Abstract
Background
Attentional deficits that accompany schizophrenia are not effectively treated by available antipsychotic medications. Disruption of NMDA receptor function is often used to model aspects of this disorder in rodents. We used the 5-choice serial reaction time task (5CSRTT) to characterize attentional deficits caused by acute administration or withdrawal from chronic administration of the NMDA receptor antagonist MK-801, and determine if they are ameliorated by haloperidol or clozapine.
Methods
Acute studies involved tests in the presence of MK-801: rats were administered haloperidol (0.008-0.125 mg/kg, SC) or clozapine (0.16-2.5 mg/kg, SC) in combination with MK-801 (0.25 mg/kg, IP) prior to daily test sessions. Chronic studies involved tests in the absence of MK-801: following daily tests, rats were administered MK-801 (0.5 mg/kg, IP) and tested 24 hr later in the absence or presence of haloperidol or clozapine.
Results
Acute MK-801 disrupted performance: it decreased accuracy while increasing omissions, premature responses, and magazine entries. Haloperidol reduced disruptive effects associated with increased activation, whereas it exacerbated other deficits. Clozapine dose-dependently attenuated several of the MK-801-induced performance deficits. Withdrawal from chronic MK-801 progressively increased omissions and response latencies and decreased premature responding, suggesting an amotivational state. Neither haloperidol nor clozapine ameliorated these performance deficits.
Discussion
Acute administration and withdrawal from chronic MK-801 administration produced distinct behavioral profiles in the 5CSRTT. Acute MK-801 impaired attention and impulse control whereas chronic MK-801 withdrawal caused signs consistent with amotivation. Haloperidol and clozapine were more effective at attenuating deficits caused by acute MK-801 administration.
Keywords: attention, schizophrenia, 5-choice serial reaction time task, MK-801, clozapine, haloperidol, behavior, rat
Introduction
Schizophrenia affects 1% of the population worldwide and is characterized by positive symptoms (hallucinations, delusions, thought disorder), negative symptoms (amotivation, flattened affect) and cognitive symptoms (impaired attention, working memory and executive functioning) (http://www.nimh.nih.gov/publicat/schizoph.cfm). Attentional deficits precede the illness onset, are stable across phases of illness, and are present in non-schizophrenic first-degree relatives of people with schizophrenia (Cornblatt et al., 2001; Chen et al., 2000). Furthermore, cognitive deficits predict patient difficulties in maintaining employment, living independently and having meaningful social interactions (Green et al., 2004). Effective treatment of such deficits could have a profound impact on the lives of people with schizophrenia.
Early experiments addressing the effects of antipsychotic medications on cognition suggested that atypical (second generation) antipsychotics were more beneficial than classical (first generation) antipsychotics (Keefe et al., 1999). These early experiments can be difficult to interpret because they often involved small sample sizes, lack of comparator groups, high doses of classical antipsychotics, and adjunctive therapy with anticholinergic drugs (Keefe et al., 2007). Recent meta-analyses suggest that classical antipsychotics may have pro-cognitive effects comparable to atypical antipsychotics (Mishara et al., 2004; Keefe et al., 2007). However, repeated cognitive assessments themselves can lead to measurable cognitive improvements, an effect that may explain the apparent pro-cognitive effects of antipsychotic treatment (Goldberg et al., 2007). Studies using animals may help resolve discrepancies in the clinical literature regarding the relative pro-cognitive efficacy of antipsychotics.
The present studies were designed to examine if a classical (haloperidol) or an atypical (clozapine) antipsychotic drug would have pro-cognitive effects as measured in the 5-choice serial reaction time task (5CSRTT). The 5CSRTT is a rodent task of attention analogous to the continuous performance task used to study attention in humans (Robbins, 2002). Because our initial studies indicated that neither haloperidol nor clozapine had pro-cognitive effects when administered alone, we then examined if they would attenuate cognitive deficits observed following acute administration of the N-methyl-D-aspartate (NMDA) receptor antagonist MK-801. Acute NMDA receptor antagonism models aspects of schizophrenia- causing both psychotic symptoms and cognitive deficits (e.g., impairments in attention and working memory) in healthy volunteers and people with schizophrenia (Lahti et al., 2001, Coyle et al., 2004; Jentsch et al., 1999). Consistent with previous reports, MK-801 administration impaired attention (Paine et al., 2007), but these effects were not substantially attenuated by either acute haloperidol or acute clozapine administration. Because acute MK-801 administration causes non-specific behavioral changes that may interfere with the ability to detect pro-cognitive effects of antipsychotic treatments (Jentsch et al., 1999), we also tested whether acute antipsychotic treatment could alleviate the deficits observed during withdrawal from chronic MK-801 administration. Withdrawal from chronic NMDA receptor antagonism impairs cognitive function in rodents (Dunn et al., 2006; Madillo et al., 2003; O'Donnell et al., 2003; Jentsch et al., 1997; Rujescu et al., 2006; Shroeder et al., 2001; Hashimoto et al., 2005) and causes pathological changes reminiscent of schizophrenia (Jentsch et al., 1997; Tsukada et al., 2005; Pratt et al., 2008; Behrens et al., 2007; Lewis et al., 2006).
Methods
Rats
Twenty-eight male Sprague-Dawley rats (Charles River Laboratories, Raleigh NC) weighing 250-300 g at the start of the experiment were housed in pairs on a 12-h/12-h light-dark cycle (lights on at 0700h). Rats were given 1 week to acclimate to the housing conditions with free access to food (Purina Rat Chow) and water. Twenty-four hours prior to the onset of training and throughout training, rats were food restricted to 85% of their free-feeding weights. Rats had free access to water while in the home cage. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996) and McLean Hospital policies.
Behavioral apparatus
Testing was conducted in 5CSRTT operant chambers that were contained in sound-attenuating ventilated cubicles (Med-Associates, St. Albans VT). Five equally spaced 2.5 × 2.5 × 2.2 cm apertures were set into a curved aluminum front wall; each aperture was fitted with a yellow LED stimulus light (6.4 mm in diameter) and an infrared detector (1.0 cm from the front of the aperture). The opposite wall was fitted with a food magazine connected to a 45-mg pellet dispenser; an infrared detector located horizontally across the magazine allowed for the detection of nose pokes into the magazine. The top of the magazine was fitted with a light (1.0 cm in diameter). The house light was located on the ceiling directly above the magazine. The sidewalls and ceiling were made of clear polycarbonate and the floor was a stainless steel grid.
