Abstract
2-Amino-3-methylimidazo[4,5-f]quinoline (IQ) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) are heterocyclic amines (HCA) derived from high temperature cooking of meat and thought to cause colon cancer in humans. Reactive nitrogen oxygen species, which are mediators of the inflammatory response, can convert these amines to the corresponding N-nitrosamines, N-NO-IQ and N-NO-MeIQx. This study was designed to evaluate whether these N-nitrosamines are genotoxic and could be responsible, in part, for the high incidence of colon cancer in individuals with colitis. Such an association would counsel reduced intake of well-done red meat by colitis patients. Mutagenicity was evaluated by reversion of a lacZ frameshift allele in three different E. coli strains. Strains DJ701 and DJ702 express recombinant (S. typhimurium) aromatic amine N-acetyltransferase; DJ702 also expresses recombinant human cytochrome P450 1A2 and NADPH-P450 reductase; and DJ2002 served as an N-acetyltransferase-negative control. In strain DJ701, N-NO-IQ and N-NO-MeIQx elicited dose-dependent mutagenicity, which was not further increased in DJ702. Neither nitrosamine was mutagenic in strain DJ2002. While both N-nitrosamines are stable for >4 hours (pH 7.4, 37°C), they react with DNA or 2′-deoxyguanosine 3′-monophosphate at lower pH (5.5) to form adducts. HOCl, a component of the inflammatory response, increased adduct formation, as measured by 32P-postlabeling. Following treatment with nuclease P1 and separation by two-dimensional thin-layer chromatography and then HPLC, N-NO-IQ and N-NO-MeIQx were shown to form the same adducts as those formed by N-OH-MeIQx or N-OH-IQ, namely N-(deoxyguanosin-8-yl) adducts. In summary, these N-nitrosamines are genotoxic and might be alternatives to their hydroxylamine analogues as activated intermediates leading to initiation of colon cancer in individuals with colitis.
Keywords: Heterocyclic amines; 2-nitrosoamino-3-methylimidazo[4, 5-f]quinoline, 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline; nitrosation; mutagenicity; nucleotide adducts
1. Introduction
Nitrosation is recognized as a chemical process with wide biological significance [1]. Both endogenous [2] and exogenous [3] compounds may be chemically modified by nitrosation. Particular attention has been given to the formation of S-nitrosation products (nitrosothiols or thionitrites), such as nitrosoglutathione and S-nitrosylated proteins [4], but N-nitrosation of amines and nitrosylation of heme also occur [5]. When the latter reaction is elicited by nitric oxide (NO), soluble guanylate cyclase is activated, with increased synthesis of cyclic GMP [6]. Cyclic GMP signaling occurs at low concentrations of NO and is important in regulating blood pressure, along with other diverse physiological functions [7]. NO, at much higher concentrations produced by inflammation, can react with superoxide to form peroxynitrite, or with oxygen to form reactive nitrogen oxygen species [8]. These reactive agents are thought to mediate pathologic pathways related to inflammation. Certain types of cancer, such as colitis-associated colon cancer [9, 10], are associated with a high level of inflammation and NO production. The cited studies suggested that nitrosation by reactive nitrogen oxygen species causes genotoxic lesions observed in patients with colitis and colon cancer.
The best-known role of nitrosation in toxicology and carcinogenesis is the formation of nitrosamines, including tobacco-specific nitrosamines [11], by the interaction of nitrite (or other nitrosating agents) with secondary or tertiary amines [12, 13], particularly under mildly acidic conditions [14, 15]. However, aromatic and heterocyclic primary amine carcinogens may also be susceptible to nitrosation reactions that generate reactive intermediates.
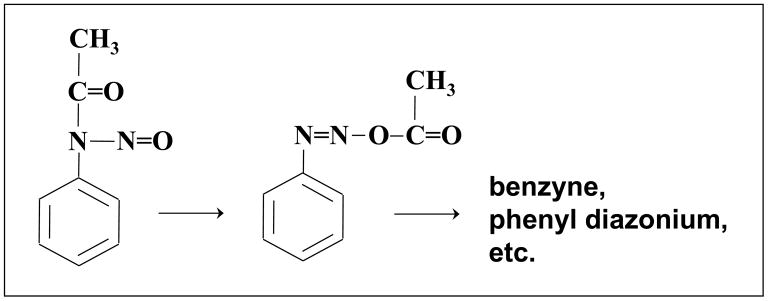
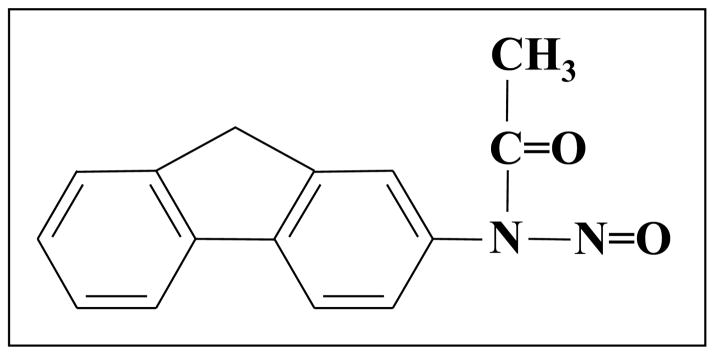
Primary aromatic amines (aniline derivatives) are rapidly diazotized and hydrolyzed by treatment with nitrous acid. Nitrosation of aromatic amides, however, yields isolable nitrosoacyl-arylamines, such as nitrosoacetanilide [16]. N-Nitrosoacetanilide decomposes by migration of the acetyl group (Fig. 1) and gives reactive species, including the diazonium ion and benzyne [17–19]. In the toxicological context, Lin and colleagues examined the nitrosation of the model aromatic amide carcinogen, N-2-fluorenylacetamide (2-acetylaminofluorene) [20–22]. The product, N-nitroso-N-2-fluorenylacetamide (Fig. 2), was isolated and characterized as an electrophilic compound that forms adducts with DNA.
Figure 1.
N-Nitrosoacetanilide: structure and decomposition pathway.
Figure 2.
N-Nitroso-N-2-fluorenylacetamide: structure.
