Abstract
Current methods for measuring renal blood flow (RBF) are time consuming and not widely available. Contrast-enhanced ultrasound (CEU) is a safe and noninvasive imaging technique suitable for assessment of tissue blood flow, which has been used clinically to assess myocardial blood flow. We tested the utility of CEU in monitoring changes in RBF in healthy volunteers. We utilized CEU to monitor the expected increase in RBF following a high protein meal in healthy adults. Renal cortical perfusion was assessed by CEU using low mechanical index (MI) power modulation Angio during continuous infusions of Definity. Following destruction of tissue microbubbles using ultrasound at a MI of 1.0, the rate of tissue replenishment with microbubbles and the plateau acoustic intensity (AI) were used to estimate the RBF velocity and cortical blood volume, respectively. Healthy adults (n = 19, mean age 26.6 yr) were enrolled. The A.β parameter of CEU, representing mean RBF increased by 42.8%from a baseline of 17.05 ± 6.23 to 23.60 ± 6.76 dB/s 2 h after the ingestion of the high-protein meal (P = 0.002). Similarly, there was a 37.3%increase in the β parameter, representing the geometric mean of blood velocity after the high protein meal (P < 0.001). The change in cortical blood volume was not significant (P = 0.89). Infusion time of Definity was 6.3 ± 2.0 min. The ultrasound contrast agent was tolerated well with no serious adverse events. CEU is a fast, noninvasive, and practical imaging technique that may be useful for monitoring renal blood velocity, volume, and flow.
Keywords: renal hemodynamics, renal cortical blood flow, high-protein diet, Definity
contrast-enhanced ultrasonography (CEU) has been well validated in cardiology to assess myocardial perfusion during echocardiography. (2, 10, 12, 13, 16, 20) Gas-filled microbubbles are injected intravenously to enhance the ultrasound image. Since these agents remain solely in the intravascular space and have a rheology similar to that of red blood cells (9, 11, 19), they are considered a good choice for the study of the microvasculature and for the quantification of tissue blood flow (8, 13, 14). Ultrasound-contrast agents are safe and well tolerated and free of any hemodynamic effects.
To quantify tissue blood flow, we have previously shown that at steady state during a continuous infusion of microbubbles, measuring the rate at which microbubbles replenish tissue after their destruction provides an assessment of tissue blood flow velocity. (22) Using QLAB software (Philips), the data are obtained from different regions of interest within the tissue. When the tissue has been completely replenished with microbubbles, the signal from the microbubbles reflects tissue blood volume. (22, 23). The product of tissue blood volume and blood flow velocity reflects tissue blood flow (22, 23).
Measurement of renal blood flow (RBF) in clinical settings has been a challenge, mainly because current methods are either invasive, rely on the use of tracers which are diffusible, have kinetics which are complicated by tubular transport or glomerular filtration, or do not allow assessment of rapid changes in RBF (1, 25). These barriers make repeated measuring of RBF and monitoring the potential changes in response to physiological or pharmacological stimuli almost impossible. At the same time, none of the current techniques are suitable for bedside testing such as in intensive care unit settings. Color and spectral Doppler are currently commonly used to assess renal perfusion noninvasively (16), but these methods are operator dependent and not suitable for all patients depending on their body habitus.
We therefore hypothesized that CEU may be a noninvasive method for quantifying changes in RBF in response to a physiological stimulus. We chose to evaluate the increase in cortical RBF in response to a meal with high animal protein content (1.5 g/kg body wt) in healthy volunteers (18).
METHODS
Patients and protocol.
