Abstract
Several studies have anecdotally reported the occurrence of altered urinary voiding patterns in rodents exposed to social stress. A recent study characterized the urodynamic and central changes in a rat model of social defeat. Here, we describe a similar voiding phenotype induced in mice by social stress and in addition we describe potential molecular mechanisms underlying the resulting bladder wall remodeling. The mechanism leading to the altered voiding habits and underlying bladder phenotype may be relevant to the human syndrome of dysfunctional voiding which is thought to have a psychological component. To better characterize and investigate social stress-induced bladder wall hypertrophy, FVB mice (6 wk old) were randomized to either social stress or control manipulation. The stress involved repeated cycles of a 1-h direct exposure to a larger aggressive C57Bl6 breeder mouse followed by a 23-h period of barrier separation over 4 wk. Social stress resulted in altered urinary voiding patterns suggestive of urinary retention and increased bladder mass. In vivo cystometry revealed an increased volume at micturition with no change in the voiding pressure. Examination of these bladders revealed increased nuclear expression of the transcription factors MEF-2 and NFAT, as well as increased expression of the myosin heavy chain B isoform mRNA. BrdU uptake was increased within the urothelium and lamina propria layers in the social stress group. We conclude that social stress induces urinary retention that ultimately leads to shifts in transcription factors, alterations in myosin heavy chain isoform expression, and increases in DNA synthesis that mediate bladder wall remodeling. Social stress-induced bladder dysfunction in rodents may provide insight into the underlying mechanisms and potential treatment of dysfunctional voiding in humans.
Keywords: dysfunctional voiding, mind-body interaction, calcineurin, NFAT, smooth muscle
the concept that murine voiding habits are affected by social rank dates back to 1973 and the work of Desjardins et al. (9) who demonstrated marked differences in micturition patterns among submissive mice exposed to a dominant cage mate. Since that time, additional reports have confirmed altered voiding patterns (16, 22), but the effect on the bladder wall itself has not been characterized. In this paper, we present data that support our contention that social stress not only results in an abnormal voiding pattern, but also leads to the remodeling and hypertrophy of the target visceral organ. This serves as an example of a mind-body interaction in which exposure to social stress leads to measurable hypertrophy and associated changes in a transcriptional pathway with concomitant changes in gene expression within the bladder wall.
Multiple signaling cascades lead to bladder wall hypertrophy; one candidate that we focused on is the calcineurin pathway. This ubiquitous pathway first described in lymphocytes enables alterations in cytosolic calcium to trigger changes in gene expression that allow for organ remodeling (8). Calcineurin is a phosphatase activated by sustained changes in the basal levels of cytosolic calcium and serves to dephosphorylate the nuclear factor of activated T cells (NFAT). Following dephosphorylation, NFAT crosses the nuclear membrane to act as a transcription factor (8). In addition to activating NFAT, the transcription factor myocyte enhancing factor 2 (MEF-2) is also partly regulated by calcineurin. This pathway has been shown to be instrumental in cardiac remodeling (23), and in particular for the development of the left ventricular wall hypertrophy that results from aortic banding (32). We previously demonstrated evidence for calcineurin activation following surgical partial bladder outlet obstruction (pBOO) in a rabbit (7) as well as a murine model (21). To study this pathway in the bladder, we began using a transgenic mouse containing an NFAT-luciferase reporter construct developed for the study of cardiac hypertrophy (32). In the course of our surgery to create pBOO in these transgenic mice, serendipitous observations revealed large and distended bladders were occasionally observed in older cohorts of nonoperated mice. Additionally, there appeared to be a correlation between the bladder size and the degree of subordination observed. These large bladders from the subordinate mice all had increased luciferase activity indicative of activation of the calcineurin pathway (since these data were obtained in the NFAT-luciferase transgenic mice, a rise in nuclear NFAT leads to a rise in luciferase activity) as well as shifts in their expression of myosin heavy chain mRNA for the A and B isoforms. These findings were strikingly similar to those we observed in mice subjected to surgically induced pBOO (1, 21).
These preliminary, albeit retrospective, data suggested that social stress, perhaps through urinary retention, engaged the calcineurin pathway which in turn leads to an increase in NFAT and ensuing bladder hypertrophy. This was an exciting finding as it implied that social cues could engage cellular signaling pathways that result in visceral remodeling. More importantly, given that dysfunctional voiding in children is thought to have a psychological basis and can present with significant bladder hypertrophy, social subordination in mice may offer a useful model of this common clinical disorder. We therefore undertook the present study to systematically characterize the impact of a randomized trial of social stress on the bladder wall and a cellular signaling pathway known to play a role in bladder hypertrophy.
MATERIALS AND METHODS
Protocol to identify C57 aggressor mice.
Six-month-old retired breeder male C57BL6 mice were obtained from commercial vendors. Aggressive males were identified by placing the mice in a cage with an FVB mouse and determining the time to the first aggressive episode. Aggressive mice were defined as those C57BL6 males who attacked the FVB mouse in under 1 min. Screening was ended if bite marks appeared on the tail of the FVB mouse. Approximately one-third of all C57BL6 mice screened were aggressive enough to meet this criteria.
