Introduction
Celiac disease is an autoimmune disease caused by the ingestion of gluten. Classically, it presents with diarrhea and failure to thrive within the first couple of years of life. Diagnosis is based on abnormalities of small intestinal biopsy. However, screening for celiac disease can be initially performed using serologic markers with very high sensitivity and specificity for disease such as IgA antibodies to tissue transglutaminase (TG). With the advent of rapid screening methods and increased awareness of celiac disease, an increasing number of individuals who are otherwise asymptomatic (or have subclinical symptoms) are being diagnosed. The clinical manifestations of celiac disease that have been identified are extensive and varied and are no longer isolated to the gastrointestinal tract. Celiac disease has been associated with many other autoimmune conditions including autoimmune thyroid disease and type 1 diabetes.
A series of seminal observations from early-recorded history has led what we now understand as a disease stemming from gluten intolerance. A condition similar to modern day celiac disease was described as early as the first century AD, where the classic symptoms of wasting and diarrhea were noted. By the 19th century, the link between diet and disease was initially appreciated when Dr. Mathew Baillie identified the benefits of a diet consisting largely of rice. In the early 20th century, a new dietary treatment was started consisting of a high-banana diet that helped reduced the previously high mortality of this condition. This diet specifically excluded bread and cereals, and became the cornerstone of therapy for celiac disease over the next several decades, although it was believed at that time that the essential component was the provision of bananas, and not from the exclusion of wheat. A breakthrough came when Professor Dicke in the Netherlands noted a paradoxical improvement in children with celiac disease during bread shortages in World War II. These same children experienced a clinical deterioration when Allied planes later dropped bread into the Netherlands. He subsequently produced a series of papers in the 1950’s that described the association of wheat and rye in the celiac condition. In the same decade, the offending component of wheat was identified by Charlotte Anderson as the “gluten mass” extracted from wheat. Physicians began performing small intestinal biopsies to aid in diagnosis of celiac disease in the 1960’s, and finally in 1997, transglutaminase was identified as the autoantigen in celiac disease (1). These observations have formed the basis for our current understanding of celiac disease and its treatment with a gluten free diet.
Clinical Presentation(Table 1)
Table 1.
Clinical Features of Celiac Disease
| Symptoms | Extraintestinal manifestations | Associated conditions |
|---|---|---|
| Gastrointestinal | Arthritis | Type 1 diabetes |
| Diarrhea | Aphthous stomatitis | Autoimmune thyroid |
| Abdominal pain | Dermatitis Herpetiformis | disease |
| Bloating | Osteoporosis/Osteopenia | Down syndrome |
| Constipation | Elevations in transaminases | Turner syndrome |
| Infertility | IgA deficiency | |
| Nutritional deficiency | Recurrent abortions | IgA nephropathy |
| Anemia –iron deficiency | Neurologic | |
| Folate deficiency | Ataxia | |
| Vitamin D deficiency | Epilepsy | |
| Rickets | Psychiatric | |
| Hypocalcemia | Anxiety | |
| Vitamin K deficiency | Depression | |
| Coagulopathy | ||
| Growth | ||
| Short stature | ||
| Delayed puberty | ||
The clinical presentation of celiac disease is remarkably varied and depends on age (2,3,4,5). The classic presentation with failure to thrive, malnutrition, diarrhea, abdominal pain and distension within the first couple of years of life represents the tip of what is commonly referred to as the “celiac disease iceberg”.
In contrast to the dramatic presentation noted typically in younger children, many patients with celiac disease present at a later age with subtle symptoms and the diagnosis of celiac disease may be delayed. Gastrointestinal symptoms may include abdominal pain, diarrhea or constipation, bloating, and excessive gas. Avoidance of foods containing gluten may also occur and a careful diet history is necessary to identify this symptom. Vitamin deficiencies due to fat malabsorption can also occur. With longer-standing disease, patients may present with profound vitamin D deficiency resulting in rickets or hypocalcemia and tetany or coagulopathy secondary to vitamin K deficiency. Anemia secondary to iron and/or folate deficiency is also observed (2,6,4,5).
Children and adolescents often present with short stature and constitutional delay of puberty. Two to 8% of children and adolescents presenting for evaluation of short stature have evidence of celiac disease (7). Once endocrine causes of short stature have been excluded, rates of celiac disease increase two- to four-fold depending upon the population and referral base studies (7). Access to previous growth points may be useful in the differentiation between constitutional delay of puberty and an underlying pathological cause of short stature such as celiac disease. Children presenting with celiac disease often will experience a decline in both height and weight growth velocity resulting in a decrease in the growth percentiles. In contrast, children presenting with constitutional delay of puberty often have low-normal growth velocity and will have no change in their growth percentiles. In the setting of declining growth percentiles or where the data are not available, the diagnosis of celiac disease should be entertained and testing with autoantibodies performed (8).
Adults have diarrhea as a major symptom of celiac disease in approximately 50% of cases (5). They may also be diagnosed in the setting of anemia or osteoporosis. Adults may be symptomatic for years prior to their diagnosis or have short stature (suggesting long-standing celiac disease). They are often initially misdiagnosed with irritable bowel syndrome and may have had multiple procedures and/or hospital admissions that can ultimately be traced to their undiagnosed celiac disease (5).
Patients identified by screening due to genetic risk factors are often asymptomatic or mildly symptomatic for celiac disease (9). This is the population of individuals with celiac disease that is rapidly growing due to increased screening efforts.
Extra-intestinal Manifestations (Table 1)
We will use the phrase extra-intestinal manifestations of celiac disease to refer to conditions that are associated with celiac disease and are at least partially responsive to a gluten free diet. The distinction from conditions that are associated with celiac disease can be difficult and categorization is not necessarily exact.