The protocol was approved by the Institutional Review Board (IRB) at the University of Virginia. Healthy adult volunteers older than 18 yr of age were eligible for participation in this study. All subjects underwent a thorough history and physical examination as well as blood tests, including a basic metabolic panel, measurement of liver enzymes, complete blood count, and a urinalysis as well as a 12-lead electrocardiogram (ECG). Exclusion criteria were history of or active signs and symptoms of any organ system diseases, chronic use of any medications, including over-the counter drugs, pregnancy, lactation, or abnormal serological, urine, or ECG findings. After signing the informed consent form, study participants were asked to present to the General Clinical Research Center at the University of Virginia after an overnight fast. A 20-gauge peripheral intravenous catheter was inserted into the forearm for administration of Definity microbubbles. Imaging of the right kidney was performed with the patient positioned comfortably in the prone or, if needed, left decubitus position. After two-dimensional ultrasound images of the right kidney were obtained, color and spectral Doppler of the right main renal artery was performed at baseline. An infusion of 1.5 ml of Definity (Lantheus Medical Imaging, North Billerica, MA) diluted in 30 ml of normal saline was then administered at a rate of 2 ml/min for the baseline CEU study of the right kidney. Study participants then consumed a meal composed of high animal protein (1.5 g of chicken/kg body wt). After 2 h, Doppler flow measurements from the right renal artery and CEU studies were repeated. The rate of Definity infusion was kept constant throughout the study. Participants were kept in the GCRC for another hour after the completion of study procedures for observation. Vital signs were checked every 15 min, and three-lead cardiac monitoring and pulse oximetry were monitored continuously throughout the study period. A urine sample for urinalysis was collected before the subjects were discharged to assess for any potential adverse events. A follow-up visit was scheduled within 2 wk of the study day.
CEU.
All CEU studies were performed by the same individual. For the baseline CEU study, Definity was infused intravenously at a rate of 2 ml/min using a syringe pump (model AS40A, Baxter, Deerfield, IL). After reaching a steady state (∼2 min after the initiation of the infusion based on previous studies) (24), power modulation Angio (Sonos 7500, Philips Ultrasound) was used to assess renal cortical perfusion at a mechanical index (MI) of 0.1 (2). A pulse-repetition frequency of ∼2 kHz and medium line density were used. All color gain settings, focus, and depth were initially optimized and then kept constant during the study. Maximal compression was used. After a high MI destruction pulse sequence (MI = 1.0), low-MI images were acquired at >20 Hz for 15 s. All images were acquired digitally for future analyses.
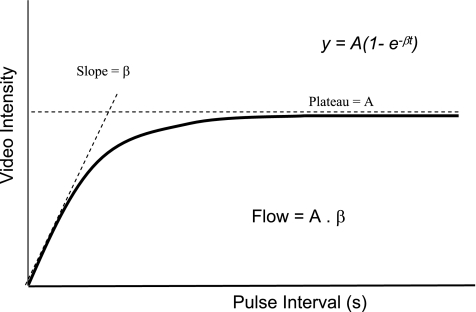
Data were transferred to an off-line computer and analyzed using Q-LAB (Philips Ultrasound). At least four large regions of interest (ROIs) were placed over the cortex of the kidney. The cortex was clearly seen as a more highly echogenic zone between the capsule of the kidney and the medulla. The medullary pyramids were identified as the darker and deeper zones within the kidney, which replenish much more slowly with microbubbles due to their much lower flow compared with the cortex (Fig. 1). Care was taken to avoid including the interlobar and arcuate arteries within the ROIs. Since filling of arteries with microbubbles occurs almost immediately after their destruction with a high-energy pulse (Fig. 1), frames from the first 0.2 s after microbubble destruction were used to select ROIs to avoid including interlobular and arcuate arteries. Acoustic intensity (AI), which is the “brightness” of pixels in the ultrasound image in decibels (dB), within each ROI was automatically measured from every frame using commercially available off-line image analysis software (QLAB) (Fig. 2, A and B). Time vs. AI plots were then generated and were fitted to an exponential function: y = A(1 − e−βt), where y is AI at time of t, A is the plateau AI representing tissue blood volume (BV), and β is the rate constant reflecting the rate of rise of AI (or the mean microbubble velocity) (Fig. 2) (12, 13).
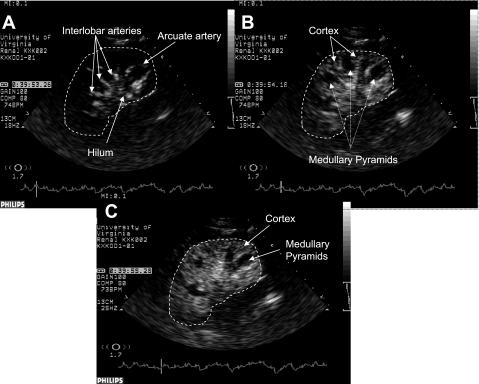
Fig. 1.
Contrast-enhanced ultrasound (CEU) images of the kidneys 0.1 (A), 1.0 (B), and 2.1 s (C) after replenishment of tissue with contrast agent (microbubbles). Note that renal hilum, interlobar, and arcuate arteries fill with contrast agent very rapidly (A) followed in a second or two by renal cortex (B). Due to low velocity and flow of blood to the medulla, complete opacification of medullary pyramids may take several seconds, making distinction between cortex and medulla easy (B and C). A white dashed line is drawn around the kidney to better demonstrate its boundaries.
