SUMMARY
Insulin resistance and elevated glucagon levels result in non-suppressible hepatic glucose production and hyperglycemia in patients with type 2 diabetes. The CREB co-activator complex controls transcription of hepatic gluconeogenic enzyme genes. Here we show that both the antidiabetic agent metformin and insulin phosphorylate the transcriptional co-activator CBP at serine 436 via PKCι/λ. This event triggers the dissociation of the CREB-CBP-TORC2 transcription complex and reduces gluconeogenic enzyme gene expression. Mice carrying a germline mutation of this CBP phosphorylation site (S436A) demonstrate resistance to the hypoglycemic effect of both insulin and metformin. Obese, hyperglycemic mice display hepatic insulin resistance, but metformin is still effective in treating the hyperglycemia of these mice since it stimulates CBP phosphorylation by bypassing the block in insulin signaling.
INTRODUCTION
Blood glucose levels are maintained within a narrow range by the liver through the opposing actions of insulin and glucagon (Biddinger and Kahn, 2006; Kitamura and Accili, 2004; Saltiel and Pessin, 2002). Impaired insulin signaling together with elevated glucagon levels result in hyperglycemia and subsequent type 2 diabetes mellitus (Baron et al., 1987; Dunning and Gerich, 2007). Inappropriate hepatic glucose production is the major cause of hyperglycemia in these patients.
Glucagon promotes hepatic glucose production by activating cAMP-PKA signaling that induces the phosphorylation of transcription factor CREB at Ser133 and dephosphorylation of TORC2 (also referenced as CRTC2), leading to the formation of the transcriptional CREB-CBP-TORC2 complex (Ravnskjaer et al., 2007; Xu et al., 2007) on a cAMP response element site (CRE). This complex increases transcription of the Ppargc1 (also termed as PGC-1α, the protein product is PGC-1α) gene and its downstream target genes such as the rate-limiting gluconeogenic enzyme genes, phosphoenolpyruvate carboxykinase (Pck1) and glucose-6-phosphatase (G6pc) (Koo et al., 2005; Ravnskjaer et al., 2007). Insulin counteracts the action of glucagon on hepatic gluconeogenesis mainly at the transcriptional level through PGC-1α (Yoon et al., 2001). The CREB-CBP-TORC2 complex binds to CRE site of Ppargc1 through transcription factor CREB, and both CBP and TORC2 are co-activators for CREB.
CBP was originally identified as a CREB binding protein that interacts with the CREB KID domain (Radhakrishnan et al., 1997; Goodman and Smolik, 2000). CBP, which contains intrinsic histone acetyltransferase (HAT) activity, also interacts with various components of the basal transcription machinery such as TATA box binding protein (TBP), TFIIB and with cell-specific transcription factors (Goodman and Smolik, 2000). TORC2, in contrast, binds to the bZIP domain of CREB in a phosphorylation-independent manner, and augments CRE-dependent transcription (Conkright et al., 2003). The phosphorylation of CREB at Ser133 is critical for the recruitment of CBP to CREB. However, both glucagon and insulin were documented to stimulate phosphorylation at this site (Koo et al., 2005). We have previously shown that insulin stimulates CBP phosphorylation at Ser436 and a germline mutation of this CBP phosphorylation site (S436A) in a mouse model leads to inappropriate activation of gluconeogenesis and glucose intolerance (Zhou et al., 2004). Insulin also stimulates the phosphorylation of TORC2 at Ser171 leading to its nuclear exclusion and degradation (Dentin et al., 2007). It remains unclear, however, which insulin signaling event determines the disassembly of CREB-CBP-TORC2 complex.
Metformin was introduced clinically in the 1950s and is widely used as a first line treatment for patients with type 2 diabetes mellitus. Metformin improves glucose metabolism mainly by suppressing hepatic glucose production (Hundal et al., 2000), but its mechanism of action remains elusive. Metformin has been reported to stimulate the phosphorylation and nuclear exclusion of TORC2 (Shaw et al., 2005); however, since TORC2 is O-glycosylated at Ser171 in the insulin resistant state (Dentin et al., 2008), phosphorylation at this site is unlikely to be the mechanism of action of metformin in patients with diabetes. Here we show that CBP phosphorylation at Ser436 by insulin and metformin requires aPKCι/λ, which has been previously reported to mediate insulin induced lipogenesis in the liver (Matsumoto et al. 2003). This event is essential for the dissociation of CREB-CBP-TORC2 complex. Only metformin is able to stimulate CBP phosphorylation in a mouse obesity model of insulin resistance, suggesting a mechanism for its therapeutic effect.
RESULTS
The essential role of CBP in cAMP-PKA mediated activation of gluconeogenic gene expression
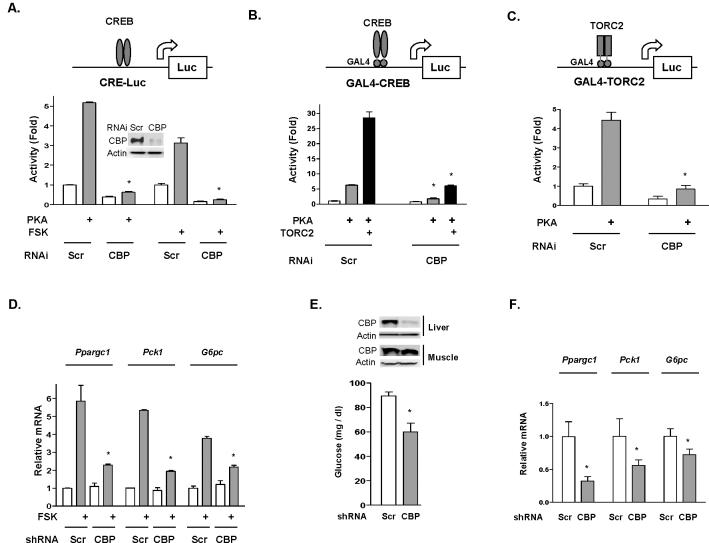
In response to the argument that recruitment of CBP to CREB target genes is unnecessary for induction of the gluconeogenic program (Koo et al., 2005), we examined the potential role of CBP in mediating CREB target gene expression. First, we used a cAMP response element (CRE)-luciferase reporter assay in a transiently transfected hepatocyte cell line. Forskolin (FSK) treatment or co-transfection with an expression vector containing the catalytic subunit of PKA increased luciferase activity by approximately 5-fold (Figure 1A). RNAi-mediated CBP knockdown abolished the effects of both PKA and FSK on the CRE-responsive reporter. We next tested GAL4 fusion constructs of full length CREB cDNA and TORC2 cDNA on a GAL4 reporter. RNAi-mediated knockdown of CBP protein levels blocked the effect of co-transfected PKA on either fusion construct (Figures 1B and 1C); additionally, knock-down of CBP blocked the effect of co-transfected TORC2 (Figure 1B). FSK stimulation of Ppargc1α, Pck1 and G6pc mRNA levels was also significantly suppressed by adenoviral shRNA-mediated knockdown of CBP in primary hepatocyte cells (Figure 1D). Furthermore, adenoviral shRNA-mediated knockdown of CBP protein in the liver of fasted mice decreased both glucose levels (Figure 1E) and the expression of gluconeogenic genes (Figure 1F). The above data indicate that CBP plays an essential role in mediating CREB target gene expression in the hepatocyte.