Arthritis involving both the peripheral and axial skeletal has been reported in as many as 25% of patients presenting with celiac disease (10). More recent reports suggest a much lower proportion of subjects with celiac disease presenting with arthritis (1%), (3). Therefore, the association is likely dependent upon the ascertainment techniques used and population of patients with celiac disease screened for arthritis. The arthritis is describe as acute and non-erosive and generally resolves with the institution of a gluten free diet. Abnormalities of the dental enamel including pitting and or grooving may be present in up to 20–70% of patients with celiac disease (11,12). The diagnosis of celiac disease may therefore first be raised by the dentist finding dental enamel hypoplasia. The recurrent aphthous stomatitis associated with celiac disease has been attributed to nutritional deficiencies and generally resolves with a gluten free diet (12,13). Abnormalities of liver transaminases occur in up to 40% of patients presenting with celiac disease, resolution of these elevations occurs in the majority of these patients upon treatment (14).
Neurologic and psychiatric disorders including depression, anxiety, irritability, peripheral neuropathy, cerebellar ataxia and migraines have all been reported (15). In a study of over 300 children (111 with celiac disease), approximately 50% of subjects in the celiac disease group had at least one neurologic abnormality, compared with 20% of control subjects (16). Hypotonia, developmental delay, epilepsy, headache, and ataxia were all reported in greater rates in subjects with celiac disease compared with controls. The majority of the reported associations did not improve with a gluten free diet (16).
Pathophysiology (Figure 1)
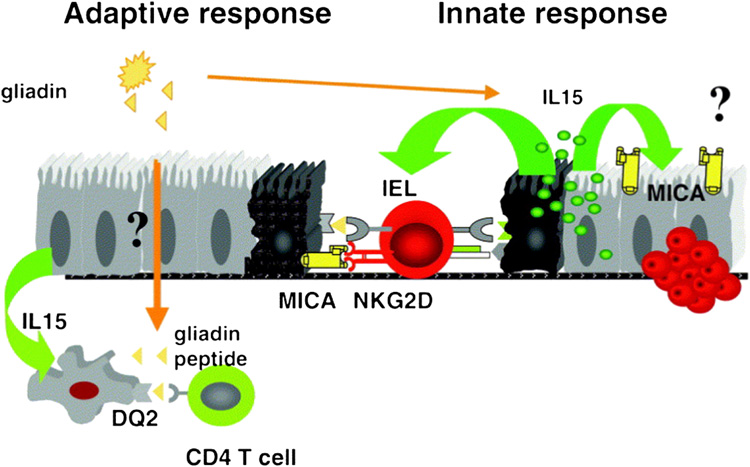
Figure 1.
Gliadin ingestion results in activation of gliadin-reactive T cells in the intestine. These CELIAC DISEASE4 T cells are hypothesized to provide immunologic help to B cells to produce TG autoantibodies, in addition to promoting a favorable cytokine milieu to help drive the celiac disease process. Gliadin ingestion is also directly toxic to the enterocytes, resulting in IL-15 release and subsequent upregulation of MIC-A on the enterocyte surface. Infiltrating intraepithelial lymphocytes also differentiate in the presence of IL-15 into lymphocyte-activated killer cells that are cytolytic to enterocytes through the NKG2D/MIC-A interaction. (From Hum Immunol. 2006 Mar;67(3):204-7. Epub 2006 Mar 31, with permission.)
Celiac disease is unique from other autoimmune diseases in that the there is a clearly identified environmental trigger (gluten), a dominant HLA contribution required for disease to occur (DQ2 or DQ8), and autoantibodies against TG are detectable in over 95% of individuals with celiac disease.
There are multiple proposed pathways involved in the pathogenesis of celiac disease that leads to enterocyte destruction and subsequent villous atrophy – all which are related to dietary gluten, the major storage protein of wheat, barley and rye (see figure 1). 1) Gliadin is a glycoprotein extract from gluten that is felt to be directly toxic to the enterocytes of individuals with celiac disease, primarily through the overexpression of IL-15 in the intestine (17,18). 2) In addition, gliadin peptides have been shown to upregulate both the stress molecule MIC-A on the surface of enterocytes and also the NKG2D receptor on the infiltrating intraepithelial lymphocytes, to promote a lymphocyte-mediated cytotoxic response against enterocytes that is also IL-15 dependent (19). 3) TG is important in the pathogenesis of celiac disease in that the enzyme crosslinks ingested gliadin and causes specific deamidation of glutamine into glutamic acid in gliadin peptides. When such deamidation occurs, the gliadin peptides are able to be more efficiently presented (in the context of MHC DQ2 molecule) to gliadin-reactive CD-4 T cells, therefore increasing its immunogenicity. Without TG, it is believed that gliadin is less immunogenic, and may not stimulate T cells as effectively (20). 4) Finally, since gliadin is unusually rich in proline residues, there is an intrinsic resistance to digestion in the intestines along with a preference for binding to DQ2 molecules. An example is a 33-amino acid residue of gliadin identified to be stable despite digestion with gastric, pancreatic, and intestinal brush-border membrane proteases, with preserved immunogenicity (21). It is believed that the absorption of such larger intact peptides of gliadin allows the immunogenic response to occur.
TG appears to play primarily a molecular role of crosslinking and deamidation of gliadin, with little evidence at this time to support an immunologic role. TG autoantibodies are proposed to occur by antigen presenting cells initially targeting the toxic gliadin peptides “inadvertently” take up TG-gliadin complexes, resulting in an immune reaction against both gliadin and TG (22). It has been proposed that TG autoantibodies play a role in disease pathogenesis, but lacks sufficient supportive evidence.
Therefore, there is a combination of activity by the innate and adaptive immune system in the generation of gliadin-reactive T cells, a cytotoxic response, and autoantibody formation.
Epidemiology (Table 2)
Table 2.
