Summary
Macrophages clear pathogens and damaged or aged cells from the blood stream via phagocytosis. Cell-surface CD47 interacts with its receptor on macrophages, SIRPα, to inhibit phagocytosis of normal, healthy cells. We find that mobilizing cytokines and inflammatory stimuli cause CD47 to be transiently up-regulated on mouse hematopoietic stem cells (HSCs) and progenitors just prior to and during their migratory phase, and that the level of CD47 on these cells determines the probability that they are engulfed in vivo. CD47 is also constitutively up-regulated on mouse and human myeloid leukemias and over-expression of CD47 on a myeloid leukemia line increases its pathogenicity by allowing it to evade phagocytosis. We conclude that CD47 up-regulation is an important mechanism that provides protection to normal HSCs during inflammation-mediated mobilization, and that leukemic progenitors co-opt this ability in order to evade macrophage killing.
Introduction
HSCs have the ability to migrate to ectopic niches in fetal and adult life via the blood stream (Christensen et al., 2004; Cumano and Godin, 2007; Morrison et al., 1995; Wright et al., 2001b). Once in the blood, HSCs home to VCAM-1+ endothelia using integrin α4β1 (Wagers et al., 2002), then navigate the vascular beds of the marrow, spleen and liver before returning to potential niches that secrete SDF-1, for which HSC have responsive CXCR4 chemokine receptors (Peled et al., 1999; Wright et al., 2002). Macrophages lining vascular sinusoids at these sites function to remove damaged cells and foreign particles from the blood stream, sensing altered surface molecules. During inflammatory responses, macrophages become more phagocytically active in order to more effectively clear offending pathogens (Wright et al., 1989). At these times, host cells are at increased risk of clearance and newly arriving stem cells might require additional protection against phagocytosis.
Like normal HSCs, leukemic stem and progenitor cells also have an intrinsic ability to migrate via the circulation to ectopic marrow sites. By analogy with the normal HSCs, the leukemic stem cells (LSCs) must also be able to navigate through macrophage lined vasculature of spleen, liver, and marrow as they travel to ectopic niches.
CD47 is an immunoglobulin-like protein that is known to interact functionally with integrins (Brown and Frazier, 2001) and thrombspondin-1 (Gao et al., 1996; Liu et al., 2001). It has been implicated in functions as diverse as neutrophil migration (Lindberg et al., 1996), axon extension (Miyashita et al., 2004), and T-cell co-stimulation (Reinhold et al., 1997). In addition, CD47 is capable of interacting with its receptor SIRPα (Jiang et al., 1999) on macrophages to negatively regulate phagocytosis (Brown and Frazier, 2001). Lack of autonomous CD47 expression results in phagocytosis of red blood cells (Oldenborg et al., 2000), as well as T-cells and whole bone marrow cells in a transplant setting (Blazar et al., 2001). Thus, CD47 functions as a “Don't eat me” signal to ensure that autologous cells are not inappropriately phagocytosed.
While the absence of CD47 is known to result in phagocytosis, the effect of differential CD47 expression is unclear. IAP+/- platelets and erythrocytes may be more prone to phagocytosis than their wild-type counterparts (Olsson et al., 2005; Olsson et al., 2007), which suggests that CD47 down-regulation might lead to clearance of these cells as they age. However, no formal exploration of the role of CD47 expression on normal or leukemic hematopoietic stem and progenitor cells, which are also physiologically migrating cells, has been undertaken. Hence, we performed experiments to understand the effect of differential CD47 expression on these cells.
Results
CD47 expression is increased in HSCs after mobilization or induced inflammation
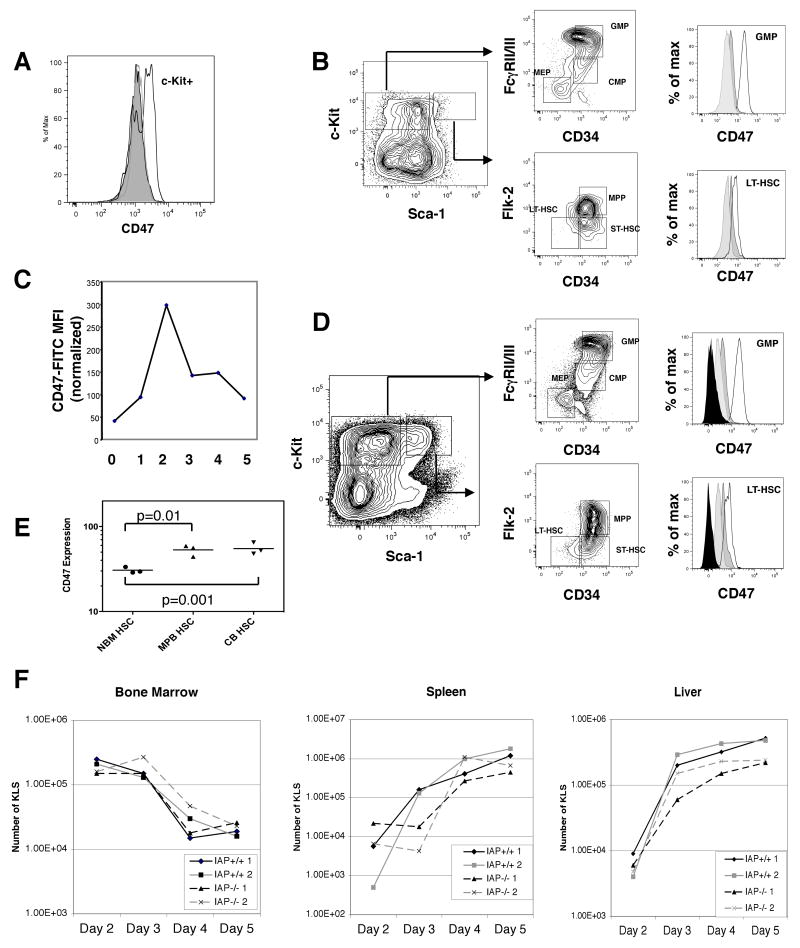
Mobilization of marrow stem and progenitor cells involves several steps in which they come into contact with macrophages. We thus utilized the cyclophosphamide/G-CSF (Cy/G) protocol (Morrison et al., 1997) to experimentally induce mobilization in mice. We found that there was a notable increase of CD47 on c-Kit+ bone marrow cells on day 2 (Figure 1a), when bone marrow HSC reach their maximum level, with CD47 increasing approximately four-fold by this time point (Figure 1b). The increase was seen at all levels of the myeloid progenitor hierarchy, as LT-HSCs as well as GMPs displayed this increase in CD47 expression (Figure 1b). By day 5, when egress from the marrow to distant marrow/spleen/liver sites had nearly stopped, the levels of CD47 on the marrow HSC/progenitors had returned to nearly normal levels. In Figure 1c, the mean fluorescence intensity of CD47 expression on GMPs is shown on days 0 to 5 of mobilization.
Figure 1. Mobilization or inflammation induces CD47 up-regulation in hematopoietic stem and progenitor cells.
(A) Expression level of CD47 on c-Kit+ cells is shown for day 2 Cy/G mobilized BM. (B) Myeloid progenitor and stem cell gates are shown for day 2 mobilized BM. Histograms on right show level of CD47 expression in marrow LT-HSC and GMP for steady-state (light gray shaded histogram), day 2 mobilized (black line), and day 5 mobilized (dark gray shaded histogram). (C) Relative MFI of CD47 for GMP on days 0-5 of Cy/G mobilization. Results were normalized so that steady state GMPs had MFI 100. (D) Myeloid progenitor and stem cell gates are shown for day 2 BM post-LPS treatment. Histograms show level of CD47 expression on day 2 post-LPS (black line), day 5 post-LPS (dark gray shaded histogram), steady state (light gray shaded histogram), and IAP-/- (black shaded histogram). (E) MFI for human CD47 on HSCs from human bone marrow (NBM), cord blood CB), or mobilized peripheral blood (MPB). (F) Evaluation of KLS cells in the hematopoetic organs of IAP+/+ and IAP-/- mice mobilized on days 2 through 5. Two mice are analyzed per genotype per day.
These results predicted that mobilized human HSC in the blood would also have increased levels of CD47 on their cell surface. We examined the CD47 expression level on human bone marrow, cord blood, (CB) and mobilized peripheral blood (MPB) HSCs. The results show that human HSCs in circulation have significantly higher levels of CD47 expression than sessile HSCs (Figure 1e).