Figure 1.
CBP is essential to the activation of cAMP-PKA responsive genes in hepatocyte cells. (A) PKA or forskolin (FSK, 3h) stimulates co-transfected CRE-luciferase reporter activity. RNAi mediated CBP knockdown (insert) abolishes PKA and FSK effects. Scr, scrambled RNAi. (B) GAL4-CREB (full length) and GAL4-TORC2 (C) transactivation assays. Both PKA and TORC2 effects are abolished upon CBP knockdown. (D) Adenoviral shRNA-mediated knockdown of CBP suppresses FSK stimulated transcription of endogenous Ppargc1αPck1 and G6pc in mouse primary hepatocyte cells. Primary hepatocyte cells were cultured in serum-free DMEM, and incubated with vehicle or 10 uM FSK for 6 h before the harvest. Mice with adenoviral shRNA-mediated CBP knockdown in liver demonstrates lower fasting blood glucose (E) and decreased gluconeogenic gene expression (F). mRNA measurements are normalized to 18S rRNA levels. Means ± SEM are shown. Asterisk (*) signifies that groups (CBP knockdown vs control) with same treatment are significantly different (P<0.05).
CBP phosphorylation at Ser436 by insulin and metformin is mediated through atypical protein kinase C
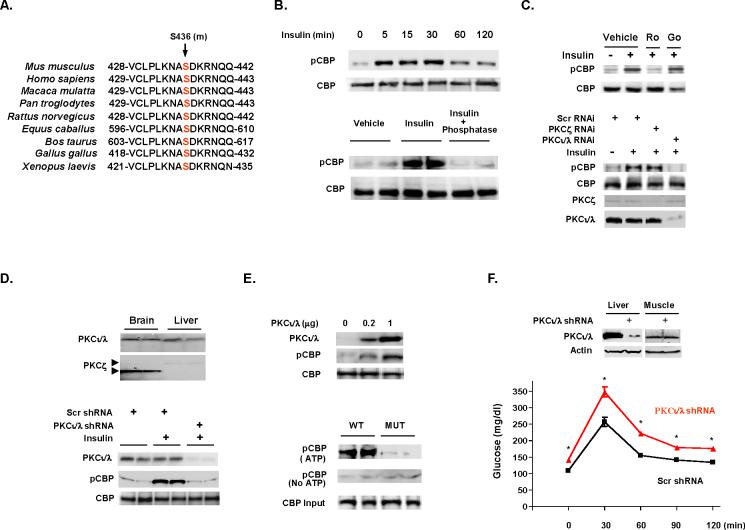
We previously reported that insulin regulates hepatic gluconeogenesis through CBP phosphorylation at Ser436 (Zhou et al., 2004). This CBP phosphorylation site is conserved in eukaryotic species (Figure 2A), suggesting that phosphorylation at this site may be a universal mechanism for controlling CBP responsive gene transcription. We therefore developed a phospho-specific CBP antiserum to this site. In a hepatocyte cell line, insulin stimulated CBP phosphorylation in a rapid (within 5 min) (Figure 2B, top) and a concentration-dependent manner (Figure S1). In fasted mice, insulin stimulated CBP phosphorylation dramatically in liver extracts, and treatment with alkaline phosphatase eliminated the phospho-CBP signal (Figure 2B, bottom). This antiserum was proven to be specific for phosphorylation of this site and does not cross-react with the related p300 protein (Figures S2 and S3). The PI3 kinase inhibitor (LY294002) blocked insulin mediated CBP phosphorylation in hepatocyte cells (Figure S4), indicating that insulin stimulates CBP phosphorylation through the PI3K pathway.
Figure 2.
Insulin induces CBP phosphorylation through aPKC. (A) CBP phosphorylation site at Ser436 (mouse) is conserved across eukaryotic species. (B) Insulin induces CBP phosphorylation in a time-dependent manner in H4IIEC3 cells (top). Hepatic CBP phosphorylation is detected after insulin administration in fasted mice and alkaline phosphatase treatment of hepatic lysates removes phosphorylation (bottom). Each lane represents an individual mouse sample. (C) Nonisoform selective PKC inhibitor Ro31-8220 (Ro), but not Go6976 (Go; inhibitor of classical and novel PKC isoforms) inhibits insulin stimulated CBP phosphorylation (top). RNAi mediated aPKCι/λ knockdown blocks insulin stimulated CBP phosphorylation (bottom). (D) Relative amount of aPKCs in brain and liver (top), adenoviral shRNA-mediated CBP knockdown in liver blocked insulin induced CBP phosphorylation (bottom). (E) Overexpression of aPKCι/λ stimulated CBP phosphorylation (top). Only immunoprecipitated CBP from wild type mouse (fasted) liver extracts can be phosphorylated by aPKCι/λ in the presence of ATP (bottom). (F) Knockdown aPKCι/λ by adenoviral shRNA in liver resulted in glucose intolerance (2 g/kg ip in 8 h fasted mice). Means ± SEM are shown. Asterisk (*) signifies p<0.05 as compared with control at each time point. Scr, scrambled RNAi.