Epidemiology of Celiac disease
| Population | Prevalence of celiac disease |
|---|---|
| Blood donors | 1:100–1:300 |
| General population | |
| Classic presentation | 1:3000–1:5000 |
| Symptomatic | 1:400–1:2000 |
| Asymptomatic screening identified | 1:100 |
| High-risk populations | |
| Type 1 diabetes | 5–10% |
| Homozygous for DRB1*0301/DQB1*0201 | 33% |
| Autoimmune thyroid disease | 8% |
| Down syndrome | 7–16% |
| Turner syndrome | 4–6% |
| First degree relatives of a patient with celiac disease | |
| General populations with DRB1*0301/DQB1*0201 | 3.2% |
Our understanding of the epidemiology of celiac disease has evolved over the last several decades with the advent of serologic tests as screening tools for celiac disease. Celiac disease was initially thought to be relatively rare with prevalence rates of approximately 1:5000 (23). These rates were based on the classic presentation of the disease and the classical presentation is now viewed only one form of celiac disease. It has become clear that these prevalence rates represent the tip of the celiac disease iceberg and the majority of people with celiac disease present with a milder, more insidious onset of symptoms. Screening of blood donors for antibodies associated with celiac disease have shown rates of positive antibodies of approximately 1:133 in non at-risk individuals (3), and certain populations as high as 1:100 (24,25). Other studies using celiac disease related antibodies followed up with small intestinal biopsy have also revealed an overall prevalence of 1% across many different populations. Most of the subjects have mild if any symptoms and would not have been identified without screening. This suggests that for every case identified through symptoms, another eight exist undetected (23). The clinical consequences of an untreated individual with subclinical celiac disease are unclear but may range from none to significant with osteoporosis, infertility and intestinal lymphomas as potential risks.
Given the background prevalence of celiac disease of 1:100, there are populations that are at an increased risk for disease, including patients with type 1 diabetes (26), autoimmune thyroid disease (both hyper- and hypothyroidism) (27,28), relatives of patients with celiac disease (3) and type 1 diabetes and patients with Turner (29,30) and Down syndromes (31). Rates of celiac disease in these populations range from 5–10%.
Serologic Testing (Table 3)
Table 3.
Serologic testing in celiac disease
| Antibody | Sensitivity | Specificity |
|---|---|---|
| Anti-gliadin | 79–90% | 82–95% |
| Anti-endomysial | 85–98% | 97–100% |
| Anti-tissue transglutaminase (IgA) | 95–98% | 94–95% |
| Anti-deamidated gliadin (IgG/IgA) | 95–98% | 94–98% |
Serologic testing can be performed in subjects in whom the diagnosis of celiac disease is entertained, such as those with malabsorption and vitamin or mineral deficiencies, osteoporosis/osteopenia, infertility or other clinical symptoms. It can also be used to screen individuals considered to be at high risk for celiac disease, such as those with type 1 diabetes or first-degree relatives of an affected individual. Finally, serologic testing can also be used to monitor therapy as antibody levels are expected to decline with treatment.
There are multiple antibodies found in celiac disease, but endomysial (EMA) and TG IgA autoantibodies are the most sensitive and specific (4). Antibodies to TG IgA is now considered to be the single best test for the diagnosis of celiac disease (32), and is gradually replacing EMA testing due to high sensitivity and specificity, ease of use, and quantitative capability. Other autoantibodies, including anti-gliadin antibodies are much less specific and are generally not used.
TG autoantibodies can be measured by radioimmunoassay or standard ELISA tests. The level of the TG antibody is associated with results of intestinal biopsy, with higher levels associated with biopsy findings consistent with celiac disease (33). TG antibodies become negative with treatment in some but not all patients, but they generally decrease on a gluten-free diet, and can take up to 2 years to resolve in some case series.
Recently, antibodies against deamidated gliadin peptides (DGP) have been identified. These antibodies are against specific deamidated gliadin peptides and are diagnostically superior to antibodies against the traditional gliadin, which underlines the importance of the deamidation process of gliadin in celiac disease (34). It is important to note that DGP antibodies reflect immunity against the dietary antigen, in contrast to antibodies against TG, which is endogenous. Antibodies against deamidated gliadin peptides parallel antibodies against TG, but resolve more quickly than TG antibodies after institution of a gluten free diet. Therefore, this may be a useful tool in the monitoring of celiac disease treatment (35).
IgA deficiency is more common in subjects with celiac disease (1 in 40) than the general population (1 in 400), which could make serologic detection of celiac disease challenging. Therefore, quantitative measurement of total IgA levels should be considered in individuals in whom celiac disease is suspected, either in conjunction with TG IgA autoantibody measurement, or following a negative test. IgG antibodies to TG and DGP can be used, but with questionable diagnostic accuracy (36). Although the presence of IgA deficiency may affect the utility of these screening tests, some patients with celiac disease and IgA deficiency will still have positive celiac disease related antibodies. When celiac disease is suspected clinically in the presence of IgA deficiency, upper intestinal endoscopy with biopsy should be considered, regardless of autoantibody results.