Endotoxins such as LPS are also thought to contribute to bone marrow mobilization (Cline and Golde, 1977) by causing activation of macrophages and a pro-inflammatory response (Fenton and Golenbock, 1998), as well as increasing the phagocytic capacity of macrophages (Wright et al., 1989). We tested whether LPS administration in mice would affect CD47 expression in stem and progenitor cells. Mirroring the pattern seen in Cy/G induced mobilization, LPS caused expansion of stem and progenitor cells by 2 days post treatment, followed by migration to the spleen and liver (Figure 1d). On day 2 after LPS administration, stem and progenitor cells in the marrow had up-regulated CD47 to a similar degree as in Cy/G mobilization. By day 5, when the inflammatory response had resolved, the levels of the protein had dropped to steady-state levels (Figure 1d).
CD47 up-regulation during mobilization is unlikely to be necessary for migration
Since CD47 was consistently up-regulated in the mobilization response, we tested the ability of stem and progenitor cells to mobilize following Cy/G. The CD47 knockout mouse has defects in migration of neutrophils to sites of inflammation (Lindberg et al., 1996) and of dendritic cells to secondary lymphoid organs (Van et al., 2006). The exact role of CD47 in migration of these cells is unknown, but it may relate to poor integrin binding in the circulation or lack of interaction with SIRPα on endothelial cells. Hence we reasoned that if CD47 was involved in the migration capacity of these cells during mobilization, then IAP-/- mice would display reduced numbers of cells in the peripheral organs after Cy/G.
We thus administered Cy/G to both wild-type and knockout mice and sacrificed mice on days 2-5. For each mouse, we analyzed the number of stem and progenitor cells in peripheral blood, marrow, spleen, and liver. We used the KLS population as a surrogate for HSCs because the number of CD34- cells drops considerably in proliferative states, making accurate calculation of LT-HSC numbers difficult.
We found that there was little difference in numbers of mobilized KLS or GMP between wild-type and IAP-/- mice (Figure 1f). There was a modest decrease in the ability of IAP-/- mice to move progenitors to the spleen by day 3 (about 10 fold less), but by days 4 and 5 they had restored normal numbers of cells to the periphery. The marrow and liver compartments in IAP-/- had no difference in HSC or GMP from wild-type mice. Additionally, there was no difference in HSC numbers in peripheral blood by day 4 (data not shown). Hence, IAP-/- mice do not have a significant mobilization defect. While this result does not support a role for CD47 in the migration phase of mobilization, it does not rule out a role for protection against phagocytosis since macrophages from IAP-/- mice also fail to properly phagocytose CD47 deficient cells, likely due to aberrant macrophage education (Wang et al., 2007).
CD47 deficient mice have no gross hematopoietic defect but IAP-/- HSCs fail to engraft wild-type mice
We examined stem and progenitor frequencies in IAP+/- and IAP-/- mice. The relative frequency of cells in the stem and myeloid progenitor compartment did not differ between these mice and wild-type mice (Figure 2a). We then tested stem cells from these mice for their ability to form colonies in an in vitro assay. We examined colony formation at day 7 and found that there was no major difference between wild-type and IAP-/- stem cells in the number and type of colonies formed (Figure 2b).
Figure 2. CD47 deficient HSCs are efficiently phagocytosed by macrophages but otherwise exhibit normal hematopoietic developmental potential.
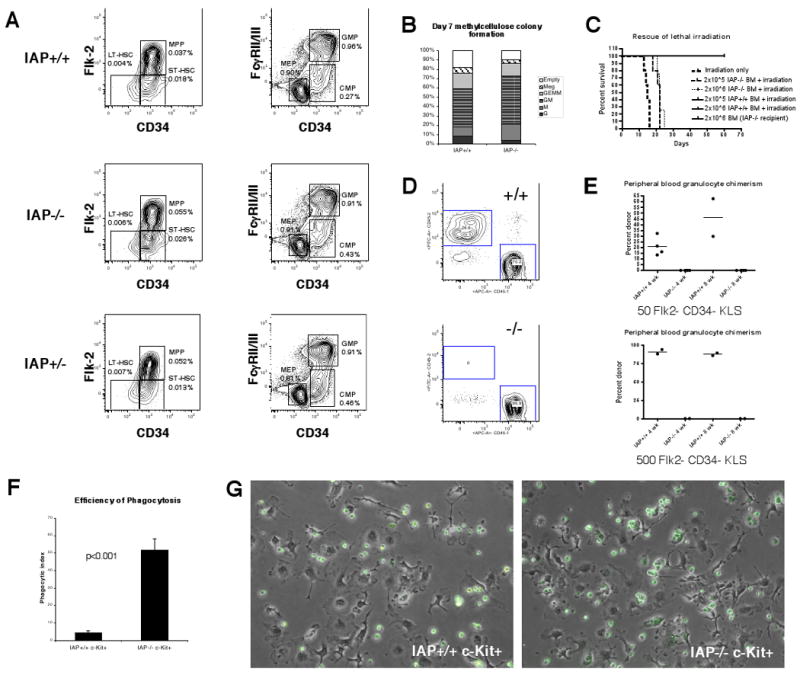
(A) Stem cells (left column) are gated on Lin- c-Kit+ Sca-1+ cells. Myeloid progenitors (right column) are gated on Lin- c-Kit+ Sca-1+ cells. Frequency in whole bone marrow is shown adjacent to each gated population. (B) Colony output on day 7 of individually sorted LT-HSC. G-granulocyte, M-macrophage, GM-granulocyte and macrophage, GEMM-granulocyte, macrophage, erythroid, and megakaryocyte, Meg- megakaryocyte. (C) Survival curve of recipient mice given lethal radiation and transplanted with the cells shown, n=5 for each group. (D) Examples of chimerism plots at 4 weeks post-transplant for IAP+/+ or IAP-/- donors. (E) Summary of chimerism analysis of mice transplanted with either 50 or 500 IAP+/+ or IAP-/- cells. (F) Results of phagocytosis assays using IAP+/+ or IAP-/- c-Kit enriched bone marrow. n=3, error bars represent 1 SD. (G) Photomicrographs of phagocytosis assays taken after 2 hours.
We also tested whether bone marrow cells from IAP-/- mice could rescue recipient mice from the effects of lethal irradiation. Typically, a dose of 2 × 105 bone marrow cells will rescue 100% of recipient mice in this assay. In agreement with previous results (Blazar et al., 2001), we found that IAP-/- bone marrow could not rescue these recipients (Figure 2c). However, administration of these cells did prolong lifespan; normally, mice die between day 12 and 15 after irradiation, but mice that received IAP-/-bone marrow lived about 7 to 10 days longer (Figure 2c). We do not yet know the reason for the prolongation of lifespan in this case.
Next, we sorted Flk-2- CD34- KLS stem cells from wild-type and IAP-/- cells and transplanted them into lethally irradiated wild-type recipients along with 2 × 105 competitor cells. None of the mice which received IAP-/- HSCs had any engraftment of donor cells, indicating that CD47 was indeed required to be expressed intrinsically for the HSC to transplant (Figure 2d-e). We speculated that this was due to phagocytosis of CD47 null cells, as has been shown for erythrocytes and T-cells. To test this, we enriched c-Kit+ cells from the bone marrow of wild-type and IAP-/- mice and co-incubated them with bone marrow derived macrophages. IAP-/- stem and progenitor cells were readily phagocytosed in this assay, whereas wild-type cells were only minimally phagocytosed (Figure 2f-g). These results were not due to increased apoptosis of the IAP-/- cells in these culture conditions, as there was no difference in Annexin V positivity between the groups (Supplementary Figure 4a).
We also tested HSC migration using the parabiosis model in which two mice are joined surgically to allow their circulatory systems to form anastamoses and a shared blood system (Wright et al., 2001b). This model allows the examination of migration in a more physiological setting since stem cells are continuously seeded into the blood stream from the marrow over time. We joined an IAP-/- mouse with a congenic wild-type GFP+ mouse. For both pairs of mice that were parabiosed, bone marrow HSC chimerism was seen in the IAP-/- mouse, but not the wild-type mouse (Supplementary Figure 1). Thus, IAP-/- HSCs are cleared even during physiological migration.
