Abstract
The phosphatidylinositol-3-kinase / Akt signaling pathway is currently one of the most exciting drug targets in oncology. However only a short time ago, the paradigm existed that drugs targeted to the four PI3K class 1 isoforms would be too toxic for use in cancer therapy due to effects on physiological signaling. Since that time studies have delineated the roles of these four isoforms in non-pathological signaling as well as their roles in cancer. An extensive effort has gone into developing agents that inhibit one or more PI3K isoforms, as well as closely related proteins implicated in cancer. These agents have proven to be tolerable and therapeutically beneficial in animal studies, and a number are in clinical testing. The agents, their properties and their molecular targets are discussed in this review.
Keywords: PI3K inhibitors, PI3K, antitumor activity
PI3K inhibitors in cancer
Developing an effective inhibitor to phosphatidylinositol-3-kinase (PI3K)/ Akt signaling has become one of the most sought after goals of pharmaceutical companies and academia alike. Such compounds are seen as possessing the potential to have a significant impact on the treatment of human disease, the largest application being in oncology, but certainly encompassing a variety of other pathological conditions. The field has gone from a handful of “archetypal” inhibitors which dominated the field for upwards of ten years, to a range of small molecules that are progressing rapidly towards, and through early clinical testing. Just a few years ago the general opinion was that broad spectrum inhibitors of the class I PI3Ks would “almost certainly have unacceptable toxicity if administered continuously” (1). Eight years later, these inhibitors now fill the oncology landscape, each with their own distinctive profile of inhibition, not only of specific PI3K isoforms but also of the PIK family of proteins to which the PI3Ks belong. Clinical testing of these agents has begun and will serve to define the optimal profile to accomplish the desired therapeutic goals while still maintaining an acceptable therapeutic index.
PIK family overview
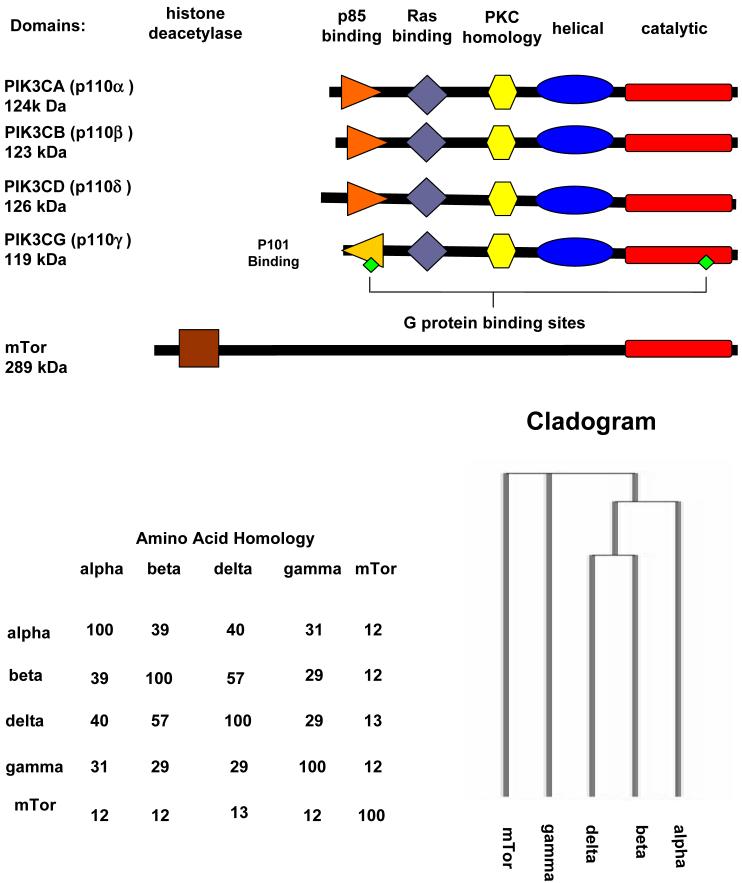
PI3Ks can be divided in to three classes. Class I PI3Ks exist as heterodimers consisting of one of four p110 catalytic subunits and one of two families of regulatory subunits (Figure 1). This class acts on PI(4,5)P2, to produce PI(3,4,5)P3 (2) and the process is reversed by the mixed function phosphatase PTEN (3) (Figure 2). Class II PI3Ks display the ability to phosphorylate PI and PI-4-P in vitro, and are generally resistant to the class I inhibitors. Class III PI3Ks phosphorylate only PI to generate PI-3-P. This class has only one member known as Vps34, which has been shown to play an essential role in trafficking of proteins form the Golgi apparatus in yeast (4). More recently, this class has been linked to autophagy and the activation of mammalian target of rapamycin (mTor) by amino acids (5). There is also a fourth class of PI3K-related enzymes which contain a catalytic core similar to the PI3Ks. This class includes enzymes involved in signal transduction and DNA damage response, such as mTor and DNA-dependent protein kinase (DNA-PK) (6).
Figure 1. Domain structure and amino acid homology of the class I PI3K catalytic subunits and mTOR.