Diagnosis (37,6) (Figure 2A and 2B)
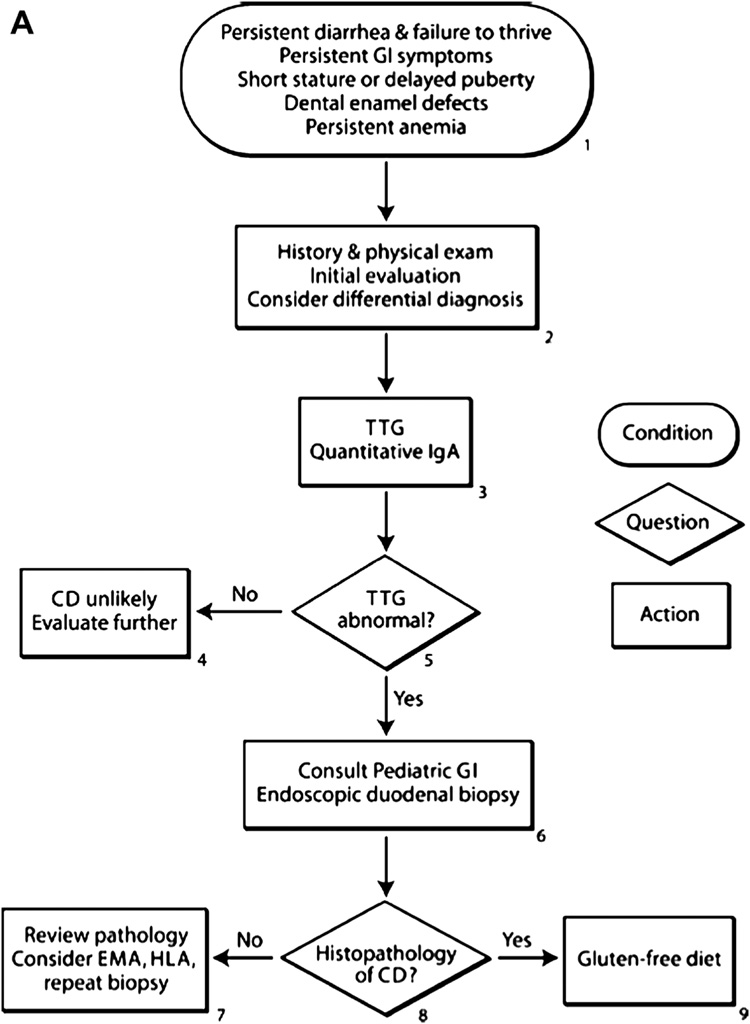
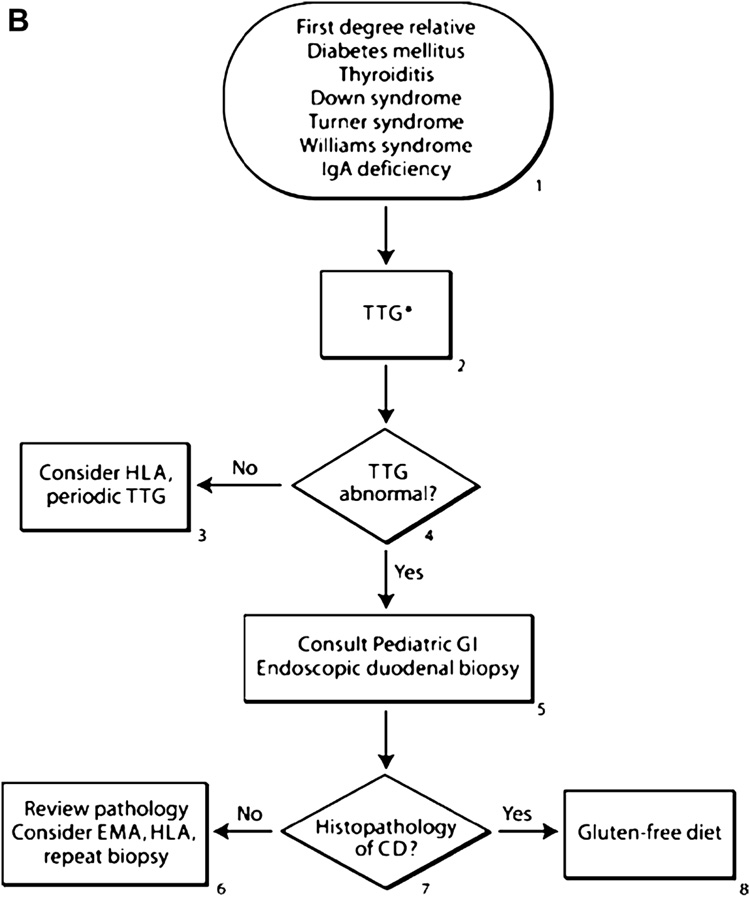
Figure 2.
Proposed algorithms for the diagnosis of celiac disease in patients with symptoms (figure 2A) or those at increased risk (figure 2B). For subjects with symptoms, an initial history and physical exam should be performed evaluating growth and for conditions associated with celiac disease. Screening with a tissue transglutaminase (TTG) antibody and then referral to a gastroenterologist are recommended for small bowel biopsy in TTG positive subjects. In subjects in groups at an increased risk, the first step is screening with TTG antibodies and then referral of positive subjects to a gastroenterologist for further evaluation.
Diagnosis of celiac disease is usually first suggested by the presence of TG autoantibodies, but established by biopsy of the small intestine by upper intestinal endoscopy. Histology will show some degree of villous atrophy and crypt hyperplasia. Intraepithelial lymphocytes are typically seen in celiac disease lesion, but their presence alone is insufficient to diagnose celiac disease. Histologic grading is based on the Marsh scoring system. Normal intestinal histology is scored a Marsh 0. The presence of intraepithelial lymphocytes alone is a Marsh 1. A biopsy specimen with crypt hyperplasia and increased numbers of intraepithelial lymphocytes is a Marsh 2. A specimen with any degree of villous atrophy is a Marsh 3 (38). A Marsh score of 2 or 3 is consistent with celiac disease. Since other conditions can yield similar histologic findings, the presence of infiltrative changes alone (Marsh type 1) on intestinal biopsy is not specific for celiac disease in children. Likewise, positive celiac disease serology (EMA or TG autoantibodies) increases the likelihood such an individual has celiac disease. A diagnosis of celiac disease is considered definitive when there is a complete resolution in symptoms on a gluten-free diet, or when there is histologic improvement on a follow-up intestinal biopsy. In addition, a positive serological test that resolves on a gluten-free diet is helpful in confirming the diagnosis (37).
Approximately 90% of patients with celiac disease will have HLA DQ2 (DQA1*0501, DQB1*0201) — which is generally in tight linkage with the DR3 haplotype identified on serologic testing, and most of the remaining patients (8% to 10%) will have HLA DQ8 (DQA1*0301, DQB1*0302) – associated with the DR4 haplotype by serologic testing (39,40). Therefore, individuals with celiac disease almost always have the DQ2 or DQ8 molecule, such that the absence of either of these molecules by genetic testing virtually excludes the possibility of celiac disease. On the other hand, the presence of either molecule does not guarantee that an individual will develop celiac disease, since DQ2 is present in approximately 30% of the general population, and either DQ2 or DQ8 is present in 40% of the general population (41,42), whereas only about 3% of the general population having DQ2 will develop celiac autoimmunity (43). There is not a large role for genetic testing in celiac disease. Most companies that perform genetic testing will test specifically for the separate A and B chains that, when together, comprise a DQ2 or DQ8 molecule. Interpretation of a genetic test can therefore be very confusing. The high-risk celiac disease allele, DQ2, requires both A1*0501 and B1*0201 chains, while DQ8 requires both A1*0301 and B1*0302 chains to form a complete and functional molecule. It is important to note that the presence of only one chain of the DQ2 or DQ8 molecule does not place an individual at increased risk for celiac disease, since the both chains are needed to form the necessary celiac disease-susceptible MHC molecule.
