Abstract
After briefly discussing human exposure to phthalates --diesters of 1,2-benzenedicarboxylic acid (phthalic acid) -- this article first presents recent findings from the Study for Future Families, a multi-center pregnancy study in which the human analogue of the phthalate syndrome was first identified. This is one of an increasing number of studies that have investigated human endpoints in relation to environmental exposure to these ubiquitous compounds. This literature, which includes a range of human health endpoints following prenatal, neonatal, childhood and adult exposures, is then summarized. At least one significant association has been reported for urinary metabolites of di-n-butyl phthalate (DBP), butylbenzyl phthalate (BzBP), diethyl phthlate (DEP) and di-isononyl phthalate (DINP) and for three of the urinary metabolites of di(2-ethylhexyl) phthalate (DEHP). Many of the findings reported in humans –most of which have been in males-- are consistent with the anti-androgenic action that has been demonstrated for several phthalates. Replication of the results described here and further mechanistic studies are needed to strengthen links between phthalates and adverse health outcomes.
Background
Diesters of 1,2-benzenedicarboxylic acid (phthalic acid), commonly referred to as phthalates, are man-made chemicals widely used in industry and commerce. This group of man-made chemicals has a wide spectrum of industrial applications and these chemicals appear, ultimately, in a wide range of consumer products, as well as in food processing and in medical applications.
Di(2-ethylhexyl) phthalate (DEHP), di-n-butyl phthalate (DBP), and benzylbutyl phthalate (BzBP) and di-isononyl phthalate (DINP) have been shown to disrupt reproductive tract development in male rodents in an anti-androgenic manner (Parks et al., 2000). Based on recent data from dose response studies with DEHP, DBP and BzBP, Howdeshell et al. reported that DBP, DEHP and BzBP were equipotent at reducing fetal testicular testosterone production (Howdeshell et al., 2008). Most adverse effects examined in animal studies have been reproductive, and findings are predominantly in males, but it should be noted that females have been less well studied. In addition, a number of non-reproductive endpoints (including some in females) have been reported in this literature, often at high doses, including; hepatic and renal effects (DBP), hepatocellular carcinoma (DEHP), anovulation and decreased fetal growth (DEHP) (Hauser and Calafat, 2005).
This review summarizes recent data on human exposure to phthalates at environmental levels and associated health endpoints in humans. Though the number of phthalates is large, many have not yet been examined in humans. After briefly discussing sources and routes of environmental exposure to these phthalates, I will present recent data on male genital endpoints from the Study for Future Families, a pregnancy cohort study in which the human analogue of the phthalate syndrome was first identified. I will then briefly review the growing body of epidemiologic studies that have investigated other health outcomes that have been associated in the literature with exposure to these ubiquitous compounds.
Exposure to phthalates
Sources of phthalates in the environment
Phthalates have been measured in residential indoor environments in both house dust and indoor air (Rudel et al., 2003). They have also been measured in foods, milk and drinking water. However, the relative contribution from the various sources and routes of exposure to phthalates is unknown (Wormuth et al., 2006).
High molecular weight phthalates, such as DEHP, are primarily used as plasticizers in the manufacture of polyvinyl chloride (PVC), which is used extensively in consumer products, flooring and wall coverings, as well as food contact applications, and medical devices (ATSDR, 2002). Exposure to DEHP can occur in the workplace (e.g. in the manufacture of DEHP or DEHP containing products, or workers using these products), during consumer use of these products, or through environmental media (food, air, water, dust). The inclusion of DEHP in products to which children are exposed (e.g. toys, bath books), particularly those used by young children, and medical devices such as intravenous tubing and blood and nutrient bags are of particular concern because of the vulnerability of these populations.
Lower molecular weight phthalates, including DBP and diethyl phthalate (DEP), are used as solvents and plasticizers for cellulose acetate, in making lacquers, varnishes, personal-care products (e.g. perfumes, lotions, and cosmetics), and coatings (ATSDR, 1995; ATSDR, 2001), including those used in making timed-release pharmaceuticals (Hauser et al., 2004a). DEP (urinary metabolite MEP) is found in population samples at levels that are often an order of magnitude higher than those of DEHP and DBP (CDC, 2003; CDC, 2005). DEP has been found in a large number of cosmetics and personal care products, and, in adults (Duty et al., 2005a) and young children (Sathyanarayana et al., 2008), linked to use of such products.
Routes of exposure to phthalates
Humans are exposed to phthalates by multiple routes, and the most likely route varies by phthalate. Exposures can be oral (e.g. DEHP via phthalate-contaminated food, water and other liquids and in children through mouthing of toys and teethers) or dermal (e.g. DEP, via cosmetics and other personal care products). Exposure can also be via inhalation; phthalates volatilize from PVC, nail polish, hair spray, and other phthalate-containing products, and parenteral (e.g. DEHP via tubing used in neonatal intensive care nurseries) (FDA, 2001). In contrast, in almost all rodent studies to date, exposure is oral. Therefore, these rodent studies may not reflect toxicity of phthalates to humans who are exposed via other routes. For example, human exposure to DEP is primarily dermal and, secondarily via inhalation. While most animal studies on DEP and MEP do not find reproductive toxicity (ATSDR, 1995), several of the human studies that have examined this phthalate have found significant adverse associations (see Table 4 and Table 5). This apparent inconsistency may be the result, at least in part, of differing exposure routes.
Table 4.