The regulatory subunits are not shown. Also shown is a cladogram showing evolutionary relationships
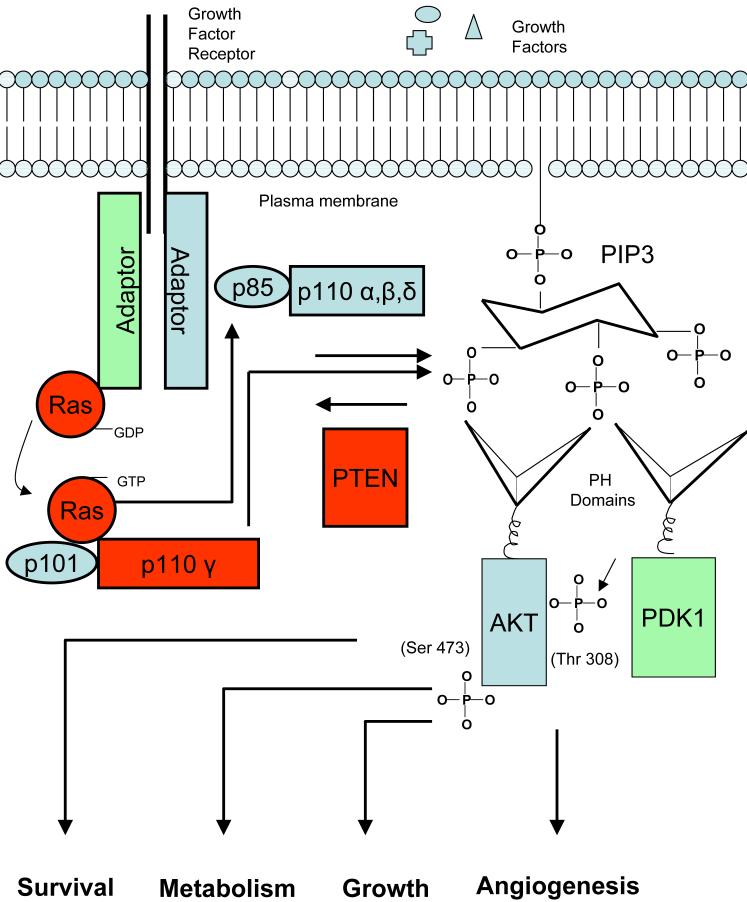
Figure 2. PI3K/Akt signaling in cells.
For an explanation see the text.
The first class 1 PI3K identified was an enzyme that co-purified with p60v-src, polyoma middle T antigen, and the PDGF receptor. Class I PI3Ks was later subdivided into class Ia consisting of the α, β and δ catalytic subunits and class 1b consisting solely of the γ catalytic subunit. The class I isoforms are activated under normal physiologic conditions upon stimulation by growth factors, either directly by the growth factor receptor or through adaptor proteins. Ligand binding results in tyrosine phosphorylation which allows docking of the SH2 domain located on the regulatory subunit, leading to activation of the lipid kinase activity of the class1a enzymes by receptors such as the epithelial growth factor receptor (EGFR). Additionally, active Ras has been shown to have the ability to activate class 1 enzymes (7). The generation of PI(3,4,5)P3 by PI3Ks allows for the recruitment to the plasma membrane of proteins containing a pleckstrin homology (PH) domain (8). Among the best characterized of these proteins is Akt, which when recruited to the plasma membrane is phosphorylated on threonine 308 by another PH domain containing protein, PDK1, and on serine 473 by PDK2 which has been identified as potentially one of at least ten proteins including DNA-PK and the rictor-mTor complex (9) (Figure 2). It should be noted that there are inhibitors of Akt itself in development that act through inhibition of kinase activity, or PH domain-dependent translocation (10). How these inhibitors will compare clinically to the emerging PI3K inhibitors will be of great interest. Examples of the multitude of targets phosphorylated by activated Akt are AS160 which regulates translocation of Glut-4 to the plasma membrane, thus, impacting glucose uptake, nuclear p27 a negative regulator of cell growth, thus, allowing cell proliferation, and inhibition of Bad, a promoter of apoptosis (11). Another downstream target of Akt is TSC2 (tuberin) which when phosphorylated by Akt disassociates from its partner TSC1 (hamartin), leading to its degradation and loss of its GTP activation activity against the small G protein Rheb which serves as a negative regulator of the PIK family member mTOR. With this negative regulation of Rheb, the mTor protein becomes active through association with raptor and other factors, stimulating TOP dependent mRNA translation through p70S6Kinase and cap-dependent translation thorough inhibition of the eiF4e repressor, 4E-BP, completing the signaling cascade known as the PI3K/Akt/mTor axis (12). Notably, inhibitors of the raptor-mTor complex including rapamycin derivatives, or rapalogs, are now approved for clinical use as antitumor agents (13) . However these inhibitors have also revealed that in some cases inhibition of mTor has the ability to activate PI3K signaling either by feedback to growth factor receptors, or by promoting the formation of an alternative mTor complex with rictor, that may serve to phosphorylate Akt, seen in both cell models and clinical samples (14). This potentially undesirable effect may be nullified through the use of direct inhibitors of mTor as opposed to inhibitors of raptor-mTor (13).
