Abstract
The discovery of the renal outer medullary K+ channel (ROMK, Kir1.1), the founding member of the inward-rectifying K+ channel (Kir) family, by Ho and Hebert in 1993 revolutionized our understanding of potassium channel biology and renal potassium handling. Because of the central role that ROMK plays in the regulation of salt and potassium homeostasis, considerable efforts have been invested in understanding the underlying molecular mechanisms. Here we provide a comprehensive guide to ROMK, spanning from the physiology in the kidney to the organization and regulation by intracellular factors to the structural basis of its function at the atomic level.
Keywords: Kir1.1; KCNJ; inward-rectifying potassium channel; Bartter's syndrome; pseudohypoaldosteronism type II; serine- and glucocorticoid-regulated kinase-1; WNK kinase; phosphatidylinositol 4,5-bisphosphate
the functional expression cloning of the renal outer medullary K+ channel (ROMK) by Ho and Hebert (50) revolutionized our understanding of renal potassium handling and opened an entirely new field of potassium channel biology. The discovery, together with the identification and molecular characterization of the bumetanide-sensitive Na+-K+-2Cl− cotransporter NKCC2 (35), and the thiazide-sensitive Na+-Cl− cotransporter NCC (36) in Hebert's laboratory, ushered in the molecular era of renal electrolyte transport. It also had a major impact outside of renal physiology and nephrology. As the defining member of a new potassium channel family, ROMK's identification revealed a channel architecture that differed from the typical voltage-dependent variety, shaping our current understanding of how potassium channel diversification is achieved. At the same time, the revelation of the ROMK molecular structure illustrated in no uncertain terms nature's conservation of the most basic potassium channel blueprint of potassium selectivity and conduction. Here we provide a comprehensive guide to ROMK, from its physiology in the kidney to an understanding of its function at the atomic level.
ROMK, the First Member of the Kir Channel Family
With the discovery of ROMK, the identification of related channels rapidly followed, revealing an entirely new potassium channel family. Encoded by 15 different “KCNJ” genes, family members share a common biophysical property. All exhibit a nonlinear current-voltage relationship, characterized by a larger inward current than outward current, and are therefore commonly referred to as the inward-rectifying potassium channels or Kir (63). They also share a common structure, distinguished by two transmembrane domains, a conserved potassium selectivity filter, and cytoplasmic NH2- and COOH-terminal domains (Fig. 1). Functional Kir channels are formed by homomeric or heteromeric assembly of four subunits. Although members of the Kir channel family exhibit a number of common functional properties and a similar body plan, they are individually specialized for their pivotal roles in a wide range of physiological processes, including controlling membrane excitability, neuronal signaling, heart rate, vascular tone, hormone secretion, and epithelial salt transport. Importantly, defective Kir forms are known to cause human disease, including inherited disorders of cardiac arrhythmias, hyperinsulinemic hypoglycemia, neonatal diabetes, and renal salt-wasting.
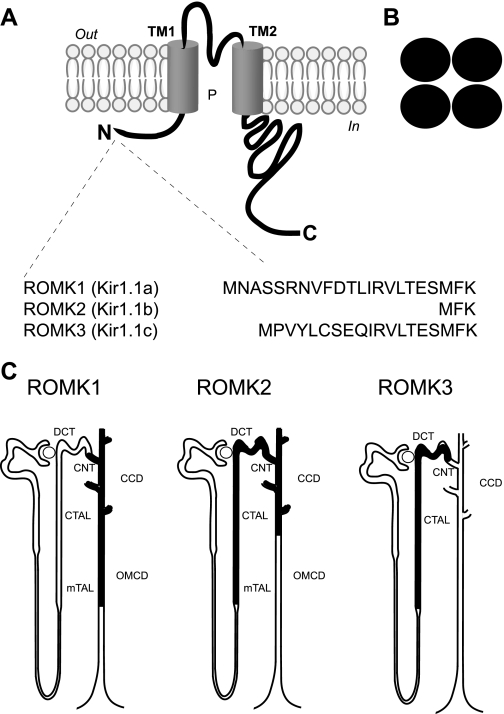
Fig. 1.
Renal outer medullary K+ channel (ROMK) isoforms. ROMK shares a common structure with other inward-rectifying K+ (Kir) channels, typified by 2 transmembrane domains, a conserved potassium selectivity filter, and cytoplasmic NH2- and COOH-terminal domain structures (A) and tetrameric assembly (B). ROMK1–3 isoforms (aka Kir1.1a, -b, -c) have different NH2-terminal structures (C) and are differently expressed along the nephron. DCT, distal convoluted tubule; CNT, connecting tubule; CCD, cortical collecting duct; cTAL, cortical thick ascending limb; OMCD, outer medullary collecting duct; mTAL, medullary thick ascending limb.
Function of ROMK in the Kidney
ROMK is the renal potassium secretory channel.
There should be little doubt that ROMK (aka Kir1.1, product of the KCNJ1 gene) encodes the pore-forming subunit of the kidney's major potassium secretory channel (147) (Fig. 2). ROMK channels comprise the major apical membrane conductance in the thick ascending limb (TAL) and mediate the K+ efflux that is required by the Na+-K+-2Cl− cotransporter NKCC2 for NaCl transport. They also contribute to the TAL transepithelial current flow and potential difference, important for paracellular Na+ and Ca2+ reabsorption. Under most conditions in cortical collecting duct (CCD) principal cells, these same channels represent the predominant and highly regulated K+ secretory pathway essential for K+ homeostasis (45). Patch-clamp studies of TAL cells and CCD principal cells previously characterized apical Kir channels with distinctive biophysical, regulatory, and pharmacological properties, including weak inward rectification, high channel open probability (Po), Ba2+ sensitivity, tetraethylammonium and quinidine insensitivity, and modulation by intracellular pH (pHi), calcium, arachidonic acid, and vasopressin (10, 33, 144, 148–150). Moreover, these channels also exhibited inhibition by intracellular ATP and the sulfonylurea glibenclamide, activation by cAMP-dependent protein kinase, and inhibition by protein kinase C (64, 143, 145, 148).
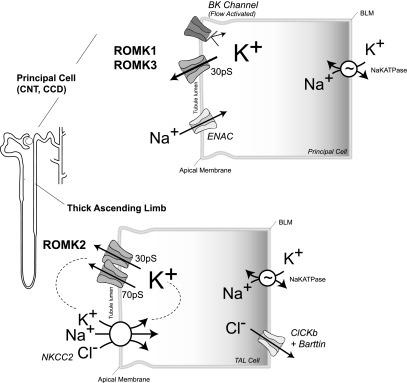
Fig. 2.
ROMK encodes a major potassium secretory channel in the kidney. In distal nephron principal cells, ROMK1 and ROMK3 form the predominant 30-pS potassium channel. The high density and activity of these channels permit avid potassium flux into the tubular lumen. Together with the flow-dependent “BK” channel (shown in low-flow setting), ROMK channels are exquisitely regulated, adjusting potassium excretion to precisely match normal variations in dietary potassium intake. In thick ascending limb (TAL), potassium efflux through the ROMK2 channel is required to safeguard the efficient turnover of the Na+-K+-2Cl− cotransporter (NKCC). Potassium that is brought into the cell by NKCC2 recycles across the apical membrane through ROMK channels. In the TAL, ROMK2 encodes the 30-pS channel; the 70-pS channel is formed by ROMK2 and yet-to-be identified subunit(s). ENaC, epithelial Na+ channel; BLM, basolateral membrane.
Immunodetectable ROMK protein is found on the apical membrane and cytoplasmic compartments of the TAL, distal convoluted tubule (DCT), connecting tubule (CNT), and CCD (60, 93, 162), where these unique potassium secretory channels are expressed (33, 148, 149). Moreover, heterologous expression studies in Xenopus oocytes revealed that the biophysical and pharmacological properties of ROMK (∼35 pS, high Po) are nearly indistinguishable from those of the predominant 35-pS secretory channel found in the TAL and CCD (50, 96, 102a). Finally, ROMK knockout studies in mice established a definitive link between ROMK and the renal secretory channel. Indeed, ablation of the ROMK gene (79) eliminates the 35-pS channel in the mouse TAL and CCD (82). Interestingly, the other TAL potassium secretory channel, which exhibits ROMK-like characteristics except for its higher single-channel conductance (70 pS) (150), is also lost in the ROMK-null mouse, making it likely that ROMK is also a critical subunit of the 70-pS K+ channel.
ROMK splice variants have different physiological roles.
