ability of infusion of the stable PGI2 analogue epoprostenol to induce headache [24].
Conclusion
Using immunohistochemistry COX-1 and COX-2 were found to be constitutively expressed in the intracranial dura mater of the rat. Double labeling revealed the expression of COX-1 in vascular endothelial cells and dural mast cells and COX-2 expression in dural macrophages and dural axons, likely nociceptive afferents. We suggest that meningeal COX-1 and COX-2 could serve as potential target for NSAIDs in aborting headaches of intracranial origin such as migraine.
Acknowledgments
Supported by NIH grants NS046502, NS032534, NS051484 and a grant from the National Headache Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 2.Chopra B, Giblett S, Little JG, Donaldson LF, Tate S, Evans RJ, Grubb BD. Cyclooxygenase-1 is a marker for a subpopulation of putative nociceptive neurons in rat dorsal root ganglia. Eur J Neurosci. 2000;12:911–920. doi: 10.1046/j.1460-9568.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- 3.Dou W, Jiao Y, Goorha S, Raghow R, Ballou LR. Nociception and the differential expression of cyclooxygenase-1 (COX-1), the COX-1 variant retaining intron-1 (COX-1v), and COX-2 in mouse dorsal root ganglia (DRG) Prostaglandins Other Lipid Mediat. 2004;74:29–43. doi: 10.1016/j.prostaglandins.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 5.Ghilardi JR, Svensson CI, Rogers SD, Yaksh TL, Mantyh PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill JM, Lukiw WJ, Gebhardt BM, Higaki S, Loutsch JM, Myles ME, Thompson HW, Kwon BS, Bazan NG, Kaufman HE. Gene expression analyzed by microarrays in HSV-1 latent mouse trigeminal ganglion following heat stress. Virus Genes. 2001;23:273–280. doi: 10.1023/a:1012517221937. [DOI] [PubMed] [Google Scholar]
- 7.Inoue A, Iwasa M, Nishikura Y, Ogawa S, Nakasuka A, Nakata Y. The long-term exposure of rat cultured dorsal root ganglion cells to bradykinin induced the release of prostaglandin E2 by the activation of cyclooxygenase-2. Neurosci Lett. 2006;401:242–247. doi: 10.1016/j.neulet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 8.Jakubowski M, Levy D, Goor-Aryeh I, Collins B, Bajwa Z, Burstein R. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850–861. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- 9.Khalil MH, Silverman AJ, Silver R. Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J Neurobiol. 2003;56:113–124. doi: 10.1002/neu.10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi S, Baba H, Uchida K, Kokubo Y, Kubota C, Yamada S, Suzuki Y, Yoshizawa H. Effect of mechanical compression on the lumbar nerve root: localization and changes of intraradicular inflammatory cytokines, nitric oxide, and cyclooxygenase. Spine. 2005;30:1699–1705. doi: 10.1097/01.brs.0000171910.97937.0e. [DOI] [PubMed] [Google Scholar]
- 11.Linton MF, Fazio S. Cyclooxygenase-2 and inflammation in atherosclerosis. Curr Opin Pharmacol. 2004;4:116–123. doi: 10.1016/j.coph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Ma W, Eisenach JC. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur J Neurosci. 2002;15:1037–1047. doi: 10.1046/j.1460-9568.2002.01940.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayer S, Izydorczyk I, Reeh PW, Grubb BD. Bradykinin-induced nociceptor sensitisation to heat depends on cox-1 and cox-2 in isolated rat skin. Pain. 2007;130:14–24. doi: 10.1016/j.pain.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 14.Nemade RV, Lewis AI, Zuccarello M, Keller JT. Immunohistochemical localization of endothelial nitric oxide synthase in vessels of the dura mater of the Sprague-Dawley rat. Neurosci Lett. 1995;197:78–80. doi: 10.1016/0304-3940(95)11887-3. [DOI] [PubMed] [Google Scholar]
- 15.Pfaffenrath V, Scherzer S. Analgesics and NSAIDs in the treatment of the acute migraine attack. Cephalalgia. 1995;15(Suppl 15):14–20. doi: 10.1111/J.1468-2982.1995.TB00043.X. [DOI] [PubMed] [Google Scholar]
- 16.Prior MJ, Cooper KM, May LG, Bowen DL. Efficacy and safety of acetaminophen and naproxen in the treatment of tension-type headache. A randomized, double-blind, placebo-controlled trial. Cephalalgia. 2002;22:740–748. doi: 10.1046/j.1468-2982.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- 17.Reddy ST, Wadleigh DJ, Herschman HR. Transcriptional regulation of the cyclooxygenase-2 gene in activated mast cells. J Biol Chem. 2000;275:3107–3113. doi: 10.1074/jbc.275.5.3107. [DOI] [PubMed] [Google Scholar]
- 18.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin T, Lee Y, Sim KB. Involvement of cyclooxygenase-1 and -2 in the sciatic nerve of rats with experimental autoimmune neuritis. Immunol Invest. 2003;32:123–130. doi: 10.1081/imm-120022973. [DOI] [PubMed] [Google Scholar]
- 20.Smith TR, Sunshine A, Stark SR, Littlefield DE, Spruill SE, Alexander WJ. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache. 2005;45:983–991. doi: 10.1111/j.1526-4610.2005.05178.x. [DOI] [PubMed] [Google Scholar]
- 21.Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95:1298–1306. doi: 10.1152/jn.01293.2005. [DOI] [PubMed] [Google Scholar]
- 22.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384:560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 23.Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- 24.Wienecke T, Olesen J, Oturai PS, Ashina M. Prostacyclin (epoprostenol) induces headache in healthy subjects. Pain. 2008;139:106–116. doi: 10.1016/j.pain.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 26.Zeilhofer HU, Brune K. Analgesic strategies beyond the inhibition of cyclooxygenases. Trends Pharmacol Sci. 2006;27:467–474. doi: 10.1016/j.tips.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]