The 5-choice serial reaction time task (5CSRTT)
We trained the rats as described previously (Paine et al., 2007). Sessions started with the delivery of 1 food pellet (45-mg, Bio-Serv, Frenchtown NJ); the first trial commenced upon retrieval. A nose poke into the magazine initiated a 5-sec inter-trial interval (ITI) and illumination of a house light. At the end of the ITI, a 1.0-sec light stimulus was presented at the rear of one of the five stimulus locations (apertures). Rats had up to 5 sec (limited hold) to make a response. A response in the illuminated aperture (correct response) triggered delivery of 1 food pellet and illumination of the magazine light, which remained illuminated for 5 sec following pellet delivery. Nose pokes in the remaining apertures during the limited hold were considered incorrect responses and triggered a 5-sec time-out during which the house light was extinguished. Similarly, failure to respond during the limited hold (i.e., an omission) triggered a 5-sec time-out. The subsequent trial was initiated at the end of the time-out period. Responses occurring prior to stimulus presentation (i.e., during the ITI) were considered premature responses and also triggered a 5-sec time-out; the same trial was re-started at the end of the time-out period. Responses occurring during the time-out period had no programmed consequences. Each session ended after 90 trials or 30 min. Performance measures of interest were: % accuracy ((correct responses/ [correct + incorrect responses])*100), % omissions ([omissions/ trials completed]*100), premature responses, magazine entries, correct response latency (the time from the stimulus onset to a correct response) and reward latency (the time from a correct response to the collection of the food). Testing began when accuracy was greater than 60% and omissions were fewer than 20% for 3 consecutive days.
Experiment 1. Effects of acute antipsychotic administration on performance
Upon reaching criterion performance, rats were tested following administration of haloperidol (n=8, 0.0-0.25 mg/kg) or clozapine (n=6, 0.0-2.50 mg/kg). Drugs were administered subcutaneously (SC) 60 min prior to testing. Doses of haloperidol and clozapine were administered in an ascending order with the exception that vehicle was administered last. To avoid potential carry-over effects rats were given a minimum of two drug-free days between drug tests and had to perform at criterion on the session preceding drug-testing.
Experiment 2. Effects of acute MK-801 administration on performance
Rats from Experiment 1 were re-stabilized for at least 5 days and then tested following administration of either haloperidol or clozapine in combination with acute MK-801. The rats were grouped according to their previous history (e.g., rats that previously received haloperidol continued to receive haloperidol). One rat from the haloperidol group was excluded from the remainder of the experiment because his performance failed to re-stabilize. In addition, new (drug naïve) rats were added to each condition (4/group); pre-testing baseline data from these animals did not differ from those that had been tested in Experiment 1. Haloperidol (n=11, 0.0-0.063 mg/kg, SC) and clozapine (n=10, 0.0-1.25 mg/kg, SC) were administered 60 min prior to testing. MK-801 (0.25 mg/kg, IP) was administered 30 min prior to testing; this dose impairs attention (% accuracy) in the 5CSRTT (Paine et al., 2007). To avoid potential order effects of repeated MK-801 administration, doses of haloperidol and clozapine were administered in a pseudo-random order (each dose was given only once). As above, rats were allowed a minimum of two drug-free days before drug tests and had to perform at criterion on the session preceding drug testing.
Experiment 3. Effects of chronic MK-801 exposure on performance
Rats were re-stabilized for at least 5 days and then the effects of a regimen of repeated MK-801 administration on performance in the 5CSRTT was assessed. As in Experiment 2, the rats were grouped according to their previous history. For 12 consecutive days, rats were administered MK-801 (0.5 mg/kg, IP) ∼30 min after their daily test sessions such that rats were tested in the absence of MK-801. MK-801 (0.5 mg/kg) was administered because this dose is known to cause schizophrenia-like pathologies in rats (Kondziella et al., 2006; Hashimoto et al., 2007). On days 6, 8, 10 and 12 rats were pretreated with haloperidol (n=11, 0.0-0.016 mg/kg, SC) or clozapine (n=11; 0.0-0.32 mg/kg, SC) 60 min prior to test sessions. One rat from the clozapine group was excluded from this portion of the experiment because his performance failed to re-stabilize. Two rats in the clozapine group were drug naïve prior to chronic MK-801 administration; data from these animals did not differ from those that had been tested in the earlier experiments. Doses of haloperidol and clozapine were administered in a pseudo-random order.
Drugs
Drugs were purchased from Sigma (St. Louis, MO). (+)-MK-801 hydrogen maleate was dissolved in saline. Haloperidol was dissolved in 75% dimethyl sulfoxide (DMSO). Clozapine was dissolved in a minimum amount of 0.1N HCl, adjusted to pH 6.0 with 0.1N NaOH, and then diluted with saline. Drugs were administered in a volume of 1.0 ml/kg.
Statistics
To ensure that there were no carry-over drug effects or any systematic effects of repeated testing, performance on baseline sessions in experiments 1 and 2 was subjected to repeated measures analyses of variance. Baseline sessions for each drug test session consisted of the training session on the day preceding that test session.
Data from the drug testing sessions were also analyzed using a repeated measures ANOVA with dose (Experiment 1), drug treatment (Experiments 2 and 3) or day (Experiment 3) as the within subjects factor. For Experiment 3, baseline values were calculated by averaging the 3 days preceding drug treatment. All significant main effects were examined further using Fisher's protected t-tests.
Results
Experiment 1. Effects of acute antipsychotic administration on performance
i) Haloperidol
The 0.25 mg/kg dose of haloperidol was excluded from statistical analyses because it profoundly disrupted performance (>99% omissions). With one exception (omissions), baseline (drug-free) performance was stable across sessions [all F(5, 35) = 2.21, P > 0.08, Table I]. Omissions differed across baseline sessions [F(5,35) = 2.57, P < 0.05]: omissions were increased on baseline session 3 compared to baseline session 1 (P < 0.05, Fisher's protected t-tests). The lack of a linear trend for an increase in omissions suggests that there were no systematic effects of repeated testing or carry-over drug effects.
Table I.
