Abstract
Cyclin dependent kinase 5 (cdk5) activity is critical for development and function of the nervous system. Cdk5 activity is dependent on association with the regulators p35 and p39 whose expression is highly regulated in the developing nervous system. We have identified a small 200bp fragment of the p39 promoter that is sufficient for cell type-specific expression in neuronal cells. Mutational analysis revealed that a cluster of predicted binding sites for Sp1, AP-1/CREB/ATF and E box-binding transcription factors is essential for full activity of the p39 promoter. Electrophoretic mobility shift assays revealed that Sp1 and Sp3 bound to sequences required for p39 promoter function and chromatin immunoprecipitation assays confirmed binding of these proteins to the endogenous p39 promoter. Furthermore, depletion of either Sp1 or Sp3 by siRNA reduced expression from the p39 promoter. Our data suggest that the ubiquitously expressed transcription factors Sp1 and Sp3 regulate transcription of the cdk5 regulator p39 in neuronal cells, possibly in cooperation with tissue-specific transcription factors.
Introduction
Regulated activity of cyclin dependent kinase 5 (cdk5) is critical for the normal development and function of the central nervous system. Mice deficient in cdk5 die perinatally and show defects in neuronal migration in a number of brain compartments including the cerebral cortex, cerebellum and hippocampus [1]. Aberrant cdk5 activity has been implicated in neuronal cell death and the pathology of neurodegenerative diseases such as Alzheimer’s and amyotrophic lateral sclerosis [2, 3]. Cdk5 kinase activity is dependent on association with a regulatory partner, either p35 or p39, whose expression is highly regulated in the developing nervous system [4–6]. p35 expression is highest in post-mitotic neurons of the embryonic central nervous system, with expression peaking in migrating cells of the developing cerebral cortex [7]. In contrast, p39 expression is high in the spinal cord, peaks 1–3 weeks postnatally, and is maintained at high levels in the adult cerebellum [8–10]. The different patterns of expression of p35 and p39 may contribute to precise regulation of cdk5 activity.
It is not known whether expression of the cdk5 regulators p35 and p39 in neurons are determined by similar or different transcriptional mechanisms. We have previously identified a 17bp GC-rich element in the p35 promoter that was both necessary and sufficient for neuron-specific expression [11]. Binding of the transcription factors Sp1, Sp3 and Sp4 to the critical GC box in the p35 promoter in vitro was found to correlate with promoter function in vivo. Sp4 expression is highest in the brain [12] and the transcriptional activities of the ubiquitously expressed Sp1 and Sp3 proteins were found to be higher in terminally differentiated neurons [11, 13] suggesting that the regulated levels and activity of these GC box-binding proteins may contribute to the cell type-specific expression of p35 in post-mitotic neurons. Additional transcription factors contribute to the temporal, spatial and signal-dependent regulation of p35 in post-mitotic neurons. In cultured neurons, p35 mRNA levels are induced following activation of MAPK signalling pathways downstream of NGF and Fas, which may involve the Egr1 transcription factor [14, 15]. The POU-domain transcription factors Brn-1 and Brn-2 have also been implicated in regulation of p35 and p39 transcription as loss of both Brn-1 and Brn-2 has been shown to reduce expression of p35 and p39 in some regions of the brain [16, 17].
We have analyzed the promoter of the cdk5 regulator p39 in order to identify DNA elements and transcription factors that regulate expression of p39 in neurons. We have mapped the transcriptional start sites of the mouse p39 gene. Deletion analysis of the p39 promoter identified a 200bp GC-rich minimal promoter that is sufficient for high levels of expression in a neuroblastoma cell line (N2A), but not in a fibroblast cell line (NIH3T3). Mutational analysis of the minimal p39 promoter revealed that a tight cluster of predicted binding sites for Sp1, AP-1/CREB/ATF and E box-binding transcription factors were required for transcriptional activity in neuronal cells. Analysis of a double mutant suggests a coordinated action of transcription factors binding specific sites within the cluster. Predicted Sp1 binding sites required for promoter function were demonstrated to be required for high-affinity binding of the transcription factors Sp1 and Sp3 in vitro and these factors were shown to bind the endogenous p39 promoter in vivo. Knockdown analysis using siRNA specifically targeting either Sp1 or Sp3 revealed that both are required for full activity of the p39 promoter. These data suggest that Sp1 and Sp3 regulate expression of the neuron-specific Cdk5 regulator p39.
Materials and Methods
Primer extension analysis
Total RNA was isolated using Trizol reagent (Invitrogen). For primer extensions, radiolabeled primers were hybridized at 60°C for 90 min to 30μg total RNA isolated from 15 day old mouse brain or liver and then reverse transcribed with Superscript II reverse transcriptase (Invitrogen). The primers used in primer extensions were Primer 1 5′GCGACAGCACCGTGCCCATCCT3′ and Primer 2 5′GGAAGCAGGGGAAAGCGACAG3′.
Northern Blot analysis
For Northern analysis, 20μg total RNA isolated from N2A and 3T3 cells was denatured and resolved by gel electrophoresis on 6% formaldehyde, 1% agarose gels and then transferred to Hybond N+ membrane (Amersham Biosciences). Hybridizations with 32P-labeled p39 or GAPDH probes were carried out using Quickhyb (Statagene).
Cell Culture and Transfections
N2A and 3T3 cells were transiently transfected using LipofectAMINE (Invitrogen). 2×105 N2A or 3T3 cells were plated into 24-well tissue culture plates, and were co-transfected with 500ng luciferase reporter construct and 25ng of Renilla reporter construct pRL-SV 24h after plating. Cells were harvested 24h after transfection, and luciferase and Renilla assays were performed using the Dual Luciferase Assay kit (Promega). For knockdown assays with specific shRNA constructs, 105 cells were plated in 6 well plates and transfected with 1μg of shRNA plasmid. For knockdown efficiency test, cells were co-transfected with 200ng of pBABE puromycin resistant vector and selected 24h after transfection with 1.5μg/ml of puromycin for two more days before protein extraction. For luciferase assay cells were co-transfected with 1μg of shRNA construct, 200ng of reporter vector and 25ng of pRL-SV. Firefly and Renilla activity were analyzed 48–72h after transfection. All transfections were done in triplicate on multiple occasions.