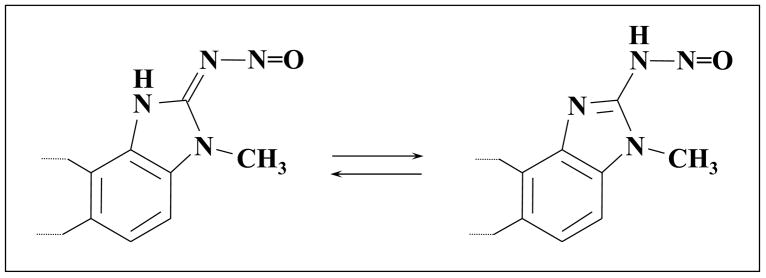
Heterocyclic amine (HCA) nitrosation has been little explored. Simonov and co-workers (Rostov State University) examined the chemistry of N-nitrosobenzimidazoles in a series of publications, beginning in 1974. Kolodyazhnaya et al. noted that stable N-nitroso products could not be obtained from unsubstituted 2-aminoimidazole or 2-amino-benzimidazole, but success was achieved with 2-amino-1-alkyl-benzimidazoles and 2-amino-1-aralkyl-benzimidazoles [23, 24]. The N-nitroso products were found to be “yellow crystalline substances … stable on prolonged storage in the dark at 20°C.” They suggested that the compounds exist primarily as nitrosoimino tautomers (Fig. 3) [25]. 2-Amino-1-methylbenzimidazole, studied by the Rostov group, is structurally equivalent to IQ, except that the terminal aromatic ring is absent (i.e., benzene rather than quinoline).
Figure 3.
Tautomeric equilibrium of N-nitrosobenzimidazoles.
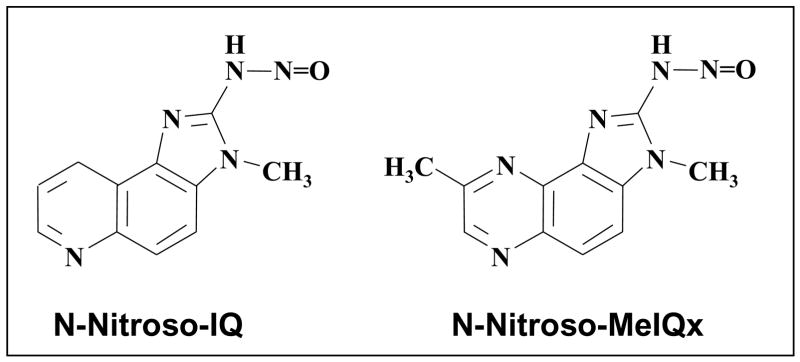
Carcinogenic HCA such as 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), which possess aminoimidazole groups, are relatively resistant to nitrosation with nitrite under acidic conditions [26]. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline (N-NO-IQ) and 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-NO-MeIQx) (Fig. 4) are formed by reaction of the amines with NO in aqueous solution at pH 7.4 [27, 28] and are stable under these conditions for > 4 h at 37°C. Under acidic conditions, N-NO-MeIQx decomposes to products in which the nitrosoamino group is replaced by –OH or –H [29]. N-NO-IQ has a longer half-life than N-NO-MeIQx, and gives rise to the deamination product but not to the corresponding alcohol [29]. N-NO-IQ activation by simulated inflame-matory conditions generates the azo compound 2,2′-azo-3,3′-dimethylimidazo[4,5-f]quinoline [30], which suggests that a nitrenium ion may be formed as a reactive intermediate. The N-nitroso compounds formed from IQ and MeIQx react with nucleophiles such as azide (although not appreciably with glutathione) and form covalent adducts with DNA [29, 31]. Enzymatic nitrosation has also been observed. Several mammalian peroxidases, such as myeloperoxidase, use NO as a physiological substrate [32] and can nitrosate IQ and MeIQx [27, 28]. In the presence of a constant flux of NO, stimulated human neutrophils produce N-NO-IQ by a myeloperoxidase-mediated reaction [28].
Figure 4.
2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline (N-NO-IQ) and 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-NO-MeIQx): structures.
In this report, we show that N-NO-IQ and N-NO-MeIQx are converted to reactive species that form covalent nucleotide adducts, and are mutagens in a biological assay that measures reversion of a lacZ frameshift allele in E. coli.
2. Materials and methods
2.1. Mutagenicity assays
Mutagenicity of heterocyclic amine derivatives was assessed with the E. coli lacZ reversion assay, following published protocols [33]. The E. coli strains used in this study incorporate a lacZ frameshift reversion mutation target with a GCGCGC repeat that is sensitive to aromatic amine-induced mutations [33]. The strains also express recombinant enzymes of xenobiotic metabolism. Many nitroaromatic compounds and heterocyclic amines are metabolically activated by cytochrome P450 (P450)-catalyzed N-oxidation to hydroxylamines [34]. The acetyl CoA-dependent O-acetylation of hydroxylamines yields reactive N-acetoxy esters. This esterification reaction is catalyzed by O-acetyltransferases, which usually also have arylamine N-acetyltransferase (NAT) activity [35, 36].
Strain DJ2002 [37] was constructed by PCR-mediated deletion of the nhoA gene, which encodes the endogenous E. coli NAT, and serves as a NAT− control. Strains DJ701 and DJ702 [38] bear plasmid pNM12 [39] and express the Salmonella typhimurium NAT enzyme at high levels. Strain DJ702 also bears a plasmid for expression of recombinant human P450 1A2 and NADPH-P450 reductase, the flavoenzyme accessory protein which transfers reducing equivalents to P450 [40].
N-Nitroso derivatives of IQ and MeIQx were prepared as previously described [27, 28]. Nitro derivatives of IQ and MeIQx were purchased from Toronto Research Chemicals, North York, Canada. Mutagens were dissolved in DMSO.
2.2. Nucleotide adduct analysis
Hydroxylamino or N-nitroso derivatives of the HCA IQ or MeIQx (60 μM) were incubated for 30 min at 37°C with 2′-deoxyguanosine 3′-monophosphate (dGp), 3 mM, and diethylenetriamine pentaacetic acid, a chelator, 0.1 mM, in 100 mM potassium phosphate buffer. Hydroxylamines were incubated at pH 7.4, while N-nitroso compounds were incubated at pH 5.5 in the presence of HOCl (0.1 mM). Following incubation, samples were placed on ice and immediately applied to Sep-Pak columns. dGp adducts were analyzed by 32P-postlabeling [41], using standard (ATP-saturating) conditions. dGp adducts were labeled with [γ-32P]ATP to form 3′,5′-bisphosphate nucleotides, using T4 polynucleotide kinase. Adducts were separated by thin-layer chromatography on PEI-cellulose plates. Samples were then scraped from the plates and treated with nuclease P1 (0.2 mg/ml), which catalyzes hydrolysis of the 3′-phosphate group of the d(32P)pGp adduct to give the d(32P)pG adduct. Samples were analyzed by HPLC, with eluent radioactivity measured using a FLO-ONE detector, as previously described [31].