CBP Ser436 is part of a potential consensus PKC phosphorylation site (435-ASDKR-439) (Figure 2A). Interestingly, the related p300 protein contains the identical amino acid sequence as CBP in the region, except that a glycine residue (Gly422) is substituted for the serine residue (Ser436). This difference is suggested to affect the length of the α4 helix in the C/H1 domain of the proteins (De Guzman et al., 2004). To define the class of PKC is responsible for CBP phosphorylation at Ser436, we treated hepatocyte cells with two PKC inhibitors. The non-isoform selective PKC specific inhibitor, Ro31-8220, blocked insulin induced CBP phosphorylation; however, an inhibitor of classical and novel PKC isoforms, Go6976, had no effect on insulin induced CBP phosphorylation (Figure 2C, top). These data suggest that insulin mediates CBP phosphorylation through atypical PKCs (aPKCs). Atypical PKCs (ζ, ι/λ) (are conserved across species and are ubiquitously expressed in most tissues (Selbie et al., 1993; Akimoto et al., 1994), and aPKCι and λ are human mouse orthologues (Nishizuka, 1995). As shown in Figure 2C (bottom), RNAi-mediated knockdown of aPKCι/λ in hepatocyte cells blocked CBP phosphorylation by insulin; in contrast, RNAi-mediated knockdown of aPKCζ had no effect on insulin induced CBP phosphorylation, suggesting that this aPKCι/λ mediates insulin-induced CBP phosphorylation. Western blot analysis of brain and liver tissue from mice demonstrates comparable amounts of aPKCι/λ in the liver and brain (Figure 2D); however, consistent with a previous finding (Selbie et al., 1993), there is significantly less aPKCζ in the liver than in the brain. Further, knockdown of aPKCι/λ in the liver using an adenoviral shRNA blocked insulin induced CBP phosphorylation in the liver (Figure 2D, bottom). In cells, transfection of increasing amount of a CMV expression vector containing aPKCι/λ led to CBP phosphorylation (Figure 2E, top). In an in vitro phosphorylation assay, purified aPKCι/λ phosphorylated CBP of liver tissue extract from fasted wild-type mice, but not from CBP mutant (S436A) mice (Figure 2E, bottom). Finally, mice injected with an adenoviral aPKCι/λ shRNA exhibited fasting hyperglycemia (Scrambled shRNA vs CBP shRNA: 109±3.3 vs 142±2.9 mg/dl, P<0.05) and glucose intolerance (Figure 2F), supporting the notion that aPKCι/λ phosphorylates CBP and modulates glucose metabolism (Chen et al., 2002).
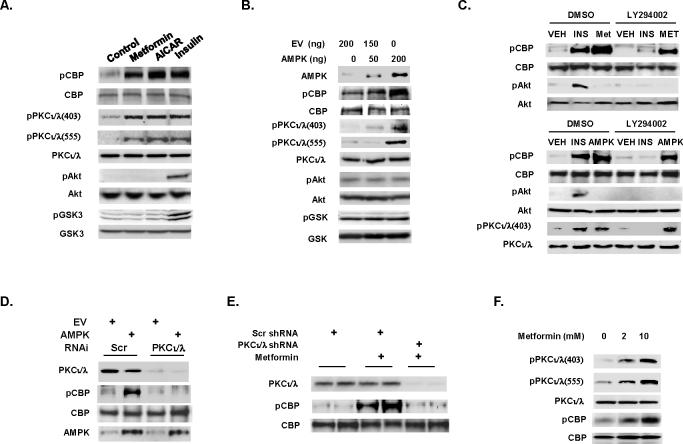
The AMPK activator, 5-aminoimidazole-4-carboxamide-1-β-D-riboside (AICAR), also activates aPKCs (Chen et al., 2002), which prompted us to evaluate the effect of AICAR and another AMPK activator, metformin, on CBP phosphorylation. Both AICAR and metformin stimulated CBP phosphorylation in a hepatocyte cell line (Figure 3A), and in contrast to insulin, did not cause phosphorylation of either AKT or GSK3. The antidiabetic effect of metformin requires AMP-activated protein kinase (AMPK) (Zhou et al., 2001; Shaw et al., 2005), and AMPK can activate aPKCs, which increase glucose transport in muscle (Chen et al., 2002). We co-transfected increasing amounts of an AMPK expression vector in hepatocyte cells, and this resulted in an increase in CBP phosphorylation (Figure 3B), and either metformin treatment or AMPK overexpression led to the CBP phosphorylation even in the presence of the PI3K inhibitor LY294002 (Figure 3C). In contrast, RNAi-mediated knockdown of aPKCι/λ blocked the ability of metformin or AMPK to stimulate CBP phosphorylation (Figure S5 and Figure 3D, respectively). In vivo, metformin like insulin stimulated CBP phosphorylation in the mouse liver (Figure 3E) and this effect was blocked in mice injected with adenoviral shRNA against aPKCι/λ.
Figure 3.
Metformin stimulates CBP phosphorylation through aPKCι/λ. (A) Metformin, AICAR, insulin and (B) AMPK overexpression stimulate CBP phosphorylation. (C) PI3K inhibitor LY294002 fails to inhibit CBP phosphorylation. CBP phosphorylation is blocked by RNAi or adenoviral shRNA-mediated knockdown aPKCι/λ in hepatocyte cells (D) or in liver (E). (F) Metformin treatment (5 h) in hepatocyte cells stimulates hepatic CBP phosphorylation in a concentration-dependent manner.
Phosphorylation plays an important role in activating PKC activity (Parekh et al., 2000; Newton, 2004). For this reason, we examined the phosphorylation status of aPKCι/λ. Metformin and AMPK overexpression stimulated phosphorylation of aPKCι/λ at Thr403 and Thr555, which are located in the activation loop of kinase domain and in the turn motif, respectively (Figures 3A-3C and 3F). Phosphorylation at Thr403 has been shown to be critical for kinase activity of aPKCι/λ (Messerschmidt et al., 2005). The PI3K inhibitor had no effect on AMPK induced aPKCι/λ phosphorylation (Figure 3C). Thus, metformin, AMPK and insulin mediate CBP phosphorylation by activated aPKCι/λ through increasing its phosphorylation at Thr403 and Thr555.