Electrophoretic mobility shift assays
Nuclear extracts were prepared as described previously [18]. 3μg nuclear extract was incubated with 100,000cpm of PCR amplified (using 32P-radiolabeled primers) p39-153/+46 or 4pmol of 32P-radiolabeled annealed oligonucleotide in a binding buffer containing 12.5mM Hepes (pH 7.9), 6.25mM MgCl2, 0.5μM ZnSO4, 10% glycerol, 0.5mM dithiothreitol, and 3μg of salmon sperm DNA at room temperature for 20min. Competitor oligonucleotides were incubated with the nuclear extract for 20 minutes at room temperature before addition of the radiolabeled probe. For supershift experiments, 2μg of anti-Sp1 (Upstate Biotechnology), anti-Sp3 (Upstate Biotechnology), or anti-Sp4 (Santa Cruz Biotechnology) antibodies were incubated with the nuclear extract at room temperature for 20 minutes before addition of the radiolabeled probe. Free probe was separated from protein-DNA complexes by electrophoresis at 200V on a 4% polyacrylamide gel in 0.5x TBE and 1% glycerol. The oligonucleotides used in the EMSA were gel-purified and annealed. The sequences of the top-strand oligonucleotides used in the EMSAs were:
WT: 5′GGGCCCAAGGGGTGGGGCTGACGCTGCAGCTGGCGCAGCTT3′;
M2: 5′GGGCAACCTTTTGGGGGCTGACGCTGCAGCTGGCGCAGCTT3′;
M3: 5′GGGCCCAAGGGGTGGGTAGTCATAGGCAGCTGGCGCAGCTT3′;
M4: 5′GGGCCCAAGGGGTGGGGCTGACGCTGCATAGTTATACGCTT3′;
Chromatin Immunoprecipitation
Chromatin immunoprecipitation was performed following Upstate Biotechnology ChIP assay protocol with modifications. Neuro2A cells near confluence were crosslinked with 1% formaldehyde for 15 min at 37°C. After washes with ice cold phosphate buffer containing protease inhibitors, cells were resuspended in SDS lysis buffer. Chromatin was sonicated to between 500 to 1000 bp and immunoprecipitated with 3 μg of either anti-Sp1 (Upstate), anti-Sp3 (Santa Cruz), or rabbit anti-IgG (Promega) polyclonal antibodies. After phenol/chloroform extraction and ethanol precipitation, sheared DNA was amplified by real-time PCR using specific primers. The real-time PCR was performed as described by the manufacturer using the iQ SYBR Green Supermix (BioRad). 10 % of total starting material was precipitated and used as input.
p39-F 5′-GACCATTTTCACCTCACTTCAACC-3′
p39-R 5′-TCTACAGTCCCTTACCTTTTCCCAC-3′
actin-F 5′-TGAGAGGGAAATCGTGCGTGAC-3′
actin-R 5′-GCTCGTTGCCAATAGTGATGACC-3′
Plasmid Constructs and DNA analysis
The indicated regions of the p39 promoter DNA were amplified by PCR and cloned upstream of luciferase in pGL2. Mutations of the p39 promoter were generated by PCR methods and verified by sequencing. Binding site predictions were carried out using web-based software at the Transfac web-site: http://transfac.gbf.de/TRANSFAC/. Short hairpin RNAs were cloned into pBS/U6 plasmid as described previously [19]. Sequences targeted in the Sp3 and Sp1 mRNAs as well as the negative control scrambled (scr) sequence are listed below:
Sp3-1: 5′GGGACCAACAACATCAAGAAG3′
Sp3-2: 5′GGGAAGACCTCACATCTGGAG3′
Sp1-1: 5′GGGTGCCAATGGCTGGCAGAT3′
Sp1-2: 5′GGGAACATCACCTTGCTACCT3′
scr: 5′GGGAATTAATATGCACAGGCC3′.
Results
Identification of the mouse p39 transcription start site
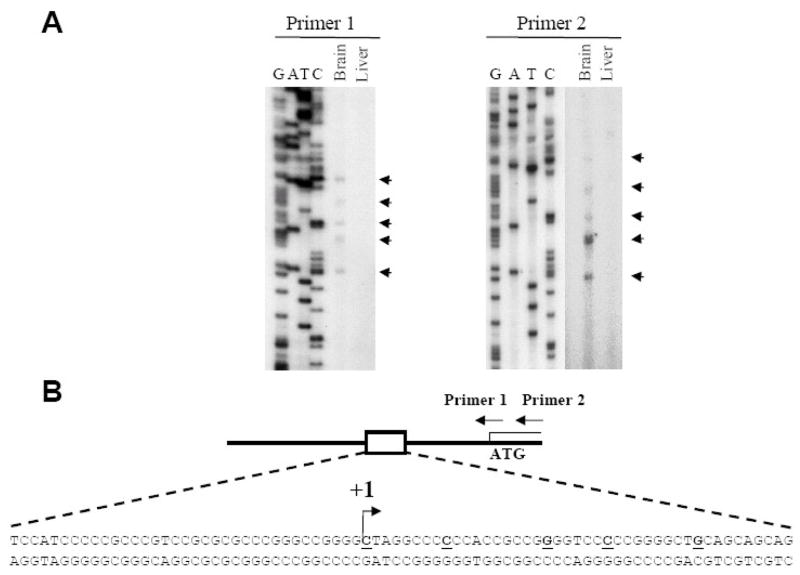
As a first step to investigate the regulation of transcription of the cdk5 activator p39, the position of the p39 promoter was identified by mapping the transcription start site. Like many neuron-specific genes, the sequences upstream of the mouse p39 ORF are highly GC-rich and do not contain a consensus TATA-box. Primer extension analysis, using primers either spanning or 3′ to the p39 ATG (Primer 1 and Primer 2) and RNA isolated from 15 day old-mouse brain, indicated that there were at least 5 transcriptional start sites located between 63 and 95 bp upstream of the ATG (Figure 1A). Transcripts of the same size were not detected using RNA isolated from 15 day old-mouse liver consistent with previous studies indicating tissue-specific expression of mouse p39 mRNA [20]. Figure 1B shows the positions of the start sites in the p39 promoter DNA sequence, with the most upstream start site indicated as +1. The p39 promoter lacks sequences resembling common core promoter elements such as initiator, DPE or BRE, which are present in many TATA-less promoters (for example the p35 promoter [11]) [21] REF. Multiple transcription start sites are a common feature of TATA-less promoters, and are found frequently in promoters of cell-type specific genes [22–24].
Figure 1. Multiple transcription start-sites upstream of the p39 ATG.
(A) The results of primer extension analysis with primers that hybridize across (Primer 1) or downstream (Primer 2) of the p39 ATG using total RNA isolated from 15 day old mouse brain or liver. Start-sites 63–95bp upstream of the ATG are indicated by arrowheads. DNA sequencing of p39 genomic DNA was performed with the same primers. (B) Schematic of the p39 promoter indicating the positions of primers used in the primer extension reactions. The 5 identified transcription start sites are indicated in bold and underlined. The most 5′ start site relative to the ATG is indicated by the arrow and the nucleotide is assigned +1.