CD47 heterozygous HSCs have reduced fitness relative to wild-type HSCs due to macrophage clearance
Our observation that CD47 expression increasea in states of stress and mobilization led us to hypothesize that HSPCs that were genetically hemizygous for CD47 might be more prone to phagocytosis and clearance by macrophages over time, as has been seen for platelets and erythrocytes (Olsson et al., 2005; Olsson et al., 2007). Hence, we asked if IAP+/- stem cells would be disadvantaged relative to wild-type stem cells in long-term contribution to hematopoiesis. We first analyzed the levels of CD47 expressed on IAP+/+, IAP+/-, and IAP-/- stem cells. FACS analysis of CD34- Flk-2-KLS stem cells revealed that the MFI of CD47 on heterozygote HSCs was indeed at roughly half the level of wild-type stem cells (Figure 3a).
Figure 3. IAP+/- HSCs have a competitive disadvantage relative to wild-type HSCs due to macrophages.
(A) MFI of CD47 on IAP+/+, IAP+/-, and IAP-/- LT-HSC. (B) Donor chimerism analysis for transplants of IAP+/+, GFP+ or IAP+/-, GFP+ marrow cells. Loss of donor chimerism is shown using Kaplan-Meier curves. (C) Experimental design for assessing effect of CD47 heterozygosity during LPS challenge. (D) Change in percent chimerism of host KLS cells compared to expected chimerism based on peripheral blood granulocytes is shown. Error bars represent 1 SD and p-values were obtained by ANOVA statistics. n=5 for IAP+/+ with clodronate (CLOD), n= 7 for IAP+/+ with control (CTRL), n=4 for IAP+/- with clodronate, n=7 for IAP+/- with control. (E) Results of in vivo phagocytosis assay comparing IAP+/+ and IAP+/- c-Kit enriched cells. Percent recipient GFP+ F4/80 cells is shown (n= 3 for each group, error bars represent 1 SD).
We then transplanted one cohort of sub-lethally irradiated recipients with 2 × 106 wild-type whole bone marrow cells, and another with the same dose of IAP+/- bone marrow cells. Such a dose would be expected to contain roughly 50-100 HSCs. Since granulocyte chimerism in the peripheral blood is a good surrogate marker of stem cell fitness (Bhattacharya et al., 2006), we analyzed cells from the blood of these recipients at periodic intervals. When wild-type marrow was transplanted into wild-type recipients, granulocyte chimerism was maintained for at least 40 weeks. However, when IAP+/-cells were transplanted, 4 out of 10 mice lost donor chimerism over time, despite having a successful engraftment initially (p=0.0297, Figure 3b, Supplementary Figure 2a). It was possible that there were fewer LT-HSC per unit of marrow in IAP+/- or IAP-/- mice compared to wild-type. To test if IAP-/- mice had fewer functional HSC, we transplanted either IAP+/+ or IAP-/- marrow cells into IAP-/- donors. We also co-transplanted IAP+/-marrow into wild-type mice together with wild-type marrow. We did not find any statistical difference in donor granulocyte chimerism for up to 12 weeks in either case (Supplementary Figures 2b-c) indicating that there was not a demonstrable deficit in stem cell numbers in IAP+/- or IAP-/- mice.
Interestingly, the IAP+/- chimeras that did not lose donor engraftment over time did not appear to have a quantitative deficit in chimerism; rather they seemed to have similar levels of donor cells as the wild-type chimeras (Supplementary Figure 2a). We thus hypothesized that physiological insults leading to chronic or acute inflammatory states that occurred in some mice, but not others, led to their losing IAP+/- donor chimerism over time, but at steady state the donor IAP+/- cells were not affected.
To test this, we created chimeras by transplanting whole bone marrow from either IAP+/+ GFP+ or IAP+/- GFP+ mice into sub-lethally irradiated wild-type syngeneic recipients. Since IAP-/- mice have no known in vivo (Lindberg et al., 1996) or in vitro (Figure 2b) defect in granulopoiesis, blood granulocyte chimerism, can be used in this setting to estimate donor HSC engraftment. After 12 weeks, contribution of donor HSCs was assessed. At this early time point, IAP+/- mice actually had a higher blood granulocyte contribution, but there was considerable variation within each group. The mice were challenged with a sublethal dose of LPS (day 0), then given clodronate or control lipsomes on day 2 and day 7. Clodronate liposomes have been shown to specifically but transiently deplete marrow, spleen, and liver macrophages when injected intravenously (Van Rooijen and Sanders, 1994). At day 14, the mice were euthanized and actual marrow KLS chimerism was assessed and compared to expected chimerism based on blood granulocyte chimerism (Figure 3d). Raw values are shown in Supplementary Table 1. The results indicate that there were more host (wild-type) KLS cells in the bone marrow of the IAP+/- chimeras than expected. When macrophages were depleted, the chimerism was at the level that would be expected based on blood granulocyte chimerism. ANOVA showed a significant interaction among IAP genotype (IAP+/+ vs. IAP +/-) and treatment with and without clodronate (CLOD vs. CTRL) (p=0.0069). When the group including the IAP+/- chimeras treated with control liposomes (IAP+/- CTRL) was excluded, there was no statistical difference between the groups (p=0.28). Analysis also showed that differences between the treatment with and without clodronate were not significant in IAP+/+ mice (p=0.20), but were significant in IAP +/- mice (p=0.011).
It was possible that these results were due to increased apoptosis of IAP+/- cells relative to IAP+/+. To test this, we measured the percent of Annexin V+ 7-AAD- KLS cells in IAP+/+ and IAP+/- mice treated with LPS in the presence of control or clodronate liposomes. There was no difference between the groups (Supplementary Figure 4b).
To establish that macrophages were indeed responsible for clearing the cells with reduced CD47 expression, we challenged donor IAP+/+, GFP+ and IAP+/-, GFP+ mice with LPS. After 3 days, we harvested bone marrow from these mice and transplanted c-Kit enriched cells intra-splenically into GFP-negative wild-type recipient mice similarly challenged with LPS 3 days prior. After 2 hours the recipient spleens were harvested and analyzed by FACS. There were significantly more F4/80+ macrophages that were GFP+ (representing macrophages containing phagocytosed GFP+ cells) in mice injected with IAP+/- cells compared to IAP+/+ (p<0.0001, Figure 3e, Supplementary Figure 3).
