Abstract
Cytokines are upregulated in a variety of inflammatory conditions and cytokine/receptor interactions can activate JAK-STAT signaling. Previous studies demonstrated upregulation of numerous cytokines in the urinary bladder following cyclophosphamide (CYP)-induced cystitis. The role of JAK-STAT signaling in urinary bladder inflammation and referred somatic sensitivity has not been addressed. The contribution of JAK-STAT signaling pathways in CYP-induced bladder hyperreflexia and referred somatic hypersensitivity was determined in CYP-treated rats using a JAK2 inhibitor, AG490. Acute (4 h; 150 mg/kg ip), intermediate (48 h; 150 mg/kg ip), or chronic (75 mg/kg ip, once every 3 days for 10 days) cystitis was induced in adult, female Wistar rats with CYP treatment. Phosphorylation status of STAT-3 was increased in urinary bladder after CYP-induced cystitis (4 h, 48 h, chronic). Blockade of JAK2 with AG490 (5–15 mg/kg ip or intravesical) significantly (P ≤ 0.05) reduced bladder hyperreflexia and hind paw sensitivity in CYP-treated rats. These studies demonstrate a potential role for JAK-STAT signaling pathways in bladder hyperreflexia and referred pain induced by CYP-induced bladder inflammation.
Keywords: somatic sensitivity, bladder hyperreflexia, cystometry, AG490
clinical symptoms of interstitial cystitis (IC)/painful bladder syndrome (PBS) include bladder and/or pelvic pain, urinary frequency, and urgency (17, 44). The pathophysiologic mechanisms of IC/PBS are not fully understood but current research hypotheses include bladder urothelial dysfunction, mast cell activation and recruitment, and neurogenic inflammation (41). Potential mediators of urinary bladder inflammation are numerous and include cytokines (19, 36, 40), chemokines (55), neuropeptides (5, 49), neuroactive compounds (4), and growth factors (51, 54, 56). Of these mediators, a number of cytokines have been implicated in animal models of urinary bladder inflammation and in IC/PBS human studies. For example, interleukin-6 (IL-6) expression is markedly increased in urine of IC/PBS patients (19, 36). Bladder inflammation in rats and mice induced by cyclophosphamide (CYP) increased transcript and protein expression in urinary bladder of several cytokines, including IL-6 (22, 40). Furthermore, the IL-6 cytokine family member leukemia inhibitory factor (LIF) is also regulated at the transcript and protein levels in the urinary bladder by CYP-induced cystitis and increased protein expression is exhibited in the urothelium, detrusor smooth muscle, and in nerve fibers of the suburothelial plexus (7) with CYP-induced cystitis.
LIF and IL-6 receptor interactions, among others (3, 12, 14, 21, 30), induce transphosphorylation of the receptor-associated Janus-activated kinases (JAK), which in turn leads to phosphorylation of the downstream signal transducer and activator of transcription (STAT) family of transcription factors (JAK-STAT pathway) (18, 30). The IL-6 family of cytokines also activates mitogen-activated protein kinase (MAPK) signaling pathways and associated transcription factors (21). Inhibition of JAK-STAT3 signaling pathway by AG490, a member of the tyrphostin family of tyrosine kinase inhibitors, reduces inflammatory changes in bronchial epithelial cells (26) and neuropathic pain stemming from peripheral nerve injury (14). Previous studies (11, 45) including those from this laboratory (10) demonstrated upregulation of phosphorylated extracellular signal-regulated kinases (ERK) in urinary bladder and lumbosacral spinal cord with CYP-induced cystitis. Mitogen-activated protein kinase kinase (MEK) inhibitors reduce voiding frequency in CYP-treated rats (10, 11).
The involvement of the JAK-STAT pathway in micturition reflexes and CYP-induced cystitis has not been addressed. The purpose of this study was to determine and quantify 1) phosphorylated (p) STAT3 expression in urinary bladder and modulation with CYP-induced cystitis, 2) urinary bladder function using conscious cystometry with intravesical instillation of AG490, an inhibitor of JAK2, one of the major STAT3 activating kinases (18), and 3) CYP-induced hind paw mechanical allodynia after administration of AG490.
MATERIALS AND METHODS
Animals
Adult female Wistar rats (200–225 g; Charles River, St. Constant, Canada) were used for this study. Rats were housed two per cage and maintained in standard laboratory conditions with free access to food and water. The University of Vermont Institutional Animal Care and Use Committee approved all animal use procedures (protocols 06-014; 08-085).