Health outcomes in infants and children associated with phthalate concentration in biological or environmental samples
| System | Timing of exposure | Sex | Outcome | Phthalate or metabolite (measured in urine unless otherwise noted) | Reference |
|---|---|---|---|---|---|
| Reproductive | Prenatal | Males/Females | Shorter gestational age at birth | MEHP (in cord blood) | (Latini et al., 2003) |
| Prenatal (mean age 12.6 months at exam) | Males | Shorter anogenital distance | MEHP, MEOHP, MEHHP, MEP, MBP | (Swan et al., 2005) and Table 1–Table 3 | |
| Reduced penile size | MEHP | ||||
| Incomplete testicular descent | MEHP, MEHHP, MEOHP | ||||
| Lactation (mean age 3 months) | Males | Increased SHBG | MEP, MBP | (Main et al., 2006) | |
| Increased LH/free T | MMP, MEP, MBP | ||||
| Increased LH | MiNP | ||||
| Decreased free T | MBP | ||||
| Early childhood | Females | Premature thelarche | DEHP (in serum) | (Colon et al., 2000) | |
| Respiratory, allergy and asthma | Childhood | Males/Females | Rhinitis and eczema | BzBP (in house dust) | (Bornehag et al., 2004) |
| Asthma | DEHP (in house dust) | ||||
| Wheezing, rhinitis and eczema | DEHP (in house dust) | (Kolarik et al., 2008) |
Table 5.
Health outcomes in adults associated with phthalate concentration in biological samples
| System | Sex | Outcome | Phthalate or metabolite (measured in urine unless otherwise noted) | Reference |
|---|---|---|---|---|
| Reproductive | Males | Increased sperm DNA damage | MEP, MEHP | (Hauser et al., 2007) |
| Increased sperm DNA damage | MEP | (Duty et al., 2003b) | ||
| Decreased sperm motility | MBP | (Duty et al., 2003a) (Hauser et al., 2006) | ||
| Decreased sperm concentration | MBP, MBzP | (Duty et al., 2003a) (Hauser et al., 2006) | ||
| Males | Decreased sperm morphology | DEHP in semen samples | (Zhang et al., 2006) | |
| Males | Decreased free T and increased LH/free T | MBP | (Pan et al., 2006) | |
| Males | Decreased FSH | MBzP | (Duty et al., 2005) | |
| Males | Decreased motility Reduced LH | MEP | (Jonsson et al., 2005) | |
| Respiratory and immune (incl. allergy and asthma) | Males | Decreased pulmonary function | MEP, MBP | (Hoppin et al., 2004) |
| Metabolic | Males | Increased waist circumference | MBzP, MEHHP MEOHP, MEP | (Stahlhut et al., 2007) |
| Increased insulin resistance | MBP, MBzP MEP | |||
| Thyroid | Males | Thyroid (decreased T3 and T4) | MEHP | (Meeker et al., 2007) |
Assessing human exposure to phthalates
Because of the silent and pervasive nature of environmental phthalate exposure, traditional epidemiological methods of exposure assessment (questionnaires, medical records, etc.) are of limited usefulness in determining individual exposure. Instead, biomarkers of exposure are preferred. Urine is the preferred matrix for phthalate determination in humans (Calafat and McKee, 2006). Because of rapid metabolism (at least in infants older than a few months) urinary metabolite levels are typically higher, and therefore more precisely measured, than levels of the parent compound found in other media. Further, by measuring metabolites, the risk of accidental contamination during collection, storage and analysis is greatly reduced. Phthalates or their metabolites have also been measured in many body fluids and matrices, including; urine, serum, saliva, seminal fluid, breast milk, amniotic fluid, meconium and even placenta.
While exposures during fetal development are more likely to result in profound and permanent alterations than postnatal exposures, conducting such studies in human populations is challenging. First, in order to estimate prenatal exposure reliably, it is necessary to assay biological samples obtained and archived during pregnancy. Second, such studies require reliable assessment of outcomes in the offspring of those pregnancies. While this is relatively easy for perinatal outcomes, in order to examine outcomes occurring in later life –such as impaired semen quality and infertility-- it is necessary to follow the pregnancy cohort for decades. Thus, logistic and financial difficulties account, at least in part, for the limited number of such studies, and the complete absence of any examination of adult effects of prenatal exposure to phthalates. On the other hand, assessing effects of postnatal or adult exposure in relation to outcomes measured concurrent with, or soon after, sample collection is more feasible.
The recent availability of sensitive, specific, and economical assays make such measures practicable for epidemiological studies (Silva et al., 2007). Moreover, international quality control programs for assays of phthalate metabolites in human samples help to maximize comparability of results across laboratories internationally.
Phthalates are rapidly metabolized in the body, with elimination half-lives of less than 24 hours (Koch et al., 2005). Several studies have examined the within-person variability of phthalate metabolites. Hoppin et al. (Hoppin et al., 2002) found that the Pearson correlation coefficient in concentration in women’s samples taken two days apart ranged from 0.5 for mBzP to 0.8 for mBP and concluded that despite their short half-lives, exposure may be sufficiently stable to assign an exposure level based on a single sample. Similarly, Hauser et al. (Hauser et al., 2004b) concluded that despite substantial day-to-day and month-to-month variability in men’s individual urinary phthalate metabolite levels, a single urine sample was moderately predictive of each subject’s exposure over 3 months. A recent review by Teitelbaum et al. (Teitelbaum et al., 2008) of sources of variability in biomarkers of environmental exposures in minority children, including phthalates, confirms that a single spot urine sample is sufficiently representative of exposure over a 6-month period to warrant its use as an exposure estimate in epidemiological studies.
The Centers for Disease Control and Prevention (CDC) has published data on levels of phthalate metabolites in a large population-based sample of the US population over 6 years of age (CDC, 2003; CDC, 2005). These data demonstrate the ubiquitous nature of these chemicals and variation in concentration by ethnicity, sex, and age. A European group (Wormuth et al., 2006) has modeled estimated phthalate levels in a European population, including infants, and estimates of exposure to DEHP and DBP in infants are about one order of magnitude higher than those in adults. A recent analysis of phthalate metabolite concentration in 163 infants (2–36 months) shows that all are exposed to one or more phthalates and 80% to seven or more. Moreover, infant levels of MEP and MBP are directly related to the number of baby care products (lotions, creams, and powders) applied by their mothers (Sathyanarayana et al., 2008).
