Abstract
Physicochemical features of a cell’s microenvironment can exert important effects on cell behavior and include the effects of matrix elasticity on cell differentiation processes, but molecular mechanisms are largely mysterious. Here we highlight recent reports of a mechanical dependence to growth factor activation, with a particular focus on release of TGFβ (Transforming Growth Factor β) from its large latent complex via forced unfolding. We discuss these processes and pathways in the contexts of matrix adhesion and fluid shearing as they might relate to stem cell differentiation and other mechanisms in development, disease, and repair.
Introduction
Fate choices of cells, including stem cells, are influenced by both soluble and insoluble factors, but many key “soluble” factors bind to matrix-associating “insoluble” complexes and at least some are regulated in their release and activation from the surrounding microenvironment. Transforming growth factor-β, TGFβ, provides the clearest recent example of regulated activation from its latent stores. Although heat is a standard bench method to release TGFβ from its latent complex, body temperature varies little from 37°C - even with extreme fever - and so heat is not a physiological mechanism for TGFβ release. Mechanical stress, on the other hand, is central to everyday life and now appears implicated in growth factor release through coupling to both cell-exerted contractile tensions in ‘stiffened’ tissues and fluid shear stresses. As reviewed here, the groundwork is being laid for understanding the intricate interplay between adherent stem cells that pull on extracellular matrix (ECM) and the regulation of growth factor by microenvironment. Throughout, the reader is encouraged to keep in mind additional cell mechanical processes such as ‘durotaxis’ (Fig.1a) in which a stiff matrix typified by a fibrotic scar tends to act like a ‘mechanical magnet’ for the firm anchorage and accumulation of cells within an otherwise soft and normal tissue. Broader implications for processes in development, disease, and regeneration will be viewed here with this perspective.
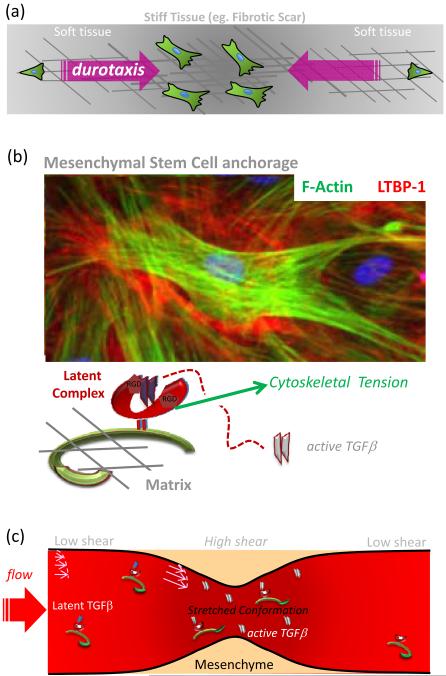
Figure 1.
(a) Durotaxis is a process in which a stiffer tissue including but not limited to a fibrotic scar tends to act like ‘mechanical magnets’ for cells. The cells are more motile in soft, normal tissue and tend to anchor in stiffened tissue where they accumulate.
(b) Latent TGFβ- Binding Protein (LTBP1) immunofluorescence in a dense culture of mesenchymal stem cells (MSC) grown on stiff gels. In such systems, current data suggests that TGFβ is released from Latent Complex via a tension-mediated mechanism. This has implications for all types of mesenchymal cells in fibrotic microenvironments. LTBP1 antibody is a gift from C. Heldin (The Ludwig Institute for Cancer Research, Uppsala, Sweden).
(c) Fluid shear forces in blood vessels can also activate Latent TGFβ in plasma, and conditions that lead to vessel narrowing and increase in the shear force result in dramatically higher amounts of activated growth factor. In this case, accumulation of tension at the Large Latent Complex is due to fluid stretching of the chain as opposed to cellular contractility against a resisting substrate.
Among the various types of stem cells, Mesenchymal Stem Cells (MSC) are notable for being isolated from sources such as bone marrow and selected for based on their adhesiveness [1]. MSC also possess the cytoskeletal machinery, motility, matrix adhesiveness, and responsiveness to TGFβ family growth factors that typify mesenchyme [2], [3] so that these cells can be considered representative. These cells also differentiate at least to a degree into various hard and soft tissue lineages that include osteoblasts, chondrocytes, skeletal myocytes, smooth muscle cells, adipocytes, and reportedly even non-mesenchymal lineages such as neurons [4]. Added to their intriguing if controversial plasticity [5] is the ability of MSCs to maintain potency even after prolonged culture on tissue culture plastic, including an impressive in vitro expansion capacity which is perhaps 500-fold or more [6]. In terms of therapy, MSC appear extremely attractive for autologous transplantation and for enhanced engraftment capabilities [7]. Despite some potential risks, initial tests suggest some usefulness in correction of connective tissue abnormalities [8], in stabilization or repair of cardiac infarcts [9], and in contributing Smooth Muscle Cells (SMC) to vascular remodeling [10].