Baseline performance on 5CSRTT during acute antipsychotic administration
| % Accuracy | % Omissions | Premature Responses | Magazine Entries | Correct Response Latency (sec) | Reward Retrieval Latency (sec) | |
|---|---|---|---|---|---|---|
| Haloperidol | ||||||
| 1 | 67.79 ± 3.41 | 4.58 ± 1.79 | 35.63 ± 8.90 | 220.75 ± 41.34 | 0.89 ± 0.08 | 1.70 ± 0.12 |
| 2 | 69.75 ± 2.88 | 8.20 ± 1.13 | 22.25 ± 5.62 | 181.13 ± 29.45 | 0.86 ± 0.06 | 1.65 ± 0.10 |
| 3 | 72.41 ± 2.46 | 10.56 ± 2.09* | 22.88 ± 3.92 | 183.5 ± 24.60 | 0.88 ± 0.06 | 1.60 ± 0.09 |
| 4 | 73.02 ± 2.98 | 5.55 ± 1.74 | 20.38 ± 4.70 | 169.25 ± 26.16 | 0.93 ± 0.08 | 1.59 ± 0.08 |
| 5 | 71.69 ± 2.82 | 7.92 ± 2.16 | 22.63 ± 4.72 | 173.88 ± 31.56 | 0.96 ± 0.06 | 1.71 ± 0.11 |
| 6 | 69.56 ± 2.93 | 5.83 ± 1.58 | 24.5 ± 7.34 | 188.63 ± 32.14 | 1.01 ± 0.06 | 1.69 ± 0.12 |
| Clozapine | ||||||
| 1 | 74.70 ± 2.43 | 8.89 ± 2.24 | 27.833 ± 6.05 | 209.83 ± 15.36 | 0.79 ± 0.03 | 1.58 ± 0.06 |
| 2 | 70.88 ± 2.55 | 6.85 ± 1.85 | 36.17 ± 8.97 | 244.67 ± 30.47 | 0.86 ± 0.06 | 1.47 ± 0.07 |
| 3 | 70.75 ± 2.30 | 9.81 ± 2.37 | 30.83 ± 8.36 | 219.50 ± 20.41 | 0.84 ± 0.05 | 1.51 ± 0.07 |
| 4 | 71.29 ± 1.03 | 6.67 ± 2.30 | 27.50 ± 6.53 | 195.83 ± 11.82 | 0.83 ± 0.06 | 1.48 ± 0.09 |
| 5 | 70.79 ± 3.41 | 6.85 ± 1.56 | 23.17 ± 4.59 | 194.17 ± 9.39 | 0.84 ± 0.05 | 1.54 ± 0.11 |
| 6 | 74.57 ± 2.80 | 7.59 ± 1.80 | 21.67 ± 8.04 | 204.17 ± 8.25 | 0.79 ± 0.05 | 1.55 ± 0.09 |
| 7 | 73.59 ± 1.53 | 6.29 ± 1.10 | 37.67 ± 8.78 | 244.5 ± 17.72 | 0.81 ± 0.05 | 1.51 ± 0.10 |
Note: Rats were administered haloperidol or clozapine with a minimum of two drug-free days between drug doses. Baseline sessions (days 1-7) consisted of the training session on the day prior to drug testing. Because drug doses were administered in an ascending order these data indicate that performance was stable across time. And, with one exception, these data indicate that there were no long-term (> 72 h) carry-over effects of acute antipsychotic administration on 5CSRTT performance. Significantly different from Haloperidol day 1,
P < 0.05 Fisher's protected t-tests.
Repeated measures ANOVAs indicated that acute haloperidol administration (Table II) affected accuracy [F(5, 35) = 5.99, P = 0.01], omissions [F(5, 35) = 141.37, P < 0.01], premature responses [F(5, 35) = 6.29, P = 0.01], magazine entries [F(5, 35) = 16.97, P < 0.01], correct response latencies [F(5, 35) = 9.22, P < 0.01], and reward retrieval latencies [F(5, 35) = 18.65, P < 0.01]. Post hoc analyses (Fisher's protected t-tests) revealed that acute haloperidol administration reduced accuracy (0.125 mg/kg, P < 0.01), premature responses (0.016-0.125 mg/kg, P's < 0.05-0.01), and magazine entries (0.032-0.125 mg/kg, P's < 0.05-0.01). In contrast, haloperidol increased omissions (0.063-0.125 mg/kg, P's < 0.01), correct response latencies (0.063-0.125 mg/kg, P's < 0.05-0.01), and reward retrieval latencies (0.063 mg/kg, P < 0.01).
Table II.
Effects of acute antipsychotic administration on performance in the 5-choice serial reaction time task
| % Accuracy | % Omissions | Premature Responses | Magazine Entries | Correct Response Latency (sec) | Reward Retrieval Latency (sec) | |
|---|---|---|---|---|---|---|
| Haloperidol | ||||||
| 0.0 | 66.78± 3.77 | 7.22 ± 2.53 | 23.5 ± 5.01 | 184.25 ± 26.27 | 0.94 ± 0.05 | 1.68 ± 0.21 |
| 0.008 | 71.08 ± 2.79 | 3.05 ± 0.88 | 27.88 ± 7.04 | 192.13 ± 22.92 | 0.86 ± 0.06 | 1.58 ± 0.09 |
| 0.016 | 72.58 ± 3.13 | 7.36 ± 2.27 | 12.75 ± 2.53* | 155.00 ± 16.94 | 0.94 ± 0.05 | 1.61 ± 0.10 |
| 0.032 | 71.99 ± 2.31 | 9.03 ± 3.67 | 10.88 ± 1.42* | 133.5 ± 11.66* | 1.21 ± 0.04 | 1.71 ± 0.09 |
| 0.063 | 63.41 ± 3.68 | 58.33 ± 6.46** | 11.63 ± 2.25* | 76.13 ± 9.88** | 1.26 ± 0.07* | 1.91 ± 0.13 |
| 0.125 | 41.64 ± 9.13** | 90.28 ± 3.40** | 3.88 ± 0.95** | 38.63 ± 10.38** | 1.64 ± 0.19** | 2.69 ± 0.24** |
| Clozapine | ||||||
| 0.0 | 71.07 ± 2.22 | 4.44 ± 1.65 | 29.33 ± 2.26 | 231.50 ± 17.25 | 0.81 ± 0.05 | 1.60 ± 0.14 |
| 0.08 | 74.08 ± 2.54 | 6.85 ± 1.61 | 29.67 ± 5.54 | 223.33 ± 26.58 | 0.78 ± 0.06 | 1.45 ± 0.04 |
| 0.16 | 70.44 ± 2.43 | 5.23 ± 2.05 | 48.50 ± 12.81* | 258.67 ± 34.00 | 0.78 ± 0.05 | 1.43 ± 0.05 |
| 0.32 | 73.71 ± 3.18 | 3.52 ± 1.45 | 34.83 ± 6.16 | 226.83 ± 21.75 | 0.81 ± 0.03 | 1.48 ± 0.06 |
| 0.63 | 69.44 ± 1.71 | 4.07 ± 2.34 | 21.17± 3.64 | 221.50 ± 13.60 | 0.90 ± 0.05 | 1.60 ± 0.08 |
| 1.25 | 67.93 ± 2.60 | 12.41 ± 2.70 | 14.00 ± 4.48 | 314.33 ± 57.28 | 1.11 ± 0.04** | 1.72 ± 0.10 |
| 2.5 | 57.64 ± 2.39** | 47.78 ± 7.40** | 14.17 ± 2.36 | 262.00 ± 25.98 | 1.50 ± 0.07** | 1.92 ± 0.09** |
Note: Haloperidol and clozapine were administered (SC) 60 min prior to testing. Significantly different from vehicle (0.0 mg/kg),
P < 0.05,
P < 0.01 Fisher's protected t-tests.
ii) Clozapine
Baseline (drug-free) performance was unaltered across sessions [all F(6, 30) < 1.90, P > 0.11, Table I] indicating that there were no systematic changes in performance over time nor any carry-over drug effects resulting from repeated clozapine administration.