Health outcomes associated with human prenatal exposure to phthalates
There were few studies conducted of human health outcomes associated with phthalate exposure prior to 2000. However, by 2000 the availability of sensitive, specific, and affordable bioassays made biomarkers feasible for use in epidemiological studies. Moreover, the growing rodent literature provided convincing data on the reproductive toxicity of several commonly used phthalates (Howdeshell et al., 2008).
We begin this discussion with the one study, to date, to have examined health outcomes in humans in relation to prenatal phthalate exposure, and the rodent data that motivated that study. We then summarize the literature on human health endpoints that have been associated with environmental exposure to phthalates.
Evidence for the “phthalate syndrome” in humans
Over the past ten years, increasingly detailed rodent studies have demonstrated that several phthalates (most notably DEHP, DBP and BzBP), when administered at the critical window for the development of the reproductive tract, disrupt the androgen-signaling pathway in males (Akingbemi et al., 2004), (Borch et al., 2004), (Foster, 2006), (Gray et al., 2000). Recently Lee et al. demonstrated the anti-androgenic effects of seven phthalates (DEHP and its metabolite, mono-2-ethyhexyl phthalate (MEHP), DBP, BzBP as well as di-isononyl phthalate (DINP), di-isodecyl phthalate (DIDP), di-n-heptyl phthalate (DnHP)) by the Hershberger assay in castrated male SD rats (Lee and Koo, 2007). This anti-androgenic action results in interference with the normal cascade of androgen-dependent outcomes, most notably shortened anogenital distance (AGD). Anogenital distance, which in newborn rodent pups is the distance from the anus to the genital tubercle, is approximately twice as long in males as females. AGD is a sensitive marker of fetal exposure to anti-androgens, including phthalates, vinclozilin and flutamide. Prior to 2005, only a single human study had evaluated AGD in males (Salazar-Martinez et al., 2004), and two other studies had evaluated AGD in female infants (Phillip et al., 1996), (Callegari et al., 1987). These studies demonstrated the sexual dimorphism of AGD in humans, consistent with rodent studies. However, these studies did not examine the relationship of AGD to chemical exposure.
Several animal studies report that DBP, DEHP and BzBP induce a marked reduction in fetal testosterone (produced by Leydig cells) and insulin-like growth factor-3 (Insl-3), resulting in a syndrome of male reproductive abnormalities, which, in addition to shortened AGD, include hypospadias, cryptorchidism and malformations of the epididymis, vas deferens, seminal vesicles, and prostate; together they comprise the “phthalate syndrome” (Welsh et al., 2008), (Foster, 2006).
While a variety of anti-androgens alter AGD in rodents, most are androgen receptor (AR) antagonists. The syndrome induced by the AR-antagonists differs from that induced by the phthalates, which inhibit the synthesis of fetal testosterone and Insl-3 (Wilson et al., 2008), (Wilson et al., 2004). A variety of potentially anti-androgenic substances including procymidone (P), linuron (L), flutamide (F) and p,p’-DDE were tested. P, L, and p,p’-DDE produced F-like profiles, that included shortened AGD but were distinct from those produced by DBP and DEHP (Gray, 2006). Thus, detection of shortened AGD appears to signal prenatal anti-androgen exposure, but knowledge of the entire profile of genital dysmorphology is necessary to pinpoint a particular etiologic agent.
Phthalates in relation to genital development in human infants
Study population
The Study for Future Families (SFF) is a multi-center pregnancy cohort study in which we collected and stored urine and serum samples obtained mid-pregnancy. The availability of these samples, allowed us to link prenatal phthalate exposure to outcomes in the children born to couples enrolled in SFF. Therefore, in 2000 we initiated the Study of Phthalates in Pregnant Women and Children (PPWC) in order to examine AGD and other genital parameters in human infants in relation to their mother’s phthalate exposure. We hypothesized that a human analogue of the “phthalate syndrome” would be found in male infants. Initial results from that study, previously published (Swan et al., 2005) are updated here.
Study methods for SFF are described in detail elsewhere (Swan et al., 2003). Briefly, couples whose pregnancy was not medically assisted were eligible to participate unless the woman or her partner was less than 18 years, either partner did not read and speak Spanish or English, or the father was unavailable or unknown. All participants completed a questionnaire and most gave blood samples. Maternal and paternal urine collection was added to the protocol in the second study year. If the participant had agreed to be recontacted (and 85% had), we invited the mother to take part in a follow-up study. The family was eligible for PPWC if the baby was 2–36 months of age when contacted, the family lived within 50 miles of the clinic, and could attend at least one study visit. Fifty-one percent of SFF subjects participated in this follow-up study. We present data on results of the physical examination in 140 boys and 153 girls from Minnesota, MN, Columbia, MO and Los Angeles, CA, and results of the phthalate analysis on the approximately 75% of these for whom both maternal urine samples and physical examination data were available. Human subject committees at all participating institutions approved both SFF and PPWC and all subjects signed informed consents for each study.
Infant measurements
At the first postnatal study visit (mean 12.8 months after delivery) a detailed examination of breast and genitals, developed for this study, was conducted under the supervision of pediatric physicians who were trained in its administration. The male examination included a description of the testes and scrotum, location of testes, measurement of the penis and AGD. All measurements (AGD and penile size) were made with a precision calipers. In males AGD is the distance (in mm) from the center of the anus to the cephalad (towards the head) base of the penis, while female AGD is the distance (in mm) from the center of the anus to the cephalad base of the clitoris.
Examiners also evaluated testicular descent, which was classified as “incomplete” if one or both testicles were found not to be “normal” or “normal retractile”. In addition, penile length and width were obtained. Neither the pediatric physicians nor the support staff had any knowledge of the mother’s phthalate concentration.
Phthalate measurements
Because SFF was not, initially, designed to examine biomarkers of prenatal exposure (but rather semen quality across four areas of the US) urine collection was begun only midway through the study. There were 106 mother-son pairs with both prenatal phthalate concentration and boy’s genital measurements. Phthalate metabolite concentrations were assayed in urine samples provided during pregnancy (mean 28.6 week of pregnancy, as measured from the last menstrual period), using a sensitive method that involves the enzymatic deconjugation of the phthalate metabolites from their glucuronidated form, automated on-line solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Silva et al., 2007).