Behavioral protocol.
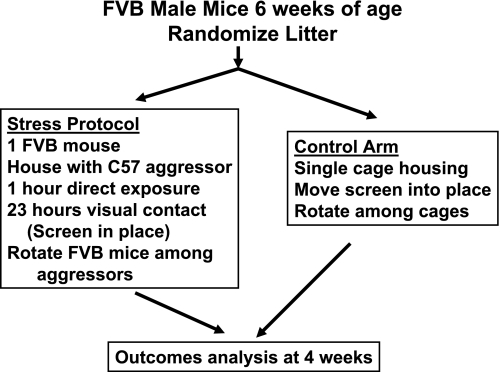
A single aggressor mouse was housed with a 6-wk-old FVB mouse for a 1-h period in a 7.5 × 6 × 5-inch space created by inserting a divider into a standard cage. After 1 h, the mice were separated by the barrier which allowed for direct visual and olfactory contact, but prevented direct physical contact. After 23 h of separation, each FVB mouse was rotated into a new cage with a new aggressor for 1 h of direct exposure, followed by a 23-h period of separation by the barrier. This cycle was repeated daily for a 1-mo period (Fig. 1). Control mice were housed individually in the same cages with barriers but were not exposed to the aggressive males. All procedures were approved by The Children's Hospital of Philadelphia institutional review board for animal care and use.
Fig. 1.
Schematic representation of the behavioral protocol used to induce social stress voiding dysfunction. The identification of aggressive C57 males from a population of retired breeders was made by observation when these mice were placed with a 6-wk-old FVB male. C57 mice were deemed aggressive if they attacked the FVB mouse in under 5 min. Approximately one-third of C57 mice screened met this criteria. Mice were separated as soon as any bite resulted in bleeding.
Voiding patterns.
In vivo voiding patterns were measured by placing stressed and control mice individually into metabolism cages (model MTB-0311, Braintree Scientific, Braintree, MA) with food and water ad libitum. These measurements were performed between days 26 and 27 of the 4-wk protocol and 6 h after the last direct exposure to the aggressive mouse. Following a 24-h acclimation period, a 12-inch Whatman filter paper was placed below the screen in the metabolism cage for an additional 12 h of monitoring. The filters were exposed to UV light to identify the urinary chromogens, and a digital image was recorded (using the Epichem3 gel documentation system). Voiding frequency and volume were determined with National Institutes of Health image analysis software and entered into an Excel data sheet.
In vivo cystometry.
In vivo cystometry was performed at the completion of 4 wk of social stress in a separate cohort of mice from the control and social stress groups. Upon completion of the 4-wk protocol, mice were anesthetized, and a PTF catheter with a blunted end (Catamount Research, St. Albans, VT) was sutured in place at the bladder dome and tunneled out the abdomen to the nape of the neck where it was then inserted into the end of a 22-gauge angiocath iv catheter. Upon determination of the optimal length, the PTF catheter was affixed to the angiocath with super glue. The angiocath was capped, after a gentle saline infusion revealed no leak at the bladder, and the abdomen was then closed in layers. The angiocath was anchored to the fascia and skin of the neck using a two to three 3–0 Vicryl sutures. Once implanted, the catheters were left undisturbed for 3 days at which point in vivo cystometry was performed by infusing saline at 10 μl/min (Catamount Research). At least four cycles of bladder filling and emptying were completed before acquiring three cystometrograms. Using the cystometry analysis software, four parameters were measured: volume at micturition, threshold pressure at the micturition volume, the voiding pressure, and the voided volume. The results of the three final cystometrograms were averaged for each individual mouse. Cystometry data were collected for seven control and eight stress mice and the results were averaged.
Tissue collection.
Upon completion of the behavioral protocol and determination of the voiding patterns, mice were weighed, anesthetized with isofluorane anesthesia, and euthanized once their bladders were excised. All bladders were weighed, divided in half (to allow for isolation of RNA and nuclear protein), and stored at −80°C.
EMSA.
Nuclear protein extracts were prepared from whole bladders following instructions from the manufacturer's kit with a minor modification of the suggested buffer volume to account for the small tissue size (Panomics, Fremont, CA). The nuclear protein levels were quantified by a modified Lowry assay (Bio-Rad, Hercules, CA). The Odyssey Infrared EMSA kit from LI-COR (Lincoln, NE) was used for all gel shift assays. All binding reactions were performed at room temperature in the dark for 20 min in a volume containing 5 μg nuclear protein and 1 μl of an infrared (IR)-labeled oligonucleotide probe. Total volume was adjusted to 20 μl with binding buffer. Using custom made IR-labeled primers and a 30-bp segment containing the NFAT consensus binding sequences from the IL-2 promoter, we were able to create an IR-NFAT oligonucleotide probe using PCR. Using identical but nonlabeled primers, we also synthesized a “cold” non-IR probe for the NFAT competition reaction. This same technique was used to construct labeled and nonlabeled oligonucleotide probes for MEF-2. To further confirm the identity of the shifted bands, nuclear protein extracts were also preincubated for 10 min with the Biotin-NFATc probe and 1 μg of NFATc3, NFATc4 (SC8405 and 1153, Santa Cruz Biotechnology, Santa Cruz, CA), or MEF2b (Ab 33540, Abcam, Cambridge, MA) antibodies and incubated for 10 min on ice followed by 10 min at room temperature before loading onto the gel. Upon completion of the binding reaction, all samples were run on a precast 5% TBE gel (Bio-Rad) at 80 V at 4°C in the dark for ∼1.5 h. Gels were scanned in the Odyssey Infrared Imaging System (Li-Cor) and bands were quantified using Odyssey software.