Acute clozapine administration (Table II) affected accuracy [F(6, 30) = 5.05, P < 0.01], omissions [F(6, 30) = 21.05, P < 0.01], premature responses [F(6, 30) = 4.74, P < 0.01], correct response latencies [F(6, 30) = 42.47, P < 0.01], and reward retrieval latencies [F(6, 30) = 8.63, P < 0.01]. Post hoc analyses revealed that clozapine decreased accuracy (2.5 mg/kg, P < 0.01) but increased omissions (2.5 mg/kg, P < 0.01), premature responses (0.16 mg/kg, P < 0.05), correct response latencies (1.25-2.5 mg/kg, P's < 0.01), and reward retrieval latencies (2.5 mg/kg, P < 0.01).
Experiment 2. Effects of acute MK-801 administration on performance
i) Haloperidol
Baseline (drug-free) performance was not altered across sessions [F(5, 50) < 1.86, P > 0.12, Table III] indicating that there were no long-term (> 72 hr) effects of acute drug administration.
Table III.
Baseline performance during acute MK-801 + Antipsychotic administration
| % Accuracy | % Omissions | Premature Responses | Magazine Entries | Correct Response Latency (sec) | Reward Retrieval Latency (sec) | |
|---|---|---|---|---|---|---|
| Haloperidol | ||||||
| 1 | 73.96 ± 3.10 | 6.36 ± 1.80 | 19.27 ± 4.06 | 210.36 ± 40.21 | 0.91 ± 0.04 | 1.62 ± 0.09 |
| 2 | 73.34 ± 2.34 | 6.36 ± 0.93 | 27.45 ± 7.51 | 224.18 ± 44.18 | 0.87 ± 0.06 | 1.61 ± 0.09 |
| 3 | 73.41 ± 2.45 | 7.45 ± 1.39 | 19.1 ± 8.30 | 226.90 ± 56.21 | 0.92± 0.05 | 1.61 ± 0.09 |
| 4 | 74.23 ± 2.66 | 7.27 ± 1.18 | 17.09 ± 4.58 | 194.27 ± 34.54 | 0.97 ± 0.05 | 1.71 ± 0.11 |
| 5 | 71.9 ± 2.63 | 7.27 ± 1.40 | 27.00 ± 5.62 | 200.64 ± 32.92 | 0.87 ± 0.05 | 1.70 ± 0.10 |
| 6 | 71.07 ± 2.64 | 8.72 ± 1.45 | 14.18 ± 2.39 | 188.091 ± 29.63 | 0.97 ± 0.05 | 1.68 ± 0.10 |
| Clozapine | ||||||
| 1 | 77.63 ± 2.19 | 7.78 ± 1.85 | 20.50 ± 6.62 | 172.60 ± 14.51 | 0.82 ± 0.04 | 1.62 ± 0.09 |
| 2 | 74.55 ± 1.44 | 6.78 ± 2.01 | 19.30 ± 4.95 | 176.80 ± 8.94 | 0.82 ± 0.04 | 1.58 ± 0.07 |
| 3 | 76.11 ± 2.42 | 7.28 ± 1.39 | 16.11 ± 4.84 | 189.22 ± 16.30 | 0.87 ± 0.05 | 1.66 ± 0.06 |
| 4 | 74.22 ± 3.42 | 9.22 ± 1.97 | 17.60 ± 5.39 | 184.30 ± 16.27 | 0.87 ± 0.05 | 1.56 ± 0.06 |
| 5 | 73.13 ± 2.54 | 6.33 ± 0.91 | 19.6 ± 5.99 | 196.10 ± 21.64 | 0.86 ± 0.05 | 1.51 ± 0.08 |
| 6 | 74.57 ± 2.65 | 7.22 ± 1.25 | 20.7 ± 4.14 | 184.22 ± 10.39 | 0.83 ± 0.05 | 1.65 ± 0.10 |
Note: Rats were administered MK-801 (0.0 or 0.25 mg/kg) in combination with haloperidol (0.0-0.0125 mg/kg, SC) or clozapine (0.0-1.25 mg/kg) with a minimum of two drug-free days between drug doses. Baseline sessions (days 1-6) consisted of the training session on the day prior to drug testing. Because drug doses were administered in a pseudo-random order, these data indicate that there were no long-term (> 72 h) carry-over effects of drug administration on 5CSRTT performance or any systematic change in performance.
The effects of haloperidol pretreatment on behavioral deficits produced by acute MK-801 are illustrated in Figure 1. Drug treatment affected accuracy [F(5, 50) = 3.96, P < 0.01, Fig. 1a], omissions [F(5, 50) = 9.90, P < 0.01, Fig. 1b], premature responses [F(5, 50) = 5.20, P < 0.01, Fig. 1c], magazine entries [F(5, 50) = 2.98, P < 0.05, Fig. 1d], correct response latency [F(5, 50) = 3.13, P < 0.05, Fig. 1e], and reward retrieval latencies [F(5, 50) = 6.48, P < 0.01, Fig. 1f]. Post hoc analyses revealed that MK-801 alone reduced accuracy and increased omissions, premature responses and magazine entries (P's < 0.05-0.01). A dose of haloperidol that affected performance when administered alone (0.063 mg/kg) reduced the MK-801-induced increase in premature responses (P < 0.05) and magazine entries (P < 0.01). High doses of haloperidol also potentiated the increase in omissions caused by acute MK-801 administration (0.032-0.063 mg/kg, P's < 0.05-0.01). Although MK-801 alone did not affect either correct response latencies or reward retrieval latencies, MK-801 in combination with haloperidol increased both correct response latencies (0.016 and 0.063 mg/kg, P's < 0.05) and reward retrieval latencies (0.032-0.063 mg/kg, P's < 0.05-0.01) compared to vehicle.
Figure 1.
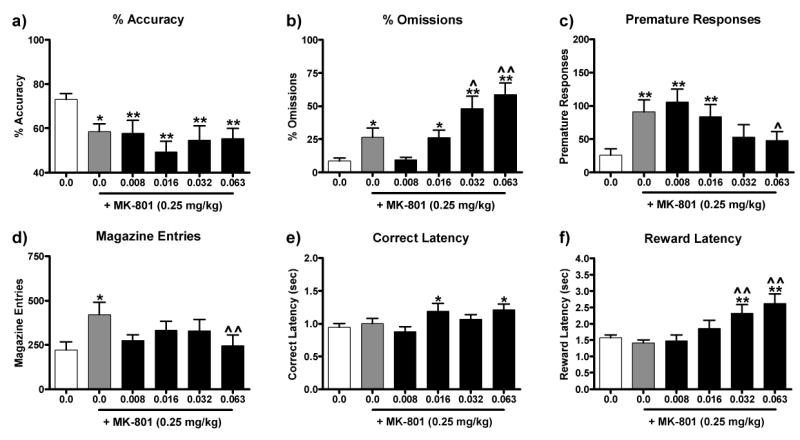
Effect of haloperidol pretreatment on behavioral deficits induced by acute MK-801 administration. Haloperidol (0.0-0.063 mg/kg, SC) was administered 60 min prior to testing and MK-801 (0.25 mg/kg, IP) was administered 30 min prior to testing. MK-801 administration (grey bar) reduced accuracy (a), increased omissions (b), premature responses (c), and magazine entries (d), but did not affect correct response latencies (e) or reward retrieval latencies (f). Haloperidol significantly attenuated the MK-801-induced increase in premature responses and magazine entries, but it exaggerated the MK-801-induced increase in omissions and reward retrieval latencies. Significantly different from 0.0 mg/kg MK-801 + 0.0 mg/kg Haloperidol, *P < 0.05, **P < 0.01, Fisher's protected t-tests. Significantly different from MK-801 (0.25 mg/kg) + Haloperidol (0.0 mg/kg), ˆP < 0.05, ˆˆP < 0.01, Fisher's protected t-tests.
ii) Clozapine
Baseline (drug-free) performance was not altered across sessions [all F(4, 45) < 1.58, P > 0.18, Table III] indicating that there were no long-term (> 72 hr) effects of acute drug administration.