Statistical analysis
Because AGD varies with age and weight, it is necessary to control for these variables in analysis. In the 2005 analysis, guided by practice in rodent studies (Hotchkiss and Vandenbergh, 2005), we divided AGD by weight to create the anogenital index. While this is common practice in rodent studies, for our babies of varying ages this method did completely eliminate confounding by weight. Therefore, in this updated analysis, we used weight percentile, a quantity that is largely independent of weight and when included as a covariate in the model eliminates residual confounding by weight. Therefore, for each infant, the expected weight for age (weight percentile) was determined by sex-specific estimates of weight percentiles in the US population (http://www.cdc.gov/growthcharts/).
The 2005 publication included AGD and phthalate results for the first visit of 85 boys. In the current analysis, we include 106 boys for whom we had measured mother’s prenatal phthalate concentration, 68 of whom had AGD measured at two visits. Because of the inclusion of multiple observations for these boys, data were analyzed using a mixed model, controlling for age and weight percentile.
We also conducted an analysis of metabolite levels in relation to a categorical (3-valued) measure of AGD. For this purpose, we compared each boy’s observed AGD to that expected based on his weight and age. We then divided boys into three categories, using the difference between the observed and expected AGD, which we call the residual AGD. Boys whose residual AGD fell in the lowest 25% were classified as having “shorter AGD”, those in the upper 25% as having “longer AGD” and others as having “intermediate AGD”. We then compared summary statistics (mean, median and geometric mean) for metabolite concentrations among these three groups of boys.
To examine joint exposure to multiple phthalates, we created a “summary phthalate score” that reflects combined exposure to the five phthalate metabolites associated with male AGD (MEHP, MEOHP, MEHHP, MBP and MEP). To construct the summary score, first the distribution of these five metabolites was divided into quartiles. The score was increased by 1 point for each additional quartile in which the metabolite fell, so each metabolite can contribute 0,1,2 or 3 points to the summary score. If the concentration of all five metabolites fell in the lowest quartile, the summary score for that woman was 0. The maximum score of 15 was assigned when all five metabolites fell in the upper quartile. We called the summary exposure “low” if the score was 0–3, “high” if the score was 12–15, and medium otherwise.
Results
In boys AGD averaged 70.4 mm (SD 10.8 mm), while in girls, AGD averaged 47.3 mm (SD 7.1 mm). Male AGD was, therefore 49% longer on average than female AGD, with a standard deviation that was greater. Thus, in humans, as in rodents, this measure is strongly sexually dimorphic, although, as can be seen in Figure 1, there is considerable overlap of the AGD distributions in males and females in this population. Salazar-Martinez et al. reported an AGD in 42 newborn males that was twice that in 45 newborn females (Salazar-Martinez 2004). In that population, the distribution of AGD in male and female were nearly non-overlapping, perhaps the result of minimal prenatal exposure to anti-androgens.
Figure 1.
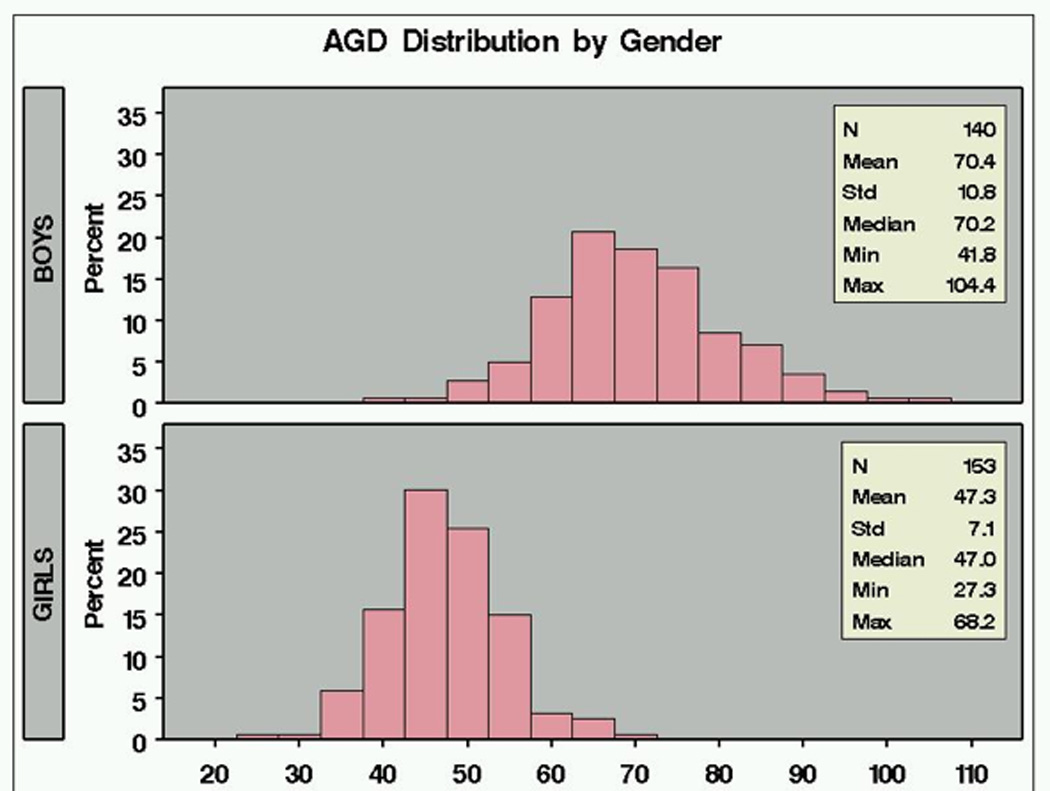
Distribution of anogenital distance (mm) in boys and girls
Table 1–Table 3 contain results of new analyses of phthalate metabolite levels in relation to AGD, penile width and testicular descent.
Table 1.