RNA isolation and PCR.
Total RNA was isolated according to the manufacturer's instructions (TRIzol, Invitrogen, Carlsbad, CA) and the final pellet was air dried and dissolved into a ribonuclease-free storage buffer (RNA Storage Solution, Ambion). The RNA concentration was determined spectrophotomically. cDNAs were produced using 1 μg total RNA with a commercially available kit (Retroscript, Ambion). cDNA (2.5 μl, 0.25 μg) was used for PCR with 1 μg each of the upstream and downstream primers. The total volume was brought to 12.5 μl with PCR grade water and then 12.5 μl PCR Master Mix (PCR Master, Roche Diagnostics GmBH, Mannheim, Germany) were added. The primers for the MHC isoforms SM-A and SM-B were: upstream 5′-CCA CAA GGG CAA GAA AGA CAG C-3′ and downstream 5′-TCC GGC GAG CAG GTA GTA GAA GA-3′. Conditions for the simultaneous amplification of SM-B (289 bp) and SM-A (268 bp) PCR products were as follows: following a 5-min denaturation at 95°C, 30 cycles consisting of 95°C for 50 s, 55°C for 1.5 min, and 72°C for 2.5 min with a final extension of 7 min at 72°C. 18S RNA was used as the internal standard against which individual myosin bands could be normalized (Quantum RNA 18S internal standards kit #1717, Ambion). PCR products were resolved by 2% agarose gel electrophoresis, visualized with ethidium bromide, and images were digitized. Individual band density was determined using image analysis software (ImageJ, version 1.36), and the expression of the SM-B and SM-A isoform mRNA normalized against the expression of the 18S ribosomal RNA standard. The signal from the myosin expression was divided by the signal from the 18S standards and the mean values ± SD were determined for the control and stress groups.
BrdU administration and staining.
In a subset of mice, BrdU was delivered in the drinking water at a concentration of 1 mg/ml. The water bottles were covered in aluminum foil, and fresh water and BrdU were added at 3-day intervals. At death, the urethra was ligated, the distended bladder was excised, and the specimen was transferred into buffered formalin. The bladders were then divided in halves, which were embedded in paraffin and 5-μm sections were cut.
Localization and quantification of BrdU synthesis within the bladder wall.
A set of five paraffin-embedded bladders from each group was analyzed with dual immunofluorescence labeling using a sheep polyclonal antibody to BrdU (Capralogics, Hardwick, MA) with a secondary antibody coupled to Texas Red, and a mouse monoclonal antibody against smooth muscle myosin heavy chain (Abcam) with a secondary antibody coupled to fluorescein. All nuclei were visualized by staining with DAPI (Sigma). Following deparaffinization, 5-μm sections were incubated at 4°C overnight with the primary antibody (1:400 dilution) and washed three times with phosphate-buffered saline. Secondary antibodies were applied at 4°C overnight, followed by an additional three washes with PBS. DAPI staining of the nuclei was carried out as per the supplier's instructions. Sections were imaged at the respective excitation wavelengths for DAPI (360 nm), FITC (480 nm), and Texas Red (595 nm) using the Leica DM400B microscope with a digital imaging camera and software (Phase 3 Image Pro, Glen Mills, PA). Images acquired from all three wavelengths were imported into Adobe photoshop and merged. In addition, the BrdU incorporation index was calculated by converting the separate DAPI and BrdU images into black and white images. This allowed us to use the image analysis software to label and then count all the nuclei as identified in the DAPI image. This same section was also viewed using the image analysis software to label and then count the sites of BrdU uptake. The total number of BrdU uptake sites was divided by the total number of nuclei identified by the DAPI stain to arrive at the BrdU labeling index.
Statistical analysis.
Comparisons between the control and social stress groups were made using paired or unpaired Student's t-tests with significance accepted as P < 0.05.