A 200 bp fragment of the p39 promoter is sufficient to direct cell type-specific expression
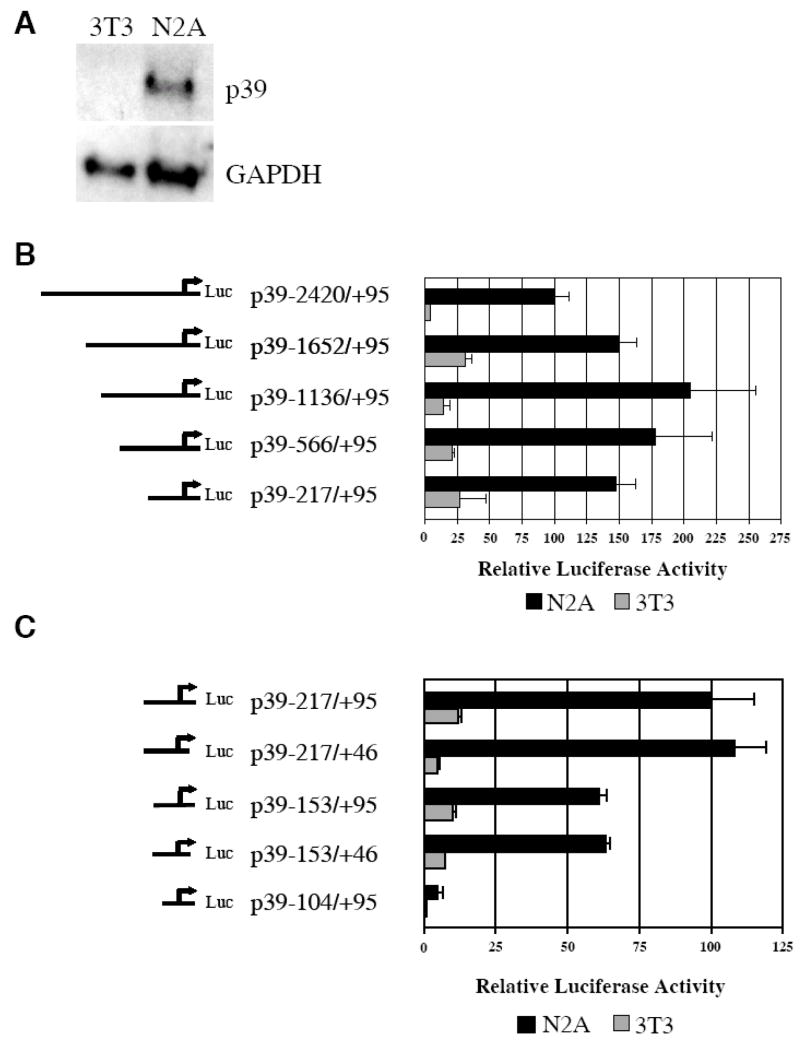
The mouse neuroblastoma cell line N2A was used to examine p39 expression and promoter activity. Northern analysis revealed high levels of endogenous p39 mRNA in total RNA extracted from cultured N2A cells but not in total RNA isolated from cultured NIH3T3 fibroblasts (Figure 2A). Thus, N2A and 3T3 cells provide suitable model cell lines for studying the mechanisms that regulate cell type-specific expression of p39.
Figure 2. p39 promoter sequences between −153 and +46 are sufficient for high levels of cell type-specific activity.
(A) Neuroblastoma N2A cells express endogenous p39. Northern blot analysis was performed with total RNA isolated from N2A cells and NIH3T3 fibroblasts. The blot was hybridized with radioactive probes specific for p39 or GAPDH transcripts; GAPDH was a control for RNA loading. (B) The p39 promoter from −217 to the ATG at +95 is sufficient for high levels of activity in neuronal N2A cells. Relative luciferase activity of reporter constructs containing 5′ truncations of the p39 promoter with the indicated end-points co-transfected with pRL-SV renilla reporter in N2A or 3T3 cells are shown. (C) p39 promoter sequences from −153 to +46 are sufficient for cell type-specific expression. Relative activities of additional 5′ and 3′ truncations with the indicated endpoints co-transfected with pRL-SV into N2A and 3T3 cells are shown. In each set of experiments, the relative luciferase activity of the longest construct in N2A cells was set at 100. The experiments were performed in triplicate; error bars indicate S.D..
A 2.5kb fragment of the mouse p39 upstream sequence which extends from 2.4 kb upstream of the transcription start site at +1 to the ATG at +95 was inserted upstream of the luciferase gene in pGL2-basic to generate p39-2420/+95. The relative activity of this reporter was determined following transfection of N2A and 3T3 cells. As shown in Figure 2B, p39-2420/+95 was ~20-fold more active in N2A cells compared to 3T3 cells. This result indicates that transcriptional mechanisms are important for cell type expression of p39 and provides an assay to identify DNA sequences important for p39 promoter activity in neuronal cells. Additional constructs were therefore created by making 5′ deletions in p39-2420/+95 to generate p39-1652/+95, p39-1136/+95, p39-566/+95 and p39-217/+95, all of which extend 3′ to the ATG. As shown in Figure 2B, each of these 5′ deletions of the p39 promoter retained high levels of activity in N2A cells. In fact, these 5′ deletions were slightly more active than p39-2420/+95 in both N2A and in 3T3 cells suggesting the presence of a negative regulatory sequence in the region −2420 to −1652 which is not cell type-specific. The smallest of these deletion constructs, p39-217/+95 was about 50% more active than the −2420/+95 fragment and maintains 5- to 9-fold higher expression in N2A relative to 3T3.
Further 5′ and 3′ deletions were therefore made in the p39 promoter and transiently transfected into N2A and 3T3 cells. As shown in Figure 2C, a 3′-deletion of p39-217/+95 to generate p39-217/+46 maintained high activity in N2A cells and was 20-fold more active in N2A than in 3T3 cells. Deletion of a further 50bp 5′ to generate p39-153/+95 reduced activity in N2A cells by approximately 40%, but this promoter construct remained 6-fold more active in N2A than in 3T3 cells. Deletion of 3′ sequences to generate p39-153/+46 had no effect on expression levels. Deletion of an additional 50 bp 5′, generating p39-104/+95, essentially eliminated activity in both N2A and 3T3 cells. Thus, an approximately 200bp fragment of the p39 promoter from −153 to +46 contains sequences sufficient for high levels of cell type-specific expression in a neuronal cell line.