Aberrant PI3K signaling has been found to play an important role in multiple aspects of tumorgenesis including uncontrolled proliferation, resistance to apoptosis, angiogenesis and metastatic capability. This aberrant signaling may occur through dysfunction of pathways upstream of the PI3K class I isoforms, such as mutationally activated growth factor receptors, or Ras, or activation of the pathway itself (15). The first mechanism discovered by which the PI3K/Akt pathway is directly activated was the loss or inactivation of PTEN, identified as a tumor suppressor. The inactivation is found at a high frequency in multiple tumor types and new mechanisms by which cancer cells can alter the function of PTEN continue to be found (16). Most recently mutations in the PH domain of Akt1 which causes electrostatic alterations leading to increased binding of the Akt PH domain with PI(3,4,5)P3 have been found to aberrantly activate the pathway. Thus far, the initial mutation found at amino acid 17 of the Akt PH domain has been identified in 8% of the breast tumors studied, 6% of colorectal tumors, and 2% of ovarian cancers. Larger studies to precisely determine the frequency and tumor type specificity of this mutation remain to be conducted (17).
Dissection of PI3K class I isoform signaling in normal physiological signaling and the oncogenic process
Both genetic manipulation and pharmacological inhibitors have proven valuable in distinguishing the activities of each of the PI3K isoforms in normal cellular signaling. Early studies revealed that knockout of the PI3Kα isoform resulted in embryonic lethality (18), later determined to be due to deficient migration of endothelial cells resulting in a loss of angiogenic activity (19). A conditional knockout of PI3Kα in developed mice resulted in impaired insulin-induced glucose uptake (20) similar to that seen in Akt2 knockout mice (21). Similar results were found in cultured muscle cells treated with PI3Kα specific inhibitors (22). Mice deficient in the PI3Kβ isoform also showed embryonic lethality (23). Conditional knockout of PI3Kβ in developed mice resulted in mice which were similar to PI3Kα knockouts in that they exhibited impaired insulin signaling, although this effect was found not to be dependent on Akt signaling. Additionally these PI3Kβ knockout mice were deficient in lyophosphatidic acid signaling (24). Knockout of either the γ or δ isoforms of PI3K resulted in viable mice displaying alteration in immune function. Mice deficient in PI3Kδ showed deficient antigen receptor signaling in both B and T cells, as well as inflammatory bowel disease (25). Mice with a deletion of the PI3K γ isoform had defective thymocyte survival and decreased thymus size, and showed an inability to active T cells, but unlike the knockout of PI3Kδ, no effect was seen on B cells. Additionally PI3K γ knockout mice showed a loss of the migratory ability of neutrophils that were unable to generate the respiratory burst generated by GTP coupled protein receptor signaling (26).
In the context of oncogenesis the PI3K isoforms have been found to have overlapping and unique roles. Overexpression of all four isoforms has been shown to be capable of inducing transformation in experimental models, PI3Kα and δ independently and PI3Kβ and γ with input from Ras (27). PI3Kα has been implicated in cancer cell proliferation and tumor angiogenesis (28). Additionally this isoform has been shown to assist in Ras induced transformation and to be necessary for tumor formation in a mouse model of Ras induced oncogenesis (29). More recently activating mutations in both the helical and kinase domains of PI3Kα have been identified particular in breast and colon tumors, occurring frequently in similar locations known as “hotspots” within the protein. The most common site for these hotspots is around amino acid 1047 in the kinase domain, and amino acid 545 in the helical domain (30). Notably, these mutations are been found exclusively in the PI3Kα isoform to date, and mutations induced at the same location as the hotspots in PI3Kα did not having similar effects in activating PI3Kβ (31). When a colon line, HCT-116, heterogenous for the PI3Kα hotspot mutation was given the homozygous mutation, enhanced survival was seen under stress conditions together with increased metastasis (32). The PI3Kβ isoform has been implicated as necessary for transformation induced by the loss or inactivation of the PTEN tumor suppressor both in vitro (33) and in vivo (31). The PI3Kδ isoform most prominently expressed in myeloid cells, has been shown to play an essential role in cell proliferation in acute myeloid leukemia (34), and has also been implicated in tumor angiogenesis, particularly in the context of repair after destruction of tumor blood vessels with radiation (35). The PI3K γ isoform appears to function largely in the context of the immune system and has been found to be utilized by the BCR-ABL fusion oncogene, implicated in chronic myeloid leukemia, for proliferation and drug resistance (36), and is also known to be a Ras effecter (37). By determining the specific functions of each isoform in both normal physiology and the pathology of cancer, it may be possible to predict on-target effects resulting from patient treatment with pan class I PI3K inhibitors. Additionally, as isoform specific inhibitors become available, the possibility of matching these inhibitors to specific conditions of oncogenesis is an attractive concept. However, current evidence indicates that in many cases redundancy of signaling among the PI3K isoforms may make this goal unobtainable (38).
Early PI3K inhibitors, classical and modern twists
The earliest report of a compound which showed an inhibitory effects on PI3K was the non-specific kinase inhibitor quercetin (39). The next inhibitor identified was wortmannin (40), already known at the time as an inhibitor of myosin light chain kinase (MLK) (41). Shortly thereafter a quercetin analog, LY294002, was developed with increased specificity towards PI3K (42). Wortmannin and LY294002 were both evaluated as potential agents for clinical development but quickly found to be found to be unsuitable candidates (42) .
Wortmannin is a member of a class of steroidal furanoids which includes viridin. Extensive structural studies have been performed and wortmannin has been found to bind in an irreversible fashion through an electrophilic site at the C-20 position of the furan ring to lysine 802 in the ATP catalytic site of PI3K (43). Minor modifications to the structure of wortmannin had only slight effects on the in vitro efficacy while modifications negating the electrophilicity in the furan ring rendered the compound inactive (44). Wortmannin has been found to have equally potent activity against all the class I PI3K enzymes with IC50’s in the single digit nanomolar concentration range, while inhibiting other members of the PIK family such as mTor and DNA-PK at higher concentrations of 250 and 16 nM respectively, and unrelated enzymes such as polo like kinase (PLK) and MLK with IC-50’s of 24 nM and 170 nM, respectively (45).