CD47 is up-regulated on mouse myeloid leukemias
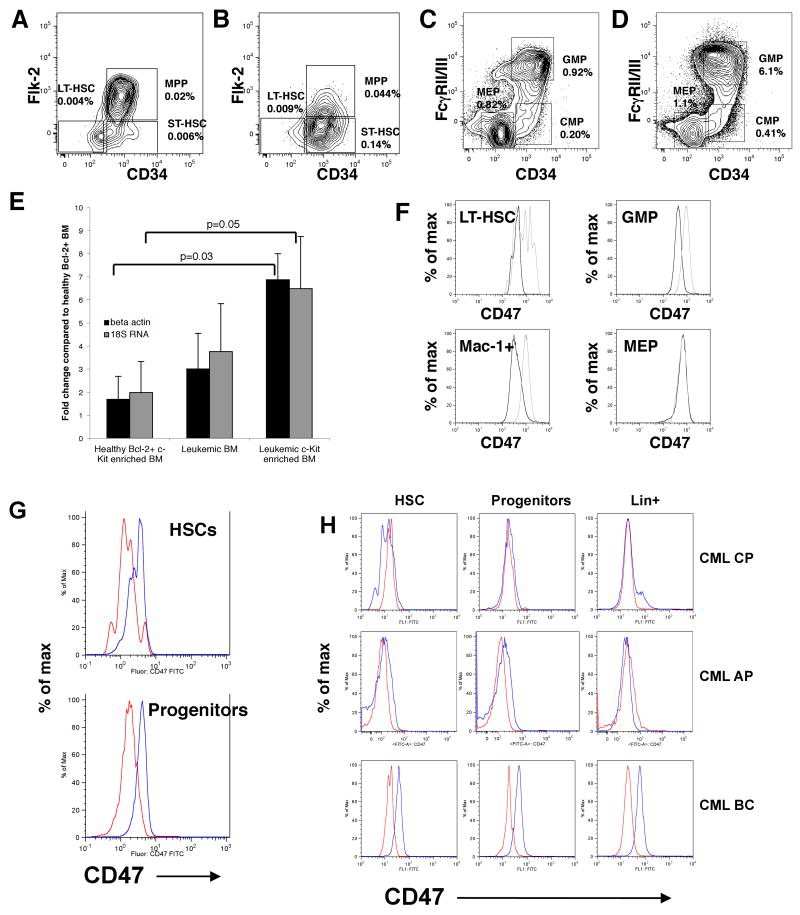
Previous microarray analysis had revealed that CD47 was significantly up-regulated in leukemic Faslpr/lpr × hMRP8bcl2 transgenic bone marrow (Traver et al., 1998) and this was confirmed in leukemic hMRP8bcr/abl × hMRP8bcl2 mice (Jaiswal et al., 2003). Transcripts for CD47 were found to be increased in leukemic hMRP8bcr/abl × hMRP8bcl2 bone marrow 3-4 fold by quantitative RT-PCR and 6-7 fold in c-Kit enriched leukemic marrow relative to healthy hMRP8bcl2+ bone marrow (Figure 4e). Leukemic mice had an expansion of the granulocyte macrophage progenitor (GMP) population as well as c-Kit+ Sca-1+ Lin-stem and progenitor subsets in the spleen relative to control mice, which were of the same genotype as leukemic mice but failed to develop disease (Figure 4a-d). Expression levels for CD47 protein were found to begin increasing in leukemic mice relative to control mice at the stage of the Flk2- CD34- c-Kit+ Sca-1+ Lin- long-term hematopoietic stem cell (LT-HSC) (Figure 4f) (Yang et al., 2005). This increased level of expression was maintained in GMP and Mac-1+ blasts, but not megakaryocyte/erythroid restricted progenitors (MEP) (Figure 4f). The increase in CD47 surface expression between leukemic and normal cells was between 3 to 20 fold, depending on the sample and fluorophore used (Supplementary figure 5c). We have observed increased CD47 expression in all mice that developed leukemia from hMRP8bcr/abl × hMRP8bcl2 primary (n=3) and secondary transplanted mice (n=3), Fas lpr/lpr × hMRP8bcl2 (Supplementary figure 4a) primary (n=14) and secondary (n=19) mice, and hMRP8bcl2 × hMRP8bcl2 primary (n=3) and secondary (n=12) mice. We have also found increased CD47 expression in mice that received p210bcr/abl retrovirally-transduced mouse bone marrow cells that developed leukemia (Supplementary figure 5b).
Figure 4. CD47 is up-regulated in murine and human myeloid leukemia.
Typical stem and progenitor plots are shown for leukemic hMRP8bcrabl × hMRP8bcl2 cells compared to control non-leukemic animals. Lin- c-Kit+ Sca-1+ gated cells from control bone marrow (A) and leukemic spleen (B) and Lin- c-Kit+ Sca-1- gated cells from control bone marrow (C) and leukemic spleen (D). Frequency is shown as a percentage of entire marrow or spleen mononuclear fraction. (E) Quantitative RT-PCR for CD47. Data are shown from 3 sets of mice transplanted with either leukemic or control hRMP8bcrabl × hMRP8bcl2 BM cells and then sacrificed 2-6 weeks later. Results were normalized to beta-actin and 18S rRNA expression. Fold change relative to control transplanted whole Bcl-2+ BM cells was determined. Error bars represent 1 SD. (F) Histograms show expression of CD47 on gated populations for leukemic (gray) and control (black) mice. (G) Comparative FACS histograms of human CD47 expression by normal (red; n=6) and acute myelogenous leukemic (AML, blue; n=6) hematopoietic stem cells (HSC; CD34+CD38-CD90+Lin-) and progenitors (CD34+CD38+Lin-). (H) Comparative FACS histograms of CD47 expression by normal (red) and chronic myelogenous leukemia (blue) hematopoietic stem cells (HSC; CD34+CD38-CD90+Lin), committed progenitors (CD34+CD38+Lin-), and Lin+ cells.
CD47 expression on normal and leukemic human stem cells
Because we found CD47 to be increased on all mouse leukemias we examined, we investigated CD47 expression on normal and dysplastic human hematopoietic cells. FACS-mediated analysis of human hematopoietic progenitor populations was performed on blood and marrow derived cells from normal cord blood and mobilized peripheral blood (n=16) and myeloproliferative disorders (MPDs) including polycythemia vera (PV; n=16), myelofibrosis (MF; n=5), essential thrombocythemia (ET; n=7), chronic myelomonocytic leukemia (CMML; n=11) and atypical chronic myeloid leukemia (aCML; n=1) as well as myeloid blast crisis [BC] phase chronic myeloid leukemia (CML; n=19), chronic phase CML (n=7) and acute myelogenous leukemia (AML; n=13). This analysis demonstrated that the granulocyte-macrophage progenitor (GMP) population was expanded in MPDs with myeloid skewed differentiation potential including atypical CML, proliferative phase CMML and acute leukemia including blast crisis CML and AML (Supplementary figure 6a, 6c). AML HSC and progenitors uniformly exhibited higher levels of CD47 expression compared with normal controls (Figure 4g); every sample from CML-BC (n=19) and AML (n=13) had elevated levels of CD47. Moreover, progression from chronic phase CML to blast crisis was associated with a significant increase in CD47 expression (Figure 4h). Using the methods described in this study, we have found that human CD47 protein expression in CML-BC increased 2.2 fold in CD90+ CD34+ CD38- Lin- cells relative to normal CB and MPB (p=6.3 × 10-5), 2.3 fold in CD90- CD34+ CD38- Lin- cells relative to normal (p=4.3 × 10-5), and 2.4 fold in CD34+ CD38+ Lin- cells (p=7.6 × 10-6) (Figures 4g, 4h, Supplementary Figure 6b). Since CB and MPB HSCs have almost double the amount of CD47 as normal marrow (Figure 1e), these figures likely underestimate the true increase in CD47 compared to normal marrow.
We also examined CD47 expression in other myeloproliferative disorders, such as polycythemia vera, post-polycythemic myeloid metaplasia with myelofibrosis, essential thrombocythemia, agnogenic myeloid metaplasia, and chronic myelomonocytic leuekmia. None of the samples we examined from these disorders displayed increased CD47 (data not shown). It is likely that the fixed, high expression of CD47 on AML cells and CML-BC cells represents a late event in malignant progression, whether by genetic or epigenetic heritably increased expression. A summary of all the human samples analyzed is shown in Supplementary Table 2.
CD47 expression can rescue the in vivo growth defect of the AML cell line MOLM-13 via a mechanism other than migration
In order to experimentally test the significance of elevated CD47 expression on myeloid leukemias, we decided to examine whether the level of CD47 expressed on myeloid leukemia could affect its pathogenicity in vivo. We first screened a panel of human AML cell lines for human CD47 expression. On the basis of this analysis, we found significant variation in the amount of cell surface CD47 (adjusted for cell size) in the varying lines (Supplementary Figures 7a-b). We then tested whether there was variation in the ability of some of these cell lines to engraft in immunocompromised mice. Human HL-60 and Kasumi-1 cells, which express moderate to high levels of human CD47, were able to engraft C57Bl/6 or Balb/c recombination activating gene 2, common gamma chain deficient (RAG2-/-, γc-/-) mice (Supplementary Figures 6c-d), which are reported to have little or no interaction between their SIRPα and human CD47 (Takenaka et al., 2007). MOLM-13 cells, which are derived from a patient with AML 5a, have low endogenous CD47 expression and were unable to engraft in either of these strains. Even when transplanted into non-obese diabetic severe combined immunodeficiency, interleukin 2 receptor common gamma chain -/- (NOG) mice that are reported to have more effective interaction between their mouse SIRPα and human CD47, and thus should be permissive for human donor cells (Takenaka et al., 2007), MOLM-13 cells failed to engraft (Supplementary Figure 7c).
Since MOLM-13 cells failed to engraft in any of the mouse models tested, we decided to use them for further analysis of the role of CD47 in leukemogenicity. MOLM-13 cells were transduced with either control (Tet) or CD47 expressing (Tet-CD47) lentiviruses (Figure 5a), and stable integrants were propagated on the basis of GFP expression. We first tested if ectopic expression of the murine CD47 (muCD47) protein could rescue the growth defect of these cells. The cells were transplanted intravenously in a competitive setting with untransduced MOLM-13 cells into T, B, and NK deficient RAG2-/-, γc-/- mice. Only cells transduced with Tet-CD47 were able to give rise to tumors in these mice, efficiently engrafting bone marrow, spleen, liver, and peripheral blood (Figures 5b-c).