Alternative splicing and promoter usage of the ROMK gene (located on human chromosome 11q24) generates three different products [ROMK1 (Kir1.1a), ROMK2 (Kir1.1b), ROMK3 (Kir1.1c)] with different NH2-terminal amino acid sequences (9, 127, 175) (Fig. 1C). Because ROMK1, ROMK2, and ROMK3 exhibit identical biophysical properties, their functional relevance is still not entirely clear. Nevertheless, they are differentially expressed along the nephron (Ref. 9; Fig. 1C) for different physiological duties (Fig. 2). ROMK2 channels in the TAL of Henle's loop are responsible for recycling potassium across the apical membrane to maintain avid NaCl reabsorption through the Na+-K+-2Cl− cotransporter, important for the urinary concentrating mechanism (Fig. 2). ROMK1 and ROMK3 channels in the distal nephron constitute an important regulated component of the potassium secretory machinery of the kidney, essential for controlling renal potassium excretion and maintaining potassium balance (37).
Biophysical signature is well suited for secretion.
The biophysical properties of ROMK channels are well suited for their role in renal potassium secretion and recycling. Upon phosphorylation or ligand binding (see below), ROMK enters a long-lived, fully active conformation. Characterized by an open state (∼10 ms) that is momentarily interrupted by brief closures (∼1 ms) and longer, but less frequent, divalent cation blocking events (<1% events of ∼40 ms), active ROMK channels are wide open (Po ∼0.9) (13). Furthermore, ROMK is weakly inward rectifying, permitting robust outward potassium flux at physiological membrane potentials. Together the two properties permit ROMK to function as a powerful conduit for avid potassium transport into the tubule lumen, important to maintain salt reabsorption in the TAL and to precisely match renal potassium excretion to dietary potassium intake.
A Multiprotein ROMK Complex
Association with ATP-binding cassette proteins.
Although ROMK shares the salient biophysical features of the small-conductance potassium channels in the TAL and collecting duct apical membrane, the absence of sensitivity to cytoplasmic adenosine triphosphate (ATP) suggested that the native channel might be more complex. Evidence from molecular reconstitution and gene knockout studies indicates that ROMK channels require an ATP-binding cassette (ABC) protein cofactor to manifest native channel properties. Coexpression of CFTR or the sulfonylurea receptor (SUR) (136) with ROMK in Xenopus oocytes leads to the formation of weakly inward-rectifying channels, which have acquired sensitivity to sulfonylurea agents (90) and ATP-dependent gating properties (119) just like the native channel (143, 145). In the kidney, CFTR plays a more important role than SUR. Indeed, expression patterns of ROMK and CFTR (19) overlap in the TAL and CCD apical membranes. Most importantly, Hebert, Lu, and coworkers (81) found that ATP and glibenclamide sensitivities of the 30-pS K+ channel in TAL and CCD cells are absent in CFTR-knockout mice, providing unequivocal evidence that the native ROMK secretory channel is regulated by CFTR. The concept has precedent, with an ever-growing body of data demonstrating that CFTR acts as a “conductance regulator,” in addition to its role as a Cl− channel (126).
The requirement of CFTR to regulate ROMK function is similar to prototypical ATP-sensitive K+ (KATP) channels (3), which are comprised of the SUR and Kir6 channel subunits (55). Although this may suggest that ATP-dependent regulation of potassium channels is generally rooted in the formation of ABC-Kir channel complexes, several important aspects of ROMK-CFTR distinguish it from classic KATP channels (49, 81, 166). Unlike Kir6-SUR channels, which assemble through direct interactions between the two subunits (125), interaction between ROMK and CFTR appears to be indirect and to require scaffolding proteins (166). Second, in contrast to the fixed and static interaction of Kir6-SUR subunits, association of CFTR with ROMK appears to be dynamic and highly regulated. Indeed, protein kinase A (PKA)-mediated phosphorylation abrogates the functional interaction between CFTR and ROMK, releasing the channel from ATP-dependent inhibition (81). Finally, the requirements for proper trafficking of ROMK channels to the plasma membrane are much different from the archetypical KATP channels. SUR and Kir6.x subunits contain endoplasmic reticulum (ER) retention/retrieval signals that are masked upon full assembly of the KATP channel complex (172). By contrast, ROMK exit from the ER is not facilitated by CFTR (166). Instead, scaffolding proteins and a phosphorylation-dependent mechanism are involved (see below).
Scaffold-dependent assembly of a multiprotein complex.
Recruitment of regulatory factors to the ROMK channel can be orchestrated by A-kinase anchor proteins (AKAPs) (1, 2) and PDZ proteins (142, 166, 168). The Na+/H+ exchange regulatory factor NHERF-2, which colocalizes with ROMK in the rat collecting duct (142), has been the focus of attention. Containing tandem PDZ domains and an ezrin/radixin/mosein/merlin (ERM) binding domain, NHERF-2 has the capacity to act as both an AKAP and a PDZ scaffold (155). ROMK preferentially interacts with the first NHERF-2 PDZ domain (166), allowing the other PDZ domain and the ERM domain to recruit regulatory factors to the channel. The ERM binding domain engages the actin-binding AKAP ezrin, likely to juxtapose PKA with the channel for efficient phosphorylation (154). CFTR (135) and serine- and glucocorticoid-regulated kinase (SGK)-1 (100) both interact with NHERF-2 via the second PDZ domain. Consequently, NHERF-2 facilitates the assembly of ternary complexes containing ROMK and CFTR or SGK-1 for optimal channel regulation.
Regulation of ROMK
Regulation of gating.
Factors that influence the activity of ROMK channels in the TAL and collecting duct have profound effects on the renal concentrating mechanism and potassium excretion. A growing body of evidence indicates that ROMK channels are activated and maintained in a high-Po state by the concerted actions of PKA phosphorylation, phosphatidylinositol 4,5-bisphosphate (PIP2) interaction, ATP binding, and pHi.
PROTEIN KINASE A.
Like the native secretory channel (146), ROMK activity is dependent on PKA phosphorylation (92, 163). In fact, activation of ROMK by PKA is thought to underlie the regulation of renal potassium transport by vasopressin (10). Biochemical approaches identified three separate PKA phosphoacceptor sites within the cytoplasmic NH2 (S44) and COOH termini (S219 and S313) (163). All must be phosphorylated for full channel function (88). Phosphorylation of the NH2-terminal residue, S44, is absolutely required for cell surface expression (99, 165, 167). Phosphorylation of the two COOH-terminal sites, by contrast, maintain the channel in a high-Po state (88). Present evidence indicates that phosphorylation of these residues controls the channel opening process by modulating cytoplasmic ligand-dependent gating of the channel [pH (69), ATP (81) or PIP2 (75)].
PHOSPHATIDYLINOSITOL 4,5-BISPHOSPHATE.
With the discovery of ROMK and other members of the Kir channel family, a completely unappreciated and novel mode of channel regulation emerged. After a report that generation of PIP2 by ATP-dependent lipid kinases activates KATP channels in the heart (48), Huang et al. (52) explored the mechanism in several recombinant Kir channels. Remarkably, they found that PIP2 directly binds to ROMK, and this is absolutely necessary for channel opening. The seminal observation provoked a torrent of intense research activity, revealing the central role that PIP2 plays in the regulation of a wide range of ion channels and ligand-gated receptors (51).
It is now clear that PIP2 is an essential Kir channel cofactor, required to maintain the open state of all known inward-rectifying potassium channels. Because the apparent PIP2 binding affinity varies widely among different Kir channels, different Kir subtypes differ in the way PIP2 regulates them. ROMK tightly interacts with PIP2 (52). Consequently, it is not as susceptible to physiological changes in PIP2 levels as other Kir channels (25). Nevertheless, activation of PIP2 hydrolysis by PKC inhibits ROMK channel activity in Xenopus oocytes and was suggested as a mechanism (170), among others (132), to explain how PKC modulates ROMK channels in the collecting duct (146). Formation of PIP2 through membrane-bound phosphoinositide kinases is required to maintain ROMK activity in vivo (80), but it remains to be determined whether ROMK activity in the kidney is controlled by physiological modulation of PIP2 levels.
ATP.
ROMK is not a classic KATP channel like those found in pancreatic islet cells, but similar to the potassium secretory channels in the TAL and CCD (145), it is inhibited by cytoplasmic ATP so long as it is expressed with CFTR (81, 119). Because ATP-dependent modulation occurs in the physiological range of ATP levels (K1/2 ∼1 mM) and is relieved by ADP, ROMK activity is likely to be directly coupled to the activity of Na+-K+-ATPase (156). Consider, for example, the sequence of cellular events that might accompany an elevation of plasma potassium after ingestion of a potassium-rich meal. Although measurements still need to be made, stimulation of Na+-K+-ATPase activity by the substrate action of potassium (94) could conceivably lower the ratio of intracellular ATP to ADP and thereby release ROMK from inhibition to augment potassium excretion. Furthermore, because PKA abrogates the functional interaction between CFTR and ROMK, Hebert and coworkers (81) proposed that the distribution of open and ATP-inhibited ROMK channels in apical membranes may be regulated by hormones to modulate potassium secretion in concert with homeostatic demands.