Matrix Elasticity, Stem Cell differentiation, and Matrix-bound Growth Factor
Even in simplified in vitro model systems, the molecular and microenvironmental cues necessary to induce differentiation are not easily identified. For example, by mimicking the ‘softness’ or elasticity E of different tissues with inert gel systems coated with collagen-I, initial studies suggest that matrix elasticity can direct lineage specification of MSC [11••]. However, microarray results in the same study further suggested the expression of highly relevant members of the TGFβ superfamily, including Bone Morphogenetic Proteins (BMP) and Myostatin (GDF8). Such potent growth factors are likely to contribute to any apparent lineage-inducing signals, even with small (∼pico-Molar) relative expression/activation differences imparted by stiffness modulation.
Like other members of the superfamily, TGFβ is a potent, pleiotropic growth factor, and it is expressed as an inactive precursor in complex with its Latency Associate Peptide, LAP, from which it must dissociate in order to bind to its receptor. TGFβ family proteins act to induce development of contractile phenotypes in a variety of mesenchymal cell types (including MSC [3]). More generally, TGFβ stimulates ECM remodeling by acting on ECM protein production, crosslinking, and proteolytic processing. TGFβ is a central cytokine in tissue repair and fibrosis, playing key roles in effecting the inflammatory response as well as in the phenotypic transition of fibroblasts into fibrogenic myofibroblasts [12]. The latent complex comprised of LAP and TGFβ, sometimes called the Small Latent Complex, associates further with the large fibrillar Latent TGFβ Binding Proteins, LTBP which target the complex for secretion [13] and ECM binding [14], [15]•, and from which TGFβ can be released via a variety of mechanisms that differ according to cell type and physiological context [16] [17] [18]. Proteases that cleave LAP, Thrombospondin-1 and certain types of integrins are among the many ways TGFβ can be activated [19]. Mechanisms by which integrins activate TGFβ seem to require LTBP1 [20], [21], which gave rise to the idea that perhaps TGFβ could be activated by cell-generated traction forces [22]. The hypothesis emerged from the specific observation that LTBP1-mediated incorporation of Large Latent Complex (LLC) into matrix was necessary for αvβ6 integrin activation of TGFβ [20]. Two reports [23] ••, [24]•• over the past two years have now provided contextual evidence for tension-driven release of TGFβ.
Forced Unfolding Mediates TGFβ Release: the First Experimental Models
Wipff et al [23]•• were the first to report evidence for tension-mediated TGFß1 release by contractile myofibroblasts, which are the characteristic cell-type in stiff, fibrotic tissue. Based on their findings, tension-activated TGFß1 would serve to perpetuate the myofibroblasts’ synthesis of matrix and α-Smooth Muscle Actin during fibrotic wound healing processes. In particular, their results suggest increased stiffness of mesenchyme, including a fibrotic scar, may be necessary to provide resistance as the cells pull on and accumulate tension in LTBP1 (Fig.1b).
Force-mediated activation of TGFβ was also recently reported by Ahamed et al [24]••, but this time for LTBP1-associated Latent Complex in serum. Under static conditions at 37°C, very little active TGFβ could be detected in serum, whereas controlled fluid shear as well as simple stirring were shown to dramatically activate TGFβ in a manner dependent on LTBP1. This large macromolecular assembly seems to be stretched out under flow like any large polymer [25], and this extension seems to stress the complex sufficiently to catalyze release of growth factor. Indeed, the first reaction-coupled studies of single molecule extension by Atomic Force Microscopy (AFM) had clearly shown that forced unfolding would - in an all or none fashion - gate the reduction of disulfides that are buried within immunoglobulin adhesion receptors [26]. In the case of TGFβ release, increased activation with both shear stress and time is characteristic of forced unfolding, and in this case the dependence on shear appears roughly linear and without a threshold. Lower stresses would therefore take a longer time for any given level of TGFβ activation from the ∼ nM of total serum concentration. Indeed, Wang et al [27] detected an increase in active but not total TGFβ1 in the plasma of patients with coronary artery disease, and demonstrated a positive correlation with the number of stenotic vessels in a patient. For the same flow rate, constricted vessels will always have a higher shear stress and therefore higher TGFβ (Fig.1c). Such effects seem likely to couple into mesenchymal remodeling in development (e.g. heart valves) as well as in disease.