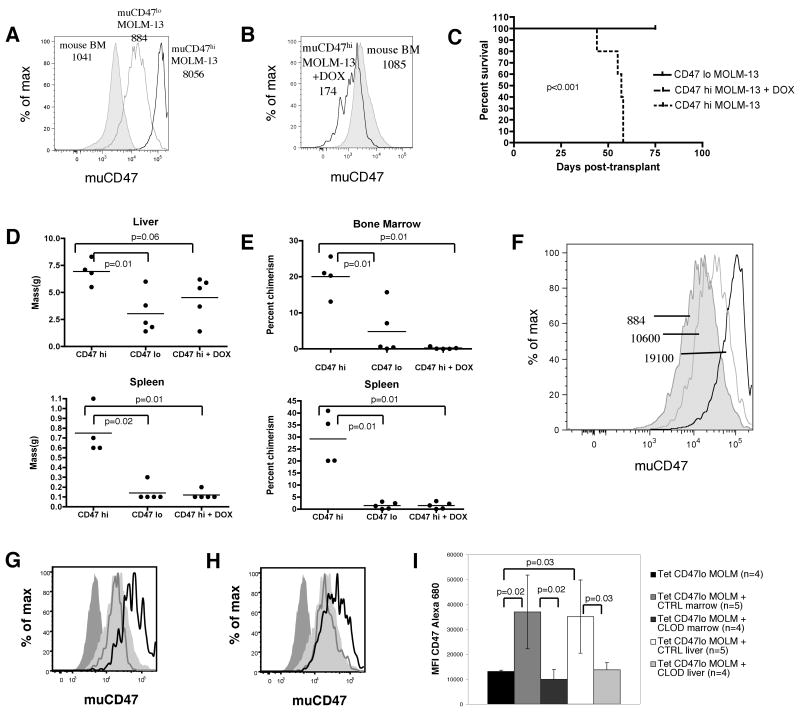
Figure 5. Over-expression of murine CD47 rescues the growth defect of MOLM-13 myeloid leukemia cells.
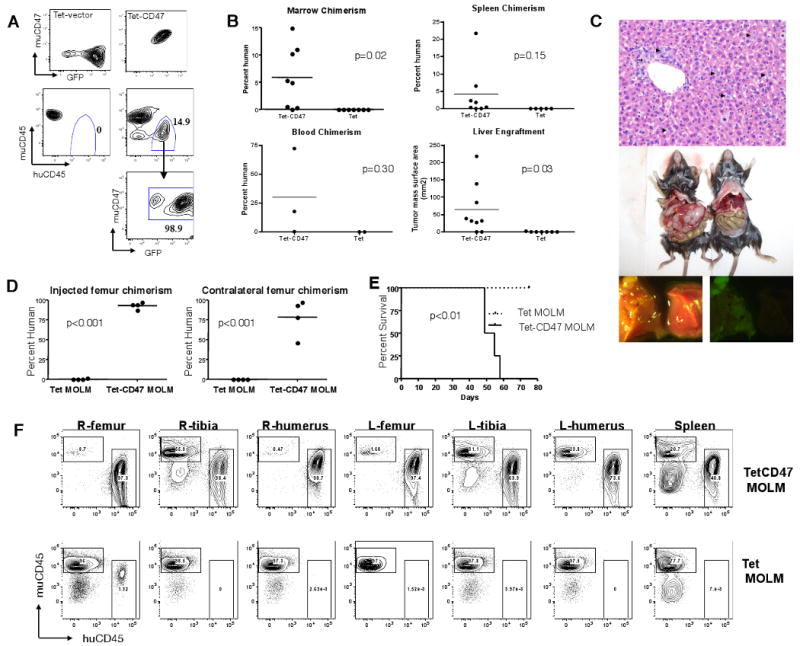
(A) GFP and human CD45 chimerism for mice transplanted with untransduced MOLM-13 cells (5×105 and either 5×105 Tet (n=6) or Tet-CD47 MOLM-13 (n=8) cells. (B) MOLM-13 chimerism in hematopoietic tissues was determined by human CD45 chimerism and measurement of tumour lesion size. (C) Hematoxylin and eosin sections of Tet-CD47 MOLM-13 transplanted liver (200×) (top panel). Periportal (arrow) and sinusoidal (arrowhead) tumor infilitration is evident. Examples of liver tumor formation and hepatomegaly in Tet-CD47 MOLM-13 transplanted mice versus control transplanted mice (middle panel). GFP fluorescence demonstrates tumor nodule formation as well diffuse infilitration (bottom panel). (D) 1×106 Tet (n=5) or Tet-CD47 MOLM-13 (n=4) cells were injected into the right femur of RAG2-/-, Gc-/- mice and the tissues were analyzed 50-75 days later for chimerism of MOLM-13 cells in BM. (E) Survival curve of mice transplanted intrafemorally with Tet or Tet-CD47 MOLM-13 cells. (F) Representative FACS plots from mice transplanted intrafemorally with Tet or Tet-CD47 MOLM-13 cells. R-right, L-left.
Since CD47 has been reported to be important for the migration of hematopoietic cells, one possibility for the lack of growth of Tet MOLM-13 cells in mice was their inability to migrate to niches. To test this, Tet MOLM-13 or Tet-CD47 MOLM-13 cells were directly injected into a single femoral cavity of immunodeficient Balb/c mice. Tet-CD47 MOLM-13 cells were able to spread to all bones and other hematopoietic tissues of recipient mice and lead to death, whereas Tet MOLM-13 cells had minimal, if any, engraftment, and only at the site of injection, without morbidity by 75 days after transplant (Figures 5d-f). These results suggested a function other than or in addition to migration for CD47 in MOLM-13 engraftment.
The level of CD47 expressed on MOLM-13 cells determines tumorigenic potential
Our results up to this point had indicated that ectopically expressing muCD47 on a non-engrafting human cell line could rescue its growth defect. These experiments did not, however, answer whether the level of CD47 expressed on a leukemic cell could affect its tumor forming potential. To model the tumorigenic effect of having high versus low CD47 expression, we sorted clones of muCD47 expressing MOLM-13 cells into high and low expressors. When adjusted for cell size, CD47 density on the CD47lo MOLM-13 cells was approximately equal to mouse bone marrow cells, whereas CD47hi MOLM-13 cells had 9-fold higher expression, an increase commensurate with the change seen in CD47 expression on primary leukemic cells compared to their normal counterparts (Figure 6a-b). When high or low expressing cells were transplanted into recipients, only mice transplanted with high expressing cells succumbed to disease by 75 days of age (Figure 6c). Furthermore, organomegaly was more pronounced in these mice(Figure 6d). As expected, the infiltration of MOLM-13 cells in bone marrow and spleen of recipient mice was also much higher for mice transplanted with CD47hi MOLM-13 cells as well (Figure 6e). We also examined the level of CD47 expression in two mice that received CD47lo MOLM-13 cells but had significant marrow engraftment. In both cases, the persisting cells after 75 days had much higher levels of CD47 than the original line (Figure 6f), indicating that a strong selection pressure exists in vivo for high levels of CD47 expression on MOLM-13 leukemia cells.
Figure 6. Higher expression of CD47 on MOLM-13 cells causes increased tumorigenicity.