INTRACELLULAR PH.
Like the native channel, ROMK channel activity is exquisitely sensitive to pHi. Cytoplasmic acidification inhibits ROMK, inducing a long-lived closed state (17, 91). The reduction in channel Po is highly cooperative and occurs with an apparent acidic dissociation constant (pKa) near neutral pHi (17, 27, 91), making channel activity especially susceptible to pathophysiological changes in pHi. Perhaps more interestingly, the apparent pKa of the channel is physiologically controlled. In fact, accumulating evidence indicates that PKA (69)- and PIP2 (72)-dependent activation of ROMK work in an interdependent fashion with the pH-dependent gating mechanism. Studies of Leipziger et al. (69) demonstrating that pH sensitivity is dynamically controlled by phosphorylation provide a salient example. These investigators demonstrated that PKA phosphorylation of the COOH-terminal residues, S219 and S313, causes an acid shift in the apparent pKa, and this activates the channel at physiological pHi. Just how the chemical modification of sites within the large cytoplasmic COOH terminus is translated to the channel pHi-gating process has been the subject of great interest. As discussed below, insights from recently solved atomic resolution structures of related inward rectifier channels provide a plausible mechanism (see below).
Regulation of ROMK Surface Density
Because ROMK channels normally exhibit a very high Po, physiological modulation of channel activity, as controlled by hormones and dietary potassium, is largely achieved by regulated changes in the number of active channels on the plasmalemma. In addition to gating mechanisms that switch on and off resident channels at the apical surface, membrane trafficking mechanisms play an important role. Regulated movement of ROMK to and from the apical surface is particularly important in the distal segments of the nephron, where apical channel expression appears to be precisely regulated, adjusting potassium excretion to exactly match dietary intake. In recent years, the molecular mechanisms have begun to be elucidated.
Forward trafficking in the secretory pathway.
Adaptive changes in the distal nephron principal cell take place in response to an increase in dietary potassium, allowing a more effective and enhanced excretion of potassium (37). The response, called potassium adaptation, involves an increase in the number of active ROMK-type channels on the apical surface (102, 148). Although the traditional text book view holds that aldosterone plays a direct role, it does not appear to be sufficient (104). The mediators of the response still remain unsettled, but other adrenal hormones (102), plasma potassium itself, and other unidentified kaliuretic factors (reviewed in Ref. 103) have been implicated. Nevertheless, the boost in channel density is rapid (101) and occurs without changes in ROMK transcript abundance (34), consistent with a posttranslational mechanism. Recent studies implicate a phosphorylation-dependent trafficking process.
Indeed, cell surface expression of ROMK requires direct phosphorylation (167). A putative ER localization signal in the extreme cytoplasmic COOH terminus of ROMK normally prevents channels from reaching the plasmalemma (99, 165), that is, until phosphorylation of a cytoplasmic NH2-terminal residue, S44, creates a separate trafficking structure that effectively overrides ER retention and drives anterograde movement of the channel through the secretory pathway. Importantly, the phosphorylation-dependent trafficking site in ROMK (a R39XRXXSp motif) exhibits sequence similarity with a comparable NH2-terminal structure in the Kir2.1 channel (R44XR) that is required for constitutive traffic out of the Golgi (134), suggesting that the NH2-terminal structure in ROMK serves a related Golgi export function. The observations contribute to a new and evolving view that the cell surface density and composition of certain types of channels and receptors can be adjusted by controlling their export from the ER and Golgi (85). ROMK should prove to be an ideal model to explore the mechanisms. It will be critical to determine whether the phosphorylated structure acts as an autonomous Golgi export signal and to identify the intracellular trafficking machinery that decodes it.
Observations that phosphorylation of S44 is mediated by either PKA or the aldosterone-induced kinase SGK-1 imply that the phosphorylation-dependent trafficking process plays an important role in the upregulation of ROMK channel density by vasopressin and dietary potassium. Consistent with a central function in potassium adaptation, a rapid increase in SGK-1 is observed in the kidney after the ingestion of a large dietary potassium load (167), coincident with the early increase in ROMK channel density (104). Renal potassium excretion is impaired in SGK-1-null mice, but further studies are required to critically determine the subcellular compartment in the CCD where ROMK channels accumulate (54). Because SGK-1 must be activated by phosphorylation after it is induced by steroid hormones, it is well poised to integrate the effects of adrenal hormones and suspected kaliuretic signals (103) for an appropriate potassium adaptation response.
Endocytosis.
Membrane trafficking processes also underpin the physiological downregulation of ROMK channels. Present evidence indicates that channels are retrieved from the apical surface by clathrin-dependent endocytosis to limit urinary potassium loss in states of dietary potassium deficiency (18, 133, 169). Investigation in this area has largely centered on the underlying signal transduction mechanisms, highlighting Src (73, 74, 133) and the WNK kinases (58, 68, 70, 141).
SRC.
Members of the protein tyrosine kinase (PTK) directly phosphorylate ROMK1 at position Y337 and reduce channel surface expression through a dynamin-dependent (74) and concanavalin A-sensitive (152) process, suggesting a phosphotyrosine-dependent endocytotic mechanism. Significantly, dietary potassium restriction is accompanied by an increase in kidney cSrc and c-Yes (153) and a parallel rise in Y337 phosphorylation (74). Pharmacological studies suggest that attenuation of protein tyrosine phosphatases (133) and/or activation of PTK are necessary for physiological suppression of ROMK (153). Therefore, it seems likely that phosphorylation of tyrosine residue 337 is required for ROMK1 internalization in dietary potassium depletion.
WNK KINASES.
Since the discovery that mutations in the WNK1 and WNK4 kinase genes cause pseudohypoaldosteronism type II, a familial disorder of renal potassium retention and hypertension (158), there has been great interest in understanding how the WNKs normally control the balance between renal potassium excretion and sodium reabsorption. Although concepts about WNK signaling in the kidney are somewhat controversial and in constant flux (53), present evidence strongly suggests that WNKs control salt balance through the divergent modulation of the Na+-Cl− cotransporter NCC and the ROMK channel (57).
Work in heterologous expression systems indicates that WNK1 (68, 141), WNK3 (70), and WNK4 (58) downregulate ROMK at the cell surface by stimulating clathrin-dependent endocytosis. Similar to the endocytotic signals found in LDL receptors, the ROMK channel contains an “NPX”-type motif that is necessary for its internalization (169). Mutation in the key asparagine residue not only impairs ROMK endocytosis (169), it also renders the channels resistant to WNK kinases (58, 68, 141). A trafficking scaffold, intersectin, recruits WNK kinases to clathrin-coated pits (41), but it is still not clear how ROMK channels are similarly targeted to sites of endocytic retrieval.
The abundance and activity of WNK kinases are tightly controlled in the kidney, apparently to regulate ROMK endocytosis in concert with physiological demands. For example, WNK4 loses its ability to inhibit ROMK when it is phosphorylated by the aldosterone-induced kinase SGK-1 (116), providing an additional mechanism to enhance ROMK surface expression with dietary potassium loading. Two WNK1 isoforms (the long form, L-WNK1 and the kidney specific form, KS-WNK1) are also regulated by dietary potassium. Unlike L-WNK1, the kinase-deficient KS-WNK1 form has no inhibitory effect on ROMK (68, 141). Instead, KS-WNK1 negatively modulates L-WNK1 to suppress channel endocytosis (68, 141) and enhance the apical surface expression of ROMK in the distal nephron (76). Acute dietary potassium loading increases the relative abundance of KS-WNK1 (141), while dietary potassium restriction increases the relative abundance of L-WNK1, suggesting that a WNK1 isoform switch plays an important role in the physiological regulation of ROMK apical surface density.
ROMK in Disease
Bartter's syndrome.
A central function of ROMK in human health and disease was illuminated by discovery of the genetic basis of Bartter's syndrome (5), a group of rare autosomal recessive salt-wasting nephropathies characterized by polyuria, hypokalemia, metabolic alkalosis, and hypotension. Bartter's disorders have diverse genetic origins, but they share a common pathological mechanism, namely, a severe reduction in salt reabsorption by the TAL (42). Indeed, the disease is caused by loss-of-function mutations in any one of the four major components of the NaCl reabsorptive machinery in the TAL or by gain-of-function mutations in the calcium-sensing receptor (type V) (139, 151), which normally negatively regulates sodium reabsorption in the TAL (43). Type I Bartter's disease involves the Na+-K+-2Cl− cotransporter NKCC2 (130). Type II disease is caused by inactivating mutations in ROMK (59, 128). Type III involves the basolateral Cl− channel ClCKb (61, 129). In type IV, the accessory subunit of ClCkb, Barttin, is affected (8).