RESULTS
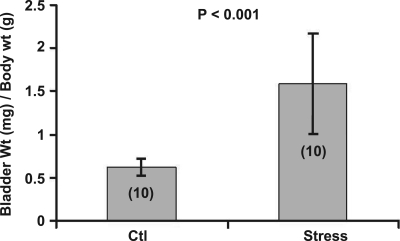
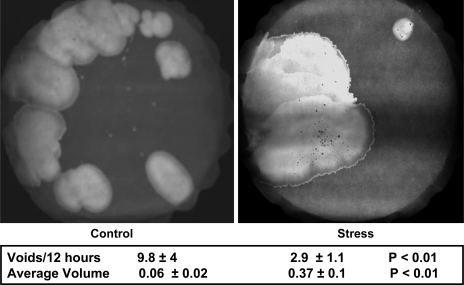
The bladder-to-body mass index (bladder mass in mg divided by the body mass in g) increased in those mice randomized to 4 wk of social stress compared with controls (P < 0.001; Fig. 2). Mice exposed to the social stress protocol demonstrated a lower voiding frequency (P < 0.05), as demonstrated by a diminished number of urine spots on the filter paper. Additionally, stressed mice exhibited an increase in average voided volume (P < 0.01) compared with controls (Fig. 3).
Fig. 2.
Bladder wall hypertrophy develops with exposure to the social stress as per the protocol outlined in Fig. 1. The y-axis represents the bladder mass (in mg) divided by the body mass (in g). Number of subjects is indicated in parentheses.
Fig. 3.
Voiding phenotype is altered by social stress with an increase seen in the average voided volume and a decrease in the frequency. These values represent the average ± SD from 10 control and 10 stressed mice.
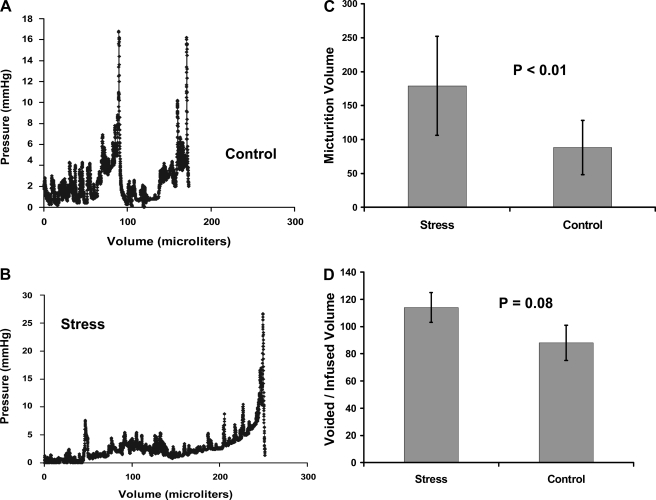
In Fig. 4, we summarize the findings and present representative tracings from the in vivo cystometry studies. The data demonstrated a statistically significant rise in bladder volume at micturition within the stress group (P < 0.01). The differences in voiding pressure were not significant (P = 0.4). There was a trend toward significance for a rise in threshold pressure within the stress group (P = 0.1). We also observed that within the control group, an average of 87% of the volume infused was voided. However, within the stress group, the voided volume on average was 113% of the infused volume (P = 0.08).
Fig. 4.
Representative in vivo cystometrograms from control (A) and stress (B) mice show a higher volume at micturition that was characteristic of the stress group. With social stress, the volume at micturition nearly doubled from 88 to 179 μl with P < 0.01 (C). There were no differences in micturition pressure or the threshold pressure at which voiding was initiated. It was noted that in the stress group, the voided volume exceeded the infused volume by 13% ; in contrast, in the control group the voided volume was 10% less than the infused volume (D). While this difference did not reach significance, it does suggest that the prolonged bladder retention induced by social stress may have resulted in postobstructive diuresis. These bar graphs represent means ± SD of cystometry recordings from 8 stressed and 7 control mice.
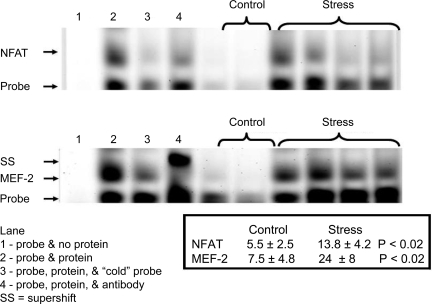
Social stress altered the expression of the transcription factors NFAT and MEF-2 in bladder. Figure 5 shows gel shift assays in which there is an increase in the expression of NFAT induced by social stress relative to controls which we were able to quantify via the fluorescent tags on the oligonucleotide probe (P < 0.02). With the application of “cold” oligonucleotide probes (lane 3 in each gel), the signals were lost implying that the band shown corresponded to the transcription factor of interest. The application of a monoclonal antibody against NFATc3 leads to a loss of signal (lane 4) similar to that we reported in our study of anatomic pBOO (21), but it did not result in a supershift. A similar finding was noted when we tested the antibody against NFATc4 (data not shown). We suspect that in bladder smooth muscle, this antibody recognizes an epitope near the oligonucleotide binding site that results in its displacement, and hence the loss of signal. In contrast, with the application of a monoclonal antibody against MEF-2b, we observed a supershift as the antibody NFAT complex and its oligonucleotide form a higher molecular weight complex. These changes in the gel shift assay serve to confirm along with the results of lanes run with “cold probe” that these bands reflect the expression of the transcription factors of interest.
Fig. 5.