AGD in relation to phthalate metabolite concentration1
| Monoester Metabolite | Coefficient of log10 metabolite concentration | P-value of coefficient | % Change in AGD for IQ2 increase in metabolite concentration |
|---|---|---|---|
| MEHP | −3.503 | 0.017 | −4.4% |
| MEHHP | −4.977 | 0.002 | −3.9% |
| MEOHP | −5.126 | 0.001 | −4.5% |
| MEP | −2.934 | 0.005 | −4.0% |
| MBP | −3.255 | 0.049 | −3.2% |
| MiBP | −2.9562 | 0.097 | −3.5% |
| MMP | −4.0002 | 0.053 | −3.6% |
| MBzP | −0.3129 | 0.826 | −0.4% |
| MCPP | −0.9650 | 0.591 | −1.0% |
Using a mixed model controlling for age and weight percentile
Estimated change in AGD for an increase in phthalate metabolite concentration from 25th percentile to 75th percentile
Table 3.
Penile width, testicular descent and DEHP metabolite concentration: regression analyses
| Monoester Metabolite | Coefficient of log10 metabolite concentration | P-value of coefficient | ||
|---|---|---|---|---|
| Penile width1 | Testicular descent2 | Penile width1 | Testicular descent2 | |
| MEHP | −0.782 | −1.258 | 0.005 | 0.048 |
| MEHHP | −0.530 | −1.417 | 0.080 | 0.054 |
| MEOHP | −0.466 | −1.350 | 0.120 | 0.064 |
| ΣDEHP | −0.570 | −1.447 | 0.072 | 0.052 |
Using a mixed model controlling for age and weight percentile
Using a logistic regression model controlling for age and weight percentile
Table 1 contains the estimate of the beta-coefficient for each metabolite (logarithmically transformed to normalize distributions) and its p-value in regression models that controlled for age and weight percentile. In this data set, urinary concentrations of five metabolites were significantly and inversely related to AGD; MEP, MBP and the three DEHP metabolites measured (MEHP, MEOHP and MEHHP). These results differ somewhat from our 2005 analysis, particularly in relation to DEHP metabolites, which in the prior analysis were either not significant (MEHP) or of borderline significance (MEOHP and MEHHP). In addition, MBzP, which was of borderline significance in the earlier analysis (P=0.055) is no longer associated with AGD.
As seen in Table 2, for several metabolites, most notably the DEHP metabolites (MEHP, MEHHP and MEOHP) and MEP, the mean metabolite concentration was several times greater among boys with “shorter” compared to “longer” AGD.
Table 2.
AGD and phthalate metabolite concentrations: Categorical analysis
| Monoester metabolite | Anogenital Distance Category | ||
|---|---|---|---|
| Longer (N=26) | Intermediate (N=51) | Shorter (N=29) | |
| Mean/median/geometric mean (ng/mL) | Mean/median/geometric mean (ng/mL) | Mean/median/geometric mean (ng/mL) | |
| MEHP | 4.4/2.3/2.5 | 6.4/2.9/3.1 | 12.3/6.2/5.8 |
| MEHHP | 12.0/8.2/8.0 | 18.3/10.0/9.8 | 37.9/19.8/20.9 |
| MEOHP | 10.1/7.3/7.0 | 16.2/11.7/8.7 | 33.9/21.3/19.3 |
| MEP | 191.9/80.5/78.5 | 599.2/92.7/110.2 | 910.3/277.3/252.1 |
| MBP | 21.5/11.4/12.6 | 19.2/14.2/12.1 | 39.9/26.4/24.0 |
| MiBP | 2.8/1.5/1.8 | 4.0/2.3/2.4 | 6.1/3.4/3.8 |
| MMP | 1.6/0.7/1.1 | 2.3/0.7/1.4 | 2.7/1.4/1.6 |
| MBzP | 22.0/8.3/8.8 | 20.5/5.6/8.3 | 16.8/10.8/10.5 |
| MCPP | 8.1/1.7/1.8 | 2.9/1.6/1.7 | 2.8/2.5/2.2 |
Using a mixed model controlling for age and weight percentile
Residual AGD was correlated with both penile width and length: correlations 0.31 and 0.16 (p=0.0003 and 0.059, respectively). In a mixed model controlling for age and weight percentile, penile width (but not length) was significantly associated with concentration of the sum of DEHP metabolites (ΣDEHP) and MEHP. No other metabolites were associated with penile size (Table 3).
Of boys for whom both testicular location and phthalate concentrations were available (N=119), 12 had incomplete testicular descent (10.1%). As can be seen in Table 3, higher DEHP metabolite concentration was significantly associated with a greater probability of incomplete testicular descent. No other phthalate metabolites were associated with testicular descent.
We then examined joint exposure to the five phthalate metabolites associated with AGD using the summary phthalate score. The median score was 9 with a range of 0–15. In this population, the summary phthalate was more predictive of AGD category than individual metabolite concentrations. Of the 29 boys with a “shorter” AGD, none were born to a mother with a low summary phthalate score. Conversely, of the 28 boys with a “longer” AGD, only one was born to a mother with a high summary phthalate score (p-value <0.00001). While numbers in this study were too small to distinguish dose additivity from effect additivity, this finding is consistent with the rodent studies demonstrating dose additivity of multiple phthalate exposure.
Conclusions from PPWC
In 2005 we published initial findings on boy’s AGD in relation to the concentration of mothers’ urinary phthalate metabolites (Swan et al., 2005). The updated data presented here (Table 1–Table 3) differ from the 2005 findings in three ways. First, the 2005 analysis included a smaller data set (85 boys with one visit per boy). Second, the statistical methods employed in the current analysis have been improved, as explained above. Third, the current analysis examines phthalate concentration in relation to both AGD and two other endpoints consistent with the phthalate syndrome (e.g. penile width and testicular descent). Thus, in this recent analysis, we see largely results consistent with and extending those previously published (Swan et al., 2005). In the current analysis we find significant associations between AGD and the three metabolites of DEHP, as well as their sum, which were not seen in the prior analysis. We also see a direct relationship between DEHP metabolites (most notably MEHP) and penile width, which were not seen previously. Additionally, the MEHP metabolites were significantly and inversely related to testicular descent. These findings warrant current concerns that low dose phthalate exposures affect several markers of human male genital development.