3. Results
3.1. Mutagenicity assays
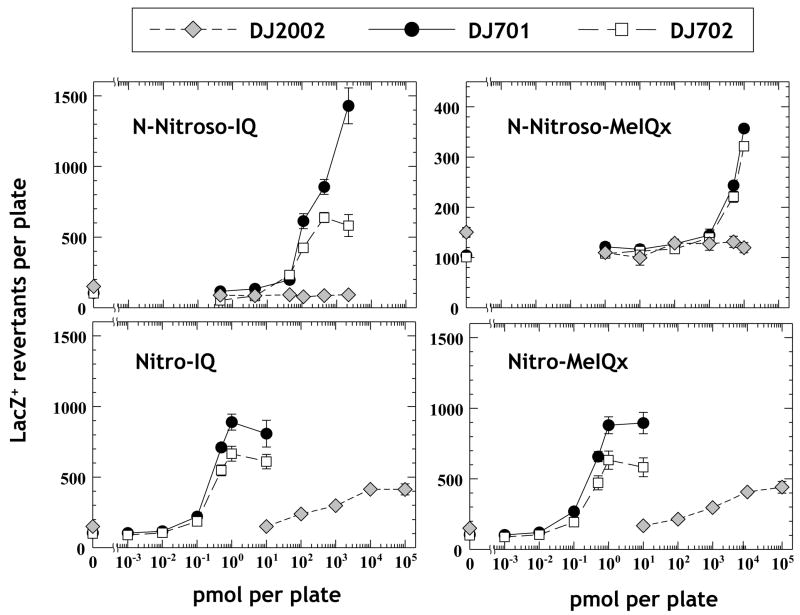
The N-nitroso derivatives of IQ and MeIQx are potent mutagens in strain DJ701, which overexpresses NAT enzyme (Fig. 5, upper panels). In strain DJ702, which also expresses a complete P450 1A2 system capable of activating HCA [40], no further increases in mutagenicity were observed. In fact, mutagenicity was somewhat lower in the P450-expressing strain. In contrast, no mutagenicity whatsoever was detectable in strain DJ2002, a strain which is devoid of NAT activity (Fig. 5).
Figure 5.
Mutagenicity of N-NO-IQ and N-NO-MeIQx in the E. coli lacZ reversion assay. Each data point represents the mean ± standard error of at least two independent triplicate experiments (at least six plates per point). The E. coli strains used were DJ2002 (NAT-minus; diamonds), DJ701 (expressing human NAT2; filled circles), and DJ702 (expressing human NAT2 and P450 1A2; open squares).
For comparison, we examined the mutagenicity of the nitro derivatives of IQ and MeIQx. As expected, these compounds were also NAT-dependent, P450-independent mutagens (Fig. 5, lower panels). In strain DJ2002, mutagenicity was far weaker, although some response was observed with each compound at extremely high doses (three or more orders of magnitude greater than in the NAT-producing strains). The NAT-dependence of the mutagenicity of nitro derivatives of heterocyclic amines such as IQ is well known [42, 43]. The nitro compounds are activated by nitroreductase-catalyzed reduction [44] to the corresponding hydroxylamines, which are then esterified to N-acetoxy esters by acetyltransferase. However, the NAT dependence of the mutagenicity of the N-nitroso derivatives was unexpected, since there is not an obvious role for acetylation in their activation (see Discussion).
3.2. Deoxyguanosine adducts
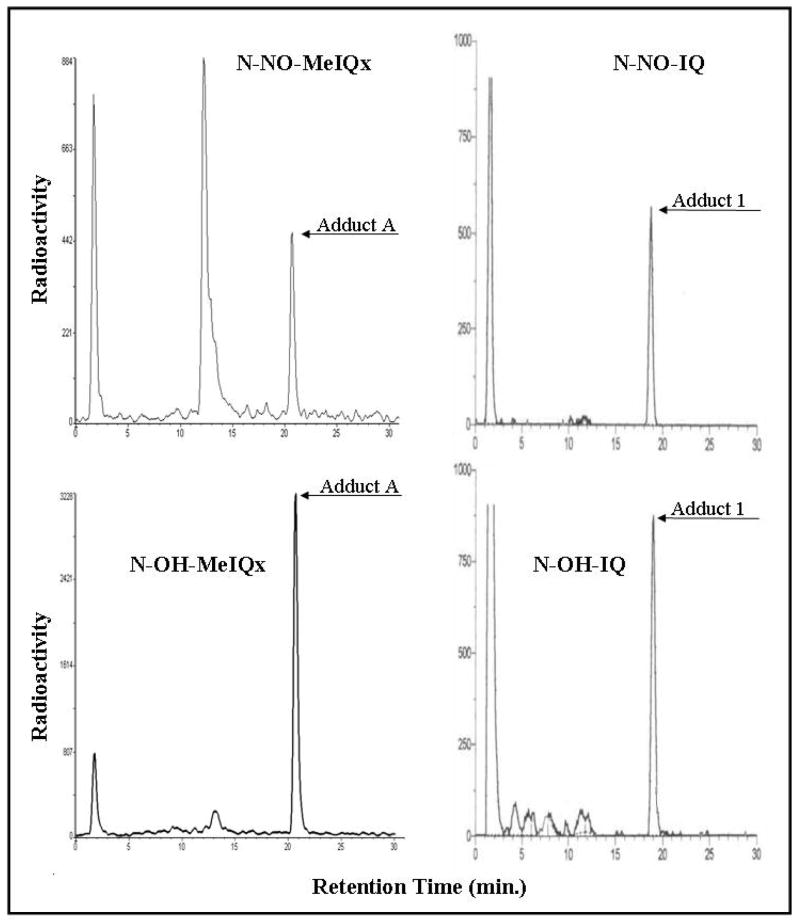
The reactions of the N-nitrosoamine derivatives of MeIQx and IQ with a deoxyguanosine nucleotide, dGp, to give covalent adducts were assessed and compared to the reactions of the hydroxylamino derivatives. The monomer dGp serves as a surrogate for dG residues in dsDNA, which are a major target for adduction by aromatic amines and HCA [45]. The N-nitrosoamines or hydroxylamines were incubated with dGp and adduct formation was analyzed following 32P-postlabeling. Adduct separation was first achieved by two-dimensional thin-layer chromatography. Adducts were then scraped off the TLC plates, treated with nuclease P1, and analyzed by HPLC (Fig. 6). Addition of the HPLC analysis step provides a greater resolving power than in our previous study [29], which relied on TLC separation alone.