Pioneering physiological studies of Greger and Schlatter (38) and Hebert, Freidman and Andreoli (44, 46) in the isolated, perfused TAL strongly suggested that apical potassium channels were required to constantly supply potassium into the tubule lumen to maintain the turnover of the Na+-K+-2Cl− cotransporter for NaCl reabsorption. The discovery that loss-of-function mutations in ROMK cause Bartter's syndrome, together with subsequent studies in ROMK-knockout mice (4, 79, 82), provided airtight genetic evidence of the transport mechanism and firmly established the molecular identity of the apical potassium channels in the TAL as ROMK.
The discovery also posed a riddle. That is, if ROMK channels in the distal nephron play a central role in potassium excretion, how can potassium wasting in Bartter's syndrome be explained? Work to address the conundrum revealed that two independent potassium secretory mechanisms rather than just one usually maintain potassium excretion. The seminal discovery that “big potassium” (BK) channels are required for flow-dependent potassium secretion (111, 115, 159) illuminated the ROMK-independent potassium secretory pathway. Bailey et al. (4) found that potassium secretion in ROMK-null mice is maintained by upregulation of a flow-dependent, iberiotoxin-sensitive pathway, establishing that compensatory overexpression of BK channels permits potassium excretion in the high-flow setting of Bartter's syndrome. In the same way, upregulation of ROMK channels preserves potassium secretion in BK channel-knockout animals (115). It would seem that the kidney has evolved separate ROMK- and BK-mediated potassium secretory mechanisms to ensure high-capacity potassium excretion and to safeguard against fatal hyperkalemia.
The clinical phenotype that is manifested in patients with inactivating mutations in ROMK is somewhat different from that observed with other forms of Bartter's syndrome (108). This is likely a consequence of the dual role that ROMK normally plays in the healthy kidney, both controlling NaCl reabsorption in the TAL and regulating distal potassium secretion. For example, potassium wasting and hypokalemia in type II Bartter's syndrome is relatively mild compared with other forms of the disease as a consequence of the diminished capacity to excrete potassium. Interestingly, newborn infants with type II Bartter's syndrome actually exhibit a transient period of urinary potassium retention and hyperkalemia before becoming normo- to hypokalemic later in life (29). Neonatal type II patients may be particularly vulnerable to potassium overload because development of BK-dependent potassium secretion is delayed (122, 160).
Over 35 different Bartter's disease mutations have been identified in ROMK. Several introduce nonsense codons or frameshifts in the NH2 terminus, producing truncated proteins with obvious loss-of-function consequences (59, 128). Over half of the missense mutations reduce or even eliminate surface expression of ROMK (107), presumably as a result of global misfolding or mistrafficking. Others alter channel opening by disrupting the potassium permeation pathway or by upsetting the way the channel is regulated by phosphorylation, PIP2 binding, or pHi. As discussed below, much has been learned about the structural basis of channel function by exploring how the disease-causing mutations alter channel gating.
Structure-Function Relationships
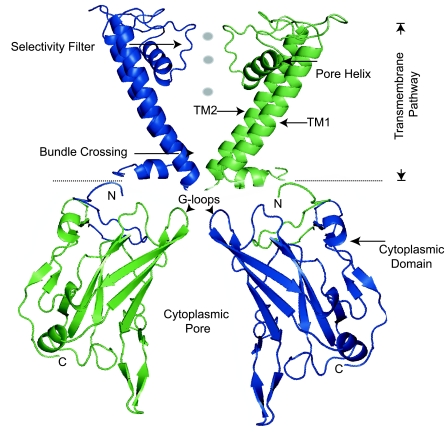
Atomic resolution structures of closely related inward-rectifying potassium channels have recently been solved (65, 97, 98, 106), revealing a common Kir body plan (Fig. 3). A unique potassium conduction pathway, involving the canonical transmembrane pore and a central cavity of a large cytoplasmic domain, characterizes inward-rectifying potassium channels. The distinctive architecture likely accounts for their specialized properties of potassium conduction, inward rectification, and ligand-dependent gating. Here we review and interpret extensive mutagenesis studies in the context of a homology-based atomic model of ROMK structure (Fig. 3) to best appreciate the structural basis of ROMK function in health and dysfunction in disease.
Fig. 3.
An atomic model of ROMK was developed with known Kir channel structures and an iterative optimization algorithm (28). The major body of ROMK is depicted in 2 subunits (amino acids 34–356 of ROMK1 are shown; residues 60–64 and 185–191 are not resolved). Images were rendered with PyMol software. TM1, TM2, first and second transmembrane domains.
Transmembrane potassium conduction pathway.
POTASSIUM SELECTIVITY.
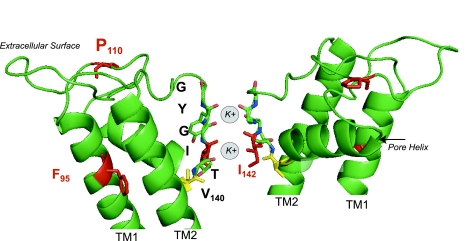
Four ROMK subunits assemble around a central pore that crosses the plasmalemma. Linkers connecting the two transmembrane domains project from the extracellular space into the membrane like staves in a barrel, forming the narrowest part of the open pore conduction pathway. This structure, called the P loop, contains the conserved potassium selectivity sequence (47). Identified by a “T[V/I]GYG” motif, the sequence adopts a strand conformation in which backbone carbonyl oxygens point into the pore. Like all known potassium channels, the close juxtaposition of identical structures from each ROMK subunit creates four equally spaced binding sites for potassium (Fig. 4). Four oxygen atoms at each binding site effectively “cage” a potassium ion, similar to the way water molecules surround potassium in free solution. By mimicking the inner hydration shell of potassium, the selectivity filter creates energetically favorable sites for potassium to diffuse from the aqueous environment. Because the binding sites do not counterbalance dehydration of smaller ions, like sodium, selectivity is restricted to potassium (89). The potassium selectivity of ROMK is remarkably high, PK/Na > 20–100 (PTl > PK > PRb > PNH4) (12, 175). Of note, a Bartter mutation, I142T, in the second residue of the selectivity sequence disrupts potassium permeation (107).
Fig. 4.
ROMK potassium selectivity filter. The selectivity motif “T141IGYG” adopts a strand conformation in which backbone carbonyl oxygens (red atoms) point into the pore, mimicking the hydration shell of potassium. V140, located between the pore helix and the selectivity filter, is a major determinant of the ROMK single-channel conductance and barium block. Rapid conformational movement of T141I142 likely underpins the fast gating process of ROMK. Residues in red are mutated in Bartter's syndrome type II.
POTASSIUM CONDUCTION.
As inferred from early biophysical measurements (83), potassium moves through the ROMK pore by means of a multiple ion conduction mechanism. Viewed in the archetypal KcsA channel, pairs of potassium ions appear to simultaneously occupy different binding sites at the selectivity filter, depending on its conformation (118). Rapid cycles of potassium entry, binding, and conformational change, together with electrostatic repulsion of potassium ions (89), allow potassium to move through the pore with extraordinary throughput (∼108 K+ ions/s) and remarkable selectivity (6, 89).
As a consequence of potassium binding at the selectivity filter, the P-loop region is a major determinant of the ROMK single-channel conductance (16). Although the selectivity filter is highly conserved, rates of potassium binding, release, and passage likely vary among different potassium channel types, giving rise to differences in their single-channel conductances. In Kir channels, the P-loop residues that are responsible for maintaining the structure and flexibility of the selectivity filter are thought to influence this. Like other Kir channels, the ROMK P loop contains a highly conserved glutamate-arginine residue pair (E136-R147), which maintains the rigid bowlike structure of the selectivity filter (20, 164). Neighboring nonconserved residues flanking the salt bridge likely affect the ROMK signature single-channel conductance. In particular, V140, located between the pore helix and the selectivity filter (Fig. 4), contribute to differences in the single-channel conductance between ROMK and its close cousin, Kir2.1 (174). V140 also influences cation selectivity and barium block.
FAST GATING.