Summary of Health outcomes in infants and children
Turning to other health outcomes in infants and children, published studies that have examined these outcomes in association with prenatal, perinatal and early childhood phthalate exposure are contained in Table 4.
Phthalates and infant hormones
Main et al. (Main et al., 2006) measured phthalate monoesters in breast milk samples collected 1–3 months after birth. Gonadotropins, sex-hormone binding globulin (SHBG), testosterone, and inhibin-b were measured in babies’ serum samples collected at 3 months. All phthalate monoesters were found in breast milk. MEP and MBP were positively correlated with SHBG (r = 0.323, p = 0.002 and r = 0.272, p = 0.01, respectively); MMP, MEP, and MBP with the ratio of LH to free testosterone (r = 0.21–0.323, p = 0.002–0.044) and MiNP with luteinizing hormone (r = 0.243, p = 0.019). MBP was negatively correlated with free testosterone (r = −0.22, p = 0.033).
The results on male genital development from PPWC and these data on neonatal male hormone concentrations in relation to phthalate exposure are consistent and together suggest that the phthalate syndrome originally identified in rodents (Gray et al., 2006) may also be occurring in male babies whose mothers were exposed to higher levels of one or more phthalates during pregnancy.
Phthalates and gestational age at delivery
Only one published study to date has examined gestational age in relation to phthalate exposure. Latini et al. (Latini et al., 2003) measured serum DEHP and MEHP in the cord blood of 84 infants born in Brindisi, Italy and examined concentration in relation to gestational age (which was not defined). DEHP and MEHP were each present in 65 of 84 (77.4%) of the examined samples. Infants for whom MEHP was detectable in cord blood were categorized as “MEHP-positive” (with a similar definition for DEHP). MEHP-positive newborns showed a significantly lower gestational age compared with MEHP-negative infants (38.16 ± 2.34 vs. 39.35 ± 1.35; t = −2.163, p = 0.033. No associations were found for DEHP, and no significant relations were observed between either DEHP or MEHP and birth weight.
Phthalates and premature thelarche
Colon et al. (Colon et al., 2000) compared serum levels of phthalates in 41 Puerto Rican girls (median age 20 months) with premature thelarche (premature breast development) and 35 pediatric controls (median age 46 months). The largest differences between cases and controls were between serum DEHP concentration in cases and controls; 450 and 70 ng/ml, respectively (p < 0.05). There are concerns regarding several aspects of the study design (McKee and Toxicology Research Task, 2004) including potential sample contamination, the large age difference between cases and controls, and the fact that exposure was measured concurrent with diagnosis.
Phthalates in relation to respiratory function, asthma and allergy
A number of studies have examined respiratory function, asthma, and allergy in children in relation to the use of PVC-containing products in the home or phthalates in house dust (Bornehag et al., 2004), (Jaakkola et al., 1999; Jaakkola et al., 2000) although none has used biomarkers to define exposure. Two cross-sectional studies found associations between allergic symptoms and house dust levels of BzBP and DEHP (Bornehag et al., 2004; Kolarik et al., 2008). In the future, biomarker studies will provide more precise measures of exposure and clarify the strength of these relationships.
Summary of health outcomes in adults
Studies that have examined health outcomes in adults in relation to recent phthalate exposure are summarized in Table 5.
Phthalates in relation to semen quality and sex hormones in males
Duty, Hauser and colleagues published a series of studies that examined urinary phthalate levels and semen characteristics, sperm DNA damage, and serum reproductive hormones in men attending an infertility clinic (Duty et al., 2005; Duty et al., 2003a; Duty et al., 2003b; Hauser et al., 2004). There were dose–response relationships of MBP with low sperm concentration (odds ratio per quartile, adjusted for age, abstinence time, and smoking status (1.0, 3.1, 2.5, 3.3; P for trend = 0.04) and motility (1.0, 1.5, 1.5, 1.8; P for trend = 0.04). There was suggestive evidence of an association between the highest MBzP quartile and low sperm concentration (1.0, 1.1, 1.1, 1.9; P for trend = 0.13). There were no relationships of MEP, MMP or the DEHP metabolites with these semen parameters. These results differed from those reported by Jonsson et al. (Jonsson et al., 2005), who did not observe any significant associations between semen quality and phthalate exposure in a study of young healthy military recruits in Sweden. However, several differences were noted between the US and Swedish studies (Hauser et al., 2006), including the age and fertility status of the study populations. A small study in Shanghai (Zhang et al., 2006) examined concentration of three phthalates (DEHP, DBP and DEP) in semen samples. No associations were seen with sperm concentration. All three phthalates were positively associated with sample liquefaction time, and DEHP was significantly correlated to “rate of malformation” (presumably impaired morphology).
In the US population studied by Hauser and Duty there was evidence of sperm DNA damage as assessed by the neutral comet assay, in association with higher exposure to MEP and MEHP (Hauser et al., 2007) (Duty et al., 2003). Duty et al. (Duty et al., 2005) also examined the relationships between urinary levels of phthalate metabolites and serum levels of FSH, LH, SHBG, testosterone, and inhibin-B in this population. They found that, after adjusting for age, body mass index, and time of day that the blood sample was drawn, only MBzP was associated with a decrease in FSH. In addition, there was an increase of borderline significance in inhibin-B associated with MBP. No other phthalate metabolites were associated with hormones in this population. However, Pan et al. (Pan et al., 2006) reported a significant decrease in free testosterone, and increased LH to free testosterone ratio in association with MBP concentration in an occupationally exposed cohort.