LY294002 has a significantly lower potency for the class I PI3Ks than does wortmannin, having an IC50 in the 1-20μM concentration range (42). This was later found to directly overlap the range necessary to inhibit other members of the PIK family such as mTor and DNA-PK. LY294002 has been found to inhibit additional kinases such as caesin kinase 2 and Pim, and to have other PI3K independent effects such as the inhibition of calcium signaling. Additionally, LY294002 had unfavorable pharmacologic properties of insolubility and a poor half life in animals Recent studies looking in more detail at the activity of LY294002 both in enzymatic assays and in cells, have shown that its affinity for some targets is higher than its affinity for the class I PI3Ks (46), leading one study to conclude that its use as a tool to study PI3K signaling should be discontinued (47). Despite these inadequacies, both wortmannin and LY294002 proved to be valuable tools for the early study of PI3K inhibition, most importantly showing that shutting down class I PI3K signaling was not instantly toxic to cells or to animals, and thus might have a therapeutic benefit in cancer. On the other hand they also set back the development of PI3K inhibitors because of associated toxicities which resulted from off-target effects which would not be fully defined until recently (46) .
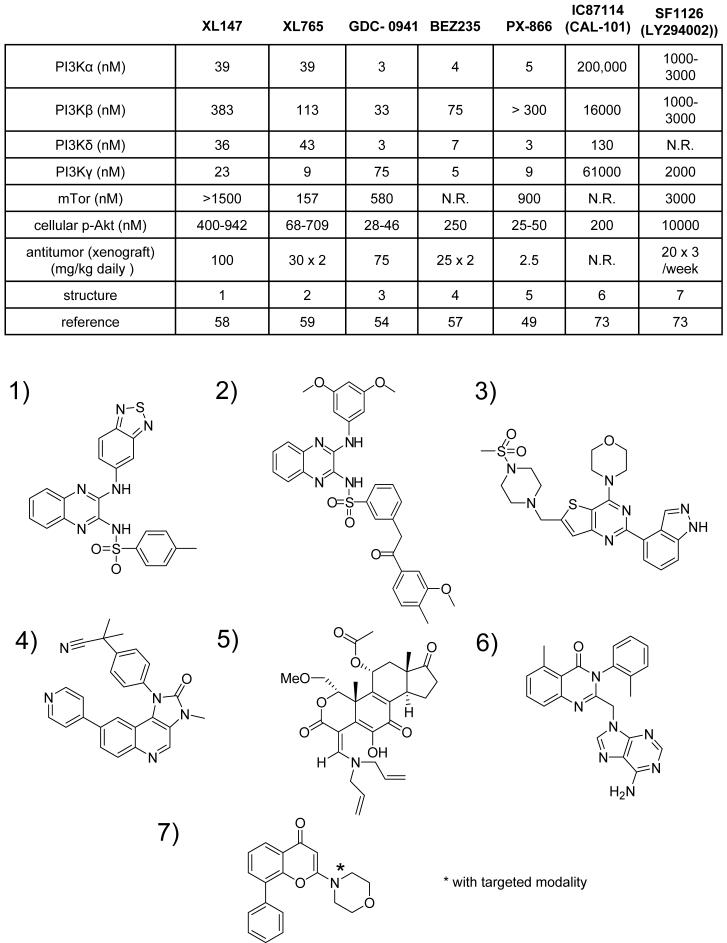
Prodrugs of wortmannin have been developed in attempts to extend its half-life in biological systems and analogs created which improve its pharmacologic properties, such as extending its half life, and favorably altering it’s selectively profile. Attempts to directly utilize the antiproliferative effects of wortmannin have used wortmannin conjugated to polyethylene glycol to delay its breakdown in biological systems (48) . Additionally modifications of wortmannin at its active C20 position through opening of its furan ring have yielded compounds which not only extend its half life but also have increased the selectively for particular PI3K isoforms. Such a compound is PX-866 which was found to have selectivity for the α,δ and γ class I PI3K isoforms while inhibiting the β isoform at higher concentrations, and showing decreased selectivity for mTor (49, 50) (Figure 3). PX-866 is currently in early clinical testing by Oncothyreon. PX-866 is the only irreversible PI3K inhibitor currently being developed clinically and has sustained oral activity against PI3K at low oral doses. Derivative compounds have been synthesized including WAY-266176 and WAY-266175 which have a modification to the C-20 position in 17-hydroxywortmannin, a wortmannin derivative (51). The irreversible PI3K inhibitors may display a unique advantage as their in vitro enzymatic and cellular activity translates closely to their in vivo activity, likely due to their irreversible inhibition of the enzyme.
Figure 3. Profiles of PI3K inhibitors in clinical trial.
The table shows the different PI3K inhibitors currently in clinical trial together with their reported activities against the PI3K α,β,γ and δ isoforms and mTOR, the concentration reported to inhibit cellular PI3K signaling measured by phospo-Akt inhibition, and the doses given to tumored animals to obtain antitumor activity, whether once or twice daily, or 3 times per week. NR is no report. The structures of the compounds are shown in the lower panel.