Fig. 2.
Graphic depiction of changes in acoustic intensity vs. time after destruction of microbubbles in the tissue with high-energy ultrasound wave.
All subjects also underwent Doppler flow studies of the main renal artery. All of the studies were performed by a single experienced vascular ultrasonographer (T. Oickle). Two-dimensional ultrasound images of the right kidney were obtained first. Doppler studies of the main renal artery were carried out using the Sonos 7500 ultrasound system (Philips) and a 3-MHz Phased Array probe. A preset abdominal/renal protocol was used with the power level set and maintained at −9 dB. The postprocessing image compression was maintained at 70 dB, while the image gain was manipulated from the preset of 55 dB to improve the overall image quality. The right renal artery was identified using color Doppler from the anterior approach by its relationship to the celiac axis and superior mesenteric artery. The right renal artery ostium was insonated at 1.6 MHz with a pulsed Doppler gate set at 7.5 mm and Doppler angle correction set at 60°. The angle correction curser was positioned parallel to the renal artery walls, obtaining velocities comparable to accepted diagnostic renal artery velocity criteria. Once three consistent Doppler waveforms were presented on-screen, the peak systolic and diastolic velocities (PSV and PDV) of one of the waveforms were measured using on-screen calipers. These measurements were taken in the same fashion pre- and postprandial. Mean Doppler velocities were calculated by the following formula: mean velocity = PDV+ 1/3 (PSV − PDV).
Study outcome.
Due to the lack of a gold standard test for repeated measurements of RBF, we chose a situation in which an increase in RBF was expected to occur. We then tested the utility of CEU in demonstrating the expected rise in RBF.
A meal rich in animal protein has been shown to increase RBF (6, 18). Therefore, the primary outcome of this study was the mean change in the A.β parameter of CEU, representing RBF, from baseline after ingestion of high protein. Secondary outcomes included change from baseline in the β and A parameters of CEU, representing blood velocity and tissue blood volume, respectively, the safety of Definity and also changes in mean blood velocity in the main renal artery by Doppler ultrasound.
Statistical analysis.
The CEU data that were collected before and after the high-protein meal were analyzed using paired Student's t-tests after transforming of the data to the natural logarithmic scale. The preprandial-to-postprandial comparisons are presented as a percentage of change in the geometric mean. The geometric mean is a location parameter similar to the arithmetic mean and the median. The geometric mean is computed simply by taking the antilog of the mean of logarithmically transformed data. P values <0.05 were considered significant. SAS 9.1.3 software (SAS) was used for statistical testing.
RESULTS
A total of 20 healthy adult volunteers were enrolled in this study. One individual was eliminated after completion of the baseline CEU study due to a mild, self-limiting back pain reaction to Definity, which precluded repeat administration of microbubbles.
Of the 19 individuals who completed the study, 10 (53%) were women, 16 (84%) were Caucasian, 2 (10%) were African American, and one (6%) was Asian. The mean age of the study participants was 26.6 (median 23, range 20–57) yr. Per our inclusion criteria, all baseline serological results were normal for all participants. The mean (±SD) infusion time for Definity at each time point was 6.3 ± 2.0 min (median, 6 min; range 3–11).
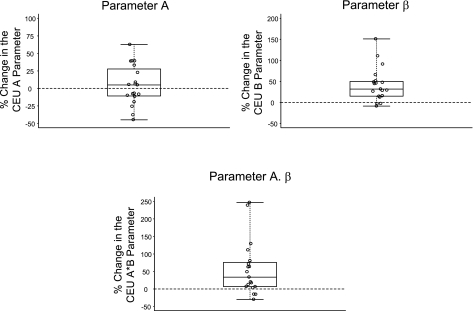
Figure 3 demonstrates frames from different stages of the CEU study. As mentioned previously, ROIs were carefully selected from renal cortex, sparing the arcuate and interlobular arteries (Figs. 1 and 4). Figure 5 shows the changes from baseline in the geometric mean for different CEU parameters. Mean renal cortical blood flow or A.β parameter by CEU increased from a mean (±SD) baseline value of 17.05 ± 6.23 to 23.60 ± 6.76 dB/s (Table. 1). This corresponds to a 42.8%(95%CI, 15.5–76.6%, P = 0.002) increase in the geometric mean of the A.β parameter after the high-protein meal compared with baseline. There was also a significant increase in the β parameter of CEU after the high-protein meal compared with baseline. The geometric mean for the β parameter increased by 37.3%(95%CI, 20.9–55.9%, P < 0.001) from a mean baseline value of 1.25 ± 0.34 to 1.68 ± 0.36 s−1 2 h after ingestion of the high-protein meal. However, there was no significant change in parameter A by CEU from baseline (0.9%increase, 95%CI, −12.0–15.7%, P = 0.892) (Table 1).