The minimal p39 promoter is highly conserved between mouse and human
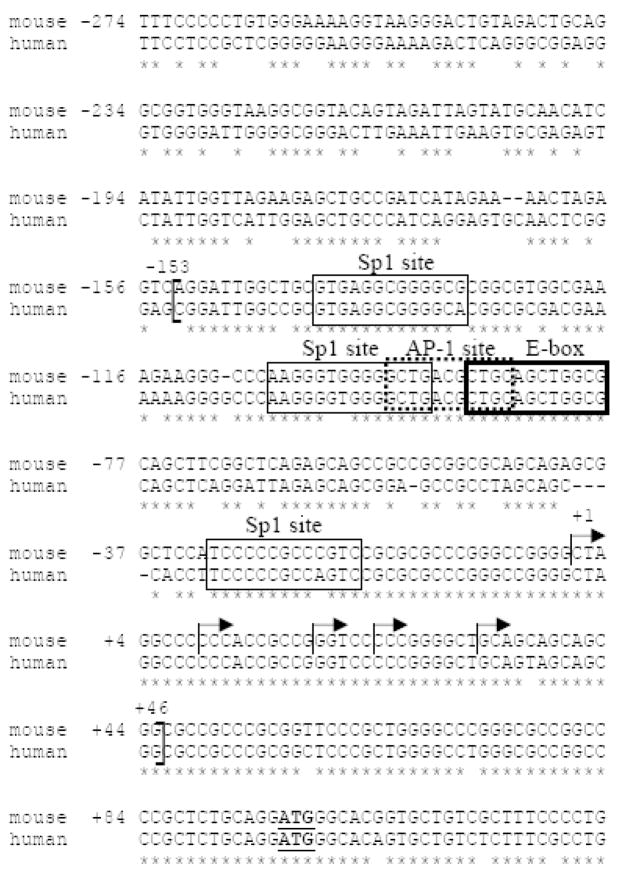
As illustrated in Figure 3, alignment of the mouse p39 promoter with sequences 5′ of the human p39 gene revealed that there is 94% identity between mouse and human promoters in the region −153 to the ATG. Notably, the region containing the mouse p39 transcription start sites is fully conserved in the human promoter. The sequence identity between mouse and human p39 decreases markedly to approximately 57% for the 200bp further upstream. Thus, the region of highest conservation upstream of the p39 ORF corresponds to the minimal p39 promoter identified in our functional studies.
Figure 3. The minimal p39 promoter sequence is highly conserved between mouse and human.
Mouse and human p39 sequences were aligned using ClustalW alignment software. Sequence identity is indicated by (*). The region between −153 and the ATG is 94% identical; sequences further upstream are not as well conserved. The five transcription start-sites identified by primer extension are indicated by forward arrows. Analysis of the minimal mouse promoter (−153/+46) (indicated by open brackets) for potential transcription factor binding sites using Transfac analysis software (http://transfac.gbf.de/TRANSFAC/) revealed 3 putative binding sites for the Sp1 family of proteins, a potential binding site for the AP-1/CREB/ATF family of proteins, and a potential E box binding site for bHLH proteins.
Mutations in a cluster of predicted binding sites for Sp1, AP1/CREB/ATF and E-box binding factors compromise p39 promoter activity
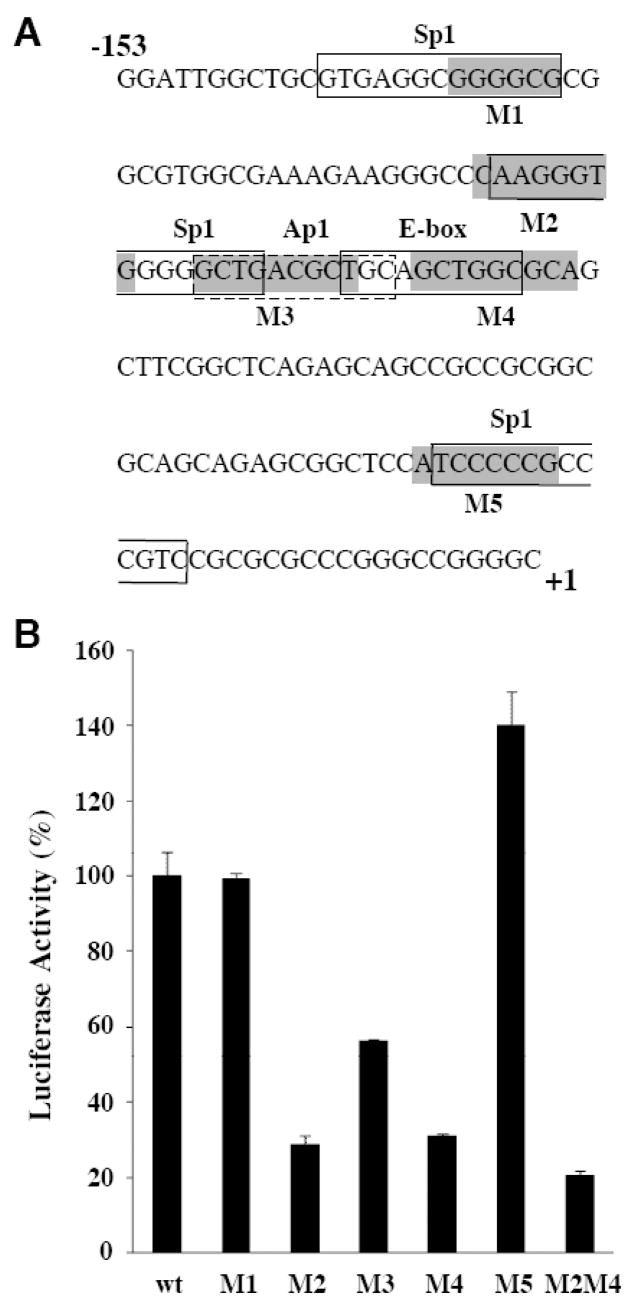
Within the −153/+46 minimal p39 promoter, there are 3 predicted binding sites for Sp1, a potential AP-1/CREB/ATF binding site, as well as a predicted E box binding site for the basic helix-loop-helix (bHLH) superfamily of transcription factors, all of which are conserved between mouse and human p39 (Figure 3). In order to determine the role of those binding sites for p39 promoter activity, they were subjected to linker scanning mutational analysis [25]. In brief, 6–10bp clustered mutations were generated using PCR to individually create M1 through M5 in the p39-153/+46 reporter (Figure 4A). The wild-type (WT) and mutated p39-153/+46 promoters were transiently transfected into N2A cells and luciferase activity relative to WT determined (Fig 4B). Mutations M2, M3 and M4, affecting the clustered Sp1, AP1/CREB/ATF and E-box binding motifs all reduced the activity of the p39 promoter in transfected N2A cells (Figure 4B). In contrast, neither M1 nor M5 mutations reduced the activity of the promoter. The M2 and M4 mutations of the p39 minimal promoter had the strongest effects, suggesting a major role for these binding sites in the activation of p39 promoter in neuronal cells. Because of the large overlap of the predicted Sp1 and the AP1/CREB/ATF sites, effects of M3 mutation in N2A cells cannot be unambiguously attributed to the AP1/CREB/ATF motif. Combined mutation of both the Sp1 and E-box binding sites (M2M4) did not further reduce p39 promoter activity (Fig 4B), suggesting that factors binding those two motifs function cooperatively to support transcription of the p39 promoter in neuronal cells.