Attempts to harness the antiproliferative effects of LY294002 have also led to the creation of a prodrug. SF1126, which consists of LY294002 linked to a RDGS integrin binding element designed to target the compound to the tumor and tumor vasculature and has shown antitumor effects on tumor xenografts (52). SF1126 is currently in early clinical development. Additionally, derivatives of LY294002 have been identified which are reported to display isoform selectivity among the class I PI3K enzymes (53). However, give the extreme non-specificity of the parent compound for different molecular target f it is difficult to envision LY294002 derivatives offering a truly selective approach to PI3K inhibition.
Recently developed inhibitors: reflecting divergent paradigms
There has been a recent a flood of PI3K inhibitors from academia and industry reflecting an intense effect to make agents with increased specificity for desired class I PI3Ks. The goal in this effort has been to maximize the therapeutic effects of the inhibitors against the effects of deregulated isoforms specific to particular cancers, thus, hopefully minimizing their total impact and increasing their therapeutic index. Many compounds have been developed with varying specificity for PI3K isoforms and other PIK family members and their selectivity profiles determined through extensive profiling (22). Despite this, few compounds have been deemed to exhibit pharmacological profiles suitable for advancement beyond preclinical testing. A concern is that although active at low nanomolar concentrations against purified PI3Ks the compounds exhibit only high nanomolar activity in cells often well in excess of their isoform selective range (Figure 3) Furthermore, although these compounds distinguish between isoforms, often at single digit nanomolar concentrations, the threshold at which they exert this distinction in cells and in vivo is unknown. Despite these concerns an early success with this strategy came with the development of a specific inhibitor of the PI3K δ isoform, CAL-101, now in early clinical trail for hematological malignancies. IC87114, the preclinical inhibitor, was found to inhibit AML proliferation and augmented the effects of a topoisomerase 2 inhibitor by the specific inhibition of the PI3Kδ isoform, with proof of selectivity established in cells as well as against the enzyme. (54). This compound is simultaneously entering the clinic for non-oncology applications. To date it is the only inhibitor in clinical trials that distinguishes between class I isoforms. The newly developed inhibitor GDC-0941 and PX-866 are reported to have selectivity for the class 1 isoforms, with varying profiles (50, 55). Which selectivity is optimal and whether the specificity seen in preclinical testing will carry into the clinic will have to be proven.
Another widely studied compound in recent years has been PI-103. This compound’s introduction brought a new paradigm of the development of PI3K inhibitors. PI-103 was found to have increased efficacy in inhibiting the growth of glioma cells due to its activity against both the class I PI3Ks and the PIK family member mTor (56), it is also notable that this compound had activity against DNA-PK (22). This proved a contrary perspective to the long held goal of achieving increased specificity against particular class 1 PI3K family members, in that perhaps with a less specific inhibitor greater antitumor effects could be achieved. There was also the observation that combined inhibition of the class I PI3Ks and mTor eliminated the increased Akt signaling that an mTor inhibitor alone often caused (57). However, PI-103 was found to have pharmacological properties unsuitable for clinical development leaving untested the concept of inhibiting multiple points in the PI-3-Kinase signaling cascade for increased efficacy. This concept has been subsequently utilized by Novartis in their selection of BEZ235 as a lead compound now in clinical trial, which was found to have activity against both the class I PI3K isoforms and mTor (58). Exelixis have advanced two compounds as potential leads, one XL147 which targets only the class I PI3Ks and Xl765 which was found to have activity against the class I PI3Ks as well as mTor (59, 60). Whether this non-specific approach will translate to clinical agents with an acceptable therapeutic index is unknown. Although other classes of kinase inhibitors have capitalized on unexpected activity against other targets which has proved useful in certain tumor types (61), this is unknown for the PI3K inhibitors. Activity against mTor may reflect broad spectrum activity against a number of additional PIK family members and unrelated targets resulting in unpredictable toxicities, which could include the cardiac toxicity seen with many other current kinase inhibitors (62).
Unanswered questions
As the PI3K inhibitors move into the clinic answers to many important concepts coming from the preclinical models are beginning to take shape. Preclinical models provide strong evidence about what may occur with this class of inhibitors but despite this, for proof-of-principle these concepts must be demonstrated in multiple clinical trials with an inhibitor deemed to be effective in order to become validated, which may then provide a guide for future prospective clinical trials. Some of the unanswered questions and the emerging results from the clinical use of PI3K inhibitors are as follows:
The first question is whether, as originally suggested, inhibition of such a ubiquitously utilized pathway will prove too toxic to achieve therapeutic benefit? The expected undesirable effects associated with inhibiting this pathway, most notably metabolic disturbance and increased blood glucose, are being seen but have been reported to be mild or treatable, at least preclinically (50), and in early clinical evaluation have been manifest only as a rise in insulin levels (63). It is also notable that a metabolic disturbance most likely arises as a result of inhibiting the PI3Kα isoform (20), and this is also the isoform which to some presents the most attractive target in the broadest range of cancers. Thus, a more specific inhibitor of this isoform is unlikely to eliminate the metabolic on-target toxic effect. It is also likely that every PI3K inhibitor in the clinic will present a subset of unique toxicities, due not only to its PI3K inhibition profile, but also its individual off-target effects.