The effects of clozapine pretreatment on behavioral deficits produced by acute MK-801 administration are shown in Figure 2. Drug treatment affected accuracy [F(5, 45) = 4.15, P < 0.01, Fig 2a], omissions [F(5, 45) = 5.20, P < 0.01, Fig 2b], premature responses, [F(5, 45) = 8.58, P < 0.01, Fig 2c], magazine entries [F(5, 45) = 7.96, P < 0.01, Fig 2d], correct response latencies [F(5, 45) = 3.23, P < 0.05, Fig 2e], and reward retrieval latencies [F(5, 45) = 3.37, P = 0.01, Fig 2f]. Post hoc analyses revealed that MK-801 alone decreased accuracy and increased omissions, premature responses, magazine entries, and correct response latencies (P's < 0.05-0.01). Clozapine administration attenuated the MK-801-induced decrease in accuracy (0.16 mg/kg, P < 0.05) and the MK-801-induced increase in omissions (0.16-0.32 mg/kg, P's < 0.05-0.01), premature responses (1.25 mg/kg, P < 0.01), magazine entries (0.16 mg/kg, 0.063-1.25 mg/kg, P's < 0.05-0.01), and correct response latencies (0.16 mg/kg, P < 0.05).
Figure 2.

Effect of clozapine pretreatment on behavioral deficits induced by acute MK-801 administration. Clozapine (0.0-1.25 mg/kg, SC) was administered 60 min prior to testing and MK-801 (0.25 mg/kg, IP) was administered 30 min prior to testing. MK-801 administration (grey bar) reduced accuracy (a), increased omissions (b), premature responses (c), magazine entries (d), and correct response latencies (e). Clozapine attenuated MK-801-induced behavioral deficits. Significantly different from 0.0 mg/kg MK-801 + 0.0 mg/kg Clozapine), *P < 0.05, **P < 0.01, Fisher's protected t-tests. Significantly different from MK-801 (0.25 mg/kg) + Clozapine (0.0 mg/kg), ˆP < 0.05, ˆˆP < 0.01 Fisher's protected t-tests.
Experiment 3. Effects of exposure to chronic MK-801 treatment on performance
Comparison of the effects of withdrawal from chronic MK-801 on 5CSRTT performance in rats from the haloperidol and clozapine treatment groups revealed that these groups were not statistically different (not shown), thus analyses were conducted on the combined data set. Chronic MK-801 affected accuracy [F(8, 184) = 3.37, P < 0.01, Fig 3a], omissions [F(8, 184) = 14.28, P < 0.01, Fig 3b], premature responses [F(8, 184) = 2.87, P = 0.01, Fig 3c], magazine entries [F(8, 184) = 2.60, P < 0.01, Fig 3d], correct response latencies [F(8, 184) = 7.59, P < 0.01, Fig 3e], and reward retrieval latencies [F(8, 184) = 3.48, P < 0.01, Fig 3f]. The latency with which withdrawal from chronic MK-801 affected performance in the 5CSRTT differed across measures. Premature responses and response latencies (both correct response and reward retrieval) were affected on test day 1 (i.e., the first day of withdrawal)). There was a transient decrease in premature responses, which waned by test day 9. In contrast, response latencies were persistently increased throughout the drug administration regimen. Omissions, magazine entries and accuracy all required repeated administration of MK-801 before differences from baseline (e.g., omissions-day 4, magazine entries- day 5, accuracy-day 7) became persistently impaired (P's < 0.05-0.01).
Figure 3.
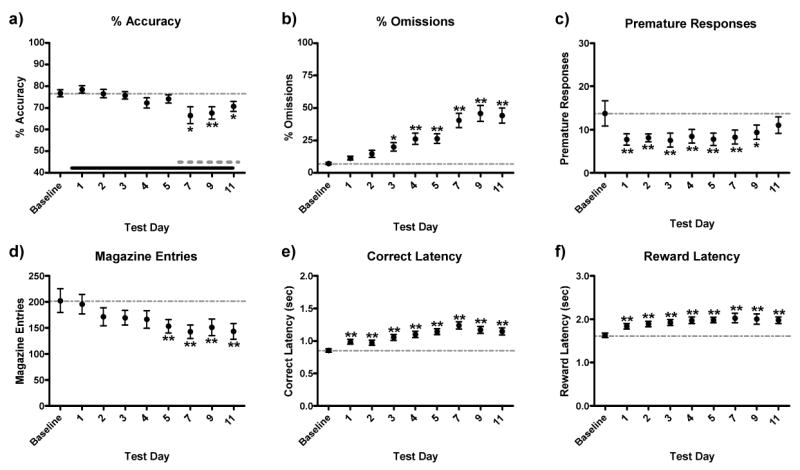
Effects of chronic MK-801 withdrawal on 5CSRTT performance. Because data from the haloperidol and clozapine groups did not differ on days when antipsychotics were not administered, these data were pooled for statistical analyses. Beginning after baseline was established, MK-801 (0.5 mg/kg, IP) was administered 30 min after daily test sessions; tests were conducted 23.5 hr later. Chronic MK-801 withdrawal decreased accuracy of responding (a), premature responses (c) and, magazine entries (d) while it increased omissions (b), correct response latencies (e), and reward retrieval latencies (f) relative to baseline. Solid black bar in (a) represents period of chronic MK-801 administration and the grey dashed bar (a) represents time period of acute intermittent antipsychotic treatment. Baseline scores were the average of the 3 test sessions preceding the initiation of MK-801 administration. Significantly different from baseline, *P < 0.05, **P < 0.01, Fisher's protected t-tests.
i) Chronic MK-801 + Haloperidol
The effect of haloperidol on the behavioral deficits caused by withdrawal from chronic MK-801 administration is illustrated in Figure 4. Drug treatment affected accuracy [F(4, 40) = 2.57, P = 0.05, Fig 4a], omissions [F(4, 40) = 9.99, P < 0.01, Fig 4b], magazine entries [F(4, 40) = 4.65, P = 0.004, Fig 4d], correct response latencies [F(4, 40) = 10.46, P < 0.01, Fig 4e], and reward retrieval latencies [F(4, 40) = 5.38, P < 0.01, Fig 4f]. Withdrawal from chronic MK-801 increased omissions, correct response latencies and reward retrieval latencies while it decreased magazine entries (P's < 0.01). Rather than attenuating the effects of withdrawal from chronic MK-801, haloperidol potentiated increases in correct response latency (0.008-0.016 mg/kg, P's < 0.05-0.01) and resulted in the emergence of a decrease in accuracy (0.004-0.016 mg/kg, P's < 0.05-0.01).