Control of hepatic gluconeogenesis through phosphorylation of CBP at Ser436 by insulin and metformin
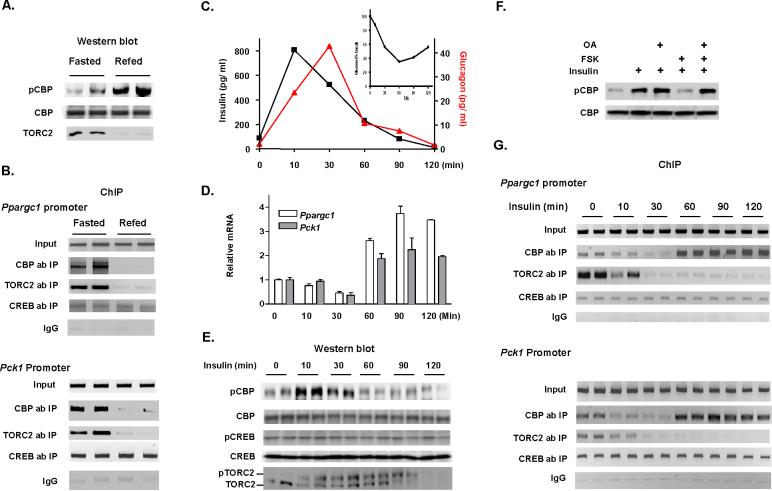
Insulin stimulates CREB phosphorylation at Ser133 (Koo et al., 2005), and stimulates TORC2 phosphorylation at Ser171 (Koo et al., 2005; Shaw et al., 2005). Alternatively, we provide evidence that insulin stimulates CBP phosphorylation at Ser436 (Figure 2B). It is important, therefore, to understand the relative importance of phosphorylation of members of the CREB-CBP-TORC2 complex. Since insulin counteracts the action of glucagon on hepatic gluconeogenesis mainly at the transcriptional level through the transcriptional co-activator PGC-1α (Yoon et al., 2001), we evaluated the binding states of CREB, CBP and TORC2 to promoters of Ppargc1α and Pck1 in liver tissue from fasted and refed mice. In this study, increased CBP phosphorylation and TORC2 degradation in refed mice (Figure 4A) coincided with the absence of both CBP and TORC2 from the Ppargc1α and Pck1 promoters as compared to fasted mice (Figure 4B). However, CREB binding to these promoters did not change, suggesting that the binding of co-activators (CBP and TORC2) to the complex determines Ppargc1α and Pck1 gene expression during fasting and refeeding.
Figure 4.
Control of hepatic gluconeogenesis is through CBP phosphorylation by insulin. (A) CBP phosphorylation increases and TORC2 protein levels decrease in hepatic lysates from fasted (16 h) or refed mice (2 h), accompanied by (B) reduced CBP and TORC2 occupancies on the CREs of Ppargc1α and Pck1 promoters. (C) Serum insulin, glucagon and blood glucose levels (inset) in fasted mice during the insulin time course experiment. (D) Relative mRNA levels of Ppargc1 and Pck1 increase as glucose recovers after insulin induced hypoglycemia. (E) Insulin rapidly stimulates CBP phosphorylation and TORC2 degradation in 16 h fasted mice. (F) Forskolin antagonizes insulin induced CBP phosphorylation which is blocked by okadaic acid in hepatocyte cells. (G) The change in CBP occupancy and reduced TORC2 on CREs of Ppargc1α and Pck1 promoters in liver ChIP assays. Each lane represents an individual mouse sample (A, B, E, G). Means ± SEM are shown (C, D).
To explore further the phosphorylation and promoter occupancy of CREB, CBP and TORC2 in the control of hepatic glucose production, we conducted insulin time course experiments in fasted wild type mice. In this experiment, blood glucose levels fell sharply after intraperitoneal administration of insulin within the first 30 min, and subsequently blood glucose levels began to rise (Figure 4C, insert). Serum insulin level reached its peak at 10 min after intraperitoneal insulin injection; we then observed robust glucagon secretion which reached a peak at 30 min after insulin exposure in response to the insulin induced hypoglycemia (Figure 4C). Hepatic mRNA expression pattern of Ppargc1α and Pck1 were very similar to the changes in blood glucose levels (Figure 4D). In liver tissue, the extent of CREB phosphorylation at Ser133 remained unchanged during the entire experiment (Figure 4E). These data are consistent with the notion that both insulin and glucagon induce phosphorylation of CREB at Ser133 (Koo et al., 2005). Insulin stimulated CBP phosphorylation within 10 min of treatment; at subsequent time points, CBP phosphorylation decreased (Figure 4E). The reduction of CBP phosphorylation at later time points after insulin treatment accompanied by robust glucagon secretion indicates glucagon may be responsible for the dephosphorylation of CBP (Figures 4C, 4E). Indeed, pretreatment of hepatocyte cells with forskolin blocked insulin-induced CBP phosphorylation (Figure S6), and the phosphatase inhibitors okadaic acid and calyculin A blocked the effect of forskolin leading to increased CBP phosphorylation (Figures 4F and S7). Consistent with previous studies (Dentin et al., 2007), the peak of TORC2 phosphorylation occurred at a later time (30 min) than peak CBP phosphorylation after insulin exposure, and TORC2 phosphorylation led to its degradation (Figure 4E).
Chromatin immunoprecipitation (ChIP) assay of hepatic tissue was then used to determine the occupancy of CREB, CBP and TORC2 on the CRE site of the Ppargc1α and Pck1 gene promoters. The binding of CBP and TORC2 started to decrease 10 min after insulin exposure and was absent from the Ppargc1α and Pck1 promoters 30 minutes after insulin treatment. In contrast to TORC2, however, CBP returned to these promoters at 60 min (Figure 4G), coinciding with an ~4-fold increase in mRNA expression of Ppargc1α and Pck1 (Figure 4D). No change in CREB binding to the Ppargc1α and Pck1 promoters was observed (Figure 4G). These data strongly support the critical role for CBP in transcriptional regulation of gluconeogenic enzyme genes.
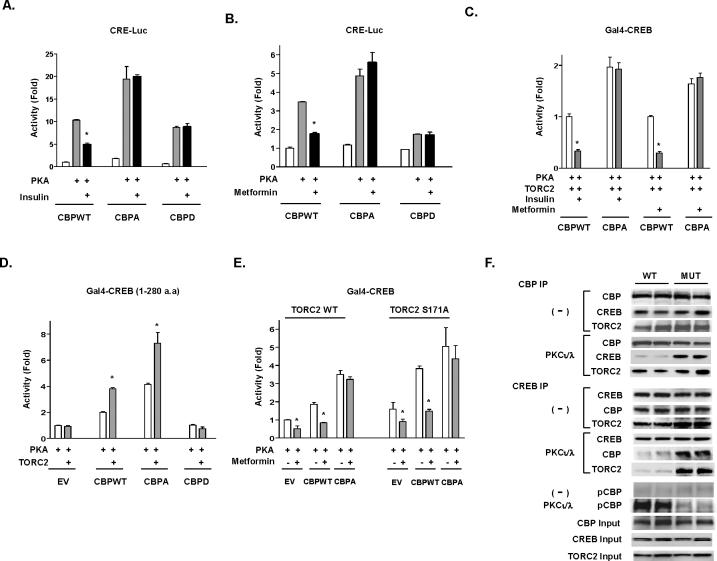
It is important to note that CBP phosphorylation was inversely correlated with CBP occupancy on Ppargc1α and Pck1 promoters, suggesting that CBP phosphorylation at Ser436 might play an important role in dissociating and reforming the CREB-CBP-TORC2 complex after insulin treatment. To test this hypothesis, we compared wild-type and mutant CBP expression vectors in transient transfection studies. We found that insulin treatment decreased the activity of a CRE-responsive reporter in cells co-transfected with wild type CBP (P<0.01), but not with CBP mutants (Figure 5A). Insulin action on the CRE-responsive reporter was abolished by treatment with a PI3 kinase (PI3K) inhibitor (LY294002) (Figure S8), suggesting that in this context insulin acts through the PI3K pathway. Metformin, like insulin, decreased CRE-mediated luciferase activity in cell cultures co-transfected with wild type CBP (P<0.01), but not CBP mutant expression vectors (Figure 5B). The above data indicate that Ser436 of CBP plays a key role in mediating the actions of insulin and metformin in the regulation of gluconeogenic enzyme gene expression.
