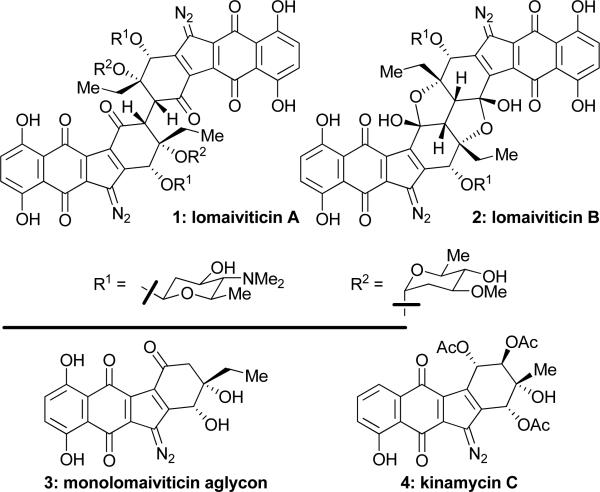
Reported in 2001, lomaiviticins A (1) and B (2, Figure 1) were isolated from Micromonospora lomaivitiensis and demonstrated striking molecular architectures and impressive antitumor and antibiotic activities against a variety of cancer cell lines and bacteria,[1] apparently exerting their action through a novel mechanism involving DNA cleavage.[2] Their chemical synthesis presents a formidable challenge, and reports describing partial solutions have already appeared.[3] In this communication, we describe the total synthesis of the monomeric aglycon unit (3, Figure 1) of the lomaiviticins, which is reminiscent of the kinamycins (i.e. kinamycin C, 4, Figure 1).
Figure 1.
Structures of lomaiviticins A (1) and B (2), their monomeric unit (3), and kinamycin C (4).
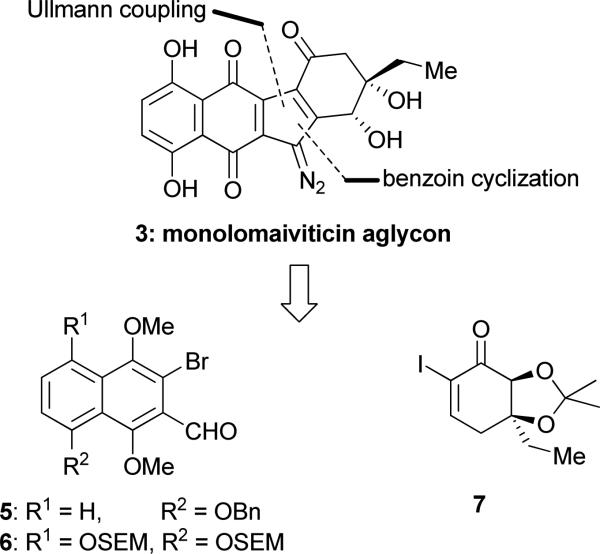
The dimeric structure of the lomaiviticins renders itself to a symmetrical retrosynthetic dissection through the center of the molecule, revealing monomeric unit 3 (see Figure 2) as a possible precursor to both 1 and 2, a scenario that might not be so dissimilar to their biosynthetic pathway. Our approach to enantiopure pre-lomaiviticin structure 3 followed our general strategy towards the kinamycins[4] (defining bromo-aldehyde 5 and iodo-enone 7 as the possible building blocks as shown in Figure 2), and involved an Ullmann coupling reaction and a benzoin-type cyclization as the main processes to construct its molecular framework. However, it required special design features (e.g. substrate 6, vide infra, in order to achieve high regiocontrol) and the development of a novel samarium-mediated allylic hydroxyl group transposition (vide infra) that allowed significant shortening of the synthetic route.
Figure 2.
Retrosynthetic analysis of lomaiviticin aglycon monomer 3.