ROMK exhibits a fast gating process, characterized by rapid transitions between the open (∼10 ms) and shortest-lived closed (∼1 ms) states (13). Because the rate of entering the short-lived closed state varies with the K+ concentration and is proportional to ROMK current amplitude, it was suspected that K+ permeation plays a key role (13–15). In fact, recent molecular simulation studies reveal that potassium occupancy in the pore has a profound influence on the structure of the selectivity filter. Cation occupancy at a particular position in the selectivity filter causes a conformational change at the position equivalent to T141-I142 in ROMK (Fig. 4), which briefly shuttles the channel into a nonconducting conformation (7, 21). The event occurs in the same time frame as a fast closure, consistent with a mechanism of fast gating. Alternatively, variable energy of potassium binding in the pore has been proposed, in which a short-lived state binds potassium so tightly that it briefly plugs the permeation pathway (15).
INWARD RECTIFICATION.
Intracellular Mg2+ (84, 96) and polyamines (77) enter the Kir channel cytoplasmic pore at depolarizing potentials, bind to a site near the selectivity filter, and plug the potassium permeation pathway (67), giving rise to inward rectification. Because of subtle differences in the chemistry of the binding site, the affinity for Mg2+ and polyamines as well as the degree of inward rectification vary widely among different members of the Kir channel family. A single amino acid within the second transmembrane domain (TM2), corresponding to N171 in ROMK, largely accounts for the rectification phenotype. A mutation, N171D, in ROMK produces strong inward rectification, whereas the reverse mutation, D172N, in the strong inward-rectifying channel Kir2.1 produces ROMK-like rectification (157). Consistent with a binding site in the transmembrane potassium conduction pathway, the side chain of N171 projects from TM2 into the pore (Fig. 5).
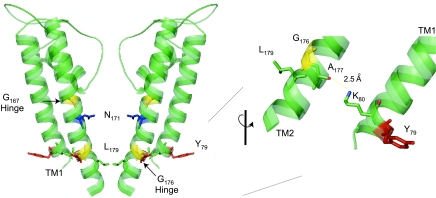
Fig. 5.
The ROMK transmembrane potassium conduction pathway contains the key determinants of inward rectification (N171) and regulated channel gating. Gating movement is thought to involve motion about the glycine hinges, pulling L179, the putative gate, out of the pore. Hydrogen bonding between K80, at the base of TM1, and A177, on TM2, stabilizes the gate. Y79 is mutated in Bartter's syndrome.
REGULATED GATING.
The transmembrane conduction pathway is also thought to contain the regulated gate that opens in response to cytoplasmic alkalization, ATP dissociation, or PIP2 interaction. The subject of Kir channel gating mechanisms is not without controversy, and much remains to be explored (26, 109, 161). Nevertheless, it is widely believed that regulated channel opening involves the movement of the pore helixes (TM2) away from the so-called “bundle crossing.” Structural homology modeling and extensive mutagenesis studies in ROMK are consistent with this idea. Glycine residues (G167 and G176; see Fig. 5) have been implicated as the flexible “hinges” that allow the TM2 helixes to pivot away from one another (121). The movement would carry the putative gate at the cytoplasmic end of TM2 away from the pore. As shown in Fig. 5, the hydrophobic alkane side chain of L179 projects from the base of TM2 into the closed pore. Replacement of this residue with small or highly polar side chains stabilizes the open state (95, 120), just as would be predicted if L179 acts as the regulated gate, occluding the conduction pathway in the closed state.
Interactions between the two transmembrane helixes also influence the regulated gating process (Fig. 5). Strong evidence from molecular modeling and mutagenesis studies suggests that hydrogen bonding between the backbone carbonyl oxygen of A177, and the ε-nitrogen of K80 on the first transmembrane domain (TM1), controls the energetics of the PIP2- and pH-dependent gating process (113, 114). A Bartter mutation that replaces A177 with threonine (107) disrupts the interaction and causes a profound alkaline shift in the pH sensitivity of ROMK (114), effectively turning off channel function at physiological pHi. Substitution of K80 with residues that are incapable of H-bonding with A177 alters gating in a similar way (113). A Bartter mutation, involving a nearby lipid-facing residue, Y79, at the base of TM1, also disrupts regulated channel opening (107). Interestingly, mutations in the comparable residues of the Kir6.2 KATP channel alter ATP-dependent gating and produce neonatal diabetes (39). In light of these observations, it seems probable that the energetics of regulated K+ channel opening are strongly influenced by the H-bonding interactions at the base of TM1 and TM2 and the interaction of TM1 with the inner lipid leaflet.
Cytoplasmic Domain
Extended pore.
The cytoplasmic regions of ROMK assemble (32, 62) to form a long water-filled pore, extending ∼30 Å from the canonical transmembrane potassium conduction pathway (Fig. 6). An antiparallel arrangement of COOH-terminal β-strands lines the inner cavity of the cytoplasmic pore with acidic residues. Electrostatic free energy calculations indicate that this architecture creates a favorable environment for efficient potassium transport and cation blocker binding (117). Remarkably, there is a high degree of concordance between residues that contributes to the favorable static field and residues known from mutagenesis studies to affect cation block and single-channel conductance. For example, acidic amino acids in the ROMK cytoplasmic pore (D254, E258, D298) largely correspond to conserved residues that control the rate of polyamine entry (66, 67). Other residues in the inner wall directly influence the transport of potassium through the cytoplasmic pore. These cytoplasmic structures act as energy barriers to K+ transport and, consequently, affect the single-channel conductance independently of structures in the transmembrane selectivity filter (16). N259 plays the most significant role (173). Importantly, the N259 side chain lines the inner wall of the pore and contributes to surface charge of this structure (117).
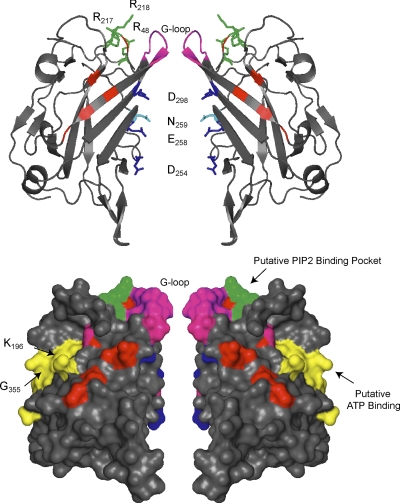
Fig. 6.
The cytoplasmic domains of ROMK. Two subunits are shown. Top: standard cartoon view. Bottom: surface rendering. Pore lining acidic residues are shown in navy blue. N259 (light blue) plays an important role in determining the single-channel conductance. The G loops (magenta) at the apex of the cytoplasmic domain likely participate in gating. Phosphatidylinositol 4,5-bisphosphate (PIP2) binding residues are shown in green. Putative ATP binding domain is shown in yellow (bottom). Note proximity of putative ATP binding residues K196 and G355. Residues mutated in Bartter's syndrome are shown in red.
Gating structure in the cytoplasmic pore.
A narrow opening at the apex of the cytoplasmic pore is formed by the coalescence of four identical loops that project from the COOH-terminal βC- and βD-strands of each subunit (Fig. 6). This structure, called the G loop, is believed to create a flexible diffusion barrier between the cytoplasmic and transmembrane pore. First recognized in Kir2.1 and Kir3.1, mutations in G-loop residues, which face into the central axis of the cytoplasmic pore (corresponding to T300 in ROMK1), alter channel gating and inward rectification (106). Analysis of Kir2.1-ROMK chimeras revealed that a comparable structure (residues 294–320) in ROMK contributes to the single-channel conductance, consistent with the notion that the G loop acts as a resistance barrier to ion flow in ROMK (173). Importantly, a Bartter mutation, A306T, that disrupts potassium conduction (107) is located in the G loop. Disease-causing mutations in other Kir channels also affect the G loop, underscoring the general importance of the structure in the inward rectifiers (86, 105).
In fact, the G loop has been implicated in regulated Kir channel gating. In Kir channel crystal structures, the G loops adopt two different conformations, depending on rigid body movements of the cytoplasmic domains (97, 106). In one state, the G loops are wide enough apart to permit the passage of a partially hydrated potassium. In the other, the G loops fold into a constricted conformation and pitch off the conduction pathway, making it too small to allow transit of even a dehydrated potassium. Present evidence is consistent with the idea that these conformational changes are physiologically relevant. Indeed, state-dependent chemical modification studies in ROMK detected a conformational movement of a G-loop residue, C308, during the pH-dependent gating process (124). Because mutagenesis and electrophysiological studies with Kir2.1 (86, 106), Kir3.2 (30, 40, 56), and Kir6.2 (112) independently mapped residues in or near the G loop as critical determinants of ligand-dependent gating, it seems likely that the G loop plays a fundamental role in a general Kir channel gating mechanism.