Figure 4. Mutation of the Sp1, AP1/CREB/ATF and E box clustered binding sites reduced the activity of the p39 promoter.
(A) The positions of 6–10bp scanning mutations (M1–M5) in the p39 promoter region are indicated by shaded boxes. Predicted transcription factor binding sites for Sp1, AP-1/CREB/ATF and E box-binding proteins are indicated. (B) Reporter constructs containing the indicated scanning mutations in the p39 promoter construct p39-153/+46 were transfected into N2A cells and the resulting luciferase activity determined. Luciferase activity of the wild-type p39-153/+46 construct was set at 100. The experiments were performed in triplicate; error bars indicate the S.D..
Sp1 and Sp3 bind to the p39 promoter both in vitro and in vivo
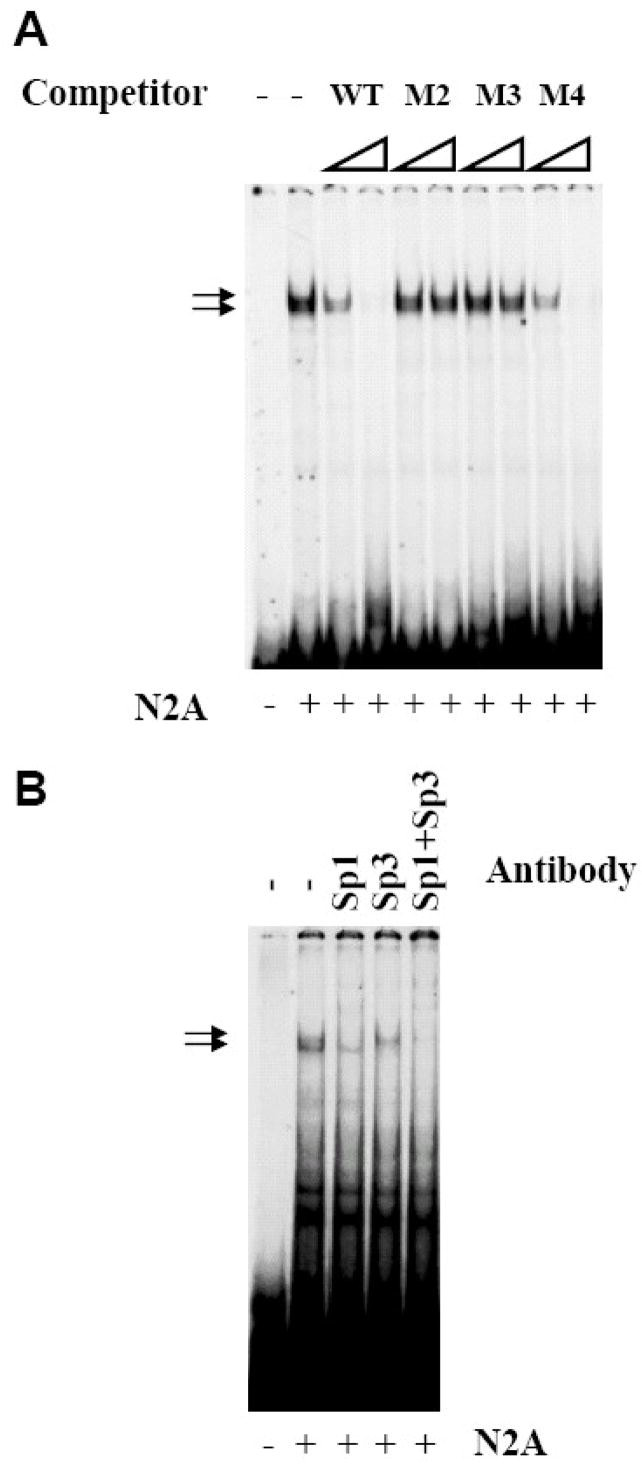
Electrophoretic mobility shift assays (EMSAs) were performed to examine protein(s) binding to functionally important regions of the p39 promoter. A 41bp oligonucleotide spanning the region which includes M2, M3 and M4 (bp −112 to −72) was used. As shown in Figure 5, protein binding to the wild-type p39 oligonucleotide produced two major closely migrating complexes. Protein/DNA complexes formed on the wild-type p39 probe are specific as unlabelled WT oligonucleotides competed for binding of both protein complexes, whereas M2 and M3 mutant oligonucleotides did not compete, even at 100-fold molar excess. In contrast, the mutant M4 fragment competed as well as WT. Since the M4 mutation reduced promoter activity (Figure 4B), this suggests that mutations at these positions alter binding of protein(s) not detectable under the gel mobility shift assay conditions used.
Figure 5. M2 and M3 mutations disrupt binding of Sp1 and Sp3.
Electrophoretic mobility shift assays (EMSA) were performed using 3μg of N2A nuclear extract (NE) and radiolabeled p39-WT oligonucleotides corresponding to nucleotides −112 to −72 spanning the clustered Sp1/Ap1/E box sites. (A) Increasing molar excess (20- and 100-fold) of the indicated cold competitor oliogonucleotides p39-WT, M2, M3, or M4 were added to the binding reaction. The two major DNA-protein complexes formed are indicated by arrows. (B) Antibodies specific for Sp1, Sp3 or both were added to the EMSA reaction as indicated.
The M2 and M3 mutations alter a predicted Sp1 binding site, and therefore interactions between the p39 promoter and the related transcription factors Sp1, Sp3, and Sp4 were investigated. Sp1 and Sp3 are ubiquitously expressed members of a transcription factor family whose DNA-binding domains are highly conserved and they bind GC boxes with similar sequence specificity and affinity [12, 26]. Addition of anti-Sp1 antibodies decreased appearance of the upper complex whereas an antibody specific to Sp3 decreased the lower complex; addition of both anti-Sp1 and anti-Sp3 antisera eliminated both complexes. Addition of antisera specific for the related Sp4 protein did not alter the protein/DNA complexes (data not shown). Thus, Sp1 and Sp3 bind to the predicted site at position −109/−94 in the p39 promoter and their binding is disrupted by the mutations M2 and M3 which severely compromise activity of the p39 promoter in neuronal cells.