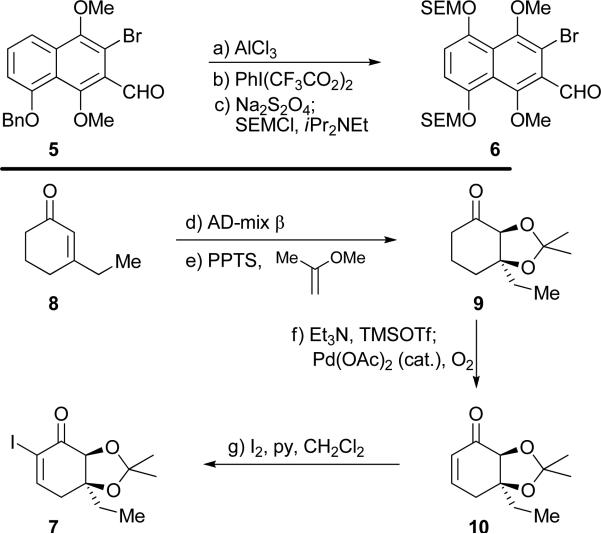
The required building blocks 6 and 7 were synthesized from starting materials 5 and 8, respectively, as summarized in Scheme 1. Thus, readily available bromo-aldehyde 5[4] was debenzylated (AlCl3, 80 % yield)[5] and selectively oxidized with PhI(CF3CO2)2[6] to the expected p-quinone (97 % yield), which was reduced with Na2S2O4 to the corresponding dihydroquinone and protected with SEM groups (SEMCl, iPr2NEt, 92 % yield for two steps) to afford bromo-aldehyde 6. On the other hand, and as shown in Scheme 1, enantioselective asymmetric dihydroxylation of enone 8[7] (AD-mix β, single recrystallization, 69 % yield, >95 % ee)[8] afforded the corresponding 1,2-diol, whose protection (2-methoxypropene, PPTS) furnished acetonide 9 in 94 % yield. Conversion of the latter to its TMS-enol ether (TMSOTf, Et3N), followed by exposure to O2 in the presence of catalytic amounts of Pd(OAc)2,[9] led to enone 10 (83 % yield), whose iodination (I2, py) afforded iodo-enone 7 in 91 % yield.
Scheme 1.
Construction of key buliding blocks bromo-aldehyde 6 and iodo-enone 7. Reagents and conditions: a) AlCl3 (1.2 equiv), CH2Cl2, 25 °C, 3 h, 80 %; b) PhI(CF3CO2)2 (3.0 equiv), MeCN, H2O, 25 °C, 30 min, 97 %; c) Na2S2O4 (5.0 equiv), EtOAc, H2O, 25 °C, 10 min; SEMCl (4.0 equiv), iPr2NEt (6.0 equiv), DMF, 25 °C, 18 h, 92 %; d) AD-mix-β (1.4 equiv), H2NSO2Me (1.0 equiv), NaHCO3 (3.0 equiv), tol., tBuOH, H2O, 0 °C, 36 h, 69 %, >95 % ee; e) 2-methoxypropene (5.0 equiv), PPTS (0.1 equiv), CH2Cl2, 25 °C, 18 h, 94 %; f) TMSOTf (1.35 equiv), Et3N (1.5 equiv), THF, 0 °C, 30 min; Pd(OAc)2 (0.1 equiv), O2 (balloon), DMSO, 25 °C, 18 h, 83 %; g) I2 (3.0 equiv), CH2Cl2, py, 25 °C, 30 min, 91 %. Bn = benzyl, MeCN = acetonitrile, OAc = acetate, SEM = 2-(trimethylsilyl) ethoxymethyl, DMF = dimethylformamide, tol = toluene, tBu = tert-butyl, PPTS = pyridinium, 4-toluenesulfonate, TMS = trimethylsilyl, Tf = trifluoromethanesulfonyl, DMSO = dimethyl sulfoxide, py = pyridine.