Given the physical proximity of the TM2 bundle crossing to the G loop (Fig. 3), it has been proposed that ligand binding (i.e., PIP2) or phosphorylation at nearby sites on the surface of the cytoplasmic domain is allosterically coupled to TM2 movement through conformational changes at the G loop (86, 97, 106). Disruption of the coupling mechanism has been proposed to explain disease-causing gating defects in Kir2.1 and Kir6.2, involving mutations in G-loop residues (86, 105, 112). Obviously, further studies are required to critically test these ideas in ROMK.
Channel modulation.
The large cytoplasmic domain structure has an enormous solvent-exposed surface area, important for docking modulators for ligand (PIP2, pH, ATP) and phosphorylation-dependent gating.
PIP2.
Systematic mutagenesis revealed a group of positively charged cytoplasmic amino acid residues (corresponding to R48, K181, K184, K186, R188, R217, K218, R311 in ROMK1) that are required for normal PIP2-dependent gating in ROMK and other Kir channels (78, 171). Mutagenic neutralization of these residues reduces channel activity and speeds up the rate of inactivation that occurs upon exposure to anti-PIP2 antibodies or polycationic chelating agents. Because the negatively charged head group of PIP2 interacts with positively charged surfaces, identification of these residues points to a conserved PIP2 interaction site. Consistent with a binding site rather than an allosteric modulator, MacGregor et al. reported (87) that a COOH-terminal ROMK peptide (amino acid residues 183–221, containing K181, K184, K186 and R188), has the capacity to directly bind PIP2. Much of the segment is not resolved in Kir crystal structures but corresponds to the initial COOH terminus that extends from TM2 and is likely to reside at the apex of the cytoplasmic domain. Mapping the other basic residues in the three-dimensional (3D) model reveals most (R48, R217, R218) cluster at the top of the cytoplasmic domain (Fig. 5), where they are predicted to face the inner lipid leaflet. Collectively, the observations indicate that PIP2 directly binds to the apical surface of the cytoplasmic domain, providing rationale for the working hypothesis that PIP2 maintains the channel in an open state by tethering the extended cytoplasmic pore to the plasma membrane.
It is noteworthy that a group of Bartter mutations (C49Y, I51T, A214V, and L220F) disrupt PIP2-dependent gating (78). Mapping the residues on the 3D ROMK model reveals that they cluster on the membrane-facing surface of the cytoplasmic domain near the putative PIP2 binding surface, making it likely that the mutations alter the PIP2 binding pocket. Similar observations with disease-causing mutations in Kir2.1 (Andersen-Tawil, Ref. 110) have been interpreted to indicate that a decrease in channel-PIP2 interactions underlies many Kir channelopathies (78).
ATP.
In a series of elegant studies, Steve Hebert's group demonstrated that ATP directly interacts with ROMK. Measurements of fluorescent ATP analog binding to purified, recombinant bacterial fusion proteins of the ROMK cytoplasmic domain revealed that the first 39 COOH-terminal amino acid residues of ROMK1 (183–221) are sufficient to support high-affinity ATP binding (138). Three arginines (R188, R203, and R217) in this segment, known to be important for modulating ATP gating, were subsequently found to be essential for the interaction (22). Because these residues also comprise part of the PIP2 interaction site, competitive binding of the two ligands at the same locale (87) offers a likely explanation for the antagonistic regulation of ROMK by PIP2 and ATP.
High-affinity ATP binding is also governed by K196 and R212 and two distal segments of the ROMK COOH terminus (G335KY and F344GKTVE) (23). Among the Kir channel family, the structures exhibit an especially high degree of similarity to classic KATP (Kir6.x) channels. Extensive mutagenesis and functional studies indicate that the corresponding structures in Kir6.2 KATP [particularly those analogous to K196 (137) and G335 (24) in ROMK] govern ATP dependent gating. Remarkably, these residues are in close proximity in the 3D structure, forming a common patch on the cytoplasmic domain surface of each subunit, consistent with a novel nucleotide-binding domain (see Fig. 5).
PH.
Identifying the structures that determine ROMK modulation by pHi has been the focus of intense investigation and the subject of some controversy. Careful and convincing studies provided multiple independent lines of evidence that lysine at position 80 acts as the pH sensor in ROMK (27). Changing K80 to methionine dramatically reduced the pH sensitivity of ROMK channels; the reverse mutation (M84K) converted a pHi-resistant channel, Kir2.1 (pKa ≤5), to a highly pH-sensitive one (pKa ∼7). Chemical modification studies indicated that K80 is accessible to the cytoplasm (27). Furthermore, 9-flurenylmethylchloroformate, a reagent that reacts with unprotonated NH2 groups on lysines to prevent proton attack, renders ROMK largely insensitive to cytoplasmic acidification (123).
With the identification of two cytoplasmic arginine residues, R41 and R311, that are required for setting the apparent pKa near neutral pH, a mechanism for the anomalous titration of K80 was put forward (123). According to this model, the pKa of K80, which would be near pH 11 if it were in free solution, is brought into the physiological range by electrostatic shielding of R41 and R311. The mechanism implied a ROMK structure in which K80, R41, and R311 were in close physical proximity (123).
The RKR triad concept quickly fell out of favor when the atomic structures of related channels were solved and ROMK modeling revealed that R41 and R311 are nowhere near K80, being over 24 Å apart (114). In the 3D model, K80 is located at the cytoplasmic tip of TM1 (Fig. 5); R41 and R311 are embedded deep within the cytoplasmic domain, where they form highly conserved intrasubunit (R41-E318) and intersubunit (R311-E302) salt bridges (71, 114). Importantly, bridge-disrupting mutations, including R311W/Q in Bartter's disease (107, 123), increase pHi sensitivity and cause channel closure at physiological pH (71, 114), underscoring the importance of the interactions in channel gating. Further investigation is required to elucidate the mechanism, particularly to explore whether these interactions control the conformation of the pH sensor or sensitize the pH-dependent gate.
The role of K80 as the titratable pH sensor has also been debated. Arguments against the idea largely stem from observations with zebrafish and pufferfish orthologs of mammalian ROMK channels (114). Because the fish channels contain an isoleucine or valine at the equivalent position to K80 yet exhibit normal pH gating in the physiological pH range, it has been suggested that the pH sensor must be located elsewhere, such as in the cytoplasmic domain (114). Others have reported that cytoplasmic histidine residues in mammalian ROMK channels (cytoplasmic pore residues H225 and H274 and the solvent-exposed surface residue H354) might collectively act as the pH sensor (11); however, these are not conserved in the zebrafish and pufferfish orthologs, either. According to this view, the locus of the pH sensor in mammalian still remains unknown.
In our view, however, the jury is still out on the role of K80. It is hard to completely dismiss all early studies, which so convincingly supported the pH sensor mechanism. Furthermore, the low homology of fish forms to mammalian ROMK channel (<60% amino acid identity) makes it difficult to make inferences about the functional equivalency of structures. It is possible, for example, that pHi modulates these channels through entirely different mechanisms. Consistent with this, replacement of K80 with either of the fish residues does not support pH gating of mammalian ROMK (114). Structural models place K80 in TM1, where it clearly is apart from the gating mechanism (see above). Given all the evidence to date, we should be open to the possibility that it also acts as the key pH sensor. Hydrogen bonding with A177 and electrostatic interaction (Fig. 5) with the lipid bilayer may be sufficient to explain how K80 is titrated at neutral pH. Obviously, further studies are required to determine whether K80 acts as both the pH sensor and the pH gate.
PKA PHOSPHORYLATION SITES.
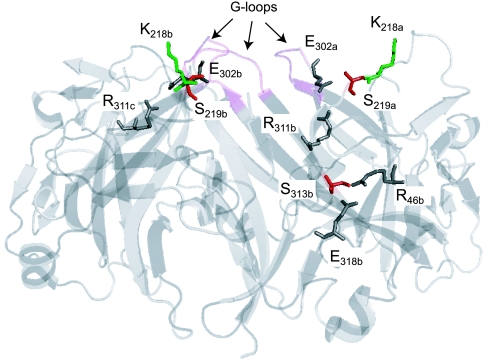
The two key PKA phosphorylation sites that are responsible for controlling channel Po are strategically located in the cytoplasmic domain (Fig. 7). S219 is situated at the apex of the domain between a PIP2 binding residue, the G loop, and an intersubunit (R311-E302) salt bridge. S313 is centrally positioned on the surface of the domain, where it is sandwiched between the intrasubunit (R41-E318) and intersubunit (R311-E302) salt bridges. The juxtaposition of the phosphorylatable serines with the PIP2 and pH gating determinants provides a structural explanation for the function coupling between PKA phosphorylation and PIP2-channel interaction (75) and pH-dependent gating (69). Importantly, several Bartter's disease-causing mutations have been identified that remove the phosphorylatable residues [S219R (128) and S313C (131)], alter nearby residues [L220F (140)], or disrupt the intersubunit salt bridge R311W (107, 123), and thereby alter the regulated gating process.