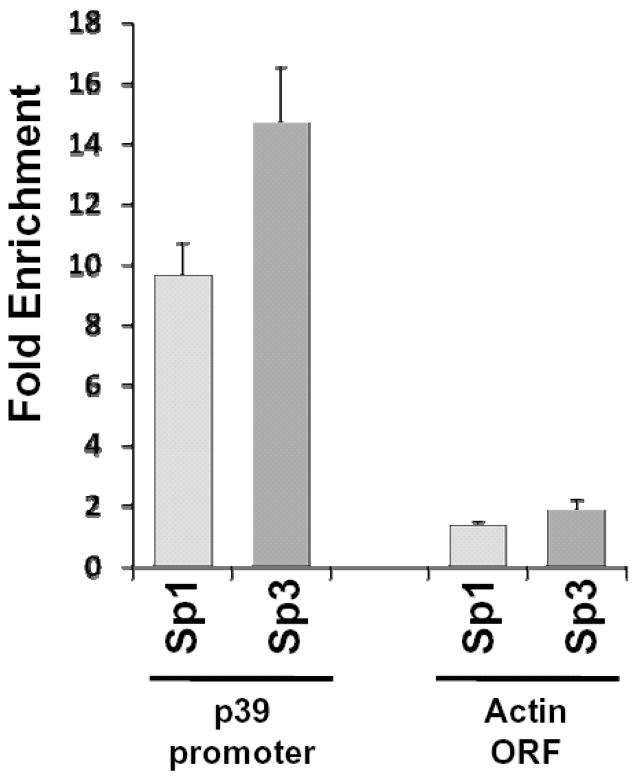
In order to validate the binding of Sp1 and Sp3 transcription factors to the p39 promoter in vivo, we performed chromatin immunoprecipitation analysis. Specific antibodies against Sp1 and Sp3, but not a non-specific IgG antibody, immunoprecipitated the endogenous p39 promoter in N2A cells, supporting a role of these transcription factors in the regulation of p39 in vivo (Fig 6). Neither Sp1 nor Sp3 were found associated with the coding region of the beta-actin gene, used as negative control for the ChIP assay.
Figure 6. Sp1 and Sp3 bind the p39 promoter in vivo.
Chromatin immunoprecipitation was performed in N2A cells using antisera specific for Sp1 and Sp3. Immunoprecipitated DNA was analyzed by real-time PCR with oligonucleotides that amplified the −368 to −240 region of the p39 promoter and, as a control a fragment within the coding region of the beta-actin gene. ChIP data represent percent input normalized to a non-immune IgG. ChIP assay was performed in triplicate.
Transcription factors Sp1 and Sp3 activate the p39 promoter
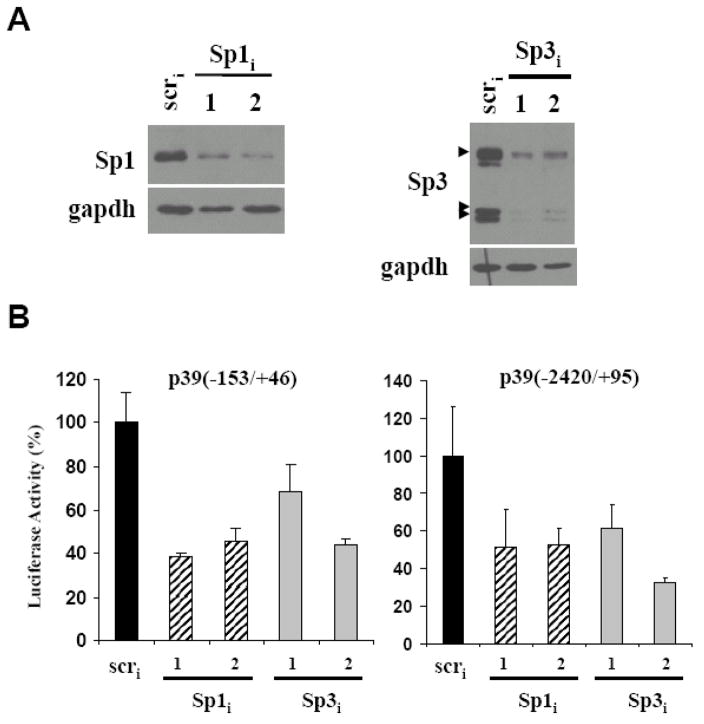
Sp1 generally functions as a transcriptional activator whereas Sp3 is both a transcriptional activator and a repressor depending on the promoter context as well as post-translational modification state [27] (For review see [28]). In order to determine the role of Sp1 and Sp3 in regulation of the p39 promoter, we generated two different shRNA constructs targeting each of these transcription factors (see Materials and Methods). Western blot analysis confirmed depletion of Sp1 or Sp3 proteins upon transfection of the specifically targeted shRNA constructs (Fig 7A and data not shown). Co-transfection of either Sp1 or Sp3 shRNA plasmids together with the minimal −153/+46 p39 promoter led to a decrease in the luciferase activity in N2A cells (Fig 7B). Similar results were observed on a larger p39 promoter fragment (−2420/+95), indicating that both Sp1 and Sp3 factors are required for maximal activity of the p39 promoter in neuronal cells.
Figure 7. Sp1 and Sp3 are required for activation of the p39 promoter.
(A) N2A cells were co-transfected with plasmid expressing shRNAs specific for either Sp1, Sp3 or control (scr). Western blot analysis with antisera specific for Sp1 (top) or Sp3 (bottom) showed transfection of the specific shRNAs reduced levels of endogenous Sp1 or Sp3 compared to the control (panel A and data not shown). Arrowheads indicate the three major Sp3 isoforms. (B) N2A cells were co-transfected with two different plasmids expressing shRNAs specific for either Sp1, Sp3 or control (scr) together with the −153/+46 or −2420/+95 p39 luciferase reporters as indicated. Luciferase activity in the scr shRNA transfected cells was set at 100. Assays were performed in triplicate; error bars indicate S.D..
Discussion
In this study we have analyzed the promoter of the cdk5 regulator p39 in order to identify DNA sequence elements and transcription factors required for transcription of p39 in neuronal cells. We have identified a small 200bp fragment of the p39 promoter that was sufficient for cell type-specific transcription in a neuronal cell line. Evolutionary conservation of non-coding sequences is an indication of conservation of functional transcription factor binding sites [29] and, consistent with this idea, the minimal mouse p39 promoter has 94% identity with sequences upstream of human p39. Mutation of clustered binding sites for Sp1, AP-1/CREB/ATF and E box-binding proteins significantly reduced the activity of the minimal p39 promoter in N2A cells, suggesting a major role for those motifs in expression of p39. Previous studies showed binding of the RE-1 Silencing Transcription factor, REST, 3′ to the p39 gene [30], suggesting that active repression may contribute to cell type-specific expression of p39. Our studies suggest that activation mechanisms also contribute to differential expression of p39 in neuronal cells. The transcription factors Sp1 and Sp3 were found to bind to the p39 promoter both in vitro and in vivo. Sp1 and Sp3 bound with high affinity to a GC box at −109/−94 and mutation of this motif reduced binding of Sp1 and Sp3 in vitro and promoter activity in vivo. In addition, RNAi-mediated knockdown of either Sp1 or Sp3 decreased the activity of the p39 promoter. These findings suggest that expression of p39 in neuronal cells is positively regulated by Sp1 and Sp3, possibly in cooperation with cell type-specific transcription factors.