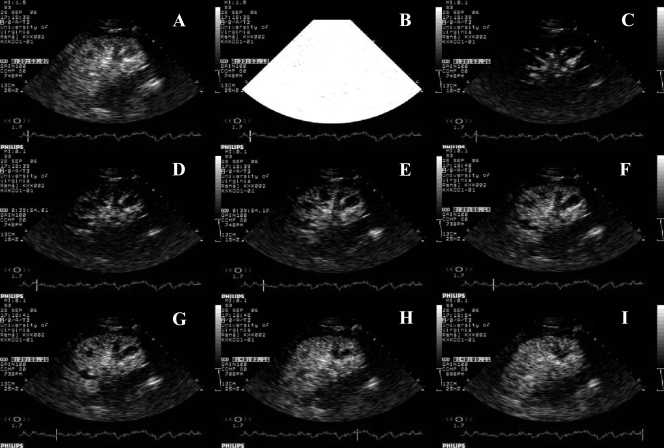
Fig. 3.
Sequential ultrasound images of the kidney before and after high-energy impulse. A: at steady state and before destruction of microbubbles. B: destruction of microbubbles in the tissue using high-energy ultrasound wave. C–I: 0.1–16 s after high-energy pulse showing gradual replenishment of kidney tissue with microbubbles. Note that the filling of large arteries occurs almost immediately after the impulse (0.13 s after high-energy pulse; C), followed by the cortex (1–2.25 s after high-energy pulse; D–G,) and much later by the medulla (10 and 16 s after high-energy pulse, respectively; H and I).
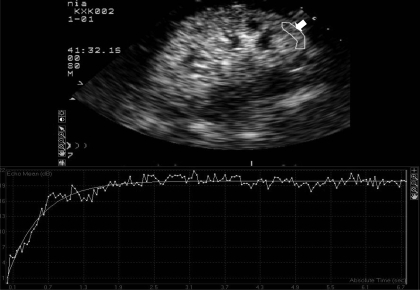
Fig. 4.
Replenishment of microbubbles in the renal cortex after destruction of microbubbles with high-energy pulse. The top section depicts the kidney 6 s after replenishment with contrast agent. The arrow points to a single region of interest (ROI) within the renal cortex. Graphic demonstration of tissue replenishment with microbubbles over time for the selected ROI using the QLAB software is shown at the bottom.
Fig. 5.
Box plots demonstrating changes from baseline in different CEU parameters after ingestion of a high-protein meal.
Table 1.
Baseline and post-high-protein meal values and ratios of geometric means (relative change) for CEU parameters
| CEU Parameter | Mean (SD) Pre | Mean (SD) Post | GM Pre | GM Post | GM Ratio | 95%CI | P Value (mean Δ = 0)* |
|---|---|---|---|---|---|---|---|
| A.β, dB/s | 17.05 (6.23) | 23.60 (6.76) | 15.77 | 22.53 | 1.428 | 1.155–1.766 | 0.002 |
| β, s−1 | 1.25 (0.34) | 1.68 (0.36) | 1.20 | 1.64 | 1.373 | 1.209–1.559 | <0.001 |
| A, dB | 13.32 (2.22) | 13.67 (3.13) | 13.13 | 13.25 | 1.009 | 0.880–1.157 | 0.892 |
CEU, contrast-enhanced untrasound; GM, geometric mean; GM ratio, ratio of GM post-high-protein diet (post) to baseline (pre); CI, confidence interval;
Δ = log(Post/Pre).
The coefficient of variation for parameters A, β, and A.β were 0.13, 0.29, and 0.33, respectively, at baseline and 0.19, 0.33, and 0.36 after the high-protein meal (Table 2).
Table 2.