Treatment
There is a clear and established treatment for celiac disease, which is lifelong avoidance of gluten, found in wheat, rye and barley. There is some controversy as to whether or not oats are safe in individuals with celiac disease, but most evidence supports the safety of oats in a celiac diet, provided there is no cross-contamination with gluten (44). Therefore, it might be wise to establish a positive response to a strict gluten-free diet before allowing the careful addition of oats to a diet. Consultation with a dietician for strict gluten-free diet education is essential in the treatment and follow-up of celiac disease. Dietary history should be carefully elicited with the dietician to look for occult sources of gluten in the diet. Individuals with active celiac disease are at risk for deficiencies of zinc, folate and iron, as well as the fat-soluble vitamins A, D, E and K. Since they are also at risk for decreased bone mineral density, screening for all of these conditions should be considered. Finally, a detailed history and exam should be obtained to look for early signs of associated autoimmune conditions such as thyroid disease, type 1 diabetes, or even pernicious anemia in an older individual. We typically repeat the TG autoantibody and DGP antibodies in 6–12 months to look for a decrease or resolution in the antibodies, and then yearly.
There are studies currently underway investigating the utility of naturally-occurring enzymes to further digest gliadin into smaller, non-pathogenic peptides (45,46). Such drug therapy is aimed at providing individuals with celiac disease safer thresholds for inadvertent gluten ingestion. Other targets for treatment include TG inhibitors, which could potentially reduce the pathogenicity of gliadin that occurs as a result of enzymatic deamidation.
Complications of celiac disease
Celiac disease has been associated with increased risk for fracture and osteoporosis, although the degree of the association varies from strong with an odds ratio for fractures of 5–7 (47) compared with control to relatively minor with an odds ratio of 1.9 (48) for fracture (23). Bone mineral density is noted to be decreased in patients with celiac disease and there is at least some improvement in the bone mineral density with institution of a gluten free diet. However, it has been shown that a gluten free diet instituted late in childhood or adolescence may not be associated with a normalized bone mineral density (49).
Celiac disease has also been associated with decreased fertility, miscarriage and infants with intrauterine growth restriction. However, there are conflicting reports with some studies showing little to no effect of undiagnosed celiac disease on fertility and outcomes of pregnancy (50), while others have shown a significant impact including pre-term birth, caesarean section, low or very low birth rate (51). Therefore, the exact impact of celiac disease on fertility and pregnancy outcomes is unknown. However, the diagnosis of celiac disease should be considered in women presenting with recurrent miscarriage, infertility and/or problems with intra-uterine growth retardation or small for gestational age babies.
Gastrointestinal malignancies including adenocarcinoma of the small intestine and particularly Non-Hodgkins lymphoma are associated with celiac disease. Adenocarcinoma of the small intestine is a rare carcinoma and the risk for this carcinoma is increased in subjects with celiac disease. However, the absolute risk for this cancer is still quite low given its rarity (52). Patients may complain of abdominal pain and/or be found to have occult bleeding and further evaluation for this carcinoma should be considered in patients with celiac disease who develop these signs or symptoms after the resolution of the acute phase of their disease (5). Non-Hodgkin’s lymphoma has also been reported to be increased in patients with celiac disease, although the absolute risk of disease is low (52), and at this time would not in itself justify the need for routine screening for celiac disease in the general population.
Associated conditions (Table 1)
The term “associated conditions” refers to conditions that are found at an increased frequency in celiac disease but that are not thought to be due to gluten ingestion.
Dermatitis herpetiformis is a skin lesion associated with celiac disease characterized by the presence of symmetric papulovesicular lesions on the arms, legs, buttocks, truck, neck and scale. Histology reveals immunofluorescence with granular deposits of IgA. Dermatitis herpetiformis often responds to a gluten free diet.
One of the most intensely studied associations with celiac disease is the association with type 1 diabetes. Approximately 5–10% of patients with type 1 diabetes have positive TG antibodies, with up to 75% having abnormalities on small intestinal biopsy (26,53). Celiac disease and type 1 diabetes share HLA risk genotypes. The highest risk HLA genotypes for type 1 diabetes DQ2 and DQ8 are found in 90% and 8–10% of people with celiac disease respectively. Therefore, the known association between the two disorders is likely largely related to shared genetic risk. Indeed, 33% of patients with type 1 diabetes homozygous for DQ2 are positive for TG autoantibodies (26). In contrast, only 1% of subjects with type 1 diabetes with neither DQ2 nor DQ8 have celiac disease related antibodies (26). Using a combination HLA genotyping and identification of a population (patients with type 1 diabetes), subgroups with very high and low risk for celiac disease can be identified.
Patients with type 1 diabetes and positive celiac disease related antibodies can be identified at onset of diabetes (54). This implies that the two diseases may have developed concurrently or at least in a similar time frame. In some patients, the diagnosis of celiac disease may precede the diagnosis of type 1 diabetes. In a report analyzing the risk for type 1 diabetes in patients with celiac disease (n=9243) are at a 3.9 increased hazard for the development of diabetes by age 20 years (55). Observations such as these have lead to hypotheses regarding a common environmental etiology for celiac disease and type 1 diabetes. Given that the environmental cause of celiac disease is known (gluten) it has been hypothesized that gluten may play an important role in the development of type 1 diabetes. Through prospective studies of high-risk infants for type 1 diabetes and celiac disease, it has been shown that early introduction (prior to 4 months of age) is associated with an increased risk for autoimmunity associated with both conditions (56,57,58). In contrast, diabetes prevention trials using a gluten free diet have not shown any efficacy in delay of diabetes onset (59). Therefore, the relationship between the two disorders is likely quite complex.