Gel shift assays were performed for nuclear factor of activated T cells (NFAT) and myocyte enhancing factor 2 (MEF-2) and quantified using the Licor IR probe sets. With social stress, there was a rise in the nuclear translocation of both NFAT and MEF-2 over that seen in controls. In the absence of nuclear protein fractions, signal was lost (lane 1) compared with the gel shift seen from nuclear proteins from a social stress bladder (lane 2). Signal intensity for both NFAT and MEF-2 was lost with the application of “cold” oligonucleotide probe to the reaction (lane 3). The specificity of the oligonucleotide probe for the transcription factors was also studied using monocloncal antibodies. With the addition of anti-NFATc3 mab, there was a loss of signal as previously described (lane 4). A similar loss of signal was seen with antibodies against NFATc4 (data not shown). With addition of the monoclonal antibody against the MEF-2b, a pronounced supershift (SS) was observed (lane 4). These values represent the average ± SD from 5 control and 5 stressed mice.
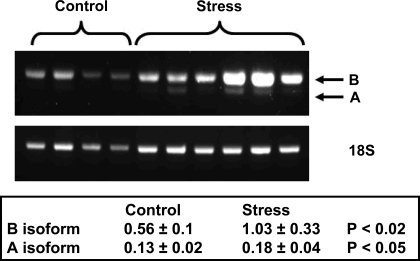
Mice subjected to social stress also showed shifts in their myosin heavy chain mRNA B and A isoform expression when normalized to 18S ribosomal RNA. The greatest increase was seen in the expression of the B isoform (Fig. 6), but there was also noted an increase in the A isoform. At this time, we do not have antibodies to allow for a Western blot analysis of the protein expression of these isoforms. The currently available murine monoclonal antibody reacts against an 8 amino acid sequence difference between the B and A isoforms and does not react in mouse bladder tissue.
Fig. 6.
Social stress leads to increased expression of the myosin heavy chain B and A mRNA isoforms. A representative gel with normalization to 18S RNA is shown. These values represent the average ± SD from 6 control (only 4 are shown) and 6 stressed mice.
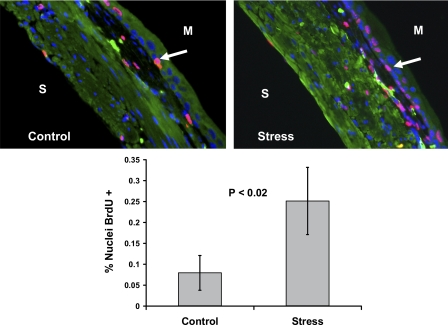
To determine whether new DNA synthesis is associated with stress-induced bladder wall remodeling, BrdU uptake was measured. In Fig. 7, there is evidence of increased BrdU uptake in the bladder wall of mice subjected to social stress. This would suggest that there is new DNA synthesis taking place as the bladder remodels itself under the new demands it faces.
Fig. 7.
Social stress leads to increased DNA synthesis in the bladder wall as measured by BrdU uptake. The values in the graph represent the means ± SD from 5 control and 5 stress mice. Images shown are color merged; therefore, nuclei staining pink are those with BrdU uptake as highlighted by the arrows. Blue nuclei are those in which no BrdU uptake is noted. S, serosal surface; M, mucosal surface.
DISCUSSION
Urologists have long understood that voiding behaviors are influenced by social stress; Desjardins et al. (9) reported that voiding patterns in mice reflect their status within the social hierarchy. In Desjardins' study it was noted that while the dominant mouse voided frequently and with small voids, the submissive mouse appeared to develop urinary retention. Recent studies have confirmed that murine voiding patterns do change in response to social stress, and these changes are accompanied in shifts in the expression of proteins secreted into the urine (16). Recently, the effects of social stress on urodynamic function in the rat have been characterized (33). This study demonstrated that increased social stress in rats leads to increased bladder mass and an altered urodynamics pattern (33) that in many ways (but not completely) resembled that seen with pBOO (25). However, to date, there has been no systematic study of the functional and molecular changes in the bladder following the exposure to social stress. In this paper, we validated the observations of Desjardins et al. and showed that associated with these changes in the voiding patterns (Fig. 3), there are clear cut changes in a molecular signaling pathway and myosin heavy chain gene expression within the bladder wall itself.
The consequences of surgically induced pBOO have been well described; however, there is a paucity of models looking at nonsurgically induced voiding dysfunction. Burnett et al. (6) described voiding dysfunction in the neuronal nitric oxide synthase (nNOS) knockout mouse, but the gene deletion was not targeted to the urinary tract (15). Subsequently, Sutherland et al. (29) pointed out these mice were also noted to be thirstier and had a vigorous diuresis. Their cystometry studies under urethane anesthesia failed to show major differences in capacity, although the leak point pressures were higher in the nNOS knockout group. Was this mouse voiding with higher pressures, or was the increase in bladder capacity and mass a reflection of volume based work? Jusuf et al. (17) studied Ncx/Hox11L.1 knockout mice and found them to have vesicourethral dysfunction with an increased voiding frequency and a lower voiding threshold pressure. Their findings also suggested alterations in the NO pathway were present in these mice. Bhattacharya et al. (4) reported on a murine model with a constitutively active point mutation in the 5-HT3A receptor subunit which demonstrated a phenotype consistent with voiding dysfunction, bladder wall hypertrophy, and upper tract changes which lead to death from uremia by 3 mo of age. Birder et al. (5) described altered bladder function in a mouse with a targeted deletion of the vanilloid receptor TRPV1. These studies offer important insights into bladder function, but the voiding dysfunction was a result of a genetic manipulation that was not bladder selective.