(A) Histograms show CD47 expression in MOLM-13 high (black), MOLM-13 low (gray), and mouse bone marrow (shaded) cells, with MFI normalized for size shown. (B) The histograms show level of CD47 expression on CD47hi MOLM-13 cells in untreated (shaded) and doxycycline treated (shaded) mice, with MFI normalized for size shown. (C) Survival of mice transplanted with 1 × 106 CD47hi, CD47lo MOLM-13 cells, or CD47hi MOLM-13 cells with doxycycline administration started after 2 weeks post-transplant. n=5 for CD47hi and CD47hi plus doxycycline. n=10 for CD47lo. (D) Liver and spleen size of mice at necropsy or 75 days after transplant with 1 × 106 CD47hi, CD47lo MOLM-13 cells, or CD47hi MOLM-13 cells with doxycycline. (E) Bone marrow and spleen chimerism of human cells in mice at necropsy or 75 days after transplant with 1 × 106 CD47hi, CD47lo MOLM-13 cells, or CD47hi MOLM-13 cells with doxycycline. (F) Murine CD47 expression on CD47lo MOLM-13 cells engrafting in bone marrow (open) compared with original cell line (shaded), with MFI normalized for size shown. (G) Bone marrow and (H) liver histograms are shown for MOLM-13 cells in mice 35 days after transplant with 1 × 106 CD47lo MOLM-13 cells. Parental Tet MOLM-13 (dark gray shaded) and Tet CD47lo MOLM-13 CD47 (light gray shaded) expression, as well as engrafting cells in the clodronate (gray line) and control (black line) cohorts is displayed in the histograms. (I) MFI of CD47 channel in engrafting MOLM-13 cells in clodronate (CLOD) or control (CTRL) treated mice is shown for bone marrow and liver. Error bars represent 1 SD.
We were also able to modulate CD47 expression with doxycycline since we utilized the Tet-responsive (Tet-OFF) promoter element to control expression of CD47 in MOLM-13 cells. Beginning two weeks after transplantation with CD47hi MOLM-13 cells, a cohort of mice was given doxycycline to extinguish CD47 expression and followed for up to 75 days post-transplant. During this time, none of the mice given doxycycline succumbed to disease or had large infiltration of MOLM-13 cells in hematopoietic organs (Figures 6d-e) other than 1-2 large extra-parenchymal masses seen in the livers. At the dose of doxycycline used in this experiment, muCD47 expression in MOLM-13 cells was reduced to levels below that of normal mouse bone marrow, but notably not completely absent (Figure 6b). Thus, a sustained high level of CD47 expression is required for robust MOLM-13 growth in hematopoietic organs.
Macrophages mediate selective pressure for CD47hi variants of MOLM-13 cells
Since we had observed that mice transplanted with CD47lo MOLM-13 cells had selection over time for higher expressing clones in vivo, we wondered if this selection pressure was mediated by macrophages. To test this, we used clodronate liposomes to deplete macrophages prior to and after transplant of CD47lo MOLM-13 cells and sacrificed mice after 5 weeks. When control liposomes were used, the level of CD47 increased significantly on the engrafted human cells. However, when clodronate containing liposomes were used, the expression of CD47 on MOLM-13 cells was maintained at the level of the parental line (Figure 6g-i). Thus, macrophages mediate selective pressure for high expressing CD47 clones in vivo.
Interestingly, when clodronate was used to deplete macrophages in the immunodeficient mice, the level of engraftment was comparable between the control Tet MOLM-13 cells and the CD47 MOLM-13 cells (Supplementary Figure 8) suggesting that depletion of macrophages could compensate for lack of CD47 expression on MOLM-13 cells as well.
Ability to evade macrophage phagocytosis correlates with CD47 expression level
We incubated Tet (vector only), bulk (unsorted) Tet-CD47, Tet-CD47hi, or Tet-CD47lo MOLM-13 cells with bone marrow derived macrophages (BMDM) for 2-24 hours and assessed phagocytosis by counting the number of ingested GFP+ cells under a microscope (Figure 7a-b) or by evaluating the frequency of GFP+ macrophages by flow cytometry (Supplementary Figure 9a). There was a strong correlation between level of CD47 expressed and the likelihood of phagocytosis (Figure 7a). When CD47lo RFP and CD47hi GFP MOLM-13 cells were co-incubated with macrophages in the same wells, the low expressing cells were far more likely to be phagocytosed (Figures 7c-d). Thus, in a mixed population of cells with varying levels of CD47 expression, the low expressiors were more likely to be cleared by macrophages over time. These results were not due to differences in apoptosis between CD47 expressing and control MOLM-13 cells, as both groups had similar levels of Annexin V positivity in vitro (Supplementary Figure 4c).
Figure 7. Increasing CD47 expression on MOLM-13 cells confers increasing ability to evade macrophage phagocytosis.
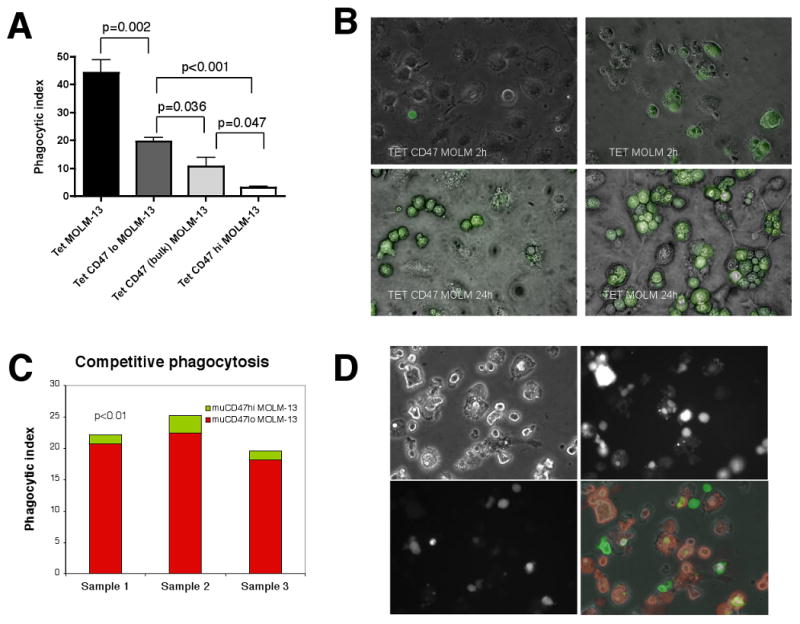
(A) Tet, Tet-CD47lo, Tet-CD47 bulk, or Tet CD47hi MOLM-13 cells were incubated with bone marrow derived macrophages (BMDM) for 2 hours and phagocytic index was determined. Error bars represent 1 SD (n=6 for each time point). (B) Photomicrographs of BMDMs incubated with Tet or Tet-CD47 MOLM-13 cells at 2 and 24 hours (400×). (C) CD47hi GFP and CD47lo MOLM-13 RFP cells were co-incubated with BMDMs for 2 hours. Phagocytic index is shown for three separate samples for CD47hi GFP (green) and CD47lo MOLM-13 RFP (red) cells. (D) Photomicrographs show brightfield (top left), RFP (top right), GFP (bottom left), and merged (bottom right) images of CD47hi GFP and CD47lo MOLM-13 RFP cells were co-incubated with BMDMs for 24 hours.
We also injected MOLM-13 cells into mice and analyzed hematopoietic organs 2 hours later for evidence of macrophage phagocytosis. Macrophages in bone marrow, spleen, and liver all had a higher GFP+ fraction when injected with Tet MOLM-13 cells as compared to muCD47 expressing cells, indicating that the muCD47- cells were more likely to be phagocytosed in vivo as well (Supplementary Figure 9b).
Finally, we asked if inhibiting macrophage SIRPα using a blocking antibody could abrogate the protection against phagocytosis that CD47 provided MOLM-13 cells. Indeed, when this receptor was blocked, CD47 expressing MOLM-13 cells were phagocytosed at the same rate as control MOLM-13 cells (Supplementary Figure 10).
Discussion
Physiological significance of CD47 up-regulation
We demonstrate here for the first time that HSPCs up-regulate CD47 in response to an insult that induces mobilization. This then leads to the surprising finding that HSPCs themselves are regulated by phagocytosis in vivo. Hence, our results indicate that protection from phagocytosis is essential for HSC survival during migration to the periphery after a strong mobilizing or inflammatory stimulus. A recent report indicates that HSPCs can circulate through the lymphatic system as well (Massberg et al., 2007). It will be interesting to see if these HSPCs up-regulate CD47 to evade the numerous sinusoidal macrophages in lymph nodes.
Since leukemias up-regulate CD47 as well, it appears they co-opt this normal physiological response to stress induced mobilization as a means to protect themselves at the expense of normal, non-neoplastic progenitors. In this manner, leukemic stem cells could quickly gain a survival advantage over normal stem cells, leading to their eventual take-over of the hematopoietic environment.