Figure 6.
32P-Postlabeling of adducts. N-OH and N-NO heterocyclic amines were incubated with 2′ deoxyguanosine 3′ monophosphate (dGp). Adducts were 32P-postlabeled and initially separated by thin-layer chromatography on PEI-cellulose plates. For analysis, samples were scraped off PEI-cellulose plates, treated with nuclease P1, and, as illustrated here, analyzed by HPLC.
2-Hydroxyamino-3-methylimidazo[4,5-f]quinoline (N-OH-IQ) and 2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline (N-OH-MeIQx) incubations each produced a single adduct peak, eluting at 21 or 19 min, respectively (Fig. 6). N-Nitroso (N-NO-MeIQx or N-NO-IQ) incubations produced major peaks eluting at the same times, consistent with identity of the products formed by the two classes of compounds. For N-NO-MeIQx and N-OH-MeIQx, the peak eluting at 13 min was present in non-nuclease P1-treated samples and likely represents a product of incomplete hydrolysis (Fig. 6). These results represent analyses of samples in series on two different chromatographic systems (two-dimensional thin-layer chromatography then HPLC) and following two distinct treatments (product formation by reaction with dGp and then enzymatic treatment of the reaction product).
By the use of a combination of techniques, including mass spectrometry, proton NMR, and 32P-postlabeling, the hydroxylamine derivatives of IQ and MeIQx were shown to form N-(3′ mono-phosphodeoxyguanosin-8-yl)-IQ [46–48] or N-(3′ monophosphodeoxyguanosin-8-yl)-MeIQx [46–48] adducts, respectively, and co-chromatography indicates that the corresponding N-nitrosamines produce the same adducts.
4. Discussion
This is the first study to demonstrate the mutagenicity of N-nitroso derivatives of HCA. Both N-NO-IQ and N-NO-MeIQx elicited dose-dependent increases in mutagenicity, which are NAT-dependent and P450-independent. Our results are consistent with these N-nitrosamines being direct-acting mutagens, although less potent than their corresponding nitro compounds (Fig. 5). The amines, MeIQx and IQ, are inactive unless activated by P450-dependent hydroxylation [49, 50]. Mutagenic HCA are also carcinogens [51], and the fact that these N-nitrosamines are mutagenic suggests that they may also cause tumor formation in vivo.
Mutagenic activation of MeIQx and IQ catalyzed by P450 1A2 [49] is consistent with formation of an N-hydroxylamino product. Binding of this reactive intermediate to DNA is facilitated by NAT, which catalyzes formation of the N-acetoxy derivative that hydrolyzes to form a nitrenium ion that binds to DNA. Both N-OH-IQ and N-OH-MeIQx have been shown to form C-8 deoxyguanosine adducts and these adducts are present in vivo [47, 48, 52]. A previous study has demonstrated N-NO-IQ formation of the same adduct as N-OH-IQ, N-(3′ monophosphodeoxy-guanosin-8-yl)-IQ [31]. This observation was reproduced in our study and extended to N-NO-MeIQx. Both N-nitrosamines form the corresponding deoxyguanosine C-8 adducts.
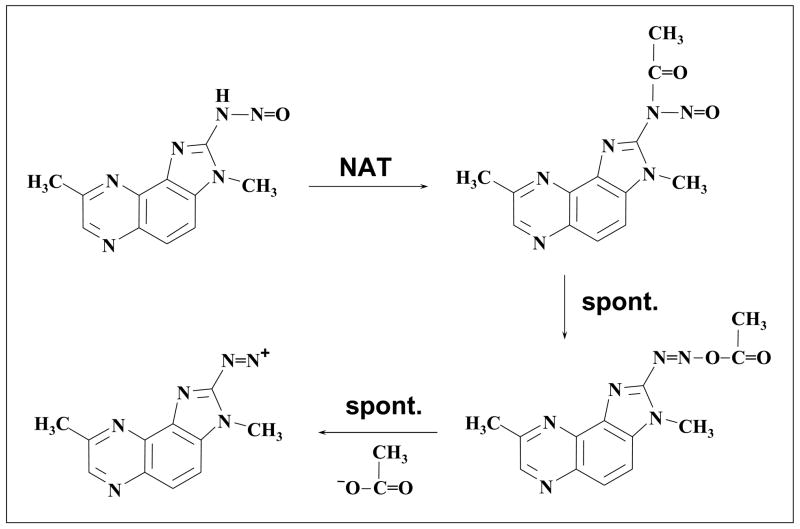
The NAT-dependent mutagenicity of the N-nitroso compounds is surprising, and the mechanism by which acetylation contributes to their activation is not known. One possibility is that the N-nitroso compounds undergo cleavage of the N-N bond and are converted, at least in part, into hydroxylamines (perhaps via nitro or C-nitroso compounds). Another possibility is that the N-nitroso compounds themselves are substrates for NAT-catalyzed acetylation, yielding N-nitrosamines (Fig. 7). An attractive feature of this hypothesis is that N-nitroso-N-2-fluorenyl-acetamide (see Introduction) provides a precedent for the potent mutagenicity of aromatic N-nitrosamines. However, the electrophilic species generated by such a pathway would presumably be diazonium (or perhaps alkyl) cations, rather than the single-nitrogen electrophiles (nitrenium ions) formed by the hydroxylamine route. This appears to be inconsistent with our adduct formation studies, which suggest that the same DNA-reactive species is formed from the hydroxylamines and the N-nitroso compounds. This discrepancy remains to be resolved.
Figure 7.