Release of TGFβ due to fluid mechanics is usefully compared to release due to matrix mechanics: fluid shear stress is measured in the same units (Pascal, Pa) as both cell or matrix tension and tissue elasticity E. A human carotid artery has a wall shear stress of about ∼1 Pa [28], and based on data of Ahamed et al, this is sufficient to accumulate ∼100 pM of active TGFβ within a couple of hours of shearing serum. Such concentrations of active TGFβ appear significant compared to Kd’s for TGFβ receptors [29], which highlights the importance to signaling, even if such concentrations constitute a small fraction of the total sequestered TGFβ in serum. In comparison to fluid stresses, the stresses or tensions applied by cells to their surrounding ECM are much higher at perhaps ∼100 Pa in soft matrices (with an elasticity E ∼ 0.1-1 kPa) and perhaps several 1000 Pa [11]•• when cells adhere to stiff matrices (of E ∼ 40 kPa) that are similar in rigidity to fibrotic matrices [30]. Dense cultures of myofibroblasts on stiff matrices appear to pull on the latent complex sufficiently and sustainably to activate about 10-20% of the total TGFβ pool, whereas softer substrates seem to elicit no net stress-activation, at least within the detection limits of the employed methods. The much higher stress scales compared to fluid shear probably reflect the fact that cell-generated stresses do not propagate deeply into matrix [11]•• and therefore activate very locally, whereas fluid stresses in a blood vessel will fill the vessel with activated growth factor as evidenced by the measurements of Wang et al. The collective results nonetheless suggest a common mechanism for highly localized growth factor activation.
Whether tethered to surrounding matrix or not, the force-catalyzed release of active TGFβ invites speculation about consequences for stem cell function in health, disease, and therapeutic interventions. In particular, damaged tissues such as infarcted hearts and stenotic vessels will generally provide a stiff fibrotic context, and stenoses in any context- including hypertension of inflammation and heart tube development - will also lead to increased shear due to the vessel narrowing.
Recapitulating the Microenvironment: Stiffness and ECM Protein Deposition
Based in part on the relevance of elasticity to growth factor release, an elucidation of the responses of mesenchymal cells (e.g. MSC) to microenvironmental cues would seem to require the use of culture substrates and scaffolds that allow for control of stiffness. Cells plated on tissue culture plastic or glass are simply unable to perturb their environment sufficiently with their contractile apparatus; the cells essentially push or pull on a brick wall. Rigidity can either exaggerate or occlude activation of signaling pathways. Considerable progress has nonetheless been made with rigid substrates in identifying mechanosensitive proteins, both in the ECM - such as Fibronectin, [31], [32] - and in the adhesions and cytoskeleton - including Focal Adhesion Kinase (FAK) [33], p130Cas [34], Filamins and Myosins [35], and also Talin [36].
Polyacrylamide gels of tunable stiffness that are coated with purified ECM ligands mimic the overall stiffnesses of tissues, including that contributed by crosslinking within ECM via lysyl oxidases, transglutaminases, and glycation. These stable gels have proven very useful in evaluating elasticity-modulated cellular processes mediated by contractility. Cellular morphology for a wide variety of cell types has been thoroughly characterized as highly responsive to matrix stiffness, and the focal adhesion field has come to many meaningful conclusions through modulation of stiffness in 2D cultures. Culture scaffolds in 3D which mimic both plane-polarized and isotropic tissue architectures might prove of further value in studies of matrix-tension interplay, [37], and decellularized embryos offer another attractive possibility [38], once methods to measure elasticity are better established. Since the matrix-secretory activity of MSC is still poorly understood, 2D gel substrates will continue to prove useful for initial characterization of tension-driven matrix assembly and establishment of growth factor deposition.