In these current studies, we set out to define a rigorously controlled randomized social stress protocol and then characterize the resulting bladder wall remodeling that occurred in the stressed cohort. The simplest measure of this remodeling is seen when the bladder mass normalized to body mass; a parameter that is significantly increased in the stressed group compared with controls. While bladder wall remodeling is undoubtedly associated with multiple signaling pathways and transcription factors, in this paper we focused on a description of the changes that take place within the calcineurin-NFAT axis. With social stress there was an increase in nuclear accumulation of NFAT as measured using the gel shift assay which correlated with the bladder hypertrophy. This is significant because we showed that downregulation of nuclear NFAT accumulation after pBOO as seen in the SERCA2 knockout mouse is also associated with minimal bladder wall hypertrophy (21). We also demonstrated a leading role for the calcineurin pathway in bladder wall hypertrophy following pBOO by using the known calcineurin inhibitor cyclosporine A (CSA) to block the development of the hypertrophy (7). In this paper, we also report a rise in the nuclear expression of another transcription factor, MEF-2, which is partly regulated by calcineurin. This finding is also of significance because the promoter region for the myosin heavy chain gene has been shown to contain a consensus sequence for MEF2-b (18) and because we also demonstrated social stress induced changes in myosin heavy chain and protein gene expression.
In addition to these changes in transcription factor expression, we also observed shifts in the expression of the mRNA for the myosin heavy chain B and A isoforms. Normal control bladder will show a predominate expression of the B isoform splice variant which is associated with higher ATP consumption and leads to a higher velocity of contraction (2). In contrast, the A isoform is associated with lower ATP consumption and a much slower velocity of contraction, and hence the muscle will generate diminished power. Both myosin heavy chain B and A isoform mRNA expression increased with social stress compared with control, although the greatest rise was seen with the B isoform. This set of observations suggests that social stress leads to alterations in transcription factor accumulation within the nucleus, with subsequent changes in gene and protein expression of a major contractile filament. We would have liked to quantify by Western blotting these shifts in the myosin B and A isoforms; however, we are limited by the antibody available. The current monoclonal antibody that recognizes the eight extra amino acids found in the B isoform was generated in a mouse and does not react with the murine myosin. It should prove possible for us to quantify these differences in isoform expression using proteomic methods.
We stated that social stress leads to bladder wall hypertrophy based on the increased bladder mass that results. However, this increase in bladder mass is also in part due to an increase in new DNA synthesis that is taking place in the urothelium and the lamina propria which are those zones of the bladder wall where the BrdU uptake is most intense. Thus, social stress results in hyperplasia, and we suspect that hypertrophy of the muscle bundles is also taking place. However, the formal definition of hypertrophy would demand a measure of multiple individual smooth muscle cell volumes (34), and these measurements cannot be made retroactively with this current method of fixation.
In the rat social stress model, Wood et al. (33) reported an increase in the volume at micturition with no changes in the voiding pressures between the stressed and control groups. These findings would suggest that the urinary retention observed develops from a decreased drive from the pontine micturition center to the parasympathetic neurons involved in reflex bladder contraction with a resulting overdistention of the bladder wall. This study was run over a 1-wk time frame, and while the cystometry patterns had changed, much less bladder wall hypertrophy was induced (33). Our cystometry data which show an increased volume at micturition and no change in the voiding pressure also suggest that a similar mechanism is operating in mice, i.e., there is suppression of the voiding reflex as opposed to detrusor sphincter dysinergia (which must by definition be accompanied by a rise in voiding pressures). While the rat model of social stress demonstrated a post void residual urine, we found this differed in our murine model of social stress. Control mice would void 10% less than the volume infused, but we were intrigued to observe that within the stress group, the voided volumes exceeded the infused volumes by an average of 13% . This suggests that there is an associated postobstructive diuresis when the stressed mice do void. Similar findings of postobstructive diuresis during urodynamic studies have been noted by our group in experimental studies of rabbit pBOO (27) as well as during human video urodynamic studies (24). We believe the reason for this postobstructive diuresis in the murine model is a reflection of the 4-wk time frame over which these mice developed a threefold increase in bladder mass which in turn allowed for the hypertrophic changes to become established.