Hypothetical mechanism for NAT2-catalyzed bioactivation of N-NO-MeIQx (shown) and N-NO-IQ. N-Acetylation of the amino N of the N-nitroso group generates a nitrosoacyl heterocyclic amine. Migration of the acetyl group from the amino N to the O atom of the nitroso group generates a reactive intermediate which decomposes by loss of acetate anion to give a diazonium ion.
The N-nitrosamine adducts were derived from incubations performed at pH 5.5 and in the presence of HOCl, conditions which simulate those generated in vivo by the inflammatory response [53–55]. For both N-NO-IQ and N-NO-MeIQx, the presence of HOCl increased C8 adduct formation more than 20-fold [29, 31]. As many as five to eight different adducts are formed by the reactions of N-NO-IQ or N-NO-MeIQx with dGp. Both N-nitrosamines demonstrate increased binding to DNA and/or adduct formation under acidic conditions. Under strongly acidic conditions, pH 2.0, increased formation of a nonpolar N-NO-IQ adduct is seen. Formation of this nonpolar adduct is blocked by azide, which reacts to form 2-azido-IQ. 2-Azido-MeIQx is formed in a similar manner from N-NO-MeIQx. This finding is consistent with a reaction sequence involving a diazonium ion and alkyl cation leading to formation of a deaminated adduct.
While the reactions of the N-nitrosamine and hydroxylamino derivatives of MeIQx/IQ will be distinct, some common adducts might be produced by the two types of activated metabolites. N-OH-IQ forms decreasing amounts of adduct as the pH is decreased, and the major deoxyguanosine adduct formed is the C8 adduct [31]. N-NO-IQ activation by inflammatory conditions yields 2,2′-azo-3,3′-dimethylimidazo[4,5-f]quinoline [30], suggesting that a nitrenium ion reactive intermediate could be formed from the N-nitrosamine. Thus, N-NO-IQ may form a nitrenium ion-like intermediate by a different mechanism than N-OH-IQ. Alternatively, radical combination reactions could be responsible for formation of the C-8 adducts by these N-nitrosamines.
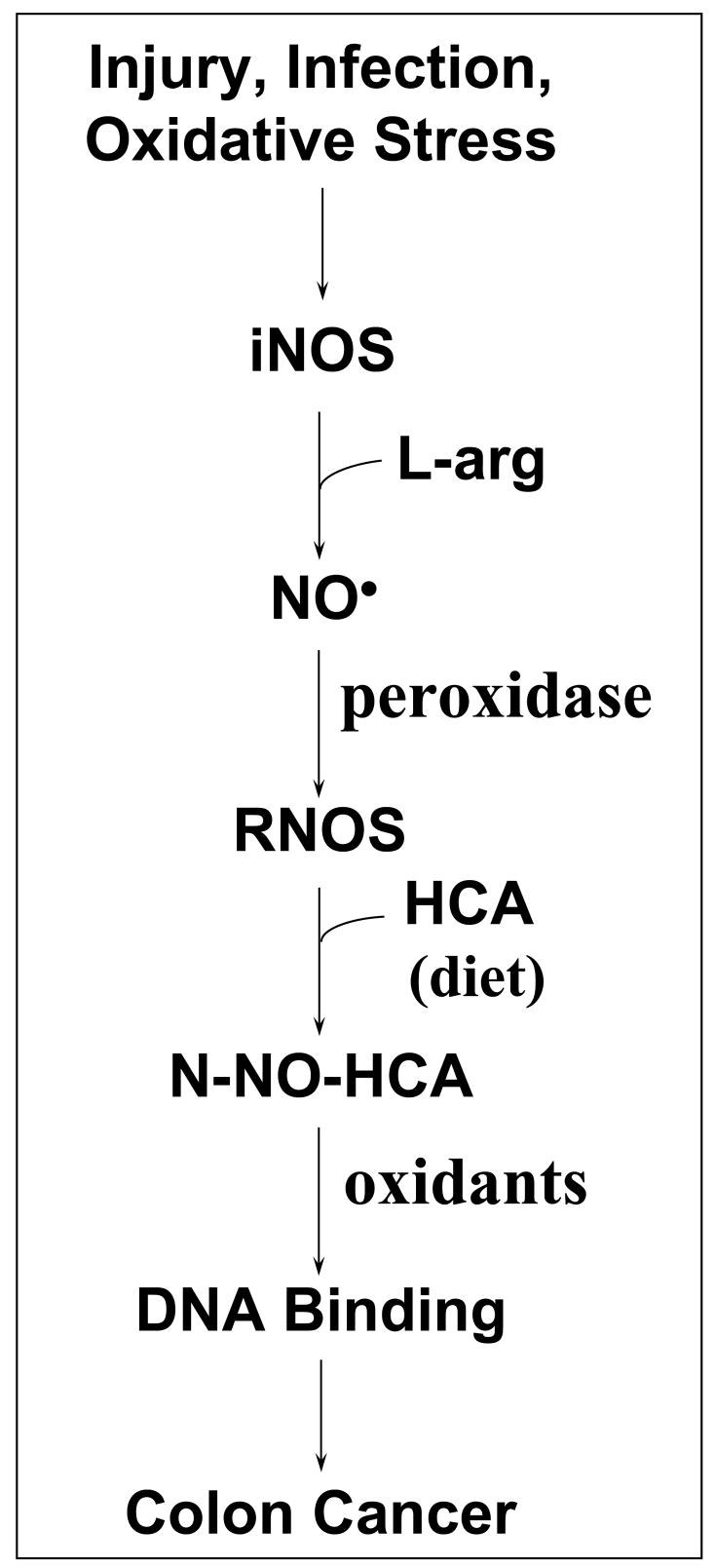
In addition to cooked red meat intake, there is also a strong association of chronic infection/inflammation, i.e., colitis, with colon cancer [56, 57]. Significant down-regulation of P450 enzymes occurs during infection/inflammation [58] and NO inhibits P450s [59], so P450-catalyzed biotransformations are unlikely to be relevant. For these reasons, we have evaluated the ability of reactive nitrogen oxygen species, components of the inflammatory response [8], to transform HCAs to genotoxic products. N-NO-IQ and N-NO-MeIQx are products of this reaction. Components in the inflammation milieu (pH 5.5 and neutrophils producing HOCl [51–53]) increase DNA binding and adduct formation by these N-nitrosamines [29, 31]. We envision nitrosation of HCAs at sites of inflammation within the colon, along with concomitant DNA adduct formation and mutagenicity (Fig. 8). This is consistent with the observation that both non-cancerous colon tissue from ulcerative colitis patients and primary colon tumor samples exhibit a statistically significant positive correlation between iNOS activity and frequency of G:C to A:T transitions at CpG sites in the p53 tumor suppressor gene [9, 10]. The present study extends our results by demonstrating that HCA-derived nitrosamines are genotoxic compounds and are potential carcinogens. In addition, preliminary data, based on a mouse model of colitis [60] have demonstrated increased colon tumors due to IQ exposure [61]. These results are consistent with our hypothesis that HCA N-nitrosamines might be alternatives to their hydroxylamine analogues as initiating agents for cancer associated with a high level of inflammation and NO production. Additional studies are needed to determine whether these nitrosamines are formed in vivo.