When it comes to addressing matrix assembly, Fibronectin (FN) is typically the first protein to consider [39], [40]. Whether MSC drive fibronectin fibril formation upon sensing resistance at a stiff substrate is an open and intriguing question, given that FN assembly precedes that of other ECM proteins and in light of the likelihood that associating proteins might bear latent growth factor complexes of TGFβ [15]•, [41]. Other mesenchymal cells such as fibroblasts engage matrix with actomyosin contractility anchored at integrins, and this leads to FN stretching at the cell surface and adhesion-mediated forced unfolding with exposure of cryptic self-assembly sites that generate long fibrils of matrix [40]. Since this process is inherently dependent on generation of tractional forces as well as on integrin-mediated interactions, manipulation of intracellular tension via soft or stiff substrata might allow for important insight into more detailed connections between mechanotransducers and rates of matrix assembly.
Tying Matrix Assembly to Mechanically Triggered Growth Factor Release: Pivotal Roles for Integrins
Tension-dependent matrix assembly is likely to coordinate growth factor release, both directly and indirectly. As reviewed recently [42]••, there are two models by which integrins can exert a direct TGFβ activating role. They can bind latent TGFβ at LAP concomitantly with proteases, thereby facilitating proteolysis at LAP residues, or they can bridge transmission of force to conformational changes in LLC, disrupting LAP/TGFβ interaction and releasing TGFβ in a protease-independent manner. The specific integrin isotype in each case as well as LLC association to a mechanically resistant ECM are key features of these models, at least within the experimental systems in which they have been examined so far. Most notably, cells that lack the α5β1 integrin were found incapable of activating TGFβ [43] via one of the first integrins identified as a tension-mediated TGFβ activator (αvβ6, [21]). α5β1 integrin is essential for Fibronectin assembly [44], which reiterates, for the case of MSC, that stiffness-dependent differences in TGFβ activation can potentially arise due to earlier differences in the assembly of the ECM-“orchestrator” Fibronectin. If that is true, it would represent one indirect way in which tension, in conjunction with integrins, would modulate TGFβ activation.
Thus, the type of integrin expressed on the cell surface could determine the mechanism of latent TGFβ activation, perhaps due to their roles in both ECM assembly and TGFβ release. Differential expression of integrin types in response to the stiffness of the environment provides yet one more level at which stiffness might affect crucial aspects of cellular processes consequential to TGFβ modulation. Moreover, differential integrin expression might even exert effects in downstream, intracellular signaling of TGFβ by altering the rate or route of TGFβ receptor endocytic recycling [45]. Whether MSC activate TGFβ via integrin binding at LAP is currently unknown, although these cells express many of the isotypes which have been found to bind LAP in over two dozen cell types (reviewed in [42] •), at times in a context-dependent manner [46]. Preliminary data from our lab indicates lower levels of TGFβ activation by MSC plated onto LLC-containing, fibroblast derived matrices upon RGD site blockage, hinting that integrin engagement of LAP by MSC does occur. Additionally, FN and Collagen- receptor integrins were found to have their expression upregulated as MSC are driven towards differentiation [47].
Implications of Mechano-regulated TGFβ for Stem cell Therapies
TGFβ has been identified in global gene expression analyses of MSC as one of three key growth factor pathways not only sufficient for MSC growth but also influential in differentiation into chondrocytes, osteocytes and adipocytes [48], [49]. The TGFβ dependence in [48] was observed at the activin receptor level, highlighting the relevance for understanding the processes upstream of receptor binding by active ligand. Furthermore, proteomic analysis of MSC treated with soluble TGFβ1 revealed notable fold increases in collagen I and related proteins, as well as in smooth muscle actin production, indicative of MSC lineage specification into SMC [3]. This becomes intriguing when contrasted with the well-characterized effect of addition of soluble TGFβ onto MSC pellet cultures or as part of a cocktail containing dexamethasone—both of which lead to chondrogenesis with expression of collagens other than type-I. Such a difference in the response of MSC to the same TGFβ treatment underscores the highly contextual nature of the wide array of responses elicited by this cytokine.
These issues highlight the likely complexities of cell therapy. One can readily envision a scenario in which MSC were to be expanded and induced down a chondrogenic lineage prior to implantation into an injured knee [50]. If the microenvironment encountered at the target site were fibrotic, then the MSC might be induced to express SMA by a local elevation of active TGFβ, and this phenotype would compromise the desired cartilage regeneration. In contrast to this, current clinical trials involving MSC have seen evidence that MSC not only preferentially home to stiffer areas, but generally act to attenuate scar formation, evoking speculation that perhaps MSC ‘handle’ the heightened TGFβ present in such microenvironments differently from local, fibrogenic cells. Hence, understanding MSC responsiveness to softness, stiffness and perhaps distinctly fibrillar adhesive microenvironments is needed if we are to develop ways to both “prepare” the target tissues and to pre-condition these stem cells.