Induction of CYP-Induced Cystitis
Rats were anesthetized under isoflurane (2%) and acute cystitis was induced with a single injection of CYP (150 mg/kg ip) and rodents were used in studies at various time points (4 h, 48 h) after treatment (8, 34, 35). Chronic CYP cystitis was induced by administration of CYP (75 mg/kg ip) once every 3 days for 10 days (8, 34, 35). Control rodents received either saline injection or no treatment. Rats were euthanized using isoflurane (5%) and a thoracotomy.
Western Blotting for pSTAT3 Expression in Whole Urinary Bladder
Whole urinary bladders (control, 4 h, 48 h and chronic; n = 5 each) were homogenized separately in tissue protein extraction agent with protease inhibitors (T-PER; Roche, Indianapolis, IN), and aliquots were removed for protein assay as previously described (35). Samples (23 μg) were suspended in sample buffer for fractionation on gels and subjected to SDS-PAGE. Proteins were transferred to nitrocellulose membranes, and efficiency of transfer was evaluated. Membranes were blocked overnight in a solution of 5% milk, 3% bovine serum albumin in Tris-buffered saline with 0.1% Tween. For immunodetection, the following antibodies were used overnight at 4°C: rabbit anti-phospho-STAT3Tyr705 (1:2,000; Cell Signaling Technology, Danvers, MA) and mouse anti-STAT3 (1:2,000; Cell Signaling Technology). Washed membranes were incubated in species-specific secondary antibodies for 2 h at room temperature for enhanced chemiluminescence detection (Pierce, Rockford, IL). Blots were exposed to Biomax film (Kodak, Rochester, NY) and developed. The intensity of each band was analyzed, and background intensities were subtracted using Un-Scan It software (Silk Scientific, Orem, UT).
Intravesical Catheter Placement
A lower midline abdominal incision was performed under general anesthesia with 2–3% isoflurane using aseptic techniques. Polyethylene tubing (PE-50, Clay Adams, Parsippany, NJ) with the end flared by heat was inserted into the dome of the bladder and secured in place with a 6–0 nylon purse string suture (28, 33, 35). The distal end of the tubing was sealed, tunneled subcutaneously, and externalized at the back of the neck, out of the animal's reach. Animals were maintained for 72 h after surgery to ensure complete recovery.
Continuous Cystometry
The effects of a JAK2 inhibitor on bladder function in control (no inflammation, n = 6) and CYP-treated rats (4 and 48 h, n = 6 each) were evaluated by intravesical infusion of AG490 [LC Laboratories, Woburn, MA (T-9142); 5 mg/kg in 10% DMSO in saline] or vehicle using conscious cystometry and continuous infusion of intravesical saline. We determined the volume that filled the bladder catheter at time of implantation. Immediately before cystometric analysis, rats were anesthetized (1–2% isoflurane) and bladders were manually emptied with the Credé maneuver. We then filled this catheter with the AG490 solution, ensured the absence of air, and displaced ∼1 ml of solution with a syringe filled with AG490 solution or vehicle (10% DMSO in saline). At the time of experimentation, the rat is anesthetized (1–2% isoflurane) to prevent voiding and expulsion of bladder contents (5, 10, 35), and ≤1 ml of AG490 solution (5 mg/kg) is infused into the bladder; the AG490 is left in place for 20 min. The concentration of AG490 chosen for this study was based on results from our somatic testing experiments and previous experiments with AG490 (12, 14). Animals were placed conscious and unrestrained in recording cages with a balance and pan for urine collection and measurement placed below (5, 10, 35). Intravesical pressure changes were recorded using a Small Animal Cystometry System (Med Associates, St. Albans, VT). Saline at room temperature was infused at a rate of 10 ml/h to elicit repetitive bladder contractions. At least four reproducible micturition cycles were recorded after an initial stabilization period of 25–30 min. Voided saline was collected to determine voided volume. Intercontraction interval, maximal voiding pressure, pressure threshold for voiding, and baseline resting pressure were measured (39). The number of nonvoiding bladder contractions (NVCs) per voiding cycle during the filling phase was determined. For these studies, NVCs were defined as rhythmic intravesical pressure rises greater than 7 cmH2O from baseline pressure without a release of fluid from the urethra (35, 56).
Exclusion Criteria
Rats were removed from study when adverse events occurred that included: ≥20% reduction in body weight postsurgery, a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of postoperative analgesics or hematuria in control rodents. In the present study, no rats were excluded from the study or from analysis due to any of these exclusion criteria. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable (47). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements (15). Rats were euthanized at the conclusion of study as previously described.