Figure 8.
Model of the postulated relationships among chronic inflammation/infection/injury, well-done red meat in the diet, and development of colon cancer. The inflammatory process leads to expression of inducible nitric oxide synthase (iNOS), which converts arginine (L-arg) to nitric oxide. NO and other factors generated during inflammation, such as peroxidases, hydrogen peroxide, hypochlorite, reactive nitrogen-oxygen species (RNOS), and acid pH (pH 5.5), facilitate this process in a temporal and spatial manner [8, 53–55]. Well-done red meat in the diet is a source of exposure to heterocyclic amines (HCA). The RNOS species nitrosate HCA to produce N-nitroso derivatives (N-NO-HCA). These N-nitroso compounds, which may be further activated by acetylation or oxidation, bind to DNA and cause damage that contributes to somatic mutation and carcinogenesis.
Acknowledgments
We wish to thank Edward W.F. Campbell for assistance with the mutagenicity assays and Priscilla Jones for adduct analysis.
This study was supported by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (P.D.J) and by the Department of Veterans Affairs (T.V.Z.) and National Cancer Institute Grant CA72613 (T.V.Z.).
Abbreviations
- dGp
2′-deoxyguanosine 3′-monophosphate
- HCA
heterocyclic amines, IQ, 2-amino-3-methylimidazo[4,5-f]quinoline
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- NAT
aromatic amine N-acetyltransferase
- N-NO-IQ
2-nitrosoamino-3-methylimidazo[4,5-f]quinoline
- N-NO-MeIQx
2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline
- N-OH-IQ
2-hydroxyamino-3-methylimidazo[4,5-f]quinoline
- N-OH-MeIQx
2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline
- P450
cytochrome P450
Footnotes
A preliminary report of this work has been presented in abstract form (Zenser, T.V., Lakshmi, V.M., Zhou, H.-j., Josephy, P.D., and Schut, H.A., Genotoxicity of 2-nitrosoamino-3-methylimidazo[4,5-f]quinoline, AACR Annual Meeting, 2004: 1123).
Conflict of interest statement
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Hughes MN. Chemistry of nitric oxide and related species. Methods Enzymol. 2008;436:3–19. doi: 10.1016/S0076-6879(08)36001-7. [DOI] [PubMed] [Google Scholar]
- 2.Miersch S, Mutus B. Protein S-nitrosation: biochemistry and characterization of protein thiol-NO interactions as cellular signals. Clin Biochem. 2005;38:777–791. doi: 10.1016/j.clinbiochem.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Kuhnle GG, Bingham SA. Dietary meat, endogenous nitrosation and colorectal cancer. Biochem Soc Trans. 2007;35:1355–1357. doi: 10.1042/BST0351355. [DOI] [PubMed] [Google Scholar]
- 4.Foster MW, McMahon TJ, Stamler JS. S-Nitrosylation in health and disease. Trends Mol Med. 2003;9:160–168. doi: 10.1016/s1471-4914(03)00028-5. [DOI] [PubMed] [Google Scholar]
- 5.Bryan NS, Rassaf T, Maloney RE, Rodriguez CM, Saijo F, Rodriguez JR, Feelisch M. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulos TL. Soluble guanylate cyclase. Curr Opin Struct Biol. 2006;16:736–743. doi: 10.1016/j.sbi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Higgs EA. Nitric oxide and the vascular endothelium. Handb Exp Pharmacol. 2006:213–254. doi: 10.1007/3-540-32967-6_7. [DOI] [PubMed] [Google Scholar]
- 8.Grisham MB, Jourd’heuil D, Wink DA. Nitric oxide. I. Physiological chemistry of nitric oxide and its metabolites: implications in inflammation. Am J Physiol. 1999;276:G315–G321. doi: 10.1152/ajpgi.1999.276.2.G315. [DOI] [PubMed] [Google Scholar]
- 9.Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Harrington AM, Shields PG, Felley-Bosco E, Hussain SP, Harris CC. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst. 1999;91:86–88. doi: 10.1093/jnci/91.1.86. [DOI] [PubMed] [Google Scholar]
- 10.Hussain SP, Amstad P, Raja K, Ambs S, Nagashima M, Bennett WP, Shields PG, Ham AJ, Swenberg JA, Marrogi AJ, Harris CC. Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: a cancer-prone chronic inflammatory disease. Cancer Res. 2000;60:3333–3337. [PubMed] [Google Scholar]
- 11.Carmella SG, Borukhova A, Desai D, Hecht SS. Evidence for endogenous formation of tobacco-specific nitrosamines in rats treated with tobacco alkaloids and sodium nitrite. Carcinogenesis. 1997;18:587–592. doi: 10.1093/carcin/18.3.587. [DOI] [PubMed] [Google Scholar]
- 12.Tricker AR. N-Nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226–268. [PubMed] [Google Scholar]
- 13.Vermeer IT, Henderson LY, Moonen EJ, Engels LG, Dallinga JW, van Maanen JM, Kleinjans JC. Neutrophil-mediated formation of carcinogenic N-nitroso compounds in an in vitro model for intestinal inflammation. Toxicol Lett. 2004;154:175–182. doi: 10.1016/j.toxlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Mirvish SS, Sams J, Hecht SS. Kinetics of nornicotine and anabasine nitrosation in relation to N′-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. J Natl Cancer Inst. 1977;59:1211–1213. doi: 10.1093/jnci/59.4.1211. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell WS, Greene JM, Plowchalk DR, deBethizy JD. The nitrosation of nicotine: a kinetic study. Chem Res Toxicol. 1991;4:513–516. doi: 10.1021/tx00023a003. [DOI] [PubMed] [Google Scholar]