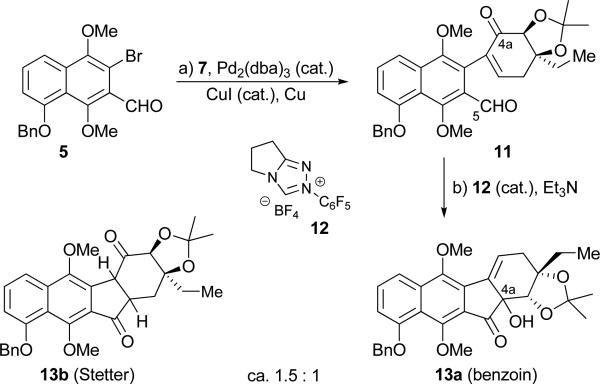
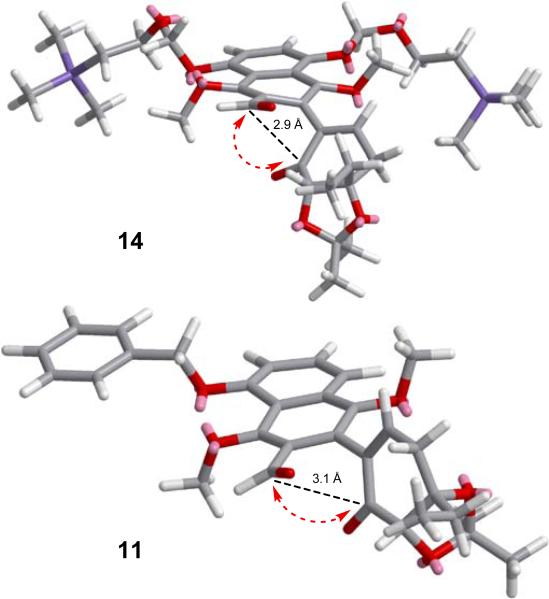
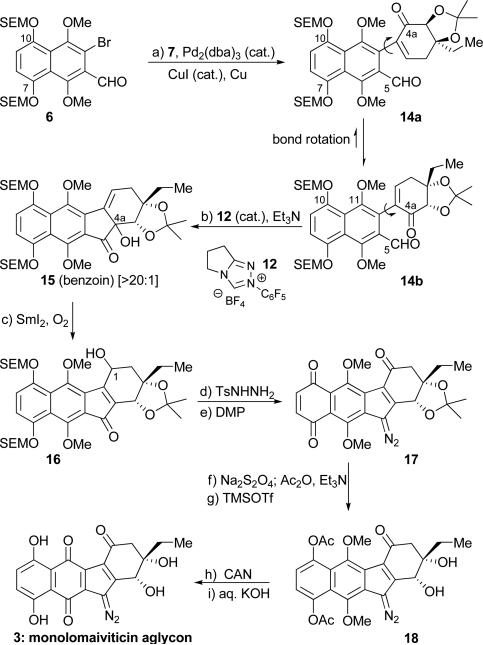
With ample quantities of 5, 6 and 7 available, we proceeded to explore ways to advance them to the target molecule (3). Our initial foray, shown in Scheme 2, involved Ullmann coupling of benzyloxy bromo-aldehyde 5 with iodo-enone 7 [(Pd2(dba)3 (cat.), CuI (cat.), Cu][10] to afford coupling product 11 in 83 % yield, whose intramolecular benzoin-type reaction with Rovis catalyst 12[11] was expected to afford desired tetracyclic structure 13a, as previously observed in the kinamycin case.[4] However, and despite its efficiency (76 % combined yield), the latter reaction [11 plus 12 (cat.), Et3N] gave a disappointing ratio of the benzoin product 13a and its isomer 13b (1,4-addition, Stetter product, 13a:13b ca. 1:1.5).[12] We attributed the formation of the latter compound to the preference of cyclization precursor ketoaldehyde 11 and its latent reaction species to reside in the shown conformation (see also Figure 3, calculated C4a–C5 distance 3.1 Å), thereby favoring the Stetter product 13b. It was to avoid this predicament that we designed coupled product 14a (from 6 and 7, utilizing the same Ullmann coupling conditions, Scheme 3), whose preferred conformation was expected to be that shown in Scheme 3 (14b, see also Figure 3, calculated C4a–C5 distance 2.9 Å) due to the bulky OSEM group exerting its influence from six carbons away.
Scheme 2.
Failure of the original synthetic plan. Reagents and conditions: a) 5 (1.0 equiv), 7 (1.5 equiv), CuI (0.4 equiv), Pd2(dba)3 (0.1 equiv), Cu (10.0 equiv), DMSO, 65 °C, 2.5 h, 83 %; b) 12 (0.2 equiv), Et3N (2.0 equiv), CH2Cl2, 42 °C, 18 h, 76 %, ca. 1:1.5 benzoin:Stetter. dba = dibenzylideneacetone.
Figure 3.
Calculated preferred conformations of 14 and 11 (Chem3D MM2).
Scheme 3.