Figure 4.
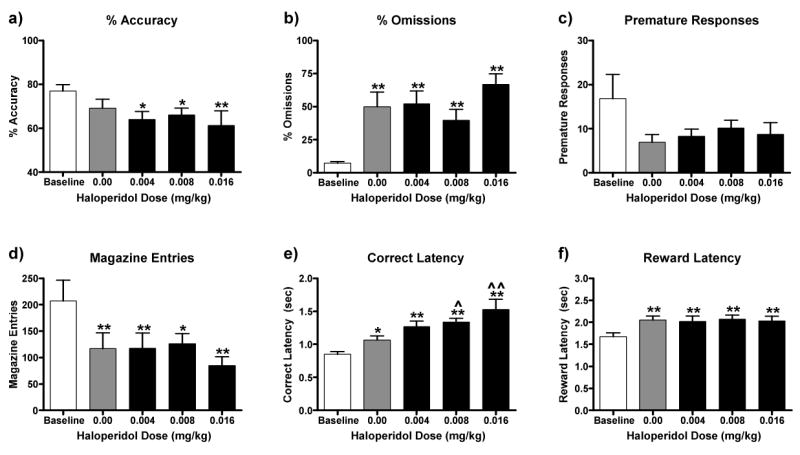
Ability of haloperidol pretreatment to attenuate performance deficits caused by exposure to a regimen of chronic MK-801 administration. MK-801 (0.5 mg/kg, IP) was administered 30 min after daily training sessions; testing occurred 23.5 hr later. On days 6, 8, 10 and 12 of MK-801 administration, haloperidol (0.0-0.016 mg/kg, SC) was administered 60 min prior to testing. Exposure to MK-801 administration alone (grey bar) did not affect accuracy (a), but it impaired accuracy in combination with haloperidol (0.004-0.016 mg/kg). Exposure to MK-801 increased omissions (c) and reward retrieval latencies (f), and decreased premature responses (c) and magazine entries (d). These effects were not altered by haloperidol (0.004-0.016 mg/kg). Exposure to MK-801 increased correct response latencies (e), and haloperidol (0.008-0.016 mg/kg) potentiated this effect. Significantly different from baseline, *P < 0.05, **P < 0.01, Fisher's protected t-tests. Significantly different from chronic MK-801 + haloperidol (0.0 mg/kg), ˆˆP < 0.01, Fisher's protected t-tests.
ii) Chronic MK-801 + Clozapine
The effect of clozapine on the behavioral deficits caused by withdrawal from chronic MK-801 administration is illustrated in Figure 5. Drug treatment affected accuracy [F(4, 40) = 3.23, P < 0.05, Fig 5a], omissions [F(4, 40) = 11.66, P < 0.01, Fig 5b], correct response latencies [F(4, 40) = 9.49, P < 0.01, Fig 5e], and reward retrieval latencies [F(4, 40) = 6.05, P < 0.01, Fig 5f]. There was a trend for drug treatment to affect magazine entries [F(4, 40) = 2.67, P = 0.07, Fig 5d]. Withdrawal from chronic MK-801 increased omissions, correct response latencies and, reward retrieval latencies while it decreased magazine entries (P's < 0.01). Clozapine significantly attenuated the increase in reward retrieval latencies (0.08 mg/kg, P < 0.05). Like haloperidol, clozapine administration resulted in the emergence of accuracy deficits (0.08-0.32 mg/kg, P's < 0.05-0.01).
Figure 5.

Ability of clozapine pretreatment to attenuate performance deficits caused by exposure to a regimen of chronic MK-801 administration. MK-801 (0.5 mg/kg, IP) was administered 30 min after daily training sessions; testing occurred 23.5 hr later. On days 6, 8, 10 and 12 of MK-801 administration, clozapine (0.08-0.63 mg/kg, SC) was administered 60 min prior to testing. Exposure to MK-801 administration alone (grey bar) did not affect accuracy (a), but it impaired accuracy in combination with clozapine (0.08-0.32 mg/kg). Exposure to MK-801 administration increased omissions (b) and correct response latencies (e), but decreased magazine entries (d). These effects were not altered by clozapine (0.08-0.32 mg/kg). Exposure to MK-801 did not affect premature responses (c), but it increased reward retrieval latencies, an effect attenuated by clozapine (0.08 mg/kg). Significantly different from baseline, *P < 0.05, **P < 0.01, Fisher's protected t-tests.
Discussion
Here we show that neither acute haloperidol nor acute clozapine are substantially pro-cognitive in the 5CSRTT. The pro-cognitive efficacy of each drug was evaluated under three conditions: alone, in combination with acute MK-801 administration, or in combination with withdrawal from chronic MK-801 administration. At high doses, both haloperidol and clozapine disrupted attention when administered alone. Haloperidol pretreatment reduced some of the disruptive effects of acute MK-801—primarily those associated with increased activation—whereas it exacerbated others. Clozapine pretreatment attenuated several acute MK-801-induced performance deficits (e.g., decreased accuracy and increased omission errors). Both haloperidol and clozapine had minimal effects on performance deficits associated with chronic MK-801 withdrawal. At least on the surface, these observations are consistent with the hypothesis that atypical antipsychotic medications have more beneficial effects on cognition than classical antipsychotic medications (Keefe et al., 1999). The modest effects of clozapine, however, underscore the need to develop more effective treatments of the attentional deficits in schizophrenia.
Effects of Acute MK-801 and Chronic MK-801 Withdrawal on the 5CSRTT
Acute MK-801 and chronic MK-801 withdrawal produced distinct behavioral profiles in the 5CSRTT. Acute MK-801 administration decreased accuracy and increased omissions, premature responses, and magazine entries—a behavioral profile that likely reflects impaired attention and inhibitory control. In contrast, chronic MK-801 withdrawal decreased response rates (i.e., increased omissions and decreased premature responding), reduced food-seeking behavior (magazine entries) and slowed response and reward retrieval latencies. This pattern of behavior may reflect reduced motivation. Although accuracy was impaired during chronic MK-801 withdrawal, this effect was not apparent until after rats started receiving intermittent antipsychotic administration, suggesting that the decrease in accuracy was not a result of withdrawal from chronic MK-801 administration per se but due to carry over effects of the antipsychotic treatment. Longer periods of MK-801 exposure without concurrent antipsychotic administration do not reduce accuracy (T. A. Paine and W. A. Carlezon, unpublished observations).