Mechanical Sensitivity Testing
To study the effects of AG490 in CYP-induced hind paw mechanical sensitivity, four groups (n = 6) of rats were used. Two groups received either 5 or 15 mg/kg ip of AG490 dissolved in 1 ml of sterile saline with 10% DMSO. A vehicle control group and a no treatment group were also included. One hour after AG490 or vehicle administration, all groups except the no treatment group received CYP (150 mg/kg ip). Four hours later, rats from all groups were tested for mechanical pain sensitivity using von Frey filament testing. Previous studies including those from this laboratory demonstrated hind paw sensitivity after CYP treatment (23, 48). The 4-h time point after CYP treatment was selected for study in this experimental series because previous studies demonstrated increased peripheral mechanical sensitivity induced by CYP treatment at this time point with a return to baseline sensitivity 48 h after CYP treatment (48).
Behavioral testing was performed during the light cycle as previously described (48). Mechanical foot withdrawal threshold was determined using a series of von Frey filaments that produce forces ranging from 0.23 to 59.0 g. Rats were placed unrestrained in a plastic cage with a metal mesh floor and permitted to acclimate to this environment (30 min) before testing. The filaments were pressed perpendicularly to the midplantar surface and held approximately for 2 s. Fibers are applied in an increasing and decreasing diameter around the response threshold, with a maximum of nine applications per paw (the up-down method) (6). The minimum interval between stimulations was 5 min. A stimulus-induced response was considered positive when the paw was sharply withdrawn, paw licking occurred, or the animal flinched upon removal of the filament. If the rat did not respond within 2 s, then the filament was removed. This procedure does not produce damage to the hind paw. All somatic testing was performed in a blinded manner with respect to treatment. The groups were decoded after data analysis.
Materials
All standard chemicals were obtained from Sigma or Fisher and were either analytical or laboratory grade.
Statistics
All values are means ± SE. Data were compared using ANOVA. Percentage data from image analysis were arcsin transformed to meet the requirements of this statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (P ≤ 0.05), the Newman-Keuls post hoc test was used to compare experimental means.
RESULTS
pSTAT3 Expression in Urinary Bladder with CYP-Induced Cystitis
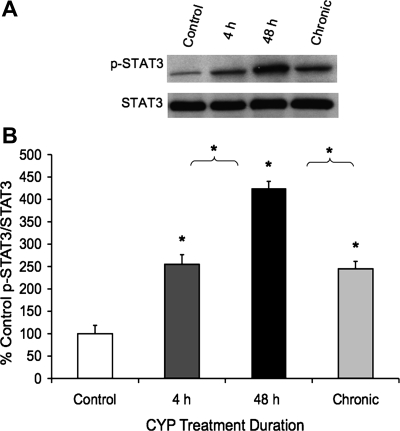
Cytokines, including members of the neuropoietic family, signal predominantly through the JAK-STAT cascades. To assess whether the increase in tissue cytokines after CYP was met with increased JAK-STAT signaling, bladder tissues from treated animals were prepared for Western blot analyses. As shown, CYP-induced cystitis (4 h, 48 h, chronic) significantly (P ≤ 0.01) increased (2.5- to 4.3-fold) STAT3 phosphorylation status in whole urinary bladder (Fig. 1). STAT3 phosphorylation status was significantly (P ≤ 0.01) greater with 48-h CYP treatment compared with either 4-h CYP or chronic CYP treatment (Fig. 1).
Fig. 1.
Upregulation of phosphorylated (p) STAT3 expression in whole urinary bladder with cyclophosphamide (CYP)-induced cystitis (4 h, 48 h, and chronic) using Western blotting techniques. A: representative example of a Western blot of whole urinary bladder (20 μg) for pSTAT3 expression in control rats and those treated with CYP for varying duration. Total STAT3 staining was also determined. B: histogram of relative pSTAT3 band density in all groups examined normalized to total STAT in the same samples presented as a percentage of control STAT3 activation (p-STAT3 expression). pSTAT3 expression in urinary bladder is significantly increased at all time points with CYP treatment. pSTAT3 expression is significantly greater at 48-h CYP treatment compared with 4-h or chronic CYP treatment. *P ≤ 0.01. Data are a summary of n = 5 for each group.
Effects of JAK2 Inhibitor, AG490, on Cystometry in Rats with and without CYP-Induced Cystitis
Control (no inflammation).
Whether the CYP-induced increase in cytokine signaling participated in altered bladder function was examined using the tyrphostin AG490 JAK2 tyrosine kinase inhibitor. In control rats, intravesical infusion of AG490 (5 mg/kg) combined with continuous fill cystometry did not affect bladder pressures and had no effect on number of NVCs per micturition cycle or the duration of the intermicturition interval (Table 1) compared with control rats treated with vehicle.
Table 1.