The second question is whether oncogenic alterations in the PI3K pathway will serve as a guide for patient selection for treatment with PI3K inhibitors? Many preclinical studies indicate that patient selection is possible, with at least one inhibitor going into a breast cancer, which one could speculate was chosen due to its high rate of PI3K mutations (64). However, there seems to be a discord with some studies finding maximal effects of PI3K inhibitors in cell types with mutations in PI3Kα (32), while others have found PI3K inhibitors to have maximal effect in lines with an inactive PTEN and modest, or unpredictable activity in lines with a mutated PI3K α (65). Some of this discrepancy may come from the use of 2-dimensional cell culture to elucidate sensitivity, as opposed to 3-dimensional cell culture or xenograft models which would serve to more accurately reflect the tumor microenivonment. At least one study has observed discrepancies in sensitivity between in vitro effects of PI3K inhibition on cell growth between 2 and 3 dimensional cell culture, as well as on cell migration, using a PI3K inhibitor currently in clinical development (66). Furthermore, it is become increasing apparent that additional mutations activating redundant pathways such as an oncogenic Ras, can confound this analysis of activity (33, 51).
A potential limitation of reversible PI3K inhibitors is that although they display potent activity against purified PI3K enzymes, they are considerably less active against cells, and their in vivo administration requires large doses, often multiple times daily, to achieve antitumor efficacy. This may be due to significantly higher levels of ATP with which they have to compete in biological systems than in the enzymatic assays, or to cellular binding and metabolism. Thus, a practical question arises whether the large doses will be acceptable to patients on long term therapy, or whether irreversible inhibitors requiring smaller and perhaps less frequent dosing, will provide a better alternative
Finally, there remains the question of which current chemotherapies will be best to combine with PI3K inhibitors, once acceptable candidates are identified? PI3K inhibitors have direct antitumor activity through their antiproliferative and antiangiogenic effects (15). Preclinical models have validated that PI3K inhibitors can enhance the effects of conventional cytotoxics and radiation (49, 59). PI3K signaling inhibits apoptosis and stimulates cell survival (11) with can allow cancer cell survival under periods in which the tumor is stressed. Thus, PI3K inhibitors may also have a role in combination therapy by facilitating apoptosis in tumors treated with cytotoxic agents or radiation. Whether this will have unacceptable adverse effects of the therapeutic window of these agents remains to be determined and may place limitations of this practice. Additionally, the concept of combining these agents with other targeted agents is proving promising . Resistance to both antibodies and small molecules targeting growth factor receptors has been shown to occur through oncogenic Ras which lies upstream of PI3K and other pathways, but also through direct alterations to the PI3K/Akt pathway itself, both through a suppression of PTEN and an activation of PI3Kα (68, 69). Preclinical data has provided strong evidence that resistance to inhibitors of growth factor receptors can be overcome with PI3K inhibitors. Additionally, as growth factor receptors and oncogenic Ras activate both the PI3K and Raf signaling cascades, in certain circumstances it may be beneficial to combine PI3K inhibitors with inhibitors already in development to various points in the Raf cascade(51). While it is well established that these pathways have redundant functions in cells (70), the increased efficacy may be offset by an increase in undesirable effects that may come with inhibiting these pathways simultaneously.
Current status and future directions
Several inhibitors of PI-3-Kinase have moved through preclinical studies and into Phase I and II clinical trials. These range from inhibitors reported to act on a single class I PI3K such CAL-101, to inhibitors of multiple class I PI3K isoforms such as PX-866, XL-147, and GDC-0941, to inhibitors acting on multiple class I isoforms and other PIK family members such as BEZ235 and XL765. Efforts to make more selective PI3K inhibitors to various PI3K isoforms have been aided by the recent identification thorough structural studies of the mechanism of inhibitors already known to be selective (70). Additionally, more detailed analysis of structural differences between the class I PI3K isoforms has recently been published (71). This information should allow for the development of compounds with a larger differential for inhibition of class I isoforms. Structural studies of the common PI3Kα mutations in cancer have also led to the concept that it may be possible to develop inhibitors with an increased selectivity for the mutant forms of the kinase (72), as has been achieved with another mutated kinase, B-Raf (73).
The ultimate answers to these questions will only come with time and more preclinical and clinical experience but will certainly provide insights for discussion and further drug development for some time to come.
Acknowledgments
Supported by NIH grants CA52995, CA17094,CA95060 and CA610159.