It is intriguing how many of the observations we made about the molecular changes in the bladder wall following social stress parallel those seen with anatomic pBOO. Following pBOO, there is an increase in bladder mass (21, 28), a rise in the nuclear expression of NFAT and MEF-2 as detected by gel shift assays (21), a marked shift in the expression of the A isoform (28), and increased DNA synthesis that is seen in the urothelium, lamina propria, and the fibroblasts that separate the smooth muscle bundles (21). However, there are differences as well. There is a much greater degree of hypertrophy induced with the surgical model and a more pronounced shift to the A isoform. We also suspect that there is a greater degree of bladder wall hypoxia that develops following surgical pBOO that drives a preferential expression of the slower A isoform. The BrdU uptake is also more intense and is extended beyond the urothelium. Following pBOO, there is intense BrdU uptake in the interstitial fibroblasts and the serosal layer of these murine bladders; these histologic findings are not seen in this social stress model. We suspect that the increased BrdU synthesis seen throughout the bladder wall following pBOO will be associated with an increase in the fibroblast population which in turn leads to enhanced deposition of extracellular matrix elements. This would suggest that pBOO should have a greater detrimental effect on bladder wall compliance.
Voiding dysfunction associated with urinary retention and urinary incontinence remains the most common reason for which pediatric urologists are consulted (11). Patients present with a wide spectrum of complaints ranging from recurrent urinary tract infections to full blown urinary incontinence. At its extreme, voiding dysfunction may be associated with bladder decompensation and upper urinary tract compromise leading to renal failure as described by Hinman and Baumann (13). Among the possible inciting events that might lead to such severe voiding dysfunction are the painful elimination associated with cystitis and or constipation. If a child associates pain with elimination, fear of elimination will develop with the resulting bladder overdistention similar to what we observed in our model. Although far less common, stressful events such as the death of a sibling or parent have been known to incite voiding dysfunction in addition to emotional or physical abuse (10). Irrespective of the exact etiology, the clinical evaluation and treatment of these patients consume a great deal of time and effort. The association of dysfunctional voiding with vesicoureteral reflux leads to increases in the cost for treating reflux (3) and is associated with higher complication rates when surgical procedures are undertaken to correct the reflux (14). Finally, we would note that recent epidemiologic studies suggest that pediatric dysfunctional voiding has been associated with the development of the overactive bladder in adult women (12).
Despite the widespread prevalence of this entity, little research has been done into the optimal management of this condition to allow for the best allocation of resources. While clinical studies described outcomes following various therapeutic approaches, there have been no experimental studies undertaken due to lack of an animal model. This is unfortunate since the pharmacologic options available to urologist today are quite limited and dated. Better pharmacologic treatment will not become available until a better understanding of the basic pathophysiology has been achieved. The stress-related neuropeptide corticotrophin-releasing factor (CRF) is a neurotransmitter found in circuits that link the brain and bladder (30, 31) and might serve as a mediator by which emotions might modulate lower urinary tract function (19, 20). We previously showed that CRF as well as its antagonists have an impact on lower urinary tract function (19). This pathway appears to be operational in the rat model of social stress model (33) in which changes in CRF mRNA and protein expression were demonstrated within Barrington's nucleus consistent with an upregulation of this neurotransmitter. The rat model is critical to the study of social stress-induced voiding dysfunction given that its larger size will allow for neural recordings to be made from Barrington's nucleus. The murine social stress model offers the benefit of being able to use genetically manipulated mice to gain further insight into the mechanisms by which these changes take place. As a consequence, we are hopeful that these tools will provide us with experimental means to develop an improved understanding of the scientific basis for this common clinical problem, with the hope that it may prove possible to ultimately expand the treatment options for these patients.
GRANTS
We acknowledge funding from The American Urological Association Foundation, The Leonard and Madlyn Abramson Endowed Chair, and National Institutes of Health Grant P50 DK-052620.
REFERENCES
- 1. Austin JC, Chacko SK, DiSanto M, Canning DA, Zderic SA. A male murine model of partial bladder outlet obstruction reveals changes in detrusor morphology, contractility and Myosin isoform expression. J Urol 172: 1524–1528, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Babu GJ, Pyne GJ, Zhou Y, Okwuchukuasanya C, Brayden JE, Osol G, Paul RJ, Low RB, Periasamy M. Isoform switching from SM-B to SM-A myosin results in decreased contractility and altered expression of thin filament regulatory proteins. Am J Physiol Cell Physiol 287: C723–C729, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Benoit RM, Wise BV, Naslund MJ, Mathews R, Docimo SG. The effect of dysfunctional voiding on the costs of treating vesicoureteral reflux: a computer model. J Urol 168: 2173–2176, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bhattacharya A, Dang H, Zhu QM, Schnegelsberg B, Rozengurt N, Cain