Phthalates in relation to respiratory function, metabolism and thyroid function in adults
Other than studies of male reproduction function, there are few human studies of phthalate effects in adults. Only one study in adults examined respiratory function. Hoppin et al. examined the association between phthalate exposure and four pulmonary function parameters in the Third National Health and Nutrition Examination Survey (NHANES, 1988–1994). After controlling for race, age, height, body mass index, and smoking, there was an inverse association between MBP and three measures of pulmonary function (FVC, FEV1, PEF), and higher MEP was associated with lower FVC and FEV1 values in men. No consistent associations were seen in females or with other phthalate metabolites (Hoppin et al., 2004).
Only a single study to date has examined markers of metabolic syndrome in relation to phthalate exposure. Stahlhut et al. (Stahlhut et al., 2007) investigated phthalate exposure and its associations with abdominal obesity and insulin resistance in adult U.S. male participants in the NHANES 1999–2002. They modeled six phthalate metabolites as predictors of waist circumference and HOMA (a measure of insulin resistance) using multiple linear regression, adjusted for age, race/ethnicity, fat and total calorie consumption, physical activity level, serum cotinine, and urine creatinine. Four metabolites were significantly associated with increased waist circumference (MBzP, MEHHP, MEOHP, and MEP) and three with increased HOMA (MBP, MBzP, and MEP).
Meeker et al. (Meeker et al., 2007) examined urinary concentrations of MEHP and other phthalate monoester metabolites in 408 male partners in subfertile couples and concluded that Urinary MEHP concentrations may be associated with altered free T4 and/or total T3 levels in adult men. They saw an inverse association between MEHP urinary concentrations and free T4 and T3 serum levels. The inverse relationship between MEHP and free T4 remained when adjusting for oxidative metabolite concentrations, which simultaneously demonstrated a suggestive positive association with free T4.
Conclusions
In the relatively short time (7–8 years) that sensitive biomarkers for assaying human phthalate exposure in humans have been available, a rapidly growing literature has demonstrated that human exposure is ubiquitous, and evidence for significant impacts on human health is mounting. As seen in this review, most studies in both the toxicological and human literature have reported associations with prenatal and postnatal phthalate exposure in males. This may be a consequence of the anti-androgenic mode of action of the most toxic of these chemicals. In addition (as was learned from the delay in detecting effects of diethylstilbestrol in females), alteration in the female genital tract are hidden and may be missed until puberty or adulthood.
Epidemiological studies may be the “gold standard” for assessing risks to human health, but these are expensive (costing upwards of one-million US dollars per study) and slow (typically a minimum of five years from conception of a human health study to results). Moreover, epidemiology is limited in its ability to draw causal inferences. Therefore, it must be informed by, and closely linked to, animal studies in which mechanism and causality can be demonstrated. While several links between rodent and human studies have been made in this area, as discussed above, these are limited by several discrepancies between human and animal testing.
Animal testing of phthalates has been, for the most part, conducted at doses far higher than those present in the ambient human environment. If dose responses are nonlinear, as has been demonstrated for other environmental chemicals, this testing strategy will not protect human health (Vom Saal al., 2005). In addition, until recently phthalates were tested singly, while humans are exposed to multiple phthalates simultaneously. Recent rodent data suggest that exposure to multiple phthalates at low doses conveys risk in a dose additive manner (Gray et al., 2006; Howdeshell et al., 2008). This suggests that the risk from a mixture of phthalates (or phthalates and other anti-androgens), whether acting by similar mechanisms or not, cannot be accurately assessed studying one chemical at a time.
The same may be true of risks from multiple exposure routes. For example, most toxicological testing of phthalate is via oral exposure. However, humans are exposed to phthalates by a multiplicity of routes. For DEP, and its metabolite MEP, human exposure is predominantly dermal. For example, Janjua et al. (Janjua et al., 2007) recently demonstrated systemic dermal uptake of MEP and MBP in male volunteers by measuring these in serum and urine following whole body topical application of cream containing these compounds. Whether differences in exposure route can account for differences in the toxicology of this phthalate in humans and rodents in not clear.
Because of these important differences in route, dose level and multiplicity of agents, it is often unclear how to interpret findings across species. For these reasons, if chemical risk assessment is to be relevant to human exposure, future assessments should incorporate more environmentally relevant scenarios, including mixtures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akingbemi BT, et al. Phthalate-induced Leydig Cell Hyperplasia is Associated with Multiple Endocrine Disturbances. PNAS. 2004;101:775–780. doi: 10.1073/pnas.0305977101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Atlanta, GA: Division of Toxicology; 1995. Toxicological profile for diethyl phthalate. [PubMed] [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Atlanta, GA: Division of Toxicology; 2001. Toxicological profile for di-n-butyl Phthalate. [PubMed] [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry. Atlanta, GA: Division of Toxicology; 2002. Toxicological profile for di(2-ethylhexyl)phthalate (DEHP) [PubMed] [Google Scholar]
- Borch J, et al. Early testicular effects in rats perinatally exposed to DEHP in combination with DEHA, apoptosis assessment and immunohistochemical studies. Reproductive Toxicology. 2004 doi: 10.1016/j.reprotox.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Bornehag C-G, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environmental Health Perspectives. 2004;112:1393–1397. doi: 10.1289/ehp.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, McKee RH. Integrating biomonitoring exposure data into the risk assessment process: phthalates [diethyl phthalate and di(2-ethylhexyl) phthalate] as a case study. Environmental Health Perspectives. 2006;114:1783–1789. doi: 10.1289/ehp.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegari C, et al. Anogenital ratio: measure of fetal virilization in premature and full-term newborn infants. The Journal of Pediatrics. 1987;111:240–243. doi: 10.1016/s0022-3476(87)80075-6. [DOI] [PubMed] [Google Scholar]
- CDC. Second national report on human exposure to environmental chemicals. Atlanta, GA: National Center for Environmental Health, Division of Laboratory Sciences; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2003
- CDC. Third national report on human exposure to environmental chemicals. Atlanta, GA: National Center for Environmental Health, Division of Laboratory Sciences; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2005
- Clark RL, et al. External genitalia abnormalities in male rats exposed in utero to finasteride, a 5 alpha-reductase inhibitor. Teratology. 1990;42:91–100. doi: 10.1002/tera.1420420111. [DOI] [PubMed] [Google Scholar]