- 16.France H, Heilbron IM, Hey DM. Nitrosoacylarylamines. Part III. A new method of preparation. J Chem Soc. 1940:369–371. [Google Scholar]
- 17.Chalfont GR, Perkins MJ. Mechanism of aromatic arylation with nitrosoacetanilide. J Am Chem Soc. 1967;89:3054–3055. [Google Scholar]
- 18.Brydon DL, Cadogan JIG, Cook J, Harger MJP, Sharp JT. Acylarylnitrosamines. Part IV. Aryne participation in decompositions of N-nitrosoacetanilide and its m- and p-t-butyl-, o-, m-, and p-chloro-derivatives in benzene. J Chem Soc B. 1971:1996–2006. [Google Scholar]
- 19.Klanderman BH, Maier DP, Clark GW, Kampmeier JA. Formation of benzyne by the decomposition of N-nitrosoacetanilide and N-(2-iodophenyl)-N-nitrosobenzamide. J Chem Soc D. 1971:1003–1004. [Google Scholar]
- 20.Lin JK, Kuo ML. N-Nitroso-N-2-fluorenylacetamide: a new direct-acting mutagen and teratogen. Mutat Res. 1988;201:117–126. doi: 10.1016/0027-5107(88)90118-2. [DOI] [PubMed] [Google Scholar]
- 21.Kuo ML, Lin JK. The relationship between DNA damage and mutation frequency in mammalian cell lines treated with N-nitroso-N-2-fluorenylacetamide. Mutat Res. 1989;212:231–239. doi: 10.1016/0027-5107(89)90074-2. [DOI] [PubMed] [Google Scholar]
- 22.Lin JK, Kuo ML. Induction of ouabain-resistance mutation and cycle-dependent transformation of C3H/10T1/2 cells by N-nitroso-2-acetylaminofluorene. Mutat Res. 1990;230:35–43. doi: 10.1016/0027-5107(90)90039-7. [DOI] [PubMed] [Google Scholar]
- 23.Kolodyazhnaya SN, Simonov AM, Podladchikova LN. Direct nitrosation of 2-amino-1-alkyl-(aralkyl)benzimidazoles. Chem Heterocyc Comp. 1974;10:1384. [Google Scholar]
- 24.Simonov AM, Kolodyazhnaya SN, Podladchikova LN. Diazo compounds of the heterocyclic series I. 2-(N-nitrosamino)benzimidazoles. Chem Heterocyc Comp. 1974;10:596–599. [Google Scholar]
- 25.Kolodyazhnaya SN, Divaeva LN, Zheltikova NN, Simonov AM. Heterocyclic diazo-compounds. 3. Structure and unusual properties of primary 2-nitroso-aminobenzimidazoles. Chem Heterocyc Comp. 1988;23:1090–1094. [Google Scholar]
- 26.Tsuda M, Negishi C, Makino R, Sato S, Yamaizumi Z, Hirayama T, Sugimura T. Use of nitrite and hypochlorite treatments in determination of the contributions of IQ-type and non-IQ-type heterocyclic amines to the mutagenicities in crude pyrolyzed materials. Mutat Res. 1985;147:335–341. doi: 10.1016/0165-1161(85)90002-0. [DOI] [PubMed] [Google Scholar]
- 27.Lakshmi VM, Hsu FF, Zenser TV. Nitric oxide-mediated nitrosation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline potentiated by hemin and myeloperoxidase. Chem Res Toxicol. 2005;18:1038–1047. doi: 10.1021/tx0500070. [DOI] [PubMed] [Google Scholar]
- 28.Lakshmi VM, Nauseef WM, Zenser TV. Myeloperoxidase potentiates nitric oxide-mediated nitrosation. J Biol Chem. 2005;280:1746–1753. doi: 10.1074/jbc.M411263200. [DOI] [PubMed] [Google Scholar]
- 29.Lakshmi VM, Hsu FF, Schut HA, Zenser TV. Stability and reactivity of 2-nitrosoamino-3,8-dimethylimidazo[4,5-f]quinoxaline. Chem Res Toxicol. 2006;19:325–333. doi: 10.1021/tx050305x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lakshmi VM, Hsu FF, Zenser TV. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline stability and reactivity. Chem Res Toxicol. 2004;17:709–716. doi: 10.1021/tx030042b. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmi VM, Schut HA, Zenser TV. 2-Nitrosoamino-3-methylimidazo[4,5-f]quinoline activated by the inflammatory response forms nucleotide adducts. Food Chem Toxicol. 2005;43:1607–1617. doi: 10.1016/j.fct.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 33.Josephy PD. The Escherichia coli lacZ reversion mutagenicity assay. Mutat Res. 2000;455:71–80. doi: 10.1016/s0027-5107(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 34.Guengerich FP. N-Hydroxyarylamines. Drug Metab Rev. 2002;34:607–623. doi: 10.1081/dmr-120005663. [DOI] [PubMed] [Google Scholar]
- 35.Dupret JM, Rodrigues-Lima F. Structure and regulation of the drug-metabolizing enzymes arylamine N-acetyltransferases. Curr Med Chem. 2005;12:311–318. doi: 10.2174/0929867053363289. [DOI] [PubMed] [Google Scholar]
- 36.Sim E, Westwood I, Fullam E. Arylamine N-acetyltransferases. Expert Opin Drug Metab Toxicol. 2007;3:169–184. doi: 10.1517/17425255.3.2.169. [DOI] [PubMed] [Google Scholar]
- 37.Josephy PD, Summerscales J, DeBruin LS, Schlaeger C, Ho J. N-Hydroxyarylamine O-acetyltransferase-deficient Escherichia coli strains are resistant to the mutagenicity of nitro compounds. Biol Chem. 2002;383:977–982. doi: 10.1515/BC.2002.104. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H, Josephy PD, Kim D, Guengerich FP. Functional characterization of four allelic variants of human cytochrome P450 1A2. Arch Biochem Biophys. 2004;422:23–30. doi: 10.1016/j.abb.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Oda Y, Yamazaki H, Watanabe M, Nohmi T, Shimada T. Development of high sensitive umu test system: rapid detection of genotoxicity of promutagenic aromatic amines by Salmonella typhimurium strain NM2009 possessing high O-acetyltransferase activity. Mutat Res. 1995;334:145–156. doi: 10.1016/0165-1161(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 40.Josephy PD, Evans DH, Parikh A, Guengerich FP. Metabolic activation of aromatic amine mutagens by simultaneous expression of human cytochrome P450 1A2, NADPH-cytochrome P450 reductase, and N-acetyltransferase in Escherichia coli. Chem Res Toxicol. 1998;11:70–74. doi: 10.1021/tx970171q. [DOI] [PubMed] [Google Scholar]