Figure 5.
Insulin and metformin modulate the activation of cAMP-PKA responsive genes through CBP phosphorylation. (A) Insulin and metformin (B) fail to suppress PKA stimulated CRE responsive transcription in the presence of CBP S436 mutants in H2.35 cells. (C) Insulin and metformin suppress PKA and TORC2 stimulated GAL4-CREB (full length) transcription in the presence of wild type CBP, but not of CBP S436A mutant in H4IIEC3 cells. (D) GAL4 fused to a shortened CREB (1-280 aa), which has the CBP binding domain but lacks the TORC2 interacting domain requires CBP WT or CBP S436A for TORC2 mediated transcription stimulation. CBP S436 phosphorylation mimicked by CBP S436D mutant abolishes TORC2 mediated stimulation. EV, empty control vector. (E) Metformin reduction of GAL4-CREB transactivation requires intact Ser436 on CBP and is independent of TORC2 S171 phosphorylation. Bars = means ± SEM. Asterisk (*) signifies p<0.01 as compared with other PKA transfected groups. (F) Hepatic lysates from overnight fasted wild type mice or CBP mutant mice were immunoprecipitated with either a CBP antibody (upper panel) or CREB antibody (lower panel), immunoprecipitates were treated with or without aPKCι/λ kinase, washed and blotted with indicated antibody.
To examine this hypothesis more directly, a hepatocyte cell line was co-transfected with wild type or mutant (S436A) CBP and TORC2 expression vectors in a GAL4-CREB (full length) reporter assay. Both insulin and metformin treatment decreased reporter activity significantly (P<0.01) in cells co-transfected with wild type CBP (Figure 5C). However, the effect of insulin and metformin on GAL4-CREB (full length) reporter activity was abolished in cells co-transfected with mutant (S436A) CBP. These data indicate that Ser436 of CBP plays a dominant role in the action of insulin and metformin on GAL4-CREB complex in this reporter assay and raise the possibility that the phosphorylation of CBP at Ser436 is the key event leading to dissociation of both CBP and TORC2 from the CREB-CBP-TORC2 complex.
TORC2 co-immunoprecipitated with CBP (Ravnskjaer et al., 2007), indicating that TORC2 might associate with CBP as well in the CREB-CBP-TORC2 complex. To test this possibility, we co-transfected wild type or CBP mutants into a hepatocyte cell line with TORC2 and a GAL4-CREB construct (1-280 a.a.), which lacks the TORC2 binding domain (Figure 5D). We found that TORC2 significantly enhanced reporter activity when co-transfected with wild type CBP and had an even greater effect when co-transfected with mutant (S436A) CBP. No effect on reporter activity was found with co-transfection of mutant (S436D) CBP, which mimics phosphorylated CBP (Figure 5D). These results suggest that TORC2 associates directly with CBP in the CREB-CBP-TORC2 complex. To examine this further, a hepatocyte cell line was co-transfected wild type or mutant (S436A) CBP and either wild type or mutant TORC2 (S171A)-a functional nuclear resident, non-phosphorylated form of TORC2 (Koo et al., 2005) in a GAL4-CREB (full length) reporter assay. Metformin antagonized the stimulatory effect of PKA and CBP, even in the presence of mutant TORC2 (S171A), while no antagonism was observed with CBP S436A (Figure 5E).
To support the hypothesis that CBP phosphorylation leads to the dissociation of the CREB-CBP-TORC2 complex, we conducted in vitro phosphorylation assays using a purified aPKCι/λ kinase preparation after immunoprecipitation of liver extracts with either a CBP or CREB antibody (Figure 5F). Without aPKCι/λ kinase treatment, CREB and TORC2 co-immunoprecipated with either wild type or mutant CBP (upper panel); and either wild type or mutant CBP and TORC2 co-immunoprecipated with CREB (lower panel). After aPKCι/λ kinase treatment, in contrast, only TORC2 co-immunoprecipitated with wild type CBP (upper panel); and both wild type CBP and TORC2 dissociated from CREB (lower panel). However, aPKCι/λ kinase treatment of mutant CBP liver extract with either antibody demonstrated an intact CREB-CBP-TORC2 complex. These results strongly support a model where CBP phosphorylation at Ser436 is the triggering event for dissociation of the CREB co-activator complex.
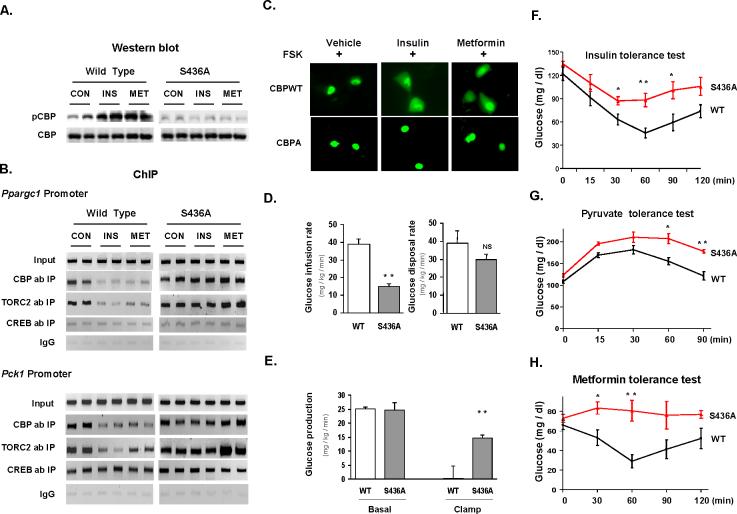
To support further this model, we determined the binding states of CBP and TORC2 in CBP S436A mutant mice treated with insulin or metformin. As expected, both insulin and metformin stimulated CBP phosphorylation in the liver of wild type mice, but not in the liver of CBP mutant mice (Figure 6A). As illustrated in Figure 6B, treatment with insulin or metformin led to CBP and TORC2 dissociation from the Ppargc1α and Pck1 promoters in wild type mice (Figure 6B, left). If the phosphorylation of CBP by insulin or metformin leads to dissociation of the CREB-CBP-TORC2 complex, then mutation of CBP (S436A) should prevent dissociation of the CREB-co-activator complex by these agents. We next preformed a ChIP assay in liver tissue from CBP mutant mice. In these mutant mice, neither insulin nor metformin affected CBP or TORC2 occupancy on the Ppargc1α and Pck1 promoter (Figure 6B, right) even though TORC2 was also phosphorylated in the CBP mutant mice by either insulin or metformin (Figure S9). To provide further evidence to support the hypothesis that CBP phosphorylation triggers the dissociation of TORC2 from the CREB co-activator complex, hepatocyte cells were co-transfected with GFP-TORC2 and either wild-type or mutant CBP S436A. Both GFP-TORC2 and CBPs are equally expressed in the hepatocyte cells in each group (data not shown). Co-expression of wild-type CBP with GFP-TORC2 resulted in nuclear localization of GFP-TORC2 after exposure to forskolin and its nuclear export after insulin or metformin treatment confirming previous reports (Koo et al., 2005; Shaw et al., 2005). Co-expression of CBPS436A and GFP-TORC2, in contrast, blocked nuclear export of GFP-TORC after these treatments (Figure 6C). These data pinpoint the crucial role of CBP phosphorylation in controlling the dissociation of the CREB co-activator complex.