Summary of cystometric parameters and numbers of observations
| Treatment | N Value | Fill Pressure, cmH2O | Threshold Pressure, cmH2O | Micturition Pressure, cmH2O | NVC/Cycle | Intermicturition Interval, s |
|---|---|---|---|---|---|---|
| No CYP + vehicle | 6 | 12.8±0.5 | 17.8±1.0 | 76.8±6.4 | 0.5±0.2 | 306.2±16.4 |
| No CYP + AG490 (5 mg/kg) | 6 | 13.6±0.4 | 16.8±1.0 | 79.8±4.0 | 0.4±0.3 | 314.8±19.3 |
| 4-h CYP + vehicle | 6 | 13.3±1.0 | 18.7±1.0 | 76.1±7.5 | 0.7±0.1 | 92.5±17.6* |
| 4-h CYP + AG490 (5 mg/kg) | 6 | 12.7±0.5 | 17.3±1.4 | 69.5±2.2 | 0.1±0.2 | 183.8±29.7† |
| 48-h CYP + vehicle | 6 | 12.8±0.9 | 17.1±1.6 | 74.5±10.8 | 0.5±0.3 | 155.9±20.4* |
| 48-h CYP + AG490 (5 mg/kg) | 6 | 12.7±1.1 | 16.0±0.5 | 72.8±11.5 | 0.1±0.2 | 235.3±25.4‡ |
Values are means ± SE. Summary of cystometric parameters and numbers of observations in control and cyclophosphamide (CYP)-treated (4 and 48 h) with and without the JAK2 inhibitor, AG490 (5 mg/kg; intravesical instillation) in conscious, unrestrained rats with continuous saline instillation is shown.
P ≤ 0.01 compared with control.
P ≤ 0.01 compared with 4-h CYP + vehicle.
P ≤ 0.01 compared with 48-h CYP + vehicle. NVC, nonvoiding contractions.
CYP treatment.
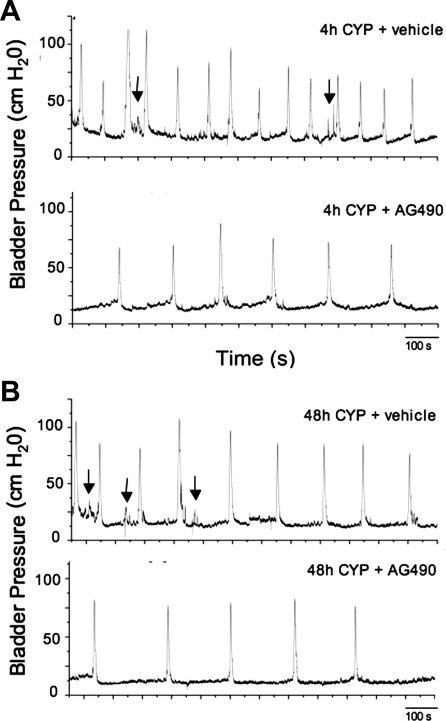
As previously demonstrated (12, 19, 45, 46) and confirmed here, CYP treatment (4 h, 48 h) significantly (P ≤ 0.001) decreased the interval between micturition events (i.e., intermicturition interval; Table 1; Fig. 2A). Intravesical infusion of AG490 (5 mg/kg) in 4-h CYP-treated rats significantly (P ≤ 0.001) increased the intermicturition interval but produced no effects on the number of NVCs or bladder pressures compared with CYP-treated (4 h) rats treated with vehicle. Similarly, intravesical infusion of AG490 (5 mg/kg) in 48-h CYP-treated rats significantly (P ≤ 0.001) increased the intermicturition interval but produced no effects on NVCs or bladder pressures (Table 1; Fig. 2B) compared with CYP-treated (48 h) rats treated with vehicle.
Fig. 2.
Bladder function recordings in CYP-treated [4 h (A) and 48 h (B)] rats treated with vehicle or the JAK2 inhibitor, AG490 (5 mg/kg; intravesical instillation). Intravesical administration of AG490 reduced voiding frequency after CYP-induced cystitis. A: continuous cystometrogram recordings in a 4-h CYP-treated + vehicle (top trace) and same rat treated with AG490 (bottom trace). Arrows point to some nonvoiding bladder contractions (NVCs). In CYP-treated rats further treated with intravesical instillation of AG490, voiding frequency was reduced. B: continuous cystometrogram recordings in a 48-h CYP-treated + vehicle (top trace) and same rat treated with AG490 (bottom trace). Arrows point to some NVCs induced by CYP treatment (see Table 1). NVCs were not significantly affected by AG490 treatment (see Table 1). With intravesical instillation of AG490 in CYP-treated rats, voiding frequency was reduced. The x-axis represents time (s) and the y-axis represents intravesical pressure (cmH2O).