The failure to observe a ‘pure’ attentional deficit during chronic MK-801 withdrawal is surprising given that early withdrawal from chronic NDMA receptor antagonism causes a number of physiological changes that mimic the pathophysiology of schizophrenia (Lewis et al., 2006). For example, the 67-kDa isoform of glutamatic acid decarboxylase and parvalbumin are reduced after chronic exposure to NMDA receptor antagonists (Pratt et al., 2008; Behrens et al., 2007). Chronic NMDA receptor antagonist regimens also reduce dopamine utilization (Jentsch et al., 1997) and extracellular glutamate concentration within the prefrontal cortex (PFC) (Tsukada et al., 2005). Importantly, withdrawal from chronic NMDA receptor antagonism also produces cognitive deficits in rodent models including deficits in conditional discrimination (Dunn et al., 2006), spatial learning (Madillo et al., 2003; O'Donnell et al., 2003), working memory (Jentsch et al., 1997; Tsukada et al., 2005), short-term memory (Rujescu et al., 2006; Shroeder et al., 2001), and novel object recognition (Hashimoto et al., 2005).
Attentional deficits following chronic drug administration regimens, however, have not been observed. Although acute NMDA receptor antagonism causes schizophrenia-like attentional deficits in humans (Lahti et al., 2001, Coyle et al., 2004; Jentsch et al., 1999), to the best of our knowledge persistent attentional deficits have not been described, even in chronic phencyclidine users (Cosgrove and Newell, 1991). In rodents, neither early withdrawal from chronic ketamine (Nelson et al., 2002) nor chronic amphetamine (Martinez et al., 2008) affects attention in a signal detection task. Similarly, early withdrawal from PCP does not affect attention in the 5CSRTT (Amitai et al., 2007). Despite the failure to observe an attentional deficit here, we tested the ability of acute antipsychotic medications to remediate the behavioral deficits caused by early withdrawal from chronic MK-801 because these deficits recapitulate aspects of the negative symptomology of schizophrenia, namely disruptions in motivation and decision making.
Effects of Antipsychotic Medications
When administered alone, neither haloperidol nor clozapine had pro-cognitive effects in the 5CSRTT. Instead, high doses of both haloperidol and clozapine disrupted all aspects of 5CSRTT performance. Similar detrimental effects of clozapine on attention have been reported previously (Martinez et al., 2008; Amitai et al., 2007; Rezvani et al., 2008).
Acute haloperidol administration did not attenuate attentional deficits caused by acute MK-801 administration. High doses of haloperidol potentiated MK-801-induced increases in omissions and reward retrieval latencies but attenuated MK-801-induced increases in premature responses and magazine entries. These doses of haloperidol increased omissions and decreased premature responses and magazine entries on their own, complicating interpretation of our findings. These haloperidol effects may reflect a generalized motivational deficit or locomotor activity impairment that, coincidently, cancels certain aspects of MK-801-induced hyperstimulation. Haloperidol reduces the impact of many types of rewards, including lateral hypothalamic brain stimulation (Benaliouad et al., 2007; Mobini et al., 2000) while producing profound motor impairments in laboratory animals and humans (O'Neill et al., 2005; Correll et al., 2004). Conceivably, the ability of haloperidol to treat some symptoms of schizophrenia may occur by normalizing states of hyperstimulation that interfere with behavior. Neither high nor low doses of haloperidol attenuated the accuracy deficit caused by acute MK-801 administration, indicating that haloperidol did not have pro-cognitive effects in the 5CSRTT under these testing conditions.
Clozapine administration had dose-dependent effects on the behavior elicited by acute MK-801 administration. Low doses of clozapine attenuated the decrease in accuracy and the increase in both omissions and magazine entries caused by acute MK-801 administration. High doses of clozapine attenuated the increase in premature responding but did not affect other performance measures. Clozapine acts at multiple receptor targets (Nasrallah, 2008); dose-dependent improvements following clozapine administration may therefore reflect dose-dependent efficacies at different receptors. As one example, clozapine actions at 5HT2A receptors might account for its dose-dependent effects: the 5-HT2A receptor antagonist M100907 improves impairments in impulse control (Higgins et al., 2003; Carli et al., 2006) and attention (Carli et al., 2006) caused by acute NMDA receptor antagonism. Consistent with our results, acute clozapine attenuates attentional and impulse control deficits caused by intra-PFC 3-(R)-2-carboxypiperazin-4-propyl-1-phosphonic acid (CPP) (Baveria et al., 2008) and attentional deficits caused by acute amphetamine in amphetamine-sensitized rats (Martinez et al., 2008). In contrast, neither acute nor chronic clozapine administration reversed behavioral deficits in the 5CSRTT caused by acute PCP administration (Amitai et al., 2007). These discrepant findings may indicate that any pro-cognitive effects of clozapine are evident only under very specific testing conditions.
Neither haloperidol nor clozapine dramatically attenuated behavioral deficits observed during chronic MK-801 withdrawal. In fact, both drugs caused the emergence of accuracy deficits and haloperidol potentiated the increase in correct response latencies. Clozapine did have one therapeutic-like effect—attenuating chronic MK-801 withdrawal-induced increase in reward retrieval latencies—raising the possibility that it might reduce amotivation associated with MK-801 withdrawal. The poor efficacy of haloperidol to ameliorate deficits caused by withdrawal from NMDA receptor antagonists has been observed using conditional discrimination (Dunn et al., 2006) and novel object recognition tasks (Hashimoto et al., 2005). Clozapine has inconsistent effects on behavioral deficits observed during withdrawal from chronic NMDA receptor antagonism: acute clozapine administration does not attenuate deficits in novel object recognition (Hashimoto et al., 2005), but attenuates deficits in conditional discrimination (Dunn et al., 2006) observed during chronic PCP withdrawal. Acute clozapine administration consistently attenuates deficits caused by chronic PCP exposure following a “washout” period (Abdul-Monim et al., 2006; Grayson et al., 2007; Jentsch et al., 1997b; Jentsch et al., 1999). In these previous studies, high doses (e.g., > 5.0 mg/kg) of clozapine are required to observe a therapeutic effect. Similar doses of clozapine were not tested here because they potentiated performance deficits in preliminary experiments and because only our low dose of clozapine (0.16 mg/kg) attenuated the effects of acute MK-801 administration.
It is unclear whether acute antipsychotic treatment is sufficient to remediate cognitive deficits in schizophrenics. In rats, subchronic but not acute clozapine attenuates deficits in novel-object recognition following chronic PCP treatment (Hashimoto et al., 2005). Chronic clozapine administration also blocks the development of attentional deficits caused by repeated PCP administration in the 5CSRTT (Amitai et al., 2007). In that previous experiment, clozapine was administered for eight days prior to the chronic PCP administration regimen and attention was measured during acute PCP intoxication. Future experiments are needed to determine whether chronic administration of clozapine would ameliorate deficits observed during chronic MK-801 withdrawal.