The regulatory mechanisms for CD47 expression in mobilization and leukemia remain unknown. Given that CD47 is up-regulated and down-regulated in such a temporally precise manner during mobilization suggests that there is tight control over its expression in normal physiology. Leukemias, however, seem to have constitutively high CD47 expression. The only published regulator of CD47 expression is the transcription factor nuclear respiratory factor 1 (Nrf1) (Chang and Huang, 2004). It remains to be determined if Nrf1 or other regulatory elements are involved in the progression of human myeloid leukemia, and if there is a common regulatory pathway that operates in mobilization as well.
Macrophages are important mediators of tumor immunosurveillance
Many examples of tumor clearance by T, B, and NK cells have been described in the literature, indicating that a healthy immune system is essential for regulating nascent tumor growth. However, to date, few examples have been produced indicating that macrophage-mediated phagocytosis can check tumor development. Collectively, our studies reveal that ectopic over-expression of CD47 can enable otherwise immunogenic tumor cells to grow rapidly in a T, B, and NK-cell deficient host. Furthermore, this is likely to reflect a mechanism used by primary human myeloid leukemias to evade the host immune system since CD47 is up-regulated in nearly all sufficiently advanced murine and human myeloid leukemias examined thus far.
This form of immune evasion is particularly important since these cancers occupy sites of high macrophage infiltration. The leukemias studied here are clonal, yet found throughout the marrow (and in mouse, spleen and liver), all tissues with abundant intra-tissue macrophages as well as macrophage-lined sinusoids at the entry site of circulating blood cells. CD47 was first cloned as an ovarian tumor cell marker, indicating that it may play a role in preventing phagocytosis of other tissue cancers as well (Campbell et al., 1992). Furthermore, solid tumors often metastasize to macrophage rich tissues such as liver, lung, bone, and lymph nodes, indicating that they must be able to escape macrophage-mediated killing in those tissues. Preventing CD47-SIRPα interaction could be doubly effective since antigens from phagocytosed tumor cells may be presented by profession antigen presenting cells such as macrophages or dendritic cells to activate an adaptive immune response, leading to further tumor destruction.
CD47 may be up-regulated because some cancers are more immunogenic than normal tissue
But why would leukemic progenitor cells require additional protection against phagocytosis? Like aged neutrophils or erythrocytes, tumor cells can display signs of cellular “damage” and/or glycosidic variations on their cell surface. There are well-established data that tumors can be recognized by NK cells via ligands that mark “stressed self” (Gasser et al., 2005). We propose that leukemic cells likewise express stress ligands that mark them for phagocytosis; by up-regulating CD47, this innate immunity check on tumor growth can be circumvented. One of these pro-phagocytic ligands, calreticulin, is known to be up-regulated in aged cells. It has been shown that its effects can be counteracted by expression of CD47 (Gardai et al., 2005). It remains to be seen if this or other stress ligands are expressed on nascent leukemic cells to mark them for destruction.
Therapeutics targeting CD47 may be clinically useful for treating cancer
Others have shown that antibodies that cross-link CD47 on the surface of chronic lymphocytic leukemia cells can induce a caspase-independent cell death (Mateo et al., 1999; Uno et al., 2007). This phenomenon, while promising, appears to function primarily by inducing apoptosis, not necessarily by promoting phagocytosis. In fact, we show in a companion paper [Majeti et al, submitted], that anti-CD47 blocked CD47hi human AML cells are phagocytosed by macrophages, without first becoming apoptotic.
In addition, our recent results in human AML indicate that increasing CD47 transcript levels are associated with shortened survival [Majeti et al, submitted], allowing the level of expression of this gene to potentially be used as an independent prognostic variable. Combined with the data presented here where we show that CD47 expression levels affect the fitness of both normal HSC and leukemic cells, we propose that CD47 is a compelling target for therapeutic strategies that reduce its functional interaction with macrophage SIRPα and thus render these cancers susceptible to innate and adaptive immune system clearance.
Experimental Procedures
Mice
CD47 deficient IAP-/- mice on a C57Bl/6 background were obtained from Eric Brown and bred onto our wild-type colony. IAP-/- mice were crossed to beta-actin GFP (Wright et al., 2001a) or CD45.1 C57Bl/6 mice from our colony to obtain GFP+ or congenic strains. hMRP8bcrabl, hMRP8bcl2, and Faslpr/lpr transgenic mice were obtained as previously described (Traver et al., 1998) (Jaiswal et al., 2003). C57Bl/6 mice from our colony were used as a source of wild-type cells. C57Bl/6 or Balb/c RAG2-/- common gamma chain (Gc)-/- mice were bred in our laboratory. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOG) mice were obtained from The Jackson Laboratory and bred in a pathogen-free environment at our facility. For transplant experiments, cells were transplanted into either immunodeficient mice given a radiation dose of 4 Gy using gamma rays from a cesium irradiator (Phillips), or CD45.2 C57Bl6/Ka mice given a radiation dose of 9.5 Gy. Sub-lethal radiation was given at a dose of 4.75 Gy. Mice were euthanized when moribund.
Cells were injected via tail vein in sterile PBS using a 27-gauge needle. Donor and recipient mice were between 6-12 weeks of age. Parabiosis was performed as previously described (Wright et al., 2001b). Mice were mobilized with cyclophosphamide (Sigma) (200 mg/kg) and G-CSF (Neupogen) (250ug/kg) as previously described (Morrison et al., 1997). Bacterial LPS from E. coli 055:B5 (Sigma) was administered at a dose of 40 mg/kg into the peritoneal cavity.
Cell lines
MOLM-13 cells were obtained from DSMZ. HL-60, Jurkat, U937, K562, and Kasumi-1 cells were obtained from ATCC. Cells were maintained in Iscove's modified Dulbecco's media (IMDM) plus 10% fetal bovine serum (FBS) (Hyclone).
Quantitative RT-PCR Analysis
Bone marrow was obtained from leukemic hMRP8bcr/abl × hMRP8bcl2 mice or hMRP8bcl2 control mice. Cells were c-Kit enriched using c-Kit microbeads and an autoMACS column (Miltenyi) and cDNA was created from RNA using standard methods (Invitrogen, SuperScript II). cDNA corresponding to approximately 1000 cells was used per PCR reaction. Quantitative PCR was performed with a SYBR green kit on an ABI Prism 7000 PCR (Applied Biosystems) machine.
Cell staining and flow cytometry
Mouse tissues were prepared and stained for stem, progenitor and peripheral blood cells as previously described (Akashi et al., 2000) (Yang et al., 2005) (Bhattacharya et al., 2006). Mouse CD47 antibody (clone mIAP301, ATCC) was assessed using biotinylated antibody produced in our lab followed by staining with streptavidin conjugated Quantum Dot 605 (Chemicon). Samples were analyzed using a FACSAria (Beckton Dickinson).
Normal human bone marrow and blood samples were obtained with informed consent from 20 – 25 year old paid donors who were hepatitis A, B, C and HIV negative by serology (All Cells). Leukemic blood and marrow cells were obtained with informed consent from previously untreated patients at Stanford University Medical Center according to IRB approved methods. HSC and progenitors were stained and identified as previously described (Manz et al., 2002). Anti-human CD47 FITC (clone B6H12, Pharmingen) was used to assess CD47 expression in all human samples. Following staining, cells were analyzed using a modified FACS Vantage (Becton Dickinson) or a FACSAria.
Engraftment of MOLM-13 cells was assessed by using anti-human CD45 PE-Cy7 (Pharmingen), anti-mouse CD45.2 APC (clone AL1-4A2 produced in our lab), and anti-mouse CD47 Alexa-680 (mIAP301, produced in our lab).
All samples were resuspended in propidium iodide containing buffer before analysis to exclude dead cells. FACS data was analyzed using FloJo software (Treestar).
Lentiviral preparation and transduction
pRRL.sin-18.PPT.Tet07.IRES.GFP.pre, CMV, VSV, and tet trans-activator (tTA) plasmids were obtained from Luigi Naldini (Vigna et al., 2002). The full-length murine cDNA for CD47 form 2 was provided by Eric Brown (Genentech). Plasmid DNA was transfected into 293T cells and supernatant harvested and concentrated by standard protocols. Cells were transduced with lentivirus for 48 hours and GFP+ cells were sorted to purity and grown for several generations to ensure stability of the transgenes.