- 41.Randerath K, Randerath E. 32P-postlabeling methods for DNA adduct detection: overview and critical evaluation. Drug Metab Rev. 1994;26:67–85. doi: 10.3109/03602539409029785. [DOI] [PubMed] [Google Scholar]
- 42.Morrison LD, Eling TE, Josephy PD. Prostaglandin H synthase-dependent formation of the direct-acting mutagen 2-nitro-3-methylimidazo[4,5-f]quinoline (nitro-IQ) from IQ. Mutat Res. 1993;302:45–52. doi: 10.1016/0165-7992(93)90089-e. [DOI] [PubMed] [Google Scholar]
- 43.Wild D, Feser W, Michel S, Lord HL, Josephy PD. Metabolic activation of heterocyclic aromatic amines catalyzed by human arylamine N-acetyltransferase isozymes (NAT1 and NAT2) expressed in Salmonella typhimurium. Carcinogenesis. 1995;16:643–648. doi: 10.1093/carcin/16.3.643. [DOI] [PubMed] [Google Scholar]
- 44.Carroll CC, Warnakulasuriyarachchi D, Nokhbeh MR, Lambert IB. Salmonella typhimurium mutagenicity tester strains that overexpress oxygen-insensitive nitroreductases nfsA and nfsB. Mutat Res. 2002;501:79–98. doi: 10.1016/s0027-5107(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 45.Turesky RJ. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol Lett. 2007;168:219–227. doi: 10.1016/j.toxlet.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 46.Snyderwine EG, Roller PP, Adamson RH, Sato S, Thorgeirsson SS. Reaction of N-hydroxylamine and N-acetoxy derivatives of 2-amino-3-methylimidazolo[4,5-f]quinoline with DNA. Synthesis and identification of N-(deoxyguanosin-8-yl)-IQ. Carcinogenesis. 1988;9:1061–1065. doi: 10.1093/carcin/9.6.1061. [DOI] [PubMed] [Google Scholar]
- 47.Turesky RJ, Rossi SC, Welti DH, Lay JOJ, Kadlubar FF. Characterization of DNA adducts formed in vitro by reaction of N-hydroxy-2-amino-3-methylimidazo[4,5-f]quinoline and N-hydroxy-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline at the C-8 and N2 atoms of guanine. Chem Res Toxicol. 1992;5:479–490. doi: 10.1021/tx00028a005. [DOI] [PubMed] [Google Scholar]
- 48.Snyderwine EG, Davis CD, Nouso K, Roller PP, Schut HA. 32P-postlabeling analysis of IQ, MeIQx and PhIP adducts formed in vitro in DNA and polynucleotides and found in vivo in hepatic DNA from IQ-, MeIQx- and PhIP-treated monkeys. Carcinogenesis. 1993;14:1389–1395. doi: 10.1093/carcin/14.7.1389. [DOI] [PubMed] [Google Scholar]
- 49.Aryal P, Yoshikawa K, Terashita T, Guengerich FP, Shimada T, Oda Y. Development of a new genotoxicity test system with Salmonella typhimurium OY1001/1A2 expressing human CYP1A2 and NADPH-P450 reductase. Mutat Res. 1999;442:113–120. doi: 10.1016/s1383-5718(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 50.Josephy PD, Batty SM, Boverhof DR. Recombinant human P450 forms 1A1, 1A2, and 1B1 catalyze the bioactivation of heterocyclic amine mutagens in Escherichia coli lacZ strains. Environ Mol Mutagen. 2001;38:12–18. doi: 10.1002/em.1045. [DOI] [PubMed] [Google Scholar]
- 51.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21:387–395. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 52.Totsuka Y, Fukutome K, Takahashi M, Takahashi S, Tada A, Sugimura T, Wakabayashi K. Presence of N2-(deoxyguanosin-8-yl)-2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (dG-C8-MeIQx) in human tissues. Carcinogenesis. 1996;17:1029–1034. doi: 10.1093/carcin/17.5.1029. [DOI] [PubMed] [Google Scholar]
- 53.Dubos RJ. The micro-environment of inflammation or Metchnikoff revisited. Lancet. 1955;269:1–5. doi: 10.1016/s0140-6736(55)93374-2. [DOI] [PubMed] [Google Scholar]
- 54.Bryant RE, Rashad AL, Mazza JA, Hammond D. beta-Lactamase activity in human pus. J Infect Dis. 1980;142:594–601. doi: 10.1093/infdis/142.4.594. [DOI] [PubMed] [Google Scholar]
- 55.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 56.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 57.Parsonnet J. Infection as a cause of human cancer. Oxford University Press; New York: 1999. Microbes and malignancy. [Google Scholar]
- 58.Morgan ET. Regulation of cytochromes P450 during inflammation and infection. Drug Metab Rev. 1997;29:1129–1188. doi: 10.3109/03602539709002246. [DOI] [PubMed] [Google Scholar]
- 59.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–123. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 60.Cooper HS, Everley L, Chang WC, Pfeiffer G, Lee B, Murthy S, Clapper ML. The role of mutant Apc in the development of dysplasia and cancer in the mouse model of dextran sulfate sodium-induced colitis. Gastroenterology. 2001;121:1407–1416. doi: 10.1053/gast.2001.29609. [DOI] [PubMed] [Google Scholar]
- 61.Clapper ML, Merkel CE, Gary MA, Cooper HS, Coudry RA, Chang W-C, Zenser T. The heterocyclic amine IQ as a promoter of colitis-associated colorectal cancer in the Apc Min/+ model of DSS-induced colitis. AACR Meeting Abstracts; 2006. p. 3482. [Google Scholar]