Although Sp1 and Sp3 are ubiquitously expressed, these transcription factors have been implicated in cell type-specific regulation of several genes in neurons [24, 31, 32]. We have previously reported that a repeated GC box that binds Sp1, Sp3 and Sp4 in vitro is necessary and sufficient for neuron-specific expression of the cdk5 regulator p35 [11]. Furthermore, our laboratory has previously shown that Sp1 and Sp3 transcriptional activity is higher in P19-derived neurons than in undifferentiated P19 cells, suggesting that regulated activity of these ubiquitous transcription factors may contribute to cell type-specific gene expression in neurons [11]. Post-translational modification of Sp1 by both phosphorylation and acetylation and modification of Sp3 by the small ubiquitin-related modifier SUMO have been shown to modulate activity of these transcription factors [27, 28, 33, 34]. It remains to be determined whether changes in post-translational modifications of Sp1 or Sp3 contribute to cell type-specific expression of the cdk5 regulators p35 and p39.
Mutations in the E box motif in the p39 promoter significantly reduced activity in neuronal N2A cells. E boxes are binding sites for the basic helix-loop-helix (bHLH) superfamily of transcription factors, which includes both ubiquitous as well as cell type-specific transcription factors. bHLH transcription factors are important regulators of several stages of neurogenesis (for review see [35]). Thus for example, proneuronal bHLH factors (e.g., Ngn1, Ngn2 and Mash) are involved in initiating neurogenesis, and neuronal differentiation bHLH factors (e.g., NeuroD) mediate terminal differentiation. It is of interest to identify which bHLH transcription factor(s) regulate p39 expression, as the spatial and temporal expression of different bHLH factors [36, 37] could contribute to the dynamic control of p39 expression during development.
The M3 mutation also led to reduced promoter activity in N2A. The M3 mutation disrupted Sp1 and Sp3 binding to the high affinity site at −109/−94 in vitro (Figure 5) and is also predicted to disrupt an AP1/CREB/ATF site. The basic-region leucine zipper (bZIP) transcription factors of the AP-1/CREB/ATF superfamily have been shown to play important roles in both cell type and signal-dependent expression of many genes in neurons [38, 39], and are candidates to contribute to the regulated activity of the p39 promoter.
Taken together, our data suggest that activity of the p39 promoter in neuronal cells is likely the result of the coordinated action of factors binding to both GC box and E box motifs, as well as perhaps the AP-1/CREB/ATF binding site. Interestingly, combined mutation of both GC-box and E-box elements did not further reduce the activity of the minimal p39 promoter in N2A cells, suggesting factors binding to the tightly clustered Sp1, AP-1/CREB/ATF and E box motifs function cooperatively to support cell type-specific transcription of p39. Although p39 is not expressed in 3T3 cells (Figure 2) we observed Sp1 and Sp3 binding to the p39 promoter in 3T3 extracts (data not shown), suggesting that Sp1/Sp3 cooperation with tissue-specific factors, or restricted modifications, could be necessary for neuron-specific expression of p39. Combinatorial activity of Sp1 and tissue-specific E box binding proteins has been previously described for other promoters. For example, myogenic bHLH proteins and Sp1 have been shown to interact as part of a multiprotein complex required for activity of the cardiac actin promoter in skeletal muscle cells [40] and Sp1 and NeuroD are implicated in the transcriptional regulation of the human secretin promoter [41, 42].
Although further analysis of the endogenous promoters is needed, current studies to identify DNA elements important for cell type-specific expression of the cdk5 activators p35 and p39 in neurons has revealed differences in the regulation of these related proteins. The p35 and p39 promoters share some features, as both minimal promoters are GC-rich and lack consensus TATA box elements, features common to many neuron-specific genes. We have reported that a discrete repeated 17bp GC box containing element which is bound by Sp1, Sp3 and Sp4 proteins in vitro is necessary and sufficient for neuron-specific expression of p35 [11]. In contrast, although the Sp1 and Sp3 transcription factors also bind to a functionally important element in the p39 promoter, in this case additional DNA elements are required for function. Our data suggests that the ubiquitously expressed Sp1 and Sp3 transcription factors function cooperatively with cell type-specific factors to promote high levels of p39 transcription. Differences in transcriptional control mechanisms may reflect the different temporal, spatial, and signal-dependent expression of p39 compared to p35, allowing for independent regulation of these activators and more precise control of cdk5 kinase activity during brain development.
Acknowledgments
We would like to thank Norman Wong (University of Calgary) and Jerry Wang (Hong Kong University of Science and Technology) for mouse p39 promoter DNAs. We thank Zhainab Khalfan for preliminary studies to establish the cell culture model used, Lily Moi for help with mouse brain and liver dissection, and Stefan Duensing for help with imaging. We are grateful to Li Huei Tsai for her input and encouragement. A.V. was supported in part by a fellowship from the Spanish Ministerio de Educacion y Ciencias. S.R. was supported in part by a Taplin Fellowship. This work was supported by awards from the William Randolph Hearst Fund, the William F. Milton Fund, and a grant from the National Institutes of Health (HD043364) to G.G..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohshima T, Ward JM, Huh CG, Longenecker G, Veeranna, Pant HC, Brady RO, Martin LJ, Kulkarni AB. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- 4.Lew J, Huang QQ, Qi Z, Winkfein RJ, Aebersold R, Hunt T, Wang JH. A brain-specific activator of cyclin-dependent kinase 5. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 5.Tsai LH, Delalle I, Caviness VS, Jr, Chae T, Harlow E. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–423. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- 6.Humbert S, Lanier LM, Tsai LH. Synaptic localization of p39, a neuronal activator of cdk5. Neuroreport. 2000;11:2213–2216. doi: 10.1097/00001756-200007140-00030. [DOI] [PubMed] [Google Scholar]
- 7.Delalle I, Bhide PG, Caviness VS, Jr, Tsai LH. Temporal and spatial patterns of expression of p35, a regulatory subunit of cyclin-dependent kinase 5, in the nervous system of the mouse. J Neurocytol. 1997;26:283–296. doi: 10.1023/a:1018500617374. [DOI] [PubMed] [Google Scholar]