Coefficient of variation for measurement of different CEU parameters at baseline and after a high-protein meal
| Time | CEU Parameter |
||
|---|---|---|---|
| A | β | A.β | |
| Baseline | 0.13 | 0.29 | 0.33 |
| Postprandial | 0.19 | 0.33 | 0.36 |
Definity was well tolerated in this study without any severe or serious adverse events. During the study period, there was a 20 mmHg increase in the systolic blood pressure (P = 0.01), but no significant changes in diastolic or mean arterial pressures, heart rate, or oxygen saturation after infusion of the ultrasound contrast agent (Table 3). No cardiac arrythmias were recorded during the study period either. One subject developed a very brief and mild episode of flushing 5 min after infusion of Definity was stopped. This problem was resolved without any interventions. Reintroduction of Definity in this subject did not cause any adverse events. Another study participant developed mild (severity of 3 in a scale of 1–10) throbbing back pain about 10 min after the Definity infusion was stopped. A physical exam, vital signs, and heart monitoring remained unchanged in this case. This episode lasted about 10 min and resolved spontaneously. A decision was made not to reintroduce Definity in this case. Predischarge urinalysis did not show any evidence of kidney damage, or any changes compared with baseline in any participant.
Table 3.
Mean (SD) and median values for vital signs at baseline and after infusion of Definity (after CEU)
| Baseline | After CEU | Change | P Value | |
|---|---|---|---|---|
| Systolic pressure, mmHg | 111 (10) | 114 (10) | 3 (8), 2 | 0.01 |
| Diastolic pressure, mmHg | 64 (9) | 66 (7) | 1 (9), 0 | 0.35 |
| Mean arterial pressure, mmHg | 80 (8) | 82 (7) | 2 (8), 1 | 0.12 |
| Heart rate, beats/min | 63 (9) | 62 (8) | −0.7 (8), −1 | 0.52 |
| Oxygen saturation,% | 99 (2) | 99 (1) | −0.1 (2), 0 | 0.61 |
With respect to Doppler velocity, there was a significant increase of 22.5%(95%CI, 13.2–32.5%, P < 0.001) in the blood flow velocity in the main renal artery after the high-protein meal compared with baseline.
DISCUSSION
The currently available methods for measuring RBF include the clearance techniques utilizing material such as PAH or a radioisotope tracer. PAH clearance involves injection of a bolus dose followed in an hour by its constant infusion and multiple timed urine and blood sample collections. Although PAH clearance in considered the gold standard method for measurement of RBF, issues with its availability for clinical use and technical difficulties have limited its application for research purposes only. Nuclear medicine techniques are cumbersome as well, mainly because of the time required for excretion of the injected radioisotopes into the urine and their clearance from the blood. More recently, computerized tomography, magnetic resonance imaging and positron emission tomography have been used to measure RBF (7, 15). The ability for dynamic imaging in addition to high spatial resolution makes these imaging modalities suitable for this purpose. However, the high cost, lack of widespread availability, toxicity related to contrast agents, and limitations of their use at the bedside make these techniques less desirable.
CEU is unique because of its safety and ease of use. CEU has been proven to be a useful imaging modality in assessing blood flow to the myocardium and is extensively used by cardiologists (2, 10, 12, 13, 16, 20). Contrast agents used for CEU behave the same as the red blood cells and do not diffuse out of the vascular space, which is an advantage compared with contrast agents used in magnetic resonance or computed tomography (13). In addition, portable ultrasound machines equipped with the most advanced technologies have made imaging in different settings ranging from outpatient clinics to intensive care units possible.
Recently, CEU has been utilized to assess RBF. Schlosser et al. (17) used CEU, with Definity as the contrast agent, to study macrovascular (main renal artery and larger vessels) and microvascular (renal cortical) blood flow in pigs. The authors found significantly higher blood velocities in the main vasculature compared with microvessels of the cortical region (17). Wei et al. (23), using SonoVue as the contrast agent, studied changes in RBF after flow-limiting mechanical blockade of the main renal arteries and in response to infusion of dopamine in dogs. In this study, RBF measured by CEU showed a significant rise after dopamine administration and a significant drop after occlusion of the main renal artery. These findings correlated very well with the renal artery flow obtained by a flow probe applied directly over the renal artery (r = 0.82, P < 0.001) (23).