Fig. 7.
Location of PKA phosphorylation sites S219 and S313 (red) and intrasubunit (R41-E318) and intersubunit salt bridges (R311-E302). Cytoplasmic domains of 3 subunits are shown. Side chains of relevant residues are highlighted. Alphabetical labeling of residues reflects subunit designations (a, b, or c). K218 (green) is a PIP2 binding residue. G loops are shown in magenta.
Coupling Ligand Binding and Phosphorylation to Gating
Just how ligand binding and posttranslational modification of the cytoplasmic domain is transmitted to the movement of the transmembrane helix remains a central unresolved question in the Kir field. Several mechanisms have been suggested. In ROMK, conformational changes in the cytoplasmic domain are associated with gating (124). The G loop and points of inter- and intrasubunit interaction (31, 32, 71, 114) have been implicated in coupling these changes to the transmembrane pore. Furthermore, because electrostatic interactions between potassium and the channel may occur over long distances, subtle conformational changes at a single site in the long cytoplasmic potassium conduction pathway can potentially affect potassium transport energetics over the entire pore (117).
Summary
The molecular mechanisms that ultimately maintain sodium and potassium homeostasis reside in the tubular epithelial cells of the kidney. Since the initial discovery of the ROMK channel, it has become clear that the predominant ionic pathways necessary for luminal K+ recycling and secretion, as well as for K+ movement across basolateral membranes, take the form of highly regulated K+-selective channels that belong to the inward-rectifier K+ channel family. ROMK represents the prototypic member. ROMK isoforms are differentially expressed along the TAL and distal nephron, where they form apical, weak inward-rectifier K+ channels. In TAL cells ROMK channels mediate the K+ efflux required by NKCC2 for NaCl transport while contributing to paracellular Na+ and Ca2+ reabsorption. Loss-of-function mutations result in type II Bartter's syndrome, confirming the dependence of NaCl reabsorption on ROMK-mediated K+ efflux. More distally, these channels establish an exquisitely regulated K+ secretory pathway essential for K+ homeostasis.
ROMK channels, similar to their inward-rectifier cousins, are extraordinarily complex molecular machines whose constituent subunits undergo complex cooperative and dramatic conformational changes, exhibit modulatory intersubunit interactions, and interact distinctively with numerous regulatory factors. A multitude of intracellular factors and processes including Mg2+, polyamines, protons, ATP, PIP2, cAMP-dependent protein kinase, protein kinase C, PTK, SGK-1, and WNK kinases (WNK4, L-WNK1) participate in the remarkably intricate regulation of these channels. The complexity of the ATP sensitivity of these channels is illustrated by their interactions with CFTR through scaffolding proteins and hence divergence from classical ATP-sensitive potassium channels. Conceptually more far-reaching has been the structure of the ROMK channel protein as a simple two-transmembrane unit flanking a “pore loop.” With four such subunits surrounding a highly conserved ion conduction pathway consisting of opposing P loops, ROMK, as well as Kir2.1 and Kir3.1, yielded at the time of their discovery the very first glimpse into nature's most basic and fundamental molecular blueprint for a self-contained potassium-selective pore. Such an architectural plan is found embedded and reproduced in all six-transmembrane, two-transmembrane, and four-transmembrane two-pore potassium channels.
GRANTS
This review was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-54231, DK-63049) and the American Heart Association (GIA0855321E).
ACKNOWLEDGMENTS
We express our appreciation of the life and work of Dr. Steven C. Hebert, who passed away unexpectedly on April 15, 2008. We lost a wonderful mentor, friend, and colleague who made remarkable contributions to the field of renal potassium and sodium transport.
REFERENCES
- 1. Ali S, Chen X, Lu M, Xu JZ, Lerea KM, Hebert SC, Wang WH. The A kinase anchoring protein is required for mediating the effect of protein kinase A on ROMK1 channels. Proc Natl Acad Sci USA 95: 10274–11028, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ali S, Wei Y, Lerea KM, Becker L, Rubin CS, Wang W. PKA-induced stimulation of ROMK1 channel activity is governed by both tethering and non-tethering domains of an A kinase anchor protein. Cell Physiol Biochem 11: 135–142, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 60: 667–687, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bartter FC, Pronove P, Gill JR, Jr, MacCardle RC. Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis: a new syndrome. Am J Med 33: 811–828, 1962 [DOI] [PubMed] [Google Scholar]
- 6. Berneche S, Roux B. Energetics of ion conduction through the K+ channel. Nature 414: 73–77, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Berneche S, Roux B. A gate in the selectivity filter of potassium channels. Structure 13: 591–600, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet 29: 310–314, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol Renal Fluid Electrolyte Physiol 268: F1132–F1140, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Cassola AC, Giebisch G, Wang W. Vasopressin increases density of apical low-conductance K+ channels in rat CCD. Am J Physiol Renal Fluid Electrolyte Physiol 264: F502–F509, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Chanchevalap S, Yang Z, Cui N, Qu Z, Zhu G, Liu C, Giwa LR, Abdulkadir L, Jiang C. Involvement of histidine residues in proton sensing of ROMK1 channel. J Biol Chem 275: 7811–7817, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Chepilko S, Zhou H, Sackin H, Palmer LG. Permeation and gating properties of a cloned renal K+ channel. Am J Physiol Cell Physiol 268: C389–C401, 1995 [DOI] [PubMed] [Google Scholar]
- 13. Choe H, Palmer LG, Sackin H. Structural determinants of gating in inward-rectifier K+ channels. Biophys J 76: 1988–2003, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choe H, Sackin H, Palmer LG. Gating properties of inward-rectifier potassium channels: effects of permeant ions. J Membr Biol 184: 81–89, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Choe H, Sackin H, Palmer LG. Permeation and gating of an inwardly rectifying potassium channel. Evidence for a variable energy well. J Gen Physiol 112: 433–446, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Choe H, Sackin H, Palmer LG. Permeation properties of inward-rectifier potassium channels and their molecular determinants. J Gen Physiol 115: 391–404, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choe H, Zhou H, Palmer LG, Sackin H. A conserved cytoplasmic region of ROMK modulates pH sensitivity, conductance, and gating. Am J Physiol Renal Physiol 273: F516–F529, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Chu PY, Quigley R, Babich V, Huang CL. Dietary potassium restriction stimulates endocytosis of ROMK channel in rat cortical collecting duct. Am J Physiol Renal Physiol 285: F1179–F1187, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Crawford I, Maloney PC, Zeitlin PL, Guggino WB, Hyde SC, Turley H, Harris A, Higgins CF. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci USA 88: 9262–9266, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dibb KM, Rose T, Makary SY, Claydon TW, Enkvetchakul D, Leach R, Nichols CG, Boyett MR. Molecular basis of ion selectivity, block, and rectification of the inward rectifier Kir3.1/Kir3.4 K+ channel. J Biol Chem 278: 49537–49548, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Domene C, Klein ML, Branduardi D, Gervasio FL, Parrinello M. Conformational changes and gating at the selectivity filter of potassium channels. J Am Chem Soc 130: 9474–9480, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Dong K, Tang L, MacGregor GG, Hebert SC. Localization of the ATP/phosphatidylinositol 4,5 diphosphate-binding site to a 39-amino acid region of the carboxyl terminus of the ATP-regulated K+ channel Kir1.1. J Biol Chem 277: 49366–49373, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Dong K, Tang LQ, MacGregor GG, Leng Q, Hebert SC. Novel nucleotide-binding sites in ATP-sensitive potassium channels formed at gating interfaces. EMBO J 24: 1318–1329, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci USA 95: 13953–13958, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J Biol Chem 279: 37271–37281, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Enkvetchakul D, Nichols CG. Gating mechanism of KATP channels: function fits form. J Gen Physiol 122: 471–480, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fakler B, Schultz JH, Yang J, Schulte U, Brandle U, Zenner HP, Jan LY, Ruppersberg JP. Identification of a titratable lysine residue that determines sensitivity of kidney potassium channels (ROMK) to intracellular pH. EMBO J 15: 4093–4099, 1996 [PMC free article] [PubMed] [Google Scholar]