Completion of the synthesis of monolomaiviticin aglycon (3). Reagents and conditions: a) 6 (1.0 equiv), 7 (1.5 equiv), CuI (0.4 equiv), Pd2(dba)3 (0.1 equiv), Cu (10.0 equiv), DMSO, 65 °C, 3 h, 69 %; b) 12 (0.2 equiv), Et3N (2.0 equiv), CH2Cl2, 42 °C, 18 h, 70 %, 3:1 mixture of diastereomers, >20:1 benzoin:Stetter; c) SmI2 (4.0 equiv), MeOH (10.0 equiv), THF, –78 °C, 5 min; –78→25 °C; O2 (balloon), 25 °C, 18 h, 76 %, 1.5:1 mixture of diastereomers; d) TsNHNH2 (5.0 equiv), aq. HCl (1 M) : iPrOH (1:100), 25 °C, 18 h, 91 %, 1:1 mixture of E:Z isomers of a 1.5:1 mixture of diastereomers; e) DMP (5.0 equiv), CH2Cl2, 25 °C, 1.5 h, 62 %; f) Na2S2O4 (5.0 equiv), EtOAc, H2O, 25 °C, 5 min; Ac2O (10.0 equiv), Et3N (10.0 equiv), DMAP (1.0 equiv), CH2Cl2, 25 °C, 20 min, 91 %; g) TMSOTf (5.0 equiv), CH2Cl2, 25 °C, 20 min, 96 %; h) CAN (3.0 equiv), MeCN, pH 7 phosphate buffer, 25 °C, 20 min, 96 %; i) aq. KOH (1 M), THF, H2O, 25 °C, 30 min, 95 %. Ts = 4-toluenesulfonyl, DMP = Dess–Martin periodinane, Ac2O = acetic anhydride, DMAP = 4-dimethylaminopyridine, CAN = cerium ammonium nitrate.
Thus, it was reasoned that by its mere presence at C10, the OSEM group would force the OMe group at C11 towards the carbonyl moiety at C4a, causing it to rotate with its carrier bicyclic ring system away and into a position to interact with the aldehyde group in the desired benzoin fashion (14b). Indeed, when ketoaldehyde 14a was heated in refluxing CH2Cl2 in the presence of catalyst 12 and Et3N, the OSEM group served its function well, with the desired benzoin product (15) forming in >20:1 selectivity (70 % yield, ca. 3:1 dr at C4a) over its Stetter counterpart. We believe that the beneficial effect of the OSEM group in this cyclization stems primarily from its steric bulk rather than its electron donating nature, since the corresponding C10, C7 bis-methoxy substrate (not shown) exhibits only a ca. 3:1 selectivity in favor of the desired benzoin product. Furthermore, it should be noted that Ullmann coupling substrates equipped at C10, C7 with bulkier groups than OSEM, such as OTES, OTIPS, and OBn, failed to couple with iodo-enone 7 under the same reaction conditions employed for 5 and 6, thus precluding them from serving as viable precursors. These observations underscore the importance of the OSEM group as a unique design feature to ensure the sequential success of both the Ullmann coupling and the benzoin-like cyclization reaction.
With the first hurdle in the synthesis behind us, we were now faced with a second challenge, that of improving our previously devised allylic alcohol transposition to convert 15 to hydroxyfluorenone 16 (Scheme 3). Although our originally employed four-step protocol[4] for this transformation formed the desired product (16) in only 42 % overall yield, it provided an important hint (in the form of trace amounts of the desired product (16) in the samarium-mediated step), namely that the sequence could be replaced with a one-step procedure. Indeed, exposure of hydroxy ketone 15 to the SmI2 conditions[13] resulted in 10 % yield of the desired rearranged alcohol 16. Our initial suspicions of this process occurring through an intramolecular delivery of oxygen were dispelled when a single diastereomer of 15 led to a mixture of epimeric alcohols (16). We soon realized that reaction of 15 with SmI2, followed by bubbling O2 through the reaction mixture generated the desired fluorenone 16 directly, and in 76 % yield (ca. 1.5:1 dr at C1).