References
- 1.Stein RC, Waterfield MD. PI3-kinase inhibition: a target for drug development? Mol Med Today. 2000 Sep;6(9):347–57. doi: 10.1016/s1357-4310(00)01770-6. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999 Nov 25;253(1):239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 3.Parsons R. Phosphatases and tumorigenesis. Curr Opin Oncol. 1998 Jan;10(1):88–91. doi: 10.1097/00001622-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 5.Backer JM. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008 Feb 15;410(1):1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 6.Kuruvilla FG, Schreiber SL. The PIK-related kinases intercept conventional signaling pathways. Chem Biol. 1999 May;6(5):R129–36. doi: 10.1016/S1074-5521(99)80070-2. [DOI] [PubMed] [Google Scholar]
- 7.Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997 Jun 23;410(1):91–5. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- 8.Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002 May;59(5):761–79. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005 Aug;289(2):E187–96. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 10.Lindsley CW, Barnett SF, Layton ME, Bilodeau MT. The PI3K/Akt pathway: recent progress in the development of ATP-competitive and allosteric Akt kinase inhibitors. Curr Cancer Drug Targets. 2008 Feb;8(1):7–18. doi: 10.2174/156800908783497096. [DOI] [PubMed] [Google Scholar]
- 11.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008 Jun 1;412(2):179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008 Feb;12(2):209–22. doi: 10.1517/14728222.12.2.209. [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006 Feb 1;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer cell. 2003 Oct;4(4):257–62. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 16.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004 Jul 15;22(14):2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 17.Carpten JD, Faber AL, Horn C, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007 Jul 26;448(7152):439–44. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 18.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999 Apr 16;274(16):10963–8. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 19.Graupera M, Guillermet-Guibert J, Foukas LC, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008 May 29;453(7195):662–6. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 20.Foukas LC, Claret M, Pearce W, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006 May 18;441(7091):366–70. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 21.Garofalo RS, Orena SJ, Rafidi K, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003 Jul;112(2):197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knight ZA, Gonzalez B, Feldman ME, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006 May 19;125(4):733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002 Mar;13(3):169–72. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 24.Jia S, Liu Z, Zhang S, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008 Aug 7;454(7205):776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okkenhaug K, Bilancio A, Farjot G, et al. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002 Aug 9;297(5583):1031–4. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki T, Irie-Sasaki J, Jones RG, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000 Feb 11;287(5455):1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 27.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006 Jan 31;103(5):1289–94. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan TL, Choi HS, Matsui A, et al. Class 1A PI3K regulates vessel integrity during development and tumorigenesis. Proc Natl Acad Sci USA. 2008 Jul 15;105(28):9739–44. doi: 10.1073/pnas.0804123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Ramjaun AR, Haiko P, et al. Binding of ras to phosphoinositide 3-kinase p110alpha is required for ras-driven tumorigenesis in mice. Cell. 2007 Jun 1;129(5):957–68. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 30.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 31.Zhao JJ, Liu Z, Wang L, Shin E, Loda MF, Roberts TM. The oncogenic properties of mutant p110alpha and p110beta phosphatidylinositol 3-kinases in human mammary epithelial cells. Proc Natl Acad Sci USA. 2005 Dec 20;102(51):18443–8. doi: 10.1073/pnas.0508988102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samuels Y, Diaz LA, Jr., Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005 Jun;7(6):561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 33.Torbett NE, Luna A, Knight ZA, et al. A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isotype-selective inhibition. Biochem J. 2008;(May 22) doi: 10.1042/BJ20080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sujobert P, Bardet V, Cornillet-Lefebvre P, et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood. 2005 Aug 1;106(3):1063–6. doi: 10.1182/blood-2004-08-3225. [DOI] [PubMed] [Google Scholar]
- 35.Geng L, Tan J, Himmelfarb E, et al. A specific antagonist of the p110delta catalytic component of phosphatidylinositol 3′-kinase, IC486068, enhances radiation-induced tumor vascular destruction. Cancer Res. 2004 Jul 15;64(14):4893–9. doi: 10.1158/0008-5472.CAN-03-3955. [DOI] [PubMed] [Google Scholar]
- 36.Hickey FB, Cotter TG. BCR-ABL regulates phosphatidylinositol 3-kinasep110gamma transcription and activation and is required for proliferation and drug resistance. J Biol Chem. 2006 Feb 3;281(5):2441–50. doi: 10.1074/jbc.M511173200. [DOI] [PubMed] [Google Scholar]
- 37.Pacold ME, Suire S, Perisic O, et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase gamma. Cell. 2000 Dec 8;103(6):931–43. doi: 10.1016/s0092-8674(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 38.Chaussade C, Rewcastle GW, Kendall JD, et al. Evidence for functional redundancy of class IA PI3K isoforms in insulin signalling. Biochem J. 2007 Jun 15;404(3):449–58. doi: 10.1042/BJ20070003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matter WF, Brown RF, Vlahos CJ. The inhibition of phosphatidylinositol 3-kinase by quercetin and analogs. Biochem Biophys Res Commun. 1992 Jul 31;186(2):624–31. doi: 10.1016/0006-291x(92)90792-j. [DOI] [PubMed] [Google Scholar]
- 40.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993 Dec 1;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakanishi S, Kakita S, Takahashi I, et al. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J Biol Chem. 1992 Feb 5;267(4):2157–63. [PubMed] [Google Scholar]