Figure 6.
CBP S436A mutation perturbs in vivo actions of insulin and metformin. (A) Insulin and metformin stimulate CBP phosphorylation in WT but not CBP S436A mouse liver. (B) CBP and TORC2 occupancies to CREs of Ppargc1α and Pck1 promoters are reduced by insulin or metformin in WT but not CBP S436A mouse liver. Each lane represents an individual mouse sample (A, B). (C) Co-transfection with S436A mutant but not WT CBP retains GFP-TORC2 in the nucleus after insulin or metformin treatment. H4IIEC3 cells were pretreated with forskolin plus 200 nM dexamethasone for 5 h, then treated with insulin or metformin for 3 h. (D) Glucose infusion rate and glucose disposal rate during a hyperinsulinemic euglycemic clamp experiment. (**p<0.01; NS, not significant). (E) Hepatic glucose production in the basal period and in response to insulin infusion during clamp experiment (n=4) (**p<0.01 as compared with control mice during the clamp). (F) Insulin tolerance test (0.35 unit/kg ip in 4 h fasted WT and CBP S436A mice (n=5)). (G) Pyruvate tolerance test (2 g/kg) in 6 h fasted WT and CBPS436A mice (n=4). (H) Metformin tolerance test in 10 h fasted WT and CBPS36A mice (n=7). *p<0.05, **p<0.01 as compared with control mice at the same time point (F,G,H). Means ± SEM are shown (D-H).
The above results offer an explanation for enhanced gluconeogenic enzyme expression, inappropriate activation of gluconeogenesis, and insulin intolerance in mutant (S436A) CBP mice (Zhou et al., 2004). Fasting blood glucose and serum insulin levels were elevated in CBP mutant mice, indicating the insulin resistance in CBP mutant mice (Zhou et al., 2004). During the hyperinsulinemic euglycemic clamp experiment, glucose infusion rates were significantly reduced in CBP mutant mice (Figure 6D, left), and glucose disposal rates were somewhat lower, but not statistically different in CBP mutant versus wild type mice (Figure 6D, right). In response to insulin, hepatic glucose production was completely suppressed in wild type control while a significant amount of glucose production remained in CBP mutant mice (Figure 6E). Moreover, CBP mutant mice exhibited both insulin and pyruvate intolerance (Figure 6F, 6G). The above data clearly demonstrate significant hepatic insulin resistance in CBP mutant mice. CBP phosphorylation was stimulated by metformin both in vitro and in vivo (Figures 3A, 3C, 3E and 3F), and metformin antagonized wild type CBP in luciferase assays (Figures 5B, 5C and 5E). As its therapeutic effect in diabetes patients occurs mainly through suppressing hepatic glucose production (Hundal et al., 2000), we attempted to determine its effects on fasting blood glucose in CBP S436A mutant and wild type mice. Treatment with metformin through intraperitoneal injection (ip) decreased blood glucose levels significantly in wild type mice (Figure 6H). However, metformin treatment had no effect on the blood glucose levels in CBP mutant mice (Figure 6H). These data clearly demonstrate that CBP Ser436 is the target site of metformin action.
Metformin not insulin promotes CBP phosphorylation in Obese Mice
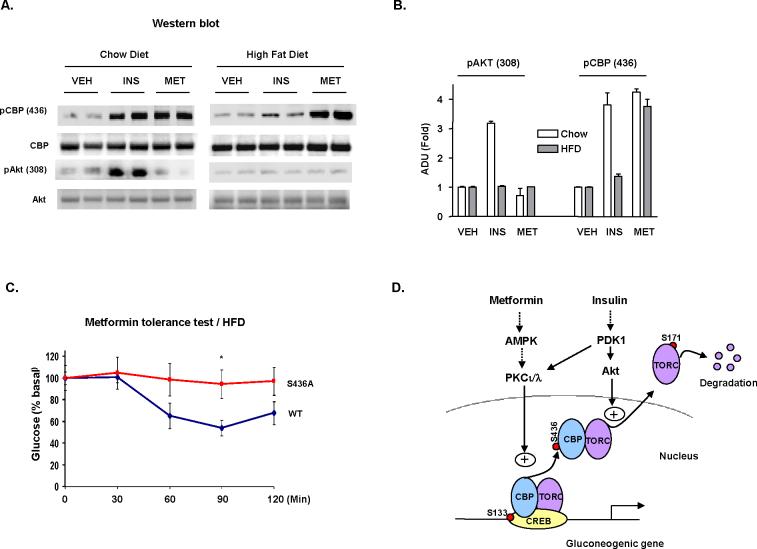
Metformin phosphorylates TORC2 at Ser171 and leads to its nuclear exclusion (Koo et al., 2005; Shaw et al., 2005); however, the same group has reported that TORC2 is O-glycosylation at Ser171 in the insulin resistance state which would preclude phosphorylation (Dentin et al., 2008). This finding argues that Ser171 of TORC2 can not be the principle site of metformin action in patients with type 2 diabetes (Shaw et al., 2005). Furthermore, metformin antagonized the stimulatory effect of PKA and CBP, even in the presence of mutant TORC2 (S171A), but not with CBPS436A co-transfection, suggesting that CBP phosphorylation is the main site of metformin action in controlling hepatic glucose production. If this is the mechanism, CBP must be phosphorylated by metformin in the setting of insulin resistance. We, therefore, placed mice on a high fat diet (HFD) for 4-month which induced hepatic insulin resistance as demonstrated by fasting hyperglycemia (wild type vs mutant: 122.5±6.4 vs 145.3±11.5 mg/dl, P<0.05) and the inability of insulin to mediate AKT phosphorylation (Figure 7A). In mice on a HFD, CBP phosphorylation was minimal after insulin treatment compared to wild type mice fed a normal chow diet (Figures 7A and 7B). In contrast and consistent with its ability to suppress hepatic glucose production in diabetes, metformin stimulated hepatic CBP phosphorylation in HFD fed mice to the same extent as mice fed a normal diet. In wild type mice on a HFD, metformin lowered the blood glucose almost to the same degree as in wild type mice fed a normal chow diet (compare Figures 6H and 7C). Again, metformin treatment had no effect on the blood glucose levels in CBP mutant mice. These results suggest a plausible mechanism for the therapeutic effect of metformin, which at molecular level results in CBP phosphorylation and a significant reduction in CREB-CBP-TORC2 responsive gene expression.