Mechanical Allodynia
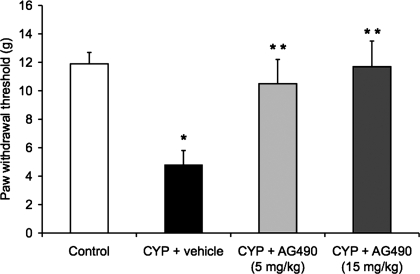
Previous studies in mice and rats demonstrate increased peripheral mechanical sensitivity after CYP-induced cystitis (23, 48). To assess whether increased cytokine/cytokine signaling mediates some of the sensory changes associated with cystitis, hind paw mechanical sensitivity to von Frey filaments in CYP-treated and control (no inflammation) rats was tested in the presence of AG490 (5, 15 mg/kg ip). After CYP treatment (4 h) and vehicle treatment, the paw withdraw threshold was significantly reduced compared with control (no inflammation; P ≤ 0.05; Fig. 3). The reduction in paw withdraw threshold was consistent with previous studies examining hind paw sensitivity after CYP-induced cystitis (23, 48). Treatment with both concentrations of AG490 (5, 15 mg/kg) produced a similar and significant (P ≤ 0.05) increase in paw withdraw threshold (Fig. 3) compared with CYP-treated rats with vehicle.
Fig. 3.
Intraperitoneal (ip) treatment with AG490, a JAK2 inhibitor, reduced referred somatic sensitivity after CYP treatment (4 h). As expected, CYP treatment + vehicle reduced paw withdraw threshold (allodynia). Pretreatment with AG490 (5 or 15 mg/kg ip) significantly reduced paw withdrawal responses induced by CYP treatment (4 h). Both concentrations of AG490 produced similar changes in paw withdraw threshold. *P ≤ 0.05 compared with control. **P ≤ 0.01 compared with CYP + vehicle. Data are a summary of n = 6 for each group.
DISCUSSION
The present studies demonstrate several novel findings with respect to the involvement of JAK-STAT signaling in CYP-induced bladder hyperreflexia and somatic hypersensitivity. Cytokine receptors signal predominantly through JAK-STAT pathways and we determined whether CYP-induced cystitis of varying duration was associated with enhanced JAK-STAT signaling. We showed that STAT3 phosphorylation/activation was increased after CYP-induced cystitis (4 h, 48 h, and chronic). Functionally, blockade of JAK2 with AG490 significantly reduced bladder hyperreflexia and hind paw sensitivity in CYP-treated rats. In aggregate, these studies demonstrate potential roles for JAK-STAT signaling pathways in contributing to bladder hyperreflexia and referred pain of CYP-induced bladder inflammation. These studies extend the list of signal transduction pathways that may represent novel targets for pharmacological intervention in bladder inflammation and referred somatic sensitivity.
IC/PBS is a chronic inflammatory bladder disease syndrome characterized by urinary frequency, urgency, suprapubic and pelvic pain (17, 44). Although the etiology and pathogenesis of IC are unknown, numerous theories including infection, autoimmune disorder, toxic urinary agents, deficiency in bladder wall lining, and neurogenic causes have been proposed (17, 25, 32, 44, 46). We hypothesized that pain associated with IC/PBS involves an alteration of visceral sensation/bladder sensory physiology. Altered visceral sensations from the urinary bladder (i.e., pain at low or moderate bladder filling) that accompany IC/PBS (17, 25, 32, 44, 46) may be mediated by many factors including changes in the properties of peripheral bladder afferent pathways such that bladder afferent neurons respond in an exaggerated manner to normally innocuous stimuli (allodynia). These changes may be mediated, in part, by inflammatory changes in the urinary bladder. Among potential mediators of inflammation, neurotrophins (e.g., nerve growth factor) have been implicated in the peripheral sensitization of nociceptors (13, 16, 38). Proinflammatory cytokines also cause sensitization of polymodal C-fibers (16) and facilitate A-beta input to the spinal cord (2, 53). Several studies from our laboratory demonstrated increased cytokine expression including IL-6 and LIF in the urinary bladder after CYP-induced cystitis (7, 40). Furthermore, a recent study also demonstrated upregulation of IL-6 transcript and protein expression in the submucosal layer of bladder after CYP treatment in mice (43).