G, Prantil R, Vorp DA, Guy N, Julius D, Ford AP, Lester HA, Cockayne DA. Uropathic observations in mice expressing a constitutively active point mutation in the 5-HT3A receptor subunit. J Neurosci 24: 5537–5548, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Burnett AL, Calvin DC, Chamness SL, Liu JX, Nelson RJ, Klein SL, Dawson VL, Dawson TM, Snyder SH. Urinary bladder-urethral sphincter dysfunction in mice with targeted disruption of neuronal nitric oxide synthase models idiopathic voiding disorders in humans. Nat Med 3: 571–574, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Clement M, Delaney DP, Austin JC, Sliwoski J, Hii GC, Canning DA, DiSanto ME, Chacko SK, Zderic SA. Activation of the calcineurin pathway is associated with detrusor decompensation: a potential therapeutic target. J Urol 176: 1225–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell 109, Suppl:S67–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Desjardins C, Maruniak JA, Bronson FH. Social rank in house mice: differentiation revealed by ultraviolet visualization of urinary marking patterns. Science 182: 939–941, 1973 [DOI] [PubMed] [Google Scholar]
- 10. Ellsworth PI, Merguerian PA, Copening ME. Sexual abuse: another causative factor in dysfunctional voiding. J Urol 153: 773–776, 1995 [PubMed] [Google Scholar]
- 11. Feldman AS, Bauer SB. Diagnosis and management of dysfunctional voiding. Curr Opin Pediatr 18: 139–147, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald MP, Thom DH, Wassel-Fyr C, Subak L, Brubaker L, Van Den Eeden SK, Brown JS. Childhood urinary symptoms predict adult overactive bladder symptoms. J Urol 175: 989–993, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hinman F, Baumann FW. Vesical and ureteral damage from voiding dyfunction in boys without neurologic or obstuctive disease. J Urol 109: 727–732, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Hinman F, Jr, Baumann FW. Complications of vesicoureteral operations from incoordination of micturition. J Urol 116: 638–643, 1976 [DOI] [PubMed] [Google Scholar]
- 15. Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75: 1273–1286, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Hurst JL, Payne CE, Nevison CM, Marie AD, Humphries RE, Robertson DH, Cavaggioni A, Beynon RJ. Individual recognition in mice mediated by major urinary proteins. Nature 414: 631–634, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Jusuf AA, Kojima S, Matsuo M, Tokuhisa T, Hatano M. Vesicourethral sphincter dysfunction in ncx deficient mice with an increased neuronal cell number in vesical ganglia. J Urol 165: 993–998, 2001 [PubMed] [Google Scholar]
- 18. Katoh Y, Molkentin JD, Dave V, Olson EN, Periasamy M. MEF2B is a component of a smooth muscle-specific complex that binds an A/T-rich element important for smooth muscle myosin heavy chain gene expression. J Biol Chem 273: 1511–1518, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Kiddoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am J Physiol Regul Integr Comp Physiol 290: R1697–R1706, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J Urol 172: 2570–2573, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Lassmann J, Sliwoski J, Chang A, Canning DA, Zderic SA. Deletion of one SERCA2 allele confers protection against bladder wall hypertrophy in a murine model of partial bladder outlet obstruction. Am J Physiol Regul Integr Comp Physiol 294: R58–R65, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Lumley LA, Sipos ML, Charles RC, Charles RF, Meyerhoff JL. Social stress effects on territorial marking and ultrasonic vocalizations in mice. Physiol Behav 67: 769–775, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen MT, Pavlock CL, Zderic SA, Carr MC, Canning DA. Overnight catheter drainage in children with poorly compliant bladders improves postobstructive diuresis and urinary incontinence. J Urol 174: 1633–1636, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Rickenbacher E, Baez MA, Hale L, Leiser SC, Zderic SA, Valentino RJ. Impact of overactive bladder on the brain: central sequelae of a visceral pathology. Proc Natl Acad Sci USA 105: 10589–10594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sambrook J, Russel DW. Gel retardation assays for DNA binding proteins. In: Molecular Cloning: A Laboratory Manual. Cold Spring, NY: Cold Spring Harbor, 2001, p. 17.13–17.17 [Google Scholar]
- 27. Stein R, Gong C, Hutcheson J, Krasnopolsky L, Canning DA, Carr M, Zderic SA. The fate of urinary bladder smooth muscle after outlet obstruction–a role for the sarcoplasmic reticulum. Adv Exp Med Biol 539: 773–790, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Su X, Stein R, Stanton MC, Zderic S, Moreland RS. Effect of partial outlet obstruction on rabbit urinary bladder smooth muscle function. Am J Physiol Renal Physiol 284: F644–F652, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Sutherland RS, Kogan BA, Piechota HJ, Bredt DS. Vesicourethral function in mice with genetic disruption of neuronal nitric oxide synthase. J Urol 157: 1109–1116, 1997 [PubMed] [Google Scholar]
- 30. Valentino RJ, Page ME, Luppi PH, Zhu Y, Van Bockstaele E, Aston-Jones G. Evidence for widespread afferents to Barrington's nucleus, a brainstem region rich in corticotropin-releasing hormone neurons. Neuroscience 62: 125–143, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Valentino RJ, Pavcovich LA, Hirata H. Evidence for corticotropin-releasing hormone projections from Barrington's nucleus to the periaqueductal gray and dorsal motor nucleus of the vagus in the rat. J Comp Neurol 363: 402–422, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang EY, Stein R, Chang S, Zheng Y, Zderic SA, Wein AJ, Chacko S. Smooth muscle hypertrophy following partial bladder outlet obstruction is associated with overexpression of nonmuscle caldesmon. Am J Pathol 164: 601–612, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]