- 8.Cai XH, Tomizawa K, Tang D, Lu YF, Moriwaki A, Tokuda M, Nagahata S, Hatase O, Matsui H. Changes in the expression of novel Cdk5 activator messenger RNA (p39nck5ai mRNA) during rat brain development. Neurosci Res. 1997;28:355–360. doi: 10.1016/s0168-0102(97)00063-1. [DOI] [PubMed] [Google Scholar]
- 9.Zheng M, Leung CL, Liem RK. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Wu DC, Yu YP, Lee NT, Yu AC, Wang JH, Han YF. The expression of Cdk5, p35, p39, and Cdk5 kinase activity in developing, adult, and aged rat brains. Neurochem Res. 2000;25:923–929. doi: 10.1023/a:1007544106645. [DOI] [PubMed] [Google Scholar]
- 11.Ross S, Tienhaara A, Lee MS, Tsai LH, Gill G. GC box-binding transcription factors control the neuronal specific transcription of the cyclin-dependent kinase 5 regulator p35. J Biol Chem. 2002;277:4455–4464. doi: 10.1074/jbc.M110771200. [DOI] [PubMed] [Google Scholar]
- 12.Supp DM, Witte DP, Branford WW, Smith EP, Potter SS. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Dev Biol. 1996;176:284–299. doi: 10.1006/dbio.1996.0134. [DOI] [PubMed] [Google Scholar]
- 13.Billon N, Carlisi D, Datto MB, van Grunsven LA, Watt A, Wang XF, Rudkin BB. Cooperation of Sp1 and p300 in the induction of the CDK inhibitor p21WAF1/CIP1 during NGF-mediated neuronal differentiation. Oncogene. 1999;18:2872–2882. doi: 10.1038/sj.onc.1202712. [DOI] [PubMed] [Google Scholar]
- 14.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3:453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 15.Desbarats J, Birge RB, Mimouni-Rongy M, Weinstein DE, Palerme JS, Newell MK. Fas engagement induces neurite growth through ERK activation and p35 upregulation. Nat Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 16.McEvilly RJ, de Diaz MO, Schonemann MD, Hooshmand F, Rosenfeld MG. Transcriptional regulation of cortical neuron migration by POU domain factors. Science. 2002;295:1528–1532. doi: 10.1126/science.1067132. [DOI] [PubMed] [Google Scholar]
- 17.Sugitani Y, Nakai S, Minowa O, Nishi M, Jishage K, Kawano H, Mori K, Ogawa M, Noda T. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16:1760–1765. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis SE, Konradi C. Analysis of DNA-Protein Interactions in the Nervous System Using the Electrophoretic Mobility Shift Assay. Methods. 1996;10:301–311. doi: 10.1006/meth.1996.0107. [DOI] [PubMed] [Google Scholar]
- 19.Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC, Shi Y. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci U S A. 2002;99:5515–5520. doi: 10.1073/pnas.082117599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nilden F, Backstrom A, Bark C. Molecular cloning and characterisation of a mouse gene encoding an isoform of the neuronal cyclin-dependent kinase 5 (CDK5) activator. Biochim Biophys Acta. 1998;1398:371–376. doi: 10.1016/s0167-4781(98)00073-6. [DOI] [PubMed] [Google Scholar]
- 21.Smale ST, Kadonaga JT. The RNA polymerase II core promoter. Annu Rev Biochem. 2003;72:449–479. doi: 10.1146/annurev.biochem.72.121801.161520. [DOI] [PubMed] [Google Scholar]
- 22.Smale ST. Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim Biophys Acta. 1997;1351:73–88. doi: 10.1016/s0167-4781(96)00206-0. [DOI] [PubMed] [Google Scholar]
- 23.Boam DS, Davidson I, Chambon P. A TATA-less promoter containing binding sites for ubiquitous transcription factors mediates cell type-specific regulation of the gene for transcription enhancer factor-1 (TEF-1) J Biol Chem. 1995;270:19487–19494. doi: 10.1074/jbc.270.33.19487. [DOI] [PubMed] [Google Scholar]
- 24.Tintrup H, Fischer M, Betz H, Kuhse J. Exonic Sp1 sites are required for neural-specific expression of the glycine receptor beta subunit gene. Biochem J. 2001;355:179–187. doi: 10.1042/0264-6021:3550179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKnight SL, Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982;217:316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- 26.Hagen G, Muller S, Beato M, Suske G. Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res. 1992;20:5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross S, Best JL, Zon LI, Gill G. SUMO-1 modification represses Sp3 transcriptional activation and modulates its subnuclear localization. Mol Cell. 2002;10:831–842. doi: 10.1016/s1097-2765(02)00682-2. [DOI] [PubMed] [Google Scholar]
- 28.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 29.Hardison RC. Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet. 2000;16:369–372. doi: 10.1016/s0168-9525(00)02081-3. [DOI] [PubMed] [Google Scholar]
- 30.Sun YM, Greenway DJ, Johnson R, Street M, Belyaev ND, Deuchars J, Bee T, Wilde S, Buckley NJ. Distinct profiles of REST interactions with its target genes at different stages of neuronal development. Mol Biol Cell. 2005;16:5630–5638. doi: 10.1091/mbc.E05-07-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heicklen-Klein A, Ginzburg I. Tau promoter confers neuronal specificity and binds Sp1 and AP-2. J Neurochem. 2000;75:1408–1418. doi: 10.1046/j.1471-4159.2000.0751408.x. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto S, Sherman K, Bai G, Lipton SA. Effect of the ubiquitous transcription factors, SP1 and MAZ, on NMDA receptor subunit type 1 (NR1) expression during neuronal differentiation. Brain Res Mol Brain Res. 2002;107:89–96. doi: 10.1016/s0169-328x(02)00440-0. [DOI] [PubMed] [Google Scholar]
- 33.Black AR, Jensen D, Lin SY, Azizkhan JC. Growth/cell cycle regulation of Sp1 phosphorylation. J Biol Chem. 1999;274:1207–1215. doi: 10.1074/jbc.274.3.1207. [DOI] [PubMed] [Google Scholar]
- 34.Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ, Ratan RR. Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:4281–4286. doi: 10.1073/pnas.0737363100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 36.Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, Alberta JA, Cheng LP, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 37.Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- 38.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 39.Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- 40.Biesiada E, Hamamori Y, Kedes L, Sartorelli V. Myogenic basic helix-loop-helix proteins and Sp1 interact as components of a multiprotein transcriptional complex required for activity of the human cardiac alpha-actin promoter. Mol Cell Biol. 1999;19:2577–2584. doi: 10.1128/mcb.19.4.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee LT, Tan-Un KC, Pang RT, Lam DT, Chow BK. Regulation of the human secretin gene is controlled by the combined effects of CpG methylation, Sp1/Sp3 ratio, and the E-box element. Mol Endocrinol. 2004;18:1740–1755. doi: 10.1210/me.2003-0461. [DOI] [PubMed] [Google Scholar]
- 42.Ray SK, Leiter AB. The Basic Helix-Loop-Helix Transcription Factor NeuroD1 Facilitates Interaction of Sp1 with the Secretin Gene Enhancer. Mol Cell Biol. 2007;27:7839–7847. doi: 10.1128/MCB.00438-07. [DOI] [PMC free article] [PubMed] [Google Scholar]