The clinical significance of celiac disease in the population with type 1 diabetes remains controversial (60). Many of the patients are asymptomatic or mildly symptomatic when identified with celiac disease related autoantibodies and they represent the portion of the iceberg that is underneath the water (i.e. silent or latent celiac disease). However, abnormalities are found in a high proportion of small intestinal biopsies of patients with diabetes and positive celiac disease related antibodies indicating that a pathologic process is occurring which may present itself clinically during adulthood or carry with it risks for complications of celiac disease including gastrointestinal malignancy or osteoporosis. Often children do not realize they were symptomatic and report that they feel better upon institution of a gluten free diet. A comparison between children with type 1 diabetes and celiac disease and those with type 1 diabetes alone confirms a lower BMI in the group with celiac disease (61) and increased proportion of symptoms (78% reporting at least one symptom compared with 13% of controls) (9). Maintaining a gluten free diet is not a trivial matter, particularly in the setting of type 1 diabetes which has its own set of dietary restrictions, and patients who have not had an obvious symptomatic course for celiac disease may thus be less motiviated to adhere to this diet.
Therefore, significant debate in the literature exists regarding the significance of positive celiac disease-related antibodies in the population with type 1 diabetes and the importance of ongoing screening and treatment with a gluten free diet. Currently, the American Diabetes Association recommends screening for celiac disease related autoantibodies at diagnosis of diabetes and with signs or symptoms of celiac disease (62,63). Our current practice is to screen at onset of diabetes and every 2 years thereafter.
Celiac disease also shares genetic risk factors with autoimmune thyroid disease. Patients with autoimmune thyroid disease are at an increased risk for celiac disease related antibodies. Patients with celiac disease are at an increased risk for autoimmune thyroid disease. In a series of 104 Dutch patients with Hashimoto’s thyroiditis, 8 (7.6%) were positive for TG autoantibodies and 5 (4.8%) had biopsies consistent with celiac disease. In this same report, researchers screened 184 patients with celiac disease and diagnosed hypothyroidism in 22 (12%) with 21% positive for antibodies associated with thyroid disease (28). This some observations holds true for pediatrics, in a series of 90 children with autoimmune thyroid disease, 7 (7.8%) were diagnosed with celiac disease after identification of positive EMA and small intestinal biopsy (27).
There are no current recommendations for screening for celiac disease in the setting of autoimmune thyroid disease, at the very least a complete review of systems should be elicited, growth and pubertal status should be monitored and any evidence for growth failure or symptoms of celiac disease should be addressed with screening for TG autoantibodies. In patients with celiac disease, it is relatively easy to screen for thyroid abnormalities and certainly should be done in the setting of poor growth, weight loss or other signs or symptoms of hypo- or hyperthyroidism.
Celiac disease has also been found at an increased rate in patients with both Turner syndrome and Down syndrome. Celiac disease related antibodies have been identified in between 4–6% of girls with Turner Syndrome, the diagnosis of celiac disease can be made as early as 4 years of age (30,29). Recently published clinical practice guidelines for the care of girls with Turner syndrome recommend screening for celiac disease beginning at age 4 with measurement of TG autoantibodies. It is recommended to repeat this screening every 2–5 years indefinitely (64). In Down Syndrome, celiac disease has been identified during childhood and at rates that are similar to those identified in Turner syndrome (7–16%) (31). Many of these children are mildly or asymptomatic. Current practice guidelines recommend screening at 2–3 years of age with TG autoantibodies with repeat screening in adolescence (65,66).
Conclusion
The clinical spectrum of celiac disease continues to evolve. What was once thought to be a rare disorder effecting young children is now recognized to be very common with a range of symptoms from asymptomatic to severely affected. Screening for celiac disease has become relatively easily with reliable antibodies against self-antigens (TG) and modified environmental antigens (DGP). Diagnosis is confirmed by small intestinal biopsy with characteristic changes graded by the Marsh score. Elimination of gluten from the diet has been the standard of care for the last half-century. Patients often have difficulty adhering to the gluten free diet, and failure of symptoms, antibody levels or pathologic changes to improve after initiating the diet may be largely due to this difficulty.
The genetic risk for celiac disease is largely related to HLA genotypes with over 90% of subjects with celiac disease positive for DQ2 and the remainder positive for DQ8. The HLA association with celiac disease is largely accountable for its link to other autoimmune diseases including type 1 diabetes and autoimmune thyroid disease, in that the majority of risk for celiac disease in these populations is related to HLA genotype. Celiac disease also carries increased risk for both type 1 diabetes and autoimmune thyroid disease. Genetic syndromes such as Turner and Down syndromes are associated with an increased risk for celiac disease. Therefore, practitioners can identify groups of subjects at high risk for celiac disease and screening with celiac disease related antibodies can be performed.