Possible mechanisms underlying the neural plasticity and bladder hyperreflexia following chronic CYP-induced cystitis (49, 50, 52) may involve alterations in neurotrophic factors in the urinary bladder (51). In addition, neuroimmune activation, including the production of cytokines, occurs after injury to the central or peripheral nervous system and cytokines are also likely to play a role in the development of pain, exacerbate pathology, or may contribute to repair strategies (1, 9, 24). Individuals with IC/PBS report a predominance of suprapubic pain as well as urethral, genital, and nongenitourinary pain. In addition, hypersensitivity to somatic stimuli has been observed in subjects with IC/PBS (20, 42). A number of reports demonstrated referred somatic hypersensitivity in animal models of urinary bladder inflammation including CYP (23, 48). In this study, we demonstrated a reduction in hind paw sensitivity in rats treated with CYP (4 h) and a reduction in CYP-induced urinary bladder hyperreflexia when rats were also treated with a JAK2 inhibitor, AG490. Tyrosine kinase inhibitors, including AG490, are a class of drugs used to block the activity of tyrosine kinases and the signaling pathways they activate to control inflammatory responses and diseases such as cancer, atherosclerosis, and psoriasis (37). Treatment with AG490 reduces cytokine production, inflammatory cell infiltration, blocks STAT3 activation and nitric oxide (NO) production, and improves survival rate in nonseptic shock and peripheral nerve injury models (12). In addition, blockade of the STAT3 pathway with AG490 attenuated mechanical allodynia and thermal hyperalgesia after peripheral nerve injury (14). In the present study, the conditions of our bladder function experiments (e.g., intravesical route of AG490, duration of exposure, and dilution of AG490 with urine production) make confirming the effects of AG490 on JAK2 signaling and pSTAT3 expression extremely challenging. We also only evaluated the acute effects of AG490 on hind paw sensitivity as previous studies demonstrated a return to baseline somatic sensitivity 48 h after CYP treatment (48) so we cannot make any conclusions about the involvement of JAK-STAT signaling in chronically maintained somatic sensitivity induced by CYP-induced cystitis. Although in previous studies in mice we determined pelvic region sensitivity after CYP-induced cystitis (48), this was not possible in the present studies in rats because the length of the von Frey filaments was not adequate to stimulate the pelvic region of the rat. Our present data suggest a role for the activation of the JAK-STAT3 pathway in referred somatic sensitivity of visceral origin of acute duration and bladder hyperreflexia with CYP-induced cystitis at acute (4 h) and intermediate (48 h) time points.
In the present study, intravesical instillation of a JAK2 inhibitor, AG490, reduced voiding frequency (i.e., increased the intercontraction interval) in CYP-treated (4 h, 48 h) rats but was without effect on bladder pressures or NVCs. Intravesical instillation of AG490 also had no effect on bladder function in control rats. We based the dose (5 mg/kg) used in the current study on our somatic testing data, which used a dose consistent with published reports (12, 14); however, this dose may not be optimal as there is no literature precedent for intravesical instillation of AG490. No effects on bladder function were observed in control rats treated with this AG490 dose; the effects were only observed on bladder function in rats treated with CYP and only on intercontraction interval, not on bladder pressure or NVCs. The absence of broad effects on all cystometric parameters and effects only in inflamed urinary bladder suggests specificity in AG490 action at the dose used in this study. The effects of AG490 were similar to those observed in our previous studies with intravesical instillation of U0126, a potent and selective MEK inhibitor (10). U0126 decreased the voiding frequency and exhibited no effects on NVCs. The lack of effect of AG490 on NVCs may be related to the concentration of AG490 tested, and/or lack of penetration to the detrusor smooth muscle with intravesical instillation. It is possible that the observed AG490 effects on bladder function in CYP-treated rats may reflect actions at the level of the urothelium and suburothelial nerve plexus. Previous studies also demonstrated that intrathecal administration of the ERK inhibitor, PD98059, also decreased voiding frequency in rats treated with CYP (200 mg/kg; 4 h) (11). The action of AG490 could be attributed to either preventing a posttranslational change mediated by the JAK-STAT signal transduction pathway or to a reduction in transcription of target genes. We did not evaluate the bladder effects of AG490 in rats treated chronically with CYP because the increased phosphorylation status of STAT3 was maximal with 48-h CYP treatment and no differences in phosphorylation status of STAT3 were observed between 4 h and chronic CYP treatment. We evaluated and demonstrated improvement in bladder function with AG490 at an acute (4 h) and intermediate (48 h) time point of bladder inflammation induced by CYP; however, whether AG490 exhibits similar effects on bladder function after chronic CYP treatment is not currently known so the relevance of these data to the chronic syndrome of PBS/IC is not known. Furthermore, it is not currently known how any of the time points evaluated (4 h, 48 h, and chronic) with CYP-induced cystitis relate to IC/PBS, thus one must always be cautious in interpretation and potential significance of these rodent data to the human IC/PBS syndrome.