Hosotani et al. (5) compared RBF obtained by CEU, radionuclide scanning using 99mtechnetium-mercaptoacetyltriglycine (99mTc-MAG3), and PAH in 16 patients with chronic kidney disease of various causes. The authors demonstrated a significant correlation (r = 0.69, P = 0.005) between CEU and RBF determined by PAH clearance. The individual RBF by CEU also correlated well with MAG3-obtained split RBF values (r = 0.67, P < 0.005) (5).
In the current study, we were able to demonstrate the utility of CEU in monitoring changes in regional RBF in response to a physiological stimulus. As shown in previous studies, CEU was safe and well tolerated. Obtaining multiple series of images was easy and quick, with an average time for a complete session lasting ∼6.3 min. The rate of adverse events was very low (5%) and mild in severity. Although there were technical difficulties in obtaining Doppler images on the main renal arteries in some cases, we did not have any technical difficulties obtaining CEU images of the kidneys. CEU was useful in monitoring the changes in the flow and velocity of blood perfusion to the renal cortex. We demonstrated a 42%increase in cortical blood flow and a 37%increase in the velocity of blood after ingestion of a high-protein meal. However, the response was variable and not all individuals had an increase in the RBF. This is consistent with the previous results reported by Hostetter (6). Repeating data analysis after removal of a few outliers with the highest changes in RBF, the increases in cortical blood flow and velocity from baseline remained significant (29%for both flow and velocity, P values of 0.006 and <0.001, respectively). Although more studies are required to validated the findings of this study, we believe CEU has great potential as a safe, versatile, and easy technique for real-time monitoring of RBF in many different clinical settings.
Our study has a few limitations. First, we did not directly test the accuracy of CEU in detecting changes in RBF. This was due to the lack of a practical and widely available gold standard for comparison. PAH clearance was initially considered as a potential gold standard against which to compare CEU findings. However, our attempts in obtaining PAH were not successful. At the same time, alterations in the excretion fraction of PAH following interventions that increase RBF make PAH clearance a less desirable method for monitoring acute changes in RBF. At the same time, simultaneous measurement of RBF using nuclear medicine techniques was not feasible since repeating the test in a 2-h period (after a high-protein meal) was not possible.
Second, although we were able to demonstrate by Doppler ultrasound a significant increase in the blood velocity in the main renal artery after ingestion of the high-protein meal, these changes did not correlate with those seen by CEU (r = 0.11, not significant). A contributing factor could be the technical difficulties encountered with Doppler ultrasound. The fact that Doppler measurements from the main renal artery were carried out only once at each time point is another potential cause. However, to definitely prove these points, further carefully designed comparative studies are required.
Although Doppler ultrasound is used in clinical settings to measure RBF, its accuracy has been questioned in recent experiments. In a study using a large-animal model, the data on RBF obtained by Doppler ultrasound was compared with an internally implanted transit-time flow probe (Transonic System) (21). The accuracy of the latter is validated and is currently widely used for the measurement of regional blood flow in animal experimentation (3, 4). In the above-mentioned study, while pharmacological interventions with dobutamine and sodium nitroprusside resulted in significant changes in RBF as measured by the flow probe, no changes were detected by Doppler ultrasound (21). There was a poor correlation between the findings obtained by the two techniques.
We have previously demonstrated a good correlation between CEU measurements and the RBF data obtained from a flow probe directly applied to the renal arteries in dogs (23). Therefore, the poor correlation between CEU and Doppler ultrasound in the current study could possibly be related to the lack of accuracy of Doppler ultrasound as was seen in the study of Wan et al. (21). Nevertheless, more clinical studies with CEU are required to better validate its utility for nephrology applications.
Finally, as mentioned above more studies with larger numbers of participants are required to confirm these findings, especially due to the relative high coefficient of variation in measuring hemodynamic indices.
We did not study medullary blood flow using CEU in this study. Unlike the renal cortex or most other organs, the medulla does not receive blood directly from a main artery. The blood delivered to the renal medulla is supplied by 10–15%of the efferent arterioles from the cortex. Therefore, changes in medullary blood flow would be a function of changes in cortical flow. As a result, replenishment of medullary tissue with microbubbles will not follow the same pattern as in most other tissues like the myocardium and the renal cortex. Currently, we are trying to develop a mathematical model to assess medullary blood flow, taking changes in cortical flow into account.
GRANTS
This work was supported in part by funds from the National Institutes of Health to K. Kalantarinia (DK-074616) and to the University of Virginia General Clinical Research Center (M01-RR00847).