In vitro phagocytosis assays
Bone marrow derived macrophages were prepared and harvested by incubation in trypsin/EDTA (Gibco) for 5 minutes and gentle scraping. Macrophages were plated at 5 × 104 cells per well in a 24-well tissue culture plate (Falcon) for 24 hours. Fresh media was added 2 hours before 2.5 × 105 target cells were added to the macrophage containing wells and incubated at 37 C° for the indicated times. After co-incubation, wells were washed thoroughly with IMDM 3 times and examined under an Eclipse T5100 (Nikon) using an enhanced green fluorescent protein (GFP) or Texas Red filter set (Nikon). Phagocytic index was calculated using the formula: phagocytic index=number of ingested cells/(number of macrophages/100). At least 200 macrophages were counted per well.
Macrophage depletion
Clodronate was a gift of Roche Diagnostics GmbH. It was encapsulated in liposomes as previously described (Van Rooijen and Sanders, 1994). Macrophages were depleted by injecting i.v. 200 μL of the final liposome preparation 48 hours prior to cell transplant. Mice were injected with 100 μL every week thereafter to maintain depletion until they were euthanized.
Statistics
Statistics were calculated using Prism 4.0 software (GraphPad Software, Inc.) The Welch corrected student's t-test was used to calculate all column statistics. Two-tailed p-values are shown. For survival curves, p-values were obtained using a logrank test. For comparison of groups, one-way ANOVA and tests of contrast were used where indicated.
Supplementary Material
Acknowledgments
The work in this study was supported in part by National Institutes of Health Grants 5R01CA086017-08, 5R01HL058770-08, 5P01DK053074-08 awarded to I.L.W., the de Villier award of the Leukemia Society, the Ludwig Institute, and a gift from the Smith Family Fund. The authors would also like to thank Christina Muscat for antibody production, Libuse Jerabek for capable laboratory management, and Eric Brown and Mette Johanssen for providing valuable advice and reagents.
Footnotes
Additional methods are available in Supplemental Data.
S.J., C.H.M.J., W.W.P., M.P.C., R.M., and I.L. W designed experiments. S.J., C.H.M.J., W.W.P., C.Y.P., M.P.C., and D.T. performed experiments and analyzed data. N.v.R. provided reagents. S.J., C.H.M.J., and I.L.W. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. The Journal of experimental medicine. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar BR, Lindberg FP, Ingulli E, Panoskaltsis-Mortari A, Oldenborg PA, Iizuka K, Yokoyama WM, Taylor PA. CD47 (integrin-associated protein) engagement of dendritic cell and macrophage counterreceptors is required to prevent the clearance of donor lymphohematopoietic cells. The Journal of experimental medicine. 2001;194:541–549. doi: 10.1084/jem.194.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Freemont PS, Foulkes W, Trowsdale J. An ovarian tumor marker with homology to vaccinia virus contains an IgV-like region and multiple transmembrane domains. Cancer Res. 1992;52:5416–5420. [PubMed] [Google Scholar]
- Chang WT, Huang AM. Alpha-Pal/NRF-1 regulates the promoter of the human integrin-associated protein/CD47 gene. The Journal of biological chemistry. 2004;279:14542–14550. doi: 10.1074/jbc.M309825200. [DOI] [PubMed] [Google Scholar]
- Christensen JL, Wright DE, Wagers AJ, Weissman IL. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MJ, Golde DW. Mobilization of hematopoietic stem cells (CFU-C) into the peripheral blood of man by endotoxin. Experimental hematology. 1977;5:186–190. [PubMed] [Google Scholar]
- Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25. [DOI] [PubMed] [Google Scholar]
- Gao AG, Lindberg FP, Finn MB, Blystone SD, Brown EJ, Frazier WA. Integrin-associated protein is a receptor for the C-terminal domain of thrombospondin. The Journal of biological chemistry. 1996;271:21–24. doi: 10.1074/jbc.271.1.21. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proc Natl Acad Sci U S A. 2003;100:10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. The Journal of biological chemistry. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaudet AL, Brown EJ. Science. Vol. 274. New York, NY: 1996. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice; pp. 795–798. [DOI] [PubMed] [Google Scholar]
- Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. The Journal of biological chemistry. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- Manz MG, Miyamoto T, Akashi K, Weissman IL. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 2002;99:11872–11877. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo V, Lagneaux L, Bron D, Biron G, Armant M, Delespesse G, Sarfati M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat Med. 1999;5:1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Ohnishi H, Okazawa H, Tomonaga H, Hayashi A, Fujimoto TT, Furuya N, Matozaki T. Promotion of neurite and filopodium formation by CD47: roles of integrins, Rac, and Cdc42. Mol Biol Cell. 2004;15:3950–3963. doi: 10.1091/mbc.E04-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Wright DE, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor induces hematopoietic stem cells to proliferate prior to mobilization. Proc Natl Acad Sci U S A. 1997;94:1908–1913. doi: 10.1073/pnas.94.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Science. Vol. 288. New York, NY: 2000. Role of CD47 as a marker of self on red blood cells; pp. 2051–2054. [DOI] [PubMed] [Google Scholar]
- Olsson M, Bruhns P, Frazier WA, Ravetch JV, Oldenborg PA. Platelet homeostasis is regulated by platelet expression of CD47 under normal conditions and in passive immune thrombocytopenia. Blood. 2005;105:3577–3582. doi: 10.1182/blood-2004-08-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Nilsson A, Oldenborg PA. Dose-dependent inhibitory effect of CD47 in macrophage uptake of IgG-opsonized murine erythrocytes. Biochem Biophys Res Commun. 2007;352:193–197. doi: 10.1016/j.bbrc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Science. Vol. 283. New York, NY: 1999. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4; pp. 845–848. [DOI] [PubMed] [Google Scholar]
- Reinhold MI, Lindberg FP, Kersh GJ, Allen PM, Brown EJ. Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway. The Journal of experimental medicine. 1997;185:1–11. doi: 10.1084/jem.185.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- Traver D, Akashi K, Weissman IL, Lagasse E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity. 1998;9:47–57. doi: 10.1016/s1074-7613(00)80587-7. [DOI] [PubMed] [Google Scholar]
- Uno S, Kinoshita Y, Azuma Y, Tsunenari T, Yoshimura Y, Iida S, Kikuchi Y, Yamada-Okabe H, Fukushima N. Antitumor activity of a monoclonal antibody against CD47 in xenograft models of human leukemia. Oncology reports. 2007;17:1189–1194. [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. Journal of immunological methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Van VQ, Lesage S, Bouguermouh S, Gautier P, Rubio M, Levesque M, Nguyen S, Galibert L, Sarfati M. Expression of the self-marker CD47 on dendritic cells governs their trafficking to secondary lymphoid organs. Embo J. 2006;25:5560–5568. doi: 10.1038/sj.emboj.7601415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna E, Cavalieri S, Ailles L, Geuna M, Loew R, Bujard H, Naldini L. Robust and efficient regulation of transgene expression in vivo by improved tetracycline-dependent lentiviral vectors. Mol Ther. 2002;5:252–261. doi: 10.1006/mthe.2002.0542. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Allsopp RC, Weissman IL. Changes in integrin expression are associated with altered homing properties of Lin(-/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Experimental hematology. 2002;30:176–185. doi: 10.1016/s0301-472x(01)00777-9. [DOI] [PubMed] [Google Scholar]
- Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci U S A. 2007;104:13744–13749. doi: 10.1073/pnas.0702881104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. The Journal of experimental medicine. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DE, Cheshier SH, Wagers AJ, Randall TD, Christensen JL, Weissman IL. Cyclophosphamide/granulocyte colony-stimulating factor causes selective mobilization of bone marrow hematopoietic stem cells into the blood after M phase of the cell cycle. Blood. 2001a;97:2278–2285. doi: 10.1182/blood.v97.8.2278. [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Science. Vol. 294. New York, NY: 2001b. Physiological migration of hematopoietic stem and progenitor cells; pp. 1933–1936. [DOI] [PubMed] [Google Scholar]
- Wright SD, Tobias PS, Ulevitch RJ, Ramos RA. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. The Journal of experimental medicine. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Bryder D, Adolfsson J, Nygren J, Mansson R, Sigvardsson M, Jacobsen SE. Identification of Lin(-)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood. 2005;105:2717–2723. doi: 10.1182/blood-2004-06-2159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.