STAT3 plays a role in the transcription control of many genes including glial fibrillary acidic protein, gp130, suppressor of cytokine signaling 3 and cyclooxygenase-2 (COX-2) (14) and STAT3 expression is altered with PACAP addition to PC12 cells (29). Previous studies demonstrated involvement of COX-2, prostaglandins, and PACAP in bladder hyperreflexia after CYP treatment (5, 27, 33). The rapid nature of the effect of AG490 in reducing acute pain hypersensitivity and bladder hyperreflexia may be attributed to a posttranslational change downstream of the activation of STAT as has been previously suggested for the rapid effects demonstrated with MEK inhibitors (31). It would be of interest in future studies to determine effects of combined JAK and MEK blockade on bladder function after CYP-induced cystitis. Furthermore, it is not known what is/are the upstream chemical mediator(s) that are activated by CYP-induced cystitis to stimulate JAK-STAT signaling. Future studies involving LIF or IL-6 null mice and subsequent responses to CYP-induced cystitis can begin to address this interesting question.
Conclusions
In summary, these studies suggest the involvement of the JAK-STAT signaling pathway in CYP-induced cystitis. Significant upregulation of pSTAT3 expression was demonstrated in the urinary bladder with CYP-induced cystitis (4 h, 48 h, and chronic). Blockade of JAK2 with AG490 significantly reduced bladder hyperreflexia and referred somatic pain induced by CYP treatment. Blockade of the JAK-STAT signaling pathway may be a novel target to improve bladder function and referred somatic sensitivity after urinary bladder inflammation.
GRANTS
This work was funded by National Institutes of Health (NIH) Grants DK-051369, DK-060481, and DK-065989. NIH Grant Number P20-RR-16435 from the COBRE Program of the National Center also supported the project for Research Resources.
Present address of B. P. Cheppudira: Dept. of Pharmacology, Univ. of Illinois at Chicago, Chicago, IL 60612.
ACKNOWLEDGEMENTS
The authors acknowledge the technical expertise and support provided by the VT Cancer Center DNA Analysis Facility.
REFERENCES
- 1. Anderson LC, Rao RD. Interleukin-6 and nerve growth factor levels in peripheral nerve and brainstem after trigeminal nerve injury in the rat. Arch Oral Biol 46: 633–640, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Baba H, Doubell TP, Woolf CJ. Peripheral inflammation facilitates A beta fiber-mediated synaptic input to the substantia gelatinosa of the adult rat spinal cord. J Neurosci 19: 859–867, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benigni F, Fantuzzi G, Sacco S, Sironi M, Pozzi P, Dinarello CA, Sipe JD, Poli V, Cappelletti M, Paonessa G, Pennica D, Panayotatos N, Ghezzi P. Six different cytokines that share GP130 as a receptor subunit induce serum amyloid A and potentiate the induction of interleukin-6 and the activation of the hypothalamus-pituitary-adrenal axis by interleukin-1. Blood 87: 1851–1854, 1996 [PubMed] [Google Scholar]
- 4. Birder LA, Wolf-Johnston A, Buffington CA, Roppolo JR, de Groat WC, Kanai AJ. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol 173: 625–629, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290: R951–R962, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Cheppudira B, Schutz K, May V, Vizzard MA. Upregulation of leukemia inhibitory factor (LIF) in urinary bladder with cyclophosphamide (CYP)-induced bladder inflammation in female rats. Soc Neurosci Abstr In press [Google Scholar]
- 8. Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F826–F836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cominelli F, Pizarro TT. Interleukin-1 and interleukin-1 receptor antagonist in inflammatory bowel disease. Aliment Pharmacol Ther 10: 49–53, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R125–R134, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Cruz CD, Avelino A, McMahon SB, Cruz F. Increased spinal cord phosphorylation of extracellular signal-regulated kinases mediates micturition overactivity in rats with chronic bladder inflammation. Eur J Neurosci 21: 773–781, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Dimitrova P, Ivanovska N. Tyrphostin AG-490 inhibited the acute phase of zymosan-induced inflammation. Int Immunopharmacol 8: 1567–1577, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Dinarello CAD. Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112: 321S–329S, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M. JAK-STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 107: 50–60, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Dorr W. Cystometry in mice–influence of bladder filling rate and circadian variations in bladder compliance. J Urol 148: 183–187, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Dray A. Inflammatory mediators of pain. Br J Anaesth 75: 125–131, 1995 [DOI] [PubMed] [Google Scholar]
- 17. Driscoll A, Teichman JMH. How do patients with interstitial cystitis present? J Urol 166: 2118–2120, 2001 [PubMed] [Google Scholar]
- 18. Dziennis S, Alkayed NJ. Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci 19: 341–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erickson DR, Xie SX, Bhavanandan VP, Wheeler MA, Hurst RE, Demers LM, Kushner L, Keay SK. A comparison of multiple urine markers for interstitial cystitis. J Urol 167: 2461–2469, 2002 [PubMed] [Google Scholar]