Concluding Remarks
Deeper insight is needed not only into the conditions under which TGFβ is released, but also conditions in which this activation results in a biochemical signal for cell behaviors such as growth, maintenance or differentiation. Growth Factor regulation as affected by local mechanics is likely to find particular relevance in understanding mesenchymal stem cell fates, since mechanical features of tissues are likely to impinge on regenerative outcomes.
Acknowledgements
NIH and NSF support are very gratefully acknowledged, as is the gift of LTBP1 antibody from C. Heldin (The Ludwig Institute for Cancer Research, Uppsala, Sweden).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Park JS, Chu JS, Krakowski A, Luo K, Chen DJ, Li S. Proteomic profiling of bone marrow mesenchymal stem cells upon transforming growth factor beta1 stimulation. J Biol Chem. 2004;279:43725–43734. doi: 10.1074/jbc.M407368200. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 6.Colter DC, Class R, Digirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdallah BM, Kassem M. The use of mesenchymal (skeletal) stem cells for treatment of degenerative diseases: current status and future perspectives. J Cell Physiol. 2009;218:9–12. doi: 10.1002/jcp.21572. [DOI] [PubMed] [Google Scholar]
- 8.Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218:237–245. doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- 9.Spees JL, Whitney MJ, Sullivan DE, Lasky JA, Laboy M, Ylostalo J, Prockop DJ. Bone marrow progenitor cells contribute to repair and remodeling of the lung and heart in a rat model of progressive pulmonary hypertension. FASEB J. 2008;22:1226–1236. doi: 10.1096/fj.07-8076com. [DOI] [PubMed] [Google Scholar]
- 10.Huang NF, Li S. Mesenchymal stem cells for vascular regeneration. Regenerative medicine. 2008;3:877–892. doi: 10.2217/17460751.3.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044.MSC plated on collagen-I coated polyacrylamide hydrogels that mimic the elasticity of brain, muscle, or bone express lineage markers of the respective lineage. The mechanical cue provided by the gels is also shown to synergize with chemical induction of differentiation.
- 12.Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845–850. doi: 10.1152/ajpgi.2000.279.5.G845. [DOI] [PubMed] [Google Scholar]
- 13.Penttinen C, Saharinen J, Weikkolainen K, Hyytiäinen M, Keski-Oja J. Secretion of human latent TGF-beta-binding protein-3 (LTBP-3) is dependent on co-expression of TGF-beta. J Cell Sci. 2002;115:3457–3468. doi: 10.1242/jcs.115.17.3457. [DOI] [PubMed] [Google Scholar]
- 14.Unsöld C, Hyytiäinen M, Bruckner-Tuderman L, Keski-Oja J. Latent TGF-beta binding protein LTBP-1 contains three potential extracellular matrix interacting domains. J Cell Sci. 2001;114:187–197. doi: 10.1242/jcs.114.1.187. [DOI] [PubMed] [Google Scholar]
- 15•.Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010.Fibronectin is once again characterized as “orchestrating” another ECM protein’s secretion-- this time, LTBP4, one of the TGFβ-complexing fibrillin family proteins. Disruption of LTBP4 resulted in increased TGFβ activity, indicating LTBP4 also has a role in the activation process.
- 16.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor beta 1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511:65–68. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 19.Annes J, Vassallo M, Munger JS, Rifkin DB. A genetic screen to identify latent transforming growth factor beta activators. Anal Biochem. 2004;327:45–54. doi: 10.1016/j.ab.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 22.Keski-Oja J, Koli K, von Melchner H. TGF-beta activation by traction? Trends Cell Biol. 2004;14:657–659. doi: 10.1016/j.tcb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23••.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042.This is the first report of purely mechanical release of TGFβ1 from matrix stores bound to matrix by LTBP1 and to the cell surface by integrin interaction with LAP. The authors detect ∼2.5 fold higher active TGFβ on stiff, fibrotic substrates in a proteolysis-independent fashion, and propose an elegant model for how such a mechanism might both work to effect the wound healing response and be a culprit in its perpetuation in fibrosis.
- 24••.Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-beta1. Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753.Dramatic TGFβ activation from Latent stores is reported upon shear, once again implicating LTBP1’s latent complex tethering. Local release of TGFβ at sites of injury would be predicted wherever restricted blood flow increases shear conditions, as occurs in vessel narrowing.