Figure 7.
High fat diet induced insulin resistance abolishes insulin but not metformin mediated CBP S436 phosphorylation. Mice fed regular or high fat (60% fat) diet for 4 months were treated with insulin or metformin. (A) In liver tissue from mice fed a high fat, insulin fails to stimulate CBP or Akt phosphorylation, whereas metformin maintains CBP phosphorylation capability. Each lane represents an individual mouse sample. (B) Desitometric analysis of pCBP (Ser436) and pAkt as in (A). (C) Metformin tolerance test in 16 h fasted mice (n=3) fed on high fat diet for 2 months. Means ± SEM are shown (B, C). *, =p<0.05 as compared to control mice at each time point. (D) Proposed model for the regulation of gluconeogenic gene expression by insulin and metformin.
DISUSSION
Hepatic gluconeogenesis is essential for the maintenance of normal blood glucose levels and is tightly regulated by the opposing actions of insulin and glucagon. Excessive hepatic glucose production, a characteristic of diabetes mellitus due to insulin deficiency/resistance and elevated glucagon levels, is responsible for the fasting hyperglycemia in diabetes. Insulin counteracts the action of glucagon on hepatic gluconeogenesis mainly at the transcriptional level through the transcriptional co-activator PGC-1α (Yoon et al., 2001). The activation of Ppargc1 gene expression by glucagon is mediated by a CREB-CBP-TORC2 complex (Ravnskjaer et al., 2007). We elucidate here a mechanism by which insulin and metformin control glucose production in the liver (Figure 7D). In the gluconeogenic CREB-CBP-TORC2 complex, CREB is phosphorylated and constitutively occupies the promoter after treatment with either glucagon or insulin (Figure 4E) (Koo et al., 2005). The binding states of CBP and TORC2 determine the transcriptional activities of CREB target genes (Figures 4B, 4G). The phosphorylation of CBP at Ser436 by insulin and metformin triggers the dissociation of CBP as well as TORC2 from CREB and disassembly of transcription machinery. In contrast, while TORC2 phosphorylation occurred as early as 10 mins after insulin treatment, peak phosphorylation of TORC2 was not observed until 30 min after treatment at a time when TORC was already completely dissociated from the promoters (Figure 4G), and consistent with a previous report (Dentin et al., 2007). After 30 min, insulin further stimulates the phosphorylation of TORC2 at Ser171 leading to its nuclear exclusion and degradation (Figures 4E, 7D) (Shaw et al., 2005; Dentin et al., 2007).
Suppression of gluconeogenesis and hepatic glucose production remains an attractive intervention for diabetes mellitus. The widely prescribed antidiabetic drug metformin lowers blood glucose levels in diabetes mainly through suppression of hepatic glucose production (Wiernsperger and Bailey, 1999; Hundal et al., 2000). It has been proposed that nuclear exclusion of TORC2 by either insulin or metformin-mediated phosphorylation is sufficient to terminate the cAMP signaling pathway acting on gluconeogenic enzyme genes (Koo et al., 2005; Shaw et al., 2005). However, the O-glycosylation of TORC2 at Ser171 in the insulin resistant state makes it unable to be phosphorylated and results in nuclear retention (Dentin et al., 2008). The present data clearly demonstrate that the triggering event for dissociation of the CREB-CBPTORC2 complex is CBP phosphorylation (Figures 4E, 5D and 5F). Thus, the effects of insulin and metformin on controlling hepatic glucose production converge on CBP phosphorylation through aPKCι/λ Atypical PKCι/λ plays a critical role in nutrient metabolism. In the fed state, the activation of aPKCι/λ by insulin phosphorylates CBP which suppresses gluconeogenesis and increases lipogenesis through the induction of SREBP-1c (Matsumoto et al., 2003). This also offers a potential explanation for why a liver-specific knockout of aPKCι/λ in mice had no effect on gluconeogenesis: reduced hepatic lipid content may have increased insulin sensitivity in these mice (Matsumoto et al., 2003). In CBP S436A mutant mice, metformin treatment did not reduce blood glucose levels, whereas insulin had some effect (Figure 6F vs 6H). This difference may reflect pathways of hepatic insulin signaling, not shared between metformin and insulin, such as the PI3K-AKT-FOX01 pathway (Kitamura and Accili, 2004, Matsumoto et al., 2007), as well as direct inhibition of PGC-1α activity by activated AKT mediated phosphorylation (Li et al., 2007). However, metformin maintains its ability to lower blood glucose levels in mice even though these alternate AKT-mediated pathways of insulin signaling appear to be disturbed following high fat feeding and induction of insulin resistance (Figure 7C). Thus, CBP phosphorylation at Ser436 by metformin is critical for its therapeutic effect and a potential target for pharmaceutical intervention.
EXPERIMENTAL PROCEDURES
Plasmids
The expression vectors for wild type CBP, CBP mutants (Zhou et al., 2004), AMPK (Hamilton et al., 2001), and PKA were as described previously (Zhou et al., 2004). Mouse WT TORC2, TORC2 S171A and aPKCι/λ were cloned into pcDNA3.1 (Invitrogen). The GFP-TORC2 expression vector was generated by inserting TORC2 cDNA into pEGFP-N1 plasmid (Clontech). GAL4-CREB and full-length TORC2 (GAL4-TORC2) were generated using standard cloning protocols. GAL4-CREB (short, 1-280 a.a.) was purchased from Stratagene. The BLOCK-iT adenoviral RNAi expresson system was used to construct adenoviral shRNA for CBP and aPKCι/λ (Invitrogen).