- 20. Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn 24: 627–632, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Gadient RA, Patterson PH. Leukemia inhibitory factor, interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem Cells 17: 127–137, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Girard BM, Malley SE, Braas KM, Waschek JA, May V, Vizzard MA. Exaggerated expression of inflammatory mediators in vasoactive intestinal polypeptide knockout (VIP−/−) mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36: 188–199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol 295: R111–R122, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill JK, Gunion-Riner L, Kulhanek D, Lessov N, Kim S, Clark WM, Dixon MP, Nishi R, Stenzel-Poore MP, Eckenstein F. Temporal modulation of cytokine expression following focal cerebral ischemia in mice. Brain Res 820: 45–54, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Ho N, Koziol JA, Parsons CL. Epidemiology of interstitial cystitis. In: Interstitial Cystitis, edited by Sant GR. Philadelphia, PA: Lippincott-Raven Publishers, 1997, p. 9–16 [Google Scholar]
- 26. Hu CP, Feng JT, Tang YL, Zhu JQ, Lin MJ, Yu ME. LIF upregulates expression of NK-1R in NHBE cells. Med Inf (Lond) 2006: 84829, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol 284: R574–R585, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide-induced cystitis. J Urol 173: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Ishido M, Masuo Y. Transcriptome of pituitary adenylate cyclase-activating polypeptide-differentiated PC12 cells. Regul Pept 123: 15–21, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Ivanenkov YA, Balakin KV, Tkachenko SE. New approaches to the treatment of inflammatory disease: focus on small-molecule inhibitors of signal transduction pathways. Drugs 9: 397–434, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci 22: 478–485, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johansson SL, Ogawa K, Fall M. The pathology of interstitial cystitis. In: Interstitial Cystitis, edited by Sant GR. Philadelphia, PA: Lippincott-Raven Publishers, 1997, p. 143–152 [Google Scholar]
- 33. Klinger MB, Dattilio A, Vizzard MA. Expression of cyclooxygenase-2 in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 293: R677–R685, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Klinger MB, Girard B, Vizzard MA. p75(NTR) expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol 507: 1379–1392, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 295: F1778–F1789, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lamale LM, Lutgendorf SK, Zimmerman MB, Kreder KJ. Interleukin-6, histamine, and methylhistamine as diagnostic markers for interstitial cystitis. Urology 68: 702–706, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science 267: 1782–1788, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature 337: 362–367, 1989 [DOI] [PubMed] [Google Scholar]
- 39. Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Methods 15: 157–167, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Malley SE, Vizzard MA. Changes in urinary bladder cytokine mRNA and protein after cyclophosphamide-induced cystitis. Physiol Genomics 9: 5–13, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Nazif O, Teichman JM, Gebhart GF. Neural upregulation in interstitial cystitis. Urology 69: 24–33, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Ness TJ. Pelvic pain in women and men: recent findings. Curr Opin Anaesthesiol 18: 555–562, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Nishii H, Nomura M, Fujimoto N, Matsumoto T. Upregulation of interleukin-6 gene expression in cyclophosphamide-induced cystitis in mice: an in situ hybridization histochemical study. Int J Urol 13: 1339–1343, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Petrone RL, Agha AH, Roy JB, Hurst RE. Urodynamic findings in patients with interstitial cystitis. J Urol 153: 290A, 1995 [Google Scholar]
- 45. Qiao LY, Gulick MA. Region-specific changes in the phosphorylation of ERK1/2 and ERK5 in rat micturition pathways following cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol 292: R1368–R1375, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sant G, Hanno PM. Interstitial cystitis: current issues and controversies in diagnosis. Urology 57: 82, 2001 [DOI] [PubMed] [Google Scholar]
- 47. Streng T, Hedlund P, Talo A, Andersson KE, Gillespie JI. Phasic nonmicturition contractions in the bladder of the anaesthetized and awake rat. BJU Int 97: 1094–1101, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36: 175–187, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21: 125–138, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Vizzard MA. Alterations in spinal Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol 278: R1027–R1039, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol 161: 273–284, 2000 [DOI] [PubMed] [Google Scholar]
- 52. Vizzard MA. Upregulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420: 335–348, 2000 [PubMed] [Google Scholar]
- 53. Woolf CJ, Doubell TP. The pathophysiology of chronic pain-increased sensitivity to low threshold A-beta fiber inputs. Curr Opin Neurobiol 4: 525–534, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci 26: 10847–10855, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yuridullah R, Corrow KA, Malley SE, Vizzard MA. Expression of fractalkine and fractalkine receptor in urinary bladder after cyclophosphamide (CYP)-induced cystitis. Auton Neurosci 126–127: 380–389, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]