- 25.Smith DE, Babcock HP, Chu S. Single polymer dynamics in steady shear flow. Science. 1999;283:1724–1721. doi: 10.1126/science.283.5408.1724. [DOI] [PubMed] [Google Scholar]
- 26.Carl P, Kwok CH, Manderson G, Speicher DW, Discher DE. Forced unfolding modulated by disulfide bonds in the Ig domains of a cell adhesion molecule. Proc Natl Acad Sci USA. 2001;98:1565–1570. doi: 10.1073/pnas.031409698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang XL, Liu SX, Wilcken DE. Circulating transforming growth factor beta 1 and coronary artery disease. Cardiovasc Res. 1997;34:404–410. doi: 10.1016/s0008-6363(97)00033-3. [DOI] [PubMed] [Google Scholar]
- 28.Stroev P, Hoskins P, Easson W. Distribution of wall shear rate throughout the arterial tree: A case study. Atherosclerosis. 2007;191:276–280. doi: 10.1016/j.atherosclerosis.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987;105:965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–268. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingham KC, Brew SA, Erickson HP. Localization of a cryptic binding site for tenascin on fibronectin. J Biol Chem. 2004;279:28132–28135. doi: 10.1074/jbc.M312785200. [DOI] [PubMed] [Google Scholar]
- 32.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mofrad MR, Golji J, Abdul Rahim NA, Kamm RD. Force-induced unfolding of the focal adhesion targeting domain and the influence of paxillin binding. Mechanics & chemistry of biosystems : MCB. 2004;1:253–265. [PubMed] [Google Scholar]
- 34.Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz M. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127:1015–1026. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson CP, Tang H-Y, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz M. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochimica et Biophysica Acta. 2009 doi: 10.1016/j.bbamcr.2009.01.012. in press. [DOI] [PubMed] [Google Scholar]
- 38.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzbauer JE, Sechler JL. Fibronectin fibrillogenesis: a paradigm for extracellular matrix assembly. Current Opinion in Cell Biology. 1999;11:622–627. doi: 10.1016/s0955-0674(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 40•.Dallas SL, Chen Q, Sivakumar P. Dynamics of assembly and reorganization of extracellular matrix proteins. Curr Top Dev Biol. 2006;75:1–24. doi: 10.1016/S0070-2153(06)75001-3.This review discusses the mechanical component of Fibronectin fibrillogenesis, which ultimately guides assembly of many other ECM components-- including the TGFβ-associating LTBPs. It also highlights how dynamic imaging of ECM proteins can prove valuable in probing their sheer complexity, which goes much beyond their traditional static structural role.
- 41•.Sivakumar P, Czirok A, Rongish BJ, Divakara VP, Wang YP, Dallas SL. New insights into extracellular matrix assembly and reorganization from dynamic imaging of extracellular matrix proteins in living osteoblasts. J Cell Sci. 2006;119:1350–1360. doi: 10.1242/jcs.02830.Dual-fluorescence time-lapse dynamic imaging of Fibronectin and LTBP1 in osteoblast matrix is shown to correlate cell motility to matrix fiber ‘rearrangement’ and assembly mechanisms.
- 42•.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012.The authors review in detail the important and interesting consequences of a crucial feature in the mechanical release of TGFβ: the TGFβ Latent Complex’s cellular connection at integrins.
- 43.Fontana L. Fibronectin is required for integrin v 6-mediated activation of latent TGF- complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 44.Sechler JL, Corbett SA, Schwarzbauer JE. Modulatory roles for integrin activation and the synergy site of fibronectin during matrix assembly. Mol Biol Cell. 1997;8:2563–2573. doi: 10.1091/mbc.8.12.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 46.Goessler UR, Bugert P, Bieback K, Sadick H, Baisch A, Hormann K, Riedel F. In vitro analysis of differential expression of collagens, integrins, and growth factors in cultured human chondrocytes. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2006;134:510–515. doi: 10.1016/j.otohns.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Goessler UR, Bugert P, Bieback K, Stern-Straeter J, Bran G, Hörmann K, Riedel F. Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med. 2008;21:271–279. [PubMed] [Google Scholar]
- 48.Ng F, Boucher S, Koh S, Sastry K, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri M, Rao M, et al. PDGF, TGF-, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 49.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 50.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(Suppl 1):S58–65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]