- 28. Fernandez-Fuentes N, Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. Comparative protein structure modeling by combining multiple templates and optimizing sequence-to-structure alignments. Bioinformatics 23: 2558–2565, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Finer G, Shalev H, Birk OS, Galron D, Jeck N, Sinai-Treiman L, Landau D. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr 142: 318–323, 2003 [DOI] [PubMed] [Google Scholar]
- 30. Finley M, Arrabit C, Fowler C, Suen KF, Slesinger PA. betaL-betaM loop in the C-terminal domain of G protein-activated inwardly rectifying K+ channels is important for G(betagamma) subunit activation. J Physiol 555: 643–657, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flagg TP, Tate M, Merot J, Welling PA. A mutation linked with Bartter's syndrome locks Kir 1.1a (ROMK1) channels in a closed state. J Gen Physiol 114: 685–700, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Flagg TP, Yoo D, Sciortino CM, Tate M, Romero MF, Welling PA. Molecular mechanism of a COOH-terminal gating determinant in the ROMK channel revealed by a Bartter's disease mutation. J Physiol 544: 351–362, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Frindt G, Zhou H, Sackin H, Palmer LG. Dissociation of K channel density and ROMK mRNA in rat cortical collecting tubule during K adaptation. Am J Physiol Renal Physiol 274: F525–F531, 1998 [DOI] [PubMed] [Google Scholar]
- 35. Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee WS, Hediger MA. Molecular cloning, primary structure, and characterization of two members of the mammalian electroneutral sodium-(potassium)-chloride cotransporter family expressed in kidney. J Biol Chem 269: 17713–17722, 1994 [PubMed] [Google Scholar]
- 36. Gamba G, Saltzberg SN, Lombardi M, Miyanoshita A, Lytton J, Hediger MA, Brenner BM, Hebert SC. Primary structure and functional expression of a cDNA encoding the thiazide-sensitive, electroneutral sodium-chloride cotransporter. Proc Natl Acad Sci USA 90: 2749–2753, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giebisch G. Renal potassium transport: mechanisms and regulation. Am J Physiol Renal Physiol 274: F817–F833, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Greger R, Schlatter E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers Arch 392: 92–94, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes 54: 2503–2513, 2005 [DOI] [PubMed] [Google Scholar]
- 40. He C, Yan X, Zhang H, Mirshahi T, Jin T, Huang A, Logothetis DE. Identification of critical residues controlling G protein-gated inwardly rectifying K+ channel activity through interactions with the beta gamma subunits of G proteins. J Biol Chem 277: 6088–6096, 2002 [DOI] [PubMed] [Google Scholar]
- 41. He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hebert SC. Bartter syndrome. Curr Opin Nephrol Hypertens 12: 527–532, 2003 [DOI] [PubMed] [Google Scholar]
- 43. Hebert SC. Calcium and salinity sensing by the thick ascending limb: a journey from mammals to fish and back again. Kidney Int Suppl : S28–S33, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Hebert SC, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle: II. determinants of the ADH-mediated increases in transepithelial voltage and in net Cl-absorption. J Membr Biol 80: 221–233, 1984 [DOI] [PubMed] [Google Scholar]
- 45. Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hebert SC, Friedman PA, Andreoli TE. Effects of antidiuretic hormone on cellular conductive pathways in mouse medullary thick ascending limbs of Henle. I. ADH increases transcellular conductance pathways. J Membr Biol 80: 201–219, 1984 [DOI] [PubMed] [Google Scholar]
- 47. Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J 66: 1061–1067, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hilgemann DW, Ball R. Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959, 1996 [DOI] [PubMed] [Google Scholar]
- 49. Ho K. The ROMK-cystic fibrosis transmembrane conductance regulator connection: new insights into the relationship between ROMK and cystic fibrosis transmembrane conductance regulator channels. Curr Opin Nephrol Hypertens 7: 49–58, 1998 [DOI] [PubMed] [Google Scholar]
- 50. Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature 362: 31–38, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Huang CL. Complex roles of PIP2 in the regulation of ion channels and transporters. Am J Physiol Renal Physiol 293: F1761–F1765, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature 391: 803–806, 1998 [DOI] [PubMed] [Google Scholar]
- 53. Huang CL, Yang SS, Lin SH. Mechanism of regulation of renal ion transport by WNK kinases. Curr Opin Nephrol Hypertens 17: 519–525, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 55. Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science 270: 1166–1170, 1995 [DOI] [PubMed] [Google Scholar]
- 56. Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, Schreibmayer W, Dessauer CW, Dascal N. Mapping the Gbetagamma-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem 278: 29174–29183, 2003 [DOI] [PubMed] [Google Scholar]
- 57. Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol 70: 329–355, 2008 [DOI] [PubMed] [Google Scholar]
- 58. Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003 [DOI] [PubMed] [Google Scholar]
- 59. International Collaborative Study Group for Bartter-like Syndromes Mutations in the gene encoding the inwardly-rectifying renal potassium channel, ROMK, cause the antenatal variant of Bartter syndrome: evidence for genetic heterogeneity. Hum Mol Genet 6: 17–26, 1997 [DOI] [PubMed] [Google Scholar]
- 60. Kohda Y, Ding W, Phan E, Housini I, Wang J, Star RA, Huang CL. Localization of the ROMK potassium channel to the apical membrane of distal nephron in rat kidney. Kidney Int 54: 1214–1223, 1998 [DOI] [PubMed] [Google Scholar]
- 61. Konrad M, Vollmer M, Lemmink HH, van den Heuvel LP, Jeck N, Vargas-Poussou R, Lakings A, Ruf R, Deschenes G, Antignac C, Guay-Woodford L, Knoers NV, Seyberth HW, Feldmann D, Hildebrandt F. Mutations in the chloride channel gene CLCNKB as a cause of classic Bartter syndrome. J Am Soc Nephrol 11: 1449–1459, 2000 [DOI] [PubMed] [Google Scholar]
- 62. Koster JC, Bentle KA, Nichols CG, Ho K. Assembly of ROMK1 (Kir 1.1a) inward rectifier K+ channel subunits involves multiple interaction sites. Biophys J 74: 1821–1829, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev 57: 509–526, 2005 [DOI] [PubMed] [Google Scholar]
- 64. Kubokawa M, Wang W, McNicholas CM, Giebisch G. Role of Ca2+/CaMK II in Ca2+-induced K+ channel inhibition in rat CCD principal cell. Am J Physiol Renal Fluid Electrolyte Physiol 268: F211–F219, 1995 [DOI] [PubMed] [Google Scholar]
- 65. Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300: 1922–1926, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Kurata HT, Cheng WW, Arrabit C, Slesinger PA, Nichols CG. The role of the cytoplasmic pore in inward rectification of Kir2.1 channels. J Gen Physiol 130: 145–155, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kurata HT, Marton LJ, Nichols CG. The polyamine binding site in inward rectifier K+ channels. J Gen Physiol 127: 467–480, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leipziger J, MacGregor GG, Cooper GJ, Xu J, Hebert SC, Giebisch G. PKA site mutations of ROMK2 channels shift the pH dependence to more alkaline values. Am J Physiol Renal Physiol 279: F919–F926, 2000 [DOI] [PubMed] [Google Scholar]
- 70. Leng Q, Kahle KT, Rinehart J, MacGregor GG, Wilson FH, Canessa CM, Lifton RP, Hebert SC. WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkalaemia, regulates the K+ channel ROMK1 (Kir1.1). J Physiol 571: 275–286, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Leng Q, MacGregor GG, Dong K, Giebisch G, Hebert SC. Subunit-subunit interactions are critical for proton sensitivity of ROMK: evidence in support of an intermolecular gating mechanism. Proc Natl Acad Sci USA 103: 1982–1987, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem 275: 10182–11019, 2001 [DOI] [PubMed] [Google Scholar]
- 73. Lin D, Sterling H, Lerea KM, Giebisch G, Wang WH. Protein kinase C (PKC)-induced phosphorylation of ROMK1 is essential for the surface expression of ROMK1 channels. J Biol Chem 277: 44278–44284, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lin DH, Sterling H, Lerea KM, Welling P, Jin L, Giebisch G, Wang WH. K depletion increases protein tyrosine kinase-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol 283: F671–F677, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci USA 96: 5820–5825, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem 284: 12198–12206, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372: 366–369, 1994 [DOI] [PubMed] [Google Scholar]
- 78. Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron 34: 933–944, 2002 [DOI] [PubMed] [Google Scholar]
- 79. Lorenz JN, Baird NR, Judd LM, Noonan WT, Andringa A, Doetschman T, Manning PA, Liu LH, Miller ML, Shull GE. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem 277: 37871–37880, 2002 [DOI] [PubMed] [Google Scholar]
- 80. Lu M, Hebert SC, Giebisch G. Hydrolyzable ATP and PIP2 modulate the small-conductance K+ channel in apical membranes of rat cortical-collecting