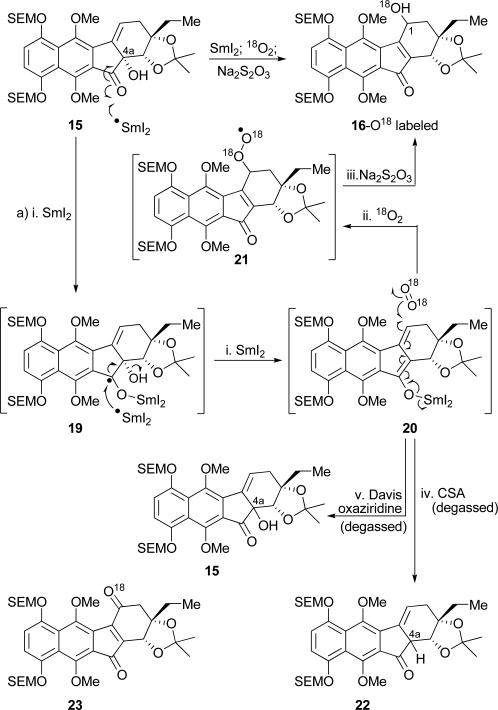
The mechanism of this remarkably regioselective oxygenation at C1 was probed through the use of 18O2, and the results are shown in Scheme 4. Thus, exposure of hydroxy ketone 15 (ca. 3:1 dr) to SmI2–MeOH likely forms extended enolate 20 (through two sequential single electron transfers). This species reacts with 18O2 regioselectively (but not stereoselectively) at C1, the most reactive position of the enolate for radical chemistry, to afford, through the intermediacy of peroxide species 21[14] and upon work-up with aq. Na2S2O3, hydroxy fluorenone 16-O18 in 76% yield, along with ketone 23-O18 (10 % yield). Although labile, the hydroperoxide species derived from aqueous work-up of 21 was detected by 1H NMR spectroscopy and mass spectrometry. Interestingly, quenching the samarium enolate 20 with CSA, or Davis’ oxaziridine, under anaerobic conditions[15] resulted in functionalization at C4a (as is typically the case with extended enolates), rather than C1, furnishing products 22 (72 % yield, ca. 1.5:1 dr)[16] and 15 (85 % yield, ca. 1.5:1 dr), respectively, as shown in Scheme 4.
Scheme 4.
18O2 Studies elucidating the samarium-induced 1,3-allylic alcohol transposition reaction (15→16). Reagents and conditions: a) i. SmI2 (4.0 equiv), MeOH (10.0 equiv), THF, –78 °C, 5 min; – 78→25 °C; ii. 18O2 (balloon), 25 °C, 18 h; iii. aq. Na2S2O3, 25 °C, 30 min, 76 %, 1.5:1 mixture of diastereomers; iv. CSA, (5.0 equiv), – 78→25 °C, 10 min, 72 % 1.5:1 mixture of diastereomers; v. Davis oxaziridine (2.0 equiv), THF, 0 °C, 2 h, 85 %, 1.5:1 mixture of diastereomers.
The final steps of the synthesis of 3 (Scheme 3) involved initial hydrazone formation within fluorenenone 16 (TsNHNH2, aq. HCl)[17] followed by an impressive performance by DMP, which concurrently oxidized the hydrazone, the secondary alcohol, and the bis-SEM aromatic system to afford diazo quinone 17 in 56 % overall yield. The quinone structural motif within the latter needed to be transposed to its proper position, an end towards which 17 was sequentially exposed to Na2S2O4 and Ac2O–Et3N (91 % yield of acetonide bis-acetate) to afford, upon treatment with TMSOTf, dihydroxy bis-acetate 18 in 96 % yield. Finally, oxidation of 18 with CAN, followed by deacetylation (aq. KOH) led to the coveted lomaiviticin aglycon monomer (–)-3 in 91 % overall yield.
The application of the developed samarium-mediated 1,3-allylic alcohol transposition technology to our kinamycin synthesis[4] reduced the previously employed sequence (involving Ac2O, Et3N, DMAP; SmI2, MeOH; Et3N; SeO2) to a single operation, and sig nificantly increased the overall yield (from 55 % over four steps to 83 % over one step, see Scheme 5).[4]
Scheme 5.
Streamlining the kinamycin samarium-mediated allylic alcohol transposition. Reagents and conditions: a) SmI2 (4.0 equiv), MeOH (10.0 equiv), THF, –78 °C, 5 min; –78→25 °C; O2 (balloon), 25 °C, 18 h, 83 %, single diastereomer.