ACKNOWLEDGMENTS
We thank Todd Oickle for assistance in performing Doppler ultrasound examinations. We also thank our certified clinical research coordinators, Cynthia Peterson and Lori Ratliff, for assistance in screening and enrolling subjects, and the General Clinical Research Center staff at the University of Virginia.
REFERENCES
- 1. Aukland K. Methods for measuring renal blood flow: total flow and regional distribution. Annu Rev Physiol 42: 543–555, 1980 [DOI] [PubMed] [Google Scholar]
- 2. Balcells E, Powers ER, Lepper W, Belcik T, Wei K, Ragosta M, Samady H, Lindner JR. Detection of myocardial viability by contrast echocardiography in acute infarction predicts recovery of resting function and contractile reserve. J Am Coll Cardiol 41: 827–833, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Bednarik JA, May CN. Evaluation of a transit-time system for the chronic measurement of blood flow in conscious sheep. J Appl Physiol 78: 524–530, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest 124: 1053–1059, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Hosotani Y, Takahashi N, Kiyomoto H, Ohmori K, Hitomi H, Fujioka H, Aki Y, Fukunaga M, Yuasa S, Mizushige K, Kohno M. A new method for evaluation of split renal cortical blood flow with contrast echography. Hypertens Res 25: 77–83, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Hostetter TH. Human renal response to meat meal. Am J Physiol Renal Fluid Electrolyte Physiol 250: F613–F618, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Huang AJ, Lee VS, Rusinek H. Functional renal MR imaging. Magn Reson Imaging Clin N Am 12: 469–86, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Jakobsen JA, Correas JM. Ultrasound contrast agents and their use in urogenital radiology: status and prospects. Eur Radiol 11: 2082–91, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res 74: 1157–1165, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Kaul S, Senior R, Dittrich H, Raval U, Khattar R, Lahiri A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation 96: 785–792, 1997 [PubMed] [Google Scholar]
- 11. Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin microbubbles within the microcirculation: a basis for their use during myocardial contrast echocardiography. Circ Res 65: 458–467, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Lepper W, Hoffmann R, Kamp O, Franke A, de Cock CC, Kuhl HP, Sieswerda GT, Dahl J, Janssens U, Voci P, Visser CA, Hanrath P. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty. Circulation 101: 2368–2374, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Lindner J, Wei K. Contrast echocardiography. Curr Probl Cardiol 27: 449–520, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr 15: 396–403, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Lorenz CH, Powers TA, Partain CL. Quantitative imaging of renal blood flow and function. Invest Radiol 27, Suppl 2: S109–S114, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Ragosta M, Camarano G, Kaul S, Powers ER, Sarembock IJ, Gimple LW. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation 89: 2562–2569, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Schlosser T, Pohl C, Veltmann C, Lohmaier S, Goenechea J, Ehlgen A, Koster J, Bimmel D, Kuntz-Hehner S, Becher H, Tiemann K. Feasibility of the flash-replenishment concept in renal tissue: which parameters affect the assessment of the contrast replenishment? Ultrasound Med Biol 27: 937–944, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Simon AH, Lima PR, Almerinda M, Alves VF, Bottini PV, de Faria JB. Renal haemodynamic responses to a chicken or beef meal in normal individuals. Nephrol Dial Transplant 13: 2261–2264, 1998 [DOI] [PubMed] [Google Scholar]
- 19. Skyba DM, Camarano G, Goodman NC, Price RJ, Skalak TC, Kaul S. Hemodynamic characteristics, myocardial kinetics and microvascular rheology of FS-069, a second-generation echocardiographic contrast agent capable of producing myocardial opacification from a venous injection. J Am Coll Cardiol 28: 1292–1300, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol 38: 19–25, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Wan L, Yang N, Hiew CY, Schelleman A, Johnson L, May C, Bellomo R. An assessment of the accuracy of renal blood flow estimation by Doppler ultrasound. Intensive Care Med 34: 1503–1510, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97: 473–483, 1998 [DOI] [PubMed] [Google Scholar]
- 23. Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol 37: 1135–1140, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation 103: 2560–2565, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Young LS, Regan MC, Barry MK, Geraghty JG, Fitzpatrick JM. Methods of renal blood flow measurement. Urol Res 24: 149–160, 1996 [DOI] [PubMed] [Google Scholar]