Cell cultures and transfection assays
Mouse hepatoma H2.35, rat hepatoma H4IIEC3 cells and primary mouse hepatocytes were exposed to forskolin (10 uM), insulin (10 nM, 10 min), metformin (10 mM), AICAR (1 mM), LY294002 (50 uM), Ro31-8220 (20 uM) or Go6876 (5 uM) as indicated. Lipofectamine 2000 was used in transfection assays. Equal total amounts of plasmid or RNAi were deployed. Transfections were repeated in both cell lines, and similar results were observed.
Animal experiments and analytical procedures
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. CBP S436A mutant mice were generated as described previously (Zhou et al., 2004). 2-6-month old mice were maintained on regular chow or high fat diet. For hepatic CBP phosphorylation measurement and metformin tolerance test, mice were injected intraperitoneally (ip) with 0.5 unit/kg insulin, 200mg/kg metformin or PBS after fasting for 10-16 h. Adenoviral shRNA knockdown experiments were conducted 3-day after mice were injected adenovirus through the tail vein. Serum insulin and glucagon were measured using a mouse endocrine panel kit (Millipore). For the hyperinsulinemic euglycemic clamp experiment, mice were fasted 18 h and then [3-3H]-glucose was infused intravenously (2.5 μCi bolus, 0.075 μCi/min). Blood samples (20ul) were collected at 70, 80, and 90 min after the initiation of 3H-glucose infusion for the measurement of 3H-glucose specific activity and blood glucose and calculation of basal hepatic glucose production. Then, regular human insulin was infused at 25 mU/kg/min and glucose levels were measured every 10 min. An infusion of 25% dextrose was adjusted to maintain the blood glucose at 100-120 mg/dl. Blood samples were again collected during the clamp for calculation of hepatic glucose production.
Immunoprecipitation and immunoblot
Phospho-Ser436 CBP antiserum was generated against phospho-CBP peptide containing amino acids 427-445 of mouse CBP protein. All other antibodies, CBP, p300 (Santa Cruz); Akt, phospho-Akt (308), GSK3, phospho-GSK3, phospho-TORC2, CREB mAb, phospho PKCς/λ (Thr410/403), PKCι, PKCς (Cell Signaling); PKCι kinase (Cell Signaling); AMPKγ TORC2 (Abcam), phospho-PKC iota (Thr555) (Millipore) were purchased. Immunoprecipitation (IP) was performed overnight at 4 °C.
Chromatin Immunoprecipitation
Mice were injected intraperitoneally with insulin, metformin or PBS. Hepatic tissue was minced and homogenized. The ChIP-IT Express assay kit (Active Motif) was used following the steps recommended by the manufacturer. Precipitated DNA was amplified by PCR using primers against relevant mouse promoters. The PCR cycle number for the input DNA and immunoprecipitates was optimized to remain in the PCR linear range.
Statistical Analyses
Statistical significance was calculated with an ANOVA test and student's t test. Significance was accepted at the level of p < 0.05 or p<0.01.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK063349 and by the Baltimore Diabetes Research and Training Center Grant P60DK079637 (F.E.W., P.I.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akimoto K, Mizuno K, Osada S, Hirai S, Tanuma S, Suzuki K, Ohno S. A new member of the third class in the protein kinase C family, PKC lambda, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J. Biol. Chem. 1994;269:12677–12683. [PubMed] [Google Scholar]
- Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36:274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- Biddinger CB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu. Rev. Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr., Farese RV. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J. Biol. Chem. 2002;277:23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol. Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- De Guzman RN, Martinez-Yamout MA, Dyson HJ, Wright PE. Interaction of the TAZ1 domain of the CREB-binding protein with the activation domain of CITED2: regulation by competition between intrinsically unstructured ligands for non-identical binding sites. J Biol Chem. 2004;279:3042–3049. doi: 10.1074/jbc.M310348200. [DOI] [PubMed] [Google Scholar]
- Dentin R, Liu Y, Koo SH, Hedrick S, Vargas T, Heredia J, Yates J, 3rd, Montminy M. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J, 3rd., Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319:1402–1405. doi: 10.1126/science.1151363. [DOI] [PubMed] [Google Scholar]
- Dunning BE, Gerich JE. The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr. Rev. 2007;28:253–283. doi: 10.1210/er.2006-0026. [DOI] [PubMed] [Google Scholar]
- Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- Hamilton SR, Stapleton D, O'Donnell JB, Jr, Kung JT, Dalal SR, Kemp BE, Witters LA. An activating mutation in the gamma1 subunit of the AMP-activated protein kinase. FEBS Lett. 2001;500:163–168. doi: 10.1016/s0014-5793(01)02602-3. [DOI] [PubMed] [Google Scholar]
- Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Accili D. New insights into the integrated physiology of insulin action. Rev. Endocr. Metab. Disord. 2004;5:143–149. doi: 10.1023/B:REMD.0000021436.91347.93. [DOI] [PubMed] [Google Scholar]
- Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–1016. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, Shimano H, Yamada N, Ohno S, Kasuga M, Noda T. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A, Macieira S, Velarde M, Bädeker M, Benda C, Jestel A, Brandstetter H, Neuefeind T, Blaesse M. Crystal structure of the catalytic domain of human atypical protein kinase C-iota reveals interaction mode of phosphorylation site in turn motif. J Mol Biol. 2005;352:918–931. doi: 10.1016/j.jmb.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as paradigm. Biochem J. 2003;370:361–371. doi: 10.1042/BJ20021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular response. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. EMBO J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan I, Pérez-Alvarado GC, Parker D, Dyson HJ, Montminy MR, Wright PE. Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell. 1997;91:741–752. doi: 10.1016/s0092-8674(00)80463-8. [DOI] [PubMed] [Google Scholar]
- Ravnskjaer K, Kester H, Liu Y, Zhang X, Lee D, Yates JR, 3rd., Montminy M. Cooperative interactions between CBP and TORC2 confer selectivity to CREB target gene expression. EMBO J. 2007;26:2880–2889. doi: 10.1038/sj.emboj.7601715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J. Biol. Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999;58(Suppl 1):31–39. doi: 10.2165/00003495-199958001-00009. [DOI] [PubMed] [Google Scholar]
- Xu W, Kasper LH, Lerach S, Jeevan T, Brindle PK. Individual CREB-target genes dictate usage of distinct cAMP-responsive coactivation mechanisms. EMBO J. 2007;26:2890–2903. doi: 10.1038/sj.emboj.7601734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XY, Shibusawa N, Naik K, Porras D, Temple K, Ou H, Kaihara K, Roe MW, Brady MJ, Wondisford FE. Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat. Med. 2004;10:633–637. doi: 10.1038/nm1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.