The ability of haloperidol and clozapine to treat cognitive symptoms of schizophrenia is controversial. While there are some reports that clozapine has more beneficial effects on cognition than haloperidol (Keefe et al., 1999), the findings are not consistent (Keefe et al., 2007; Mishara et al., 2004). Further, there is speculation that cognitive improvements following antipsychotic treatment may reflect ‘practice effects’ rather than clinically relevant symptom improvements (Goldberg et al., 2007). Our data indicate that, by the strictest definition, both haloperidol and clozapine can improve performance in the 5CSRTT under some testing conditions. The effects of haloperidol likely reflect sedation rather than improved cognition, and the effects of clozapine are modest at best. Since severe attentional deficits in schizophrenia are often associated with a poor prognosis (Green et al., 2004), our studies underscore the need to develop improved treatments.
Acknowledgments
Supported by the National Institute of Mental Health grant MH063266 (WC) and a National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (TAP). We thank Jessica Pohlman for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Monim Z, Reynolds GP, Neill JC. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behavioural Brain Research. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology (Berl) 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Baviera M, Invernizzi RW, Carli M. Haloperidol and clozapine have dissociable effects in a model of attentional performance deficits induced by blockade of NMDA receptors in the mPFC. Psychopharmacology (Berl) 2008;196:269–280. doi: 10.1007/s00213-007-0959-9. [DOI] [PubMed] [Google Scholar]
- Benaliouad F, Kapur S, Rompré PP. Blockade of 5-HT2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology. 2007;32:551–561. doi: 10.1038/sj.npp.1301136. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Faraone SV. Sustained attention deficits as markers of genetic susceptibility to schizophrenia. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2000;97:52–57. doi: 10.1002/(sici)1096-8628(200021)97:1<52::aid-ajmg7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM. Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. American Journal of Psychiatry. 2004;161:414–425. doi: 10.1176/appi.ajp.161.3.414. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Malhotra AK. Impaired attention as an endophenotype for molecular genetic studies of schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2001;105:11–15. [PubMed] [Google Scholar]
- Cosgrove J, Newell TG. Recovery in neuropsychological functions during reductions in use of phencyclidine. Journal of Clinical Psychology. 1991;47:159–169. doi: 10.1002/1097-4679(199101)47:1<159::aid-jclp2270470125>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. International Review of Neurobiology. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- Dunn MJ, Killcross S. Clozapine but not haloperidol treatment reverses sub-chronic phencyclidine-induced disruption of conditional discrimination performance. Behavioural Brain Research. 2006;175:271–277. doi: 10.1016/j.bbr.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz R, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Archives in General Psychiatry. 2007;64:1115–1122. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Grayson B, Idris NF, Neill JC. Atypical antipsychotics attenuate a sub-chronic PCP-induced cognitive deficit in the novel object recognition task in the rat. Behavioural Brain Research. 2007;184:31–38. doi: 10.1016/j.bbr.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophrenia Research. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hashimoto A, Yoshikawa M, Andoh H, Yano H, Matsumoto H, Kawaguchi M, Oka T, Kobayashi H. Effects of MK-801 on the expression of serine racemase and d-amino acid oxidase mRNAs and on the D-serine levels in rat brain. European Journal of Pharmacology. 2007;555:17–22. doi: 10.1016/j.ejphar.2006.09.062. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fujita Y, Shimizu E, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of clozapine, but not haloperidol. European Journal of Pharmacology. 2005;519:114–117. doi: 10.1016/j.ejphar.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and impulsive-type behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berl) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Roth RH. The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1999;20:201–225. doi: 10.1016/S0893-133X(98)00060-8. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Tran A, Le D, Youngren KD, Roth RH. Subchronic phencyclidine administration reduces mesoprefrontal dopamine utilization and impairs prefrontal cortical-dependent cognition in the rat. Neuropsychopharmacology. 1997;17:92–99. doi: 10.1016/S0893-133X(97)00034-1. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997b;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup S, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DA, Davis CE, Hsaio JK, Lieberman JA. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Archives of General Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bulletin. 1999;25:201–22. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Kondziella D, Brenner E, Eyjolfsson EM, Markinhuhta KR, Carlsson ML, Sonnewald U. Glial-neuronal interactions are impaired in the schizophrenia model of repeated MK801 exposure. Neuropsychopharmacology. 2006;31:1880–1887. doi: 10.1038/sj.npp.1300993. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Michaelidis T, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nature Medicine. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- Madillo S, Rinaldi A, Oliverio A, Mele A. Repeated administration of phencyclidine, amphetamine and MK-801 selectively impairs spatial learning in mice: a possible model of psychotomimetic drug-induced cognitive deficits. Behavioural Pharmacology. 2003;14:533–544. doi: 10.1097/00008877-200311000-00006. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Detection of the moderately beneficial cognitive effects of low-dose treatment with haloperidol or clozapine in an animal model of the attentional impairments in schizophrenia. Neuropsychopharmacology. 2008;33:2635–2647. doi: 10.1038/sj.npp.1301661. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biological Psychiatry. 2004;55:1012–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl) 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA. Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Molecular Psychiatry. 2008;13:27–35. doi: 10.1038/sj.mp.4002066. [DOI] [PubMed] [Google Scholar]
- National Academy Press. Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington D.C., USA: 1996. [Google Scholar]
- Nelson CL, Burk JA, Bruno JP, Sarter M. Effects of acute and repeated systemic administration of ketamine on prefrontal acetylcholine release and sustained attention performance in rats. Psychopharmacology (Berl) 2002;161:168–179. doi: 10.1007/s00213-002-1004-7. [DOI] [PubMed] [Google Scholar]
- O'Donnell J, Stemmelin J, Nitta A, Brouillette J, Quiron R. Gene expression profiling following chronic NMDA receptor blockade-induced learning deficits in rats. Synapse. 2003;50:171–180. doi: 10.1002/syn.10258. [DOI] [PubMed] [Google Scholar]
- O'Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology (Berl) 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- Paine TA, Tomasiewicz HC, Zhang K, Carlezon WA., Jr Sensitivity of the 5-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biological Psychiatry. 2007;62:687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Pradhan SH. Phencyclidine (PCP): Some human studies. Neuroscience and Biobehavioral Reviews. 1984;8:493–501. doi: 10.1016/0149-7634(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Winchester C, Egerton A, Cochran SM, Morris BJ. Modelling prefrontal cortex deficits in schizophrenia: implications for treatment. British Journal of Pharmacology. 2008;153:S465–S470. doi: 10.1038/bjp.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Tizabi Y, Getachew B, Hauser SR, Caldwell DP, Hunter C, Levin ED. Chronic nicotine and dizocilpine effects on nicotinic and NMDA glutamatergic receptor regulation: interactions with clozapine actions and attentional performance in rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2008;32:1030–1040. doi: 10.1016/j.pnpbp.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Möller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biological Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Shroeder U, Shroeder H, Schwegler H, Sabel BA. Neuroleptics ameliorate phencyclidine-induced impairments of short-term memory. British Journal of Pharmacology. 2000;130:33–40. doi: 10.1038/sj.bjp.0703171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino EF. Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in the prefrontal cortex of conscious monkeys. Neuropsychopharmacology. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]