The described chemistry provides a rapid entry into the monomeric unit of the lomaiviticins that should facilitate further synthetic, biosynthetic, and biological studies within this important area of investigation. It also demonstrates the power of remote group interactions and cascade reactions[18] in achieving control and efficiency in chemical synthesis.
Supplementary Material
Footnotes
We thank Dr. D. H. Huang and Dr. L. Pasterneck for NMR spectroscopic assistance and Dr. G. Siuzdak for mass spectrometric assistance. Financial support for this work was provided by grants from the National Institutes of Health (U.S.A.) and the Skaggs Institute for Chemical Biology, and predoctoral fellowships from the American Chemical Society and Bristol-Myers Squibb (both to A.L.N.).
Supporting information for this article is available on the WWW under http://www.angewandte.org.
References
- 1.Isolation: He H, Ding W-D, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT. J. Am. Chem. Soc. 2001;123:5362. doi: 10.1021/ja010129o.
- 2.Arya DP. Top. Heterocycl. Chem. 2006;2:129. [Google Scholar]
- 3.a Nicolaou KC, Denton RM, Lenzen A, Edmonds DJ, Li A, Milburn RR, Harrison ST. Angew. Chem. 2006;118:2130. doi: 10.1002/anie.200504466. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2006;45:2076. [Google Scholar]; b Krygowski ES, Murphy-Benenato K, Shair MD. Angew. Chem. 2008;120:1704. doi: 10.1002/anie.200704830. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2008;47:1680. [Google Scholar]
- 4.Nicolaou KC, Li H, Nold AL, Pappo D, Lenzen A. J. Am. Chem. Soc. 2007;129:10356. doi: 10.1021/ja074297d. [DOI] [PubMed] [Google Scholar]
- 5.Laatsch H. Liebigs Ann. Chem. 1985;102:1847. [Google Scholar]
- 6.Moriarty RM, Prakash O. Org. React. 2001;57:327. [Google Scholar]
- 7.Barnier J-P, Morisson V, Volle I, Blanco L. Tetrahedron: Asymmetry. 1999;10:1107. [Google Scholar]
- 8.Walsh PJ, Sharpless KB. Synlett. 1993:605. [Google Scholar]
- 9.Suzuki H, Yamazaki N, Kibayashi C. J. Org. Chem. 2001;66:1494. doi: 10.1021/jo001413l. [DOI] [PubMed] [Google Scholar]
- 10.Banwell MG, Kelly BD, Kokas OJ, Lupton DW. Org. Lett. 2003;5:2497. doi: 10.1021/ol034745w.. The addition of catalytic amounts of CuI, in our case, markedly improved the yield of the Ullmann coupling.
- 11.Kerr MS, de Alaniz JR, Rovis T. J. Org. Chem. 2005;70:5725. doi: 10.1021/jo050645n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For a review, see: Stetter H. Angew. Chem. 1976;88:695.;Angew. Chem. Int. Ed. Engl. 1976;15:639.
- 13.For reviews, see: Molander GA, Harris CR. Tetrahedron. 1998;54:3321.;Kagan HB. Tetrahedron. 2003;59:10351.;Edmonds DJ, Johnston D, Procter DJ. Chem. Rev. 2004;104:3371. doi: 10.1021/cr030017a.;Gopalaiah K, Kagan HB. New J. Chem. 2008;32:607.
- 14.For previously postulated peroxo-samarium species, see: Corey EJ, Wang Z. Tetrahedron Lett. 1994;35:539.;Fielder S, Rowan DD, Sherburn MS. Synlett. 1996:349.
- 15.Davis FA, Chen BC. Chem. Rev. 1992;92:919. [Google Scholar]
- 16.β,γ-Unsaturated ketone 22 proved rather labile and, therefore, was immediately converted (by exposure to Et3N) to its conjugated counterpart (22a, not shown) and characterized as such (see Supplementary Information).
- 17.Kumamoto T, Kitani Y, Tsuchiya H, Yamaguchi K, Seki H, Ishikawa T. Tetrahedron. 2007;63:5189. [Google Scholar]
- 18.For reviews, see: Nicolaou KC, Montagnon T, Snyder SA. Chem. Commun. 2003:551. doi: 10.1039/b209440c.;Tietze LF, Brasche G, Gericke K. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. p. 672.;Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. 2006;118:7292. doi: 10.1002/anie.200601872.;Angew. Chem. Int. Ed. 2006;45:7134.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.