- Colon I, et al. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environmental Health Perspectives. 2000;108:895–900. doi: 10.1289/ehp.108-2556932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, et al. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environmental Health Perspectives. 2005a;113:1530–1535. doi: 10.1289/ehp.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duty SM, et al. Phthalate exposure and reproductive hormones in adult men. Human Reproduction. 2005b;20:604–610. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Duty SM, et al. Phthalate exposure and human semen parameters. Epidemiology. 2003a;14:269–277. [PubMed] [Google Scholar]
- Duty SM, et al. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environmental Health Perspectives. 2003b;111:1164–1169. doi: 10.1289/ehp.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Food and Drug Administration Total Diet Study; summary of residues found ordered by pesticide market baskets 91-3--99-1. Rockville, MD: Office of Plant and Dairy Foods and Beverages; U.S. Food and Drug Administration, Center for Food Safety and Applied Nutrition. 2001
- Foster PM. Disruption of Reproductive Development in Male Rat Offspring Following in Utero Exposure to Phthalate Esters. International Journal of Andrology. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicological Sciences. 2000;58:350–365. doi: 10.1093/toxsci/58.2.350. [DOI] [PubMed] [Google Scholar]
- Gray LE, et al. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. International Journal of Andrology. 2006;29:96–104. doi: 10.1111/j.1365-2605.2005.00636.x. discussion 105-8-96-104; discussion 105-8. [DOI] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occupational and Environmental Medicine. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, et al. Medications as a source of human exposure to phthalates. Environmental Health Perspectives. 2004a;112:751–753. doi: 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, et al. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology (Cambridge, Mass.) 2006;17:682–691. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, et al. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environmental Health Perspectives. 2004b;112:1734–1740. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, et al. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Human Reproduction (Oxford, England) 2007;22:688–695. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, et al. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environmental Health Perspectives. 2002;110:515–518. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, et al. Phthalate exposure and pulmonary function. Environmental Health Perspectives. 2004;112:571–574. doi: 10.1289/ehp.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Vandenbergh JG. The anogenital distance index of mice (Mus musculus domesticus): an analysis. Contemporary Topics in Laboratory Animal Science / American Association for Laboratory Animal Science. 2005;44:46–48. [PubMed] [Google Scholar]
- Howdeshell K, Rider CV, Wilson VS, Hray LE. Mechanisms of action of phthalate esters, individually and in combination, to induce abnormal reproductive development in laboratory rats. Environmental Research. 2008 doi: 10.1016/j.envres.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the Sprague Dawley rat in a cumulative, dose additive manner. Toxicol Sci. 2008 doi: 10.1093/toxsci/kfn077. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ, et al. Interior surface materials in the home and the development of bronchial obstruction in young children in Oslo, Norway. Amer J Public Health. 1999;89:188–192. doi: 10.2105/ajph.89.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola JJ, et al. Plastic wall materials in the home and respiratory health in young children. Amer J Public Health. 2000;90:797–799. doi: 10.2105/ajph.90.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua NR, et al. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ Sci Technol. 2007;41:5564–5570. doi: 10.1021/es0628755. [DOI] [PubMed] [Google Scholar]
- Jonsson BA, et al. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16:487–493. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labeled DEHP. Archives of Toxicology. 2005;79:367–376. doi: 10.1007/s00204-004-0642-4. [DOI] [PubMed] [Google Scholar]
- Kolarik B, et al. The association between phthalates in dust and allergic diseases among Bulgarian children. Environmental Health Perspectives. 2008;116:98–103. doi: 10.1289/ehp.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environmental Health Perspectives. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BM, Koo HJ. Hershberger Assay for Antiandrogenic Effects of Phthalates. Journal of Toxicology & Environmental Health. 2007;70:1365–1370. doi: 10.1080/15287390701432285. [DOI] [PubMed] [Google Scholar]
- Main KM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee RH, Toxicology Research Task G. Phthalate exposure and early thelarche. Environmental Health Perspectives. 2004;112:A541–A543. doi: 10.1289/ehp.112-a541b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, et al. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environmental Health Perspectives. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, et al. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environmental Health Perspectives. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Phillip M, et al. Clitoral and penile sizes of full term newborns in two different ethnic groups. Journal of Pediatric Endocrinology and Metabolism. 1996;9:175–179. [PubMed] [Google Scholar]
- Rudel RA, et al. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environmental Science & Technology. 2003;37:4543–4553. doi: 10.1021/es0264596. [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E, et al. Anogenital distance in human male and female newborns: a descriptive, cross-sectional study. Environmental Health. 2004;3:8–8. doi: 10.1186/1476-069X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, et al. Baby care products: possible sources of infant phthalate exposure. Pediatrics. 2008;121:e260-8–e260-8. doi: 10.1542/peds.2006-3766. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. Quantification of 22 phthalate metabolites in human urine. Journal of Chromatography B. 2007;860:106–112. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, et al. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environmental Health Perspectives. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, et al. Geographic differences in semen quality of fertile U.S. males. Environmental Health Perspectives. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environmental Health Perspectives. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, et al. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. The Journal of Clinical Investigation. 2008;118:1479–1490. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson VS, et al. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl. 2008;31:178–187. doi: 10.1111/j.1365-2605.2007.00861.x. [DOI] [PubMed] [Google Scholar]
- Wilson VS, et al. Phthalate ester-induced gubernacular lesions are associated with reduced insl3 gene expression in the fetal rat testis. Toxicology Letters. 2004;146:207–215. doi: 10.1016/j.toxlet.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Wormuth M, et al. What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Analysis. 2006;26:803–824. doi: 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- Zhang YH, et al. Phthalate exposure and human semen quality in Shanghai: a cross-sectional study. Biomed Environ Sci. 2006;19:205–209. [PubMed] [Google Scholar]