- 42.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994 Feb 18;269(7):5241–8. [PubMed] [Google Scholar]
- 43.Walker EH, Pacold ME, Perisic O, et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000 Oct;6(4):909–19. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 44.Norman BH, Shih C, Toth JE, et al. Studies on the mechanism of phosphatidylinositol 3-kinase inhibition by wortmannin and related analogs. J Med Chem. 1996 Mar 1;39(5):1106–11. doi: 10.1021/jm950619p. [DOI] [PubMed] [Google Scholar]
- 45.Wipf P, Halter RJ. Chemistry and biology of wortmannin. Org Biomol Chem. 2005 Jun 7;3(11):2053–61. doi: 10.1039/b504418a. [DOI] [PubMed] [Google Scholar]
- 46.Gharbi SI, Zvelebil MJ, Shuttleworth SJ, et al. Exploring the specificity of the PI3K family inhibitor LY294002. Biochem J. 2007 May 15;404(1):15–21. doi: 10.1042/BJ20061489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007 Dec 15;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu T, Gu J, Yu K, et al. Pegylated wortmannin and 17-hydroxywortmannin conjugates as phosphoinositide 3-kinase inhibitors active in human tumor xenograft models. J Med Chem. 2006 Feb 23;49(4):1373–8. doi: 10.1021/jm050901o. [DOI] [PubMed] [Google Scholar]
- 49.Ihle NT, Williams R, Chow S, et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Molecular cancer therapeutics. 2004 Jul;3(7):763–72. [PubMed] [Google Scholar]
- 50.Ihle NT, Paine-Murrieta G, Berggren MI, et al. The phosphatidylinositol-3-kinase inhibitor PX-866 overcomes resistance to the epidermal growth factor receptor inhibitor gefitinib in A-549 human non-small cell lung cancer xenografts. Mol Cancer Ther. 2005 Sep;4(9):1349–57. doi: 10.1158/1535-7163.MCT-05-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu K, Toral-Barza L, Shi C, Zhang WG, Zask A. Response and determinants of cancer cell susceptibility to PI3K inhibitors: combined targeting of PI3K and Mek1 as an effective anticancer strategy. Cancer Biol Ther. 2008 Feb;7(2):307–15. doi: 10.4161/cbt.7.2.5334. [DOI] [PubMed] [Google Scholar]
- 52.Garlich JR, De P, Dey N, et al. A vascular targeted pan phosphoinositide 3-kinase inhibitor prodrug, SF1126, with antitumor and antiangiogenic activity. Cancer Res. 2008 Jan 1;68(1):206–15. doi: 10.1158/0008-5472.CAN-07-0669. [DOI] [PubMed] [Google Scholar]
- 53.Knight ZA, Chiang GG, Alaimo PJ, et al. Isoform-specific phosphoinositide 3-kinase inhibitors from an arylmorpholine scaffold. Bioorg Med Chem. 2004 Sep 1;12(17):4749–59. doi: 10.1016/j.bmc.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 54.Billottet C, Grandage VL, Gale RE, et al. A selective inhibitor of the p110delta isoform of PI 3-kinase inhibits AML cell proliferation and survival and increases the cytotoxic effects of VP16. Oncogene. 2006 Oct 26;25(50):6648–59. doi: 10.1038/sj.onc.1209670. [DOI] [PubMed] [Google Scholar]
- 55.Friedman LBMB,L, Chuckoree I.GDC-0941, a Potent, Selective, Orally Bioavaliable Inhibitor of Class 1 PI3K AACR Annual Meeting2008 April Poster Session 18, Board 9 [Google Scholar]
- 56.Fan QW, Cheng CK, Nicolaides TP, et al. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007 Sep 1;67(17):7960–5. doi: 10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogt PK, Kang S. Kinase inhibitors: vice becomes virtue. Cancer Cell. 2006 May;9(5):327–8. doi: 10.1016/j.ccr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 58.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008 Jul;7(7):1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 59.Foster PG. Potentiating the Antitumor Effects of Chemotherapy with the Selective PI3K inhibitor XL147. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2002; October 2007.p. C199. [Google Scholar]
- 60.Laird D. XL765 Targets Tumor Growth, Survival, and Angiogenesis in Preclinical Models by Dual Inhibition of PI3K and mTor. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; October 2007.p. B250. [Google Scholar]
- 61.Tuveson DA, Willis NA, Jacks T, et al. STI571 inactivation of the gastrointestinal stromal tumor c-KIT oncoprotein: biological and clinical implications. Oncogene. 2001 Aug 16;20(36):5054–8. doi: 10.1038/sj.onc.1204704. [DOI] [PubMed] [Google Scholar]
- 62.Sereno M, Brunello A, Chiappori A, et al. Cardiac toxicity: old and new issues in anti-cancer drugs. Clin Transl Oncol. 2008 Jan;10(1):35–46. doi: 10.1007/s12094-008-0150-8. [DOI] [PubMed] [Google Scholar]
- 63.Shipiro GIEG, Calvo E, Aggerwal SK. Targeting Aberrant PI3K Pathway Signaling with XL147, a Potent, Selective, and Orally Bioavaliable PI3K Inhibitor. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 2007; October 2007.p. C205. [Google Scholar]
- 64.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004 Aug;3(8):772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 65.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008 Aug 1;68(15):6084–91. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Howes AL, Chiang GG, Lang ES, et al. The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures. Mol Cancer Ther. 2007 Sep;6(9):2505–14. doi: 10.1158/1535-7163.MCT-06-0698. [DOI] [PubMed] [Google Scholar]
- 67.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008 Feb-Apr;11(12):32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008 Apr 1;26(10):1582–4. doi: 10.1200/JCO.2007.15.3700. [DOI] [PubMed] [Google Scholar]
- 69.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007 Oct;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 70.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell. 2005 Oct;8(4):287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zvelebil MJ, Waterfield MD, Shuttleworth SJ. Structural analysis of PI3-kinase isoforms: Identification of residues enabling selective inhibition by small molecule ATP-competitive inhibitors. Arch Biochem Biophys. 2008;(Jul 8) doi: 10.1016/j.abb.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 72.Amzel LM, Huang CH, Mandelker D, Lengauer C, Gabelli SB, Vogelstein B. Structural comparisons of class I phosphoinositide 3-kinases. Nat Rev Cancer. 2008;(Jul 17) doi: 10.1038/nrc2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic BRaf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008 Feb 26;105(8):3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]