Acknowledgements
Dr. Barker’s work is supported by the Juvenile Diabetes Research Foundation (JDRF grant 11-2005-15). Edwin Liu is supported by NIH K08064605.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology. 2005;128:S68–S73. doi: 10.1053/j.gastro.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 4.Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–188. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 5.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Development Conference on Celiac Disease. NIH Consens State Sci Statements. 2004;21:1–23. [PubMed] [Google Scholar]
- 7.van Rijn JC, Grote FK, Oostdijk W, et al. Short stature and the probability of celiac disease, in the absence of gastrointestinal symptoms. Arch Dis Child. 2004;89:882–883. doi: 10.1136/adc.2004.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catassi C, Fasano A. Celiac disease as a cause of growth retardation in childhood. Curr Opin Pediatr. 2004;16:445–449. doi: 10.1097/01.mop.0000133637.64414.20. [DOI] [PubMed] [Google Scholar]
- 9.Hoffenberg EJ, Emery LM, Barriga KJ, et al. Clinical features of children with screening-identified evidence of celiac disease. Pediatrics. 2004;113:1254–1259. doi: 10.1542/peds.113.5.1254. [DOI] [PubMed] [Google Scholar]
- 10.Lubrano E, Ciacci C, Ames PR, et al. The arthritis of coeliac disease: prevalence and pattern in 200 adult patients. Br J Rheumatol. 1996;35:1314–1318. doi: 10.1093/rheumatology/35.12.1314. [DOI] [PubMed] [Google Scholar]
- 11.Aguirre JM, Rodriguez R, Oribe D, et al. Dental enamel defects in celiac patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:646–650. doi: 10.1016/s1079-2104(97)90367-x. [DOI] [PubMed] [Google Scholar]
- 12.Bucci P, Carile F, Sangianantoni A, et al. Oral aphthous ulcers and dental enamel defects in children with coeliac disease. Acta Paediatr. 2006;95:203–207. doi: 10.1080/08035250500355022. [DOI] [PubMed] [Google Scholar]
- 13.Olszewska M, Sulej J, Kotowski B. Frequency and prognostic value of IgA and IgG endomysial antibodies in recurrent aphthous stomatitis. Acta Derm Venereol. 2006;86:332–334. doi: 10.2340/00015555-0087. [DOI] [PubMed] [Google Scholar]
- 14.Farre C, Esteve M, Curcoy A, et al. Hypertransaminasemia in pediatric celiac disease patients and its prevalence as a diagnostic clue. Am J Gastroenterol. 2002;97:3176–3181. doi: 10.1111/j.1572-0241.2002.07127.x. [DOI] [PubMed] [Google Scholar]
- 15.Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92–S97. doi: 10.1053/j.gastro.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Zelnik N, Pacht A, Obeid R, et al. Range of neurologic disorders in patients with celiac disease. Pediatrics. 2004;113:1672–1676. doi: 10.1542/peds.113.6.1672. [DOI] [PubMed] [Google Scholar]
- 17.Mention JJ, Ben Ahmed M, Begue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 18.Maiuri L, Ciacci C, Auricchio S, et al. Interleukin 15 mediates epithelial changes in celiac disease. Gastroenterology. 2000;119:996–1006. doi: 10.1053/gast.2000.18149. [DOI] [PubMed] [Google Scholar]
- 19.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 20.Molberg O, McAdam S, Lundin KE, et al. T cells from celiac disease lesions recognize gliadin epitopes deamidated in situ by endogenous tissue transglutaminase. Eur J Immunol. 2001;31:1317–1323. doi: 10.1002/1521-4141(200105)31:5<1317::AID-IMMU1317>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 21.Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 22.Sollid LM. Molecular basis of celiac disease. Annu Rev Immunol. 2000;18:53–81. doi: 10.1146/annurev.immunol.18.1.53. [DOI] [PubMed] [Google Scholar]
- 23.van Heel DA, West J. Recent advances in coeliac disease. Gut. 2006;55:1037–1046. doi: 10.1136/gut.2005.075119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffenberg EJ, McKenzie TL, Barriga KJ, et al. A prospective study of the Incidence of Childhood Celiac Disease. Journal of Paediatrics. 2003;143:308–314. doi: 10.1067/s0022-3476(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 25.Maki M, Mustalahti K, Kokkonen J, et al. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 26.Bao F, Yu L, Babu S, et al. One third of HLA DQ2 homozygous patients with type 1 diabetes express celiac disease associated transglutaminase autoantibodies. J Autoimmunity. 1999;13:143–148. doi: 10.1006/jaut.1999.0303. [DOI] [PubMed] [Google Scholar]
- 27.Larizza D, Calcaterra V, De Giacomo C, et al. Celiac disease in children with autoimmune thyroid disease. J Pediatr. 2001;139:738–740. doi: 10.1067/mpd.2001.118189. [DOI] [PubMed] [Google Scholar]
- 28.Hadithi M, de Boer H, Meijer JW, et al. Coeliac disease in Dutch patients with Hashimoto's thyroiditis and vice versa. World J Gastroenterol. 2007;13:1715–1722. doi: 10.3748/wjg.v13.i11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettendorf M, Doerr HG, Hauffa BP, et al. Prevalence of autoantibodies associated with thyroid and celiac disease in Ullrich-Turner syndrome in relation to adult height after growth hormone treatment. J Pediatr Endocrinol Metab. 2006;19:149–154. doi: 10.1515/jpem.2006.19.2.149. [DOI] [PubMed] [Google Scholar]
- 30.Bonamico M, Pasquino AM, Mariani P, et al. Prevalence and clinical picture of celiac disease in Turner syndrome. J Clin Endocrinol Metab. 2002;87:5495–5498. doi: 10.1210/jc.2002-020855. [DOI] [PubMed] [Google Scholar]
- 31.Hansson T, Dahlbom I, Rogberg S, et al. Antitissue transglutaminase and antithyroid autoantibodies in children with Down syndrome and celiac disease. J Pediatr Gastroenterol Nutr. 2005;40:170–174. doi: 10.1097/00005176-200502000-00016. [DOI] [PubMed] [Google Scholar]
- 32.AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977–1980. doi: 10.1053/j.gastro.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu E, Bao F, Barriga K, et al. Fluctuating transglutaminase autoantibodies are related to histologic features of celiac disease. Clin Gastroenterol Hepatol. 2003;1:356–362. doi: 10.1053/s1542-3565(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 34.Agardh D. Antibodies against synthetic deamidated gliadin peptides and tissue transglutaminase for the identification of childhood celiac disease. Clin Gastroenterol Hepatol. 2007;5:1276–1281. doi: 10.1016/j.cgh.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 35.Liu E, Li M, Emery L, et al. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 36.Lenhardt A, Plebani A, Marchetti F, et al. Role of human-tissue transglutaminase IgG and anti-gliadin IgG antibodies in the diagnosis of coeliac disease in patients with selective immunoglobulin A deficiency. Dig Liver Dis. 2004;36:730–734. doi: 10.1016/j.dld.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 37.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Marsh MN. The immunopathology of the small intestinal reaction in gluten-sensitivity. Immunol Invest. 1989;18:509–531. doi: 10.3109/08820138909112260. [DOI] [PubMed] [Google Scholar]
- 39.Farre C, Humbert P, Vilar P, et al. Serological markers and HLA-DQ2 haplotype among first-degree relatives of celiac patients. Catalonian Coeliac Disease Study Group. Dig Dis Sci. 1999;44:2344–2349. doi: 10.1023/a:1026685527228. [DOI] [PubMed] [Google Scholar]
- 40.Karell K, Louka AS, Moodie SJ, et al. HLA types in celiac disease patients not carrying the DQA1*05-DQB1*02 (DQ2) heterodimer: results from the European Genetics Cluster on Celiac Disease. Hum Immunol. 2003;64:469–477. doi: 10.1016/s0198-8859(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 41.Rewers M, Bugawan TL, Norris JM, et al. Newborn screening for HLA markers associated with IDDM: diabetes autoimmunity study in the young (DAISY) Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 42.Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 43.Hoffenberg EJ, Mackenzie T, Barriga KJ, et al. A prospective study of the incidence of childhood celiac disease. J Pediatr. 2003;143:308–314. doi: 10.1067/s0022-3476(03)00282-8. [DOI] [PubMed] [Google Scholar]
- 44.Garsed K, Scott BB. Can oats be taken in a gluten-free diet? A systematic review. Scand J Gastroenterol. 2007;42:171–178. doi: 10.1080/00365520600863944. [DOI] [PubMed] [Google Scholar]
- 45.Gass J, Bethune MT, Siegel M, et al. Combination enzyme therapy for gastric digestion of dietary gluten in patients with celiac sprue. Gastroenterology. 2007;133:472–480. doi: 10.1053/j.gastro.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Mitea C, Havenaar R, Drijfhout JW, et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for celiac disease. Gut. 2007 doi: 10.1136/gut.2006.111609. [DOI] [PubMed] [Google Scholar]
- 47.Fickling WE, McFarlane XA, Bhalla AK, et al. The clinical impact of metabolic bone disease in coeliac disease. Postgrad Med. 2001;77:33–36. doi: 10.1136/pmj.77.903.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.West J, Logan RF, Card TR, et al. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology. 2003;125:429–436. doi: 10.1016/s0016-5085(03)00891-6. [DOI] [PubMed] [Google Scholar]
- 49.Tau C, Mautalen C, De Rosa S, et al. Bone mineral density in children with celiac disease. Effect of a Gluten-free diet. Eur J Clin Nutr. 2006;60:358–363. doi: 10.1038/sj.ejcn.1602323. [DOI] [PubMed] [Google Scholar]
- 50.Tata LJ, Card TR, Logan RF, et al. Fertility and pregnancy-related events in women with celiac disease: a population-based cohort study. Gastroenterology. 2005;128:849–855. doi: 10.1053/j.gastro.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Ludvigsson JF, Montgomery SM, Ekbom A. Celiac disease and risk of adverse fetal outcome: a population-based cohort study. Gastroenterology. 2005;129:454–463. doi: 10.1016/j.gastro.2005.05.065. [DOI] [PubMed] [Google Scholar]
- 52.Green PH, Fleischauer AT, Bhagat G, et al. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–195. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 53.Barker JM, Yu J, Yu L, et al. Autoantibody "sub-specificity" in type 1 diabetes: Risk for organ specific autoimmunity clusters in distinct groups. Diabetes Care. 2005;28:850–855. doi: 10.2337/diacare.28.4.850. [DOI] [PubMed] [Google Scholar]
- 54.Peretti N, Bienvenu F, Bouvet C, et al. The temporal relationship between the onset of type 1 diabetes and celiac disease: a study based on immunoglobulin a antitransglutaminase screening. Pediatrics. 2004;113:e418–e422. doi: 10.1542/peds.113.5.e418. [DOI] [PubMed] [Google Scholar]
- 55.Ludvigsson JF, Ludvigsson J, Ekbom A, et al. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care. 2006;29:2483–2488. doi: 10.2337/dc06-0794. [DOI] [PubMed] [Google Scholar]
- 56.Norris JM, Barriga K, Klingensmith G, et al. Timing of cereal exposure in infancy and risk of islet autoimmunity. The Diabetes Autoimmunity Study in the Young (DAISY) JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 57.Ziegler AG, Schmid S, Huber D, et al. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–1728. doi: 10.1001/jama.290.13.1721. [DOI] [PubMed] [Google Scholar]
- 58.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 59.Hummel M, Bonifacio E, Naserke HE, et al. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25:1111–1116. doi: 10.2337/diacare.25.7.1111. [DOI] [PubMed] [Google Scholar]
- 60.Freemark M, Levitsky LL. Screening for Celiac Disease in Children With Type 1 Diabetes: Two views of the controversy. Diabetes Care. 2003;26:1932–1939. doi: 10.2337/diacare.26.6.1932. [DOI] [PubMed] [Google Scholar]
- 61.Simmons JH, Klingensmith GJ, McFann K, et al. Impact of celiac autoimmunity on children with type 1 diabetes. J Pediatr. 2007;150:461–466. doi: 10.1016/j.jpeds.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 62.American Diabetes Association: clinical practice recommendations 2002. Diabetes Care. 2002;25 Suppl 1:S1–S147. doi: 10.2337/diacare.25.2007.s1. S1–147. [DOI] [PubMed] [Google Scholar]
- 63.Silverstein J, Klingensmith G, Copeland K, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 64.Bondy CA. Care of girls and women with Turner syndrome: A guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 65.Van Cleve SN, Cohen WI. Part I: clinical practice guidelines for children with Down syndrome from birth to 12 years. J Pediatr Health Care. 2006;20:47–54. doi: 10.1016/j.pedhc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 66.Van Cleve SN, Cannon S, Cohen WI. Part II: Clinical Practice Guidelines for adolescents and young adults with Down Syndrome: 12 to 21 Years. J Pediatr Health Care. 2006;20:198–205. doi: 10.1016/j.pedhc.2006.02.006. [DOI] [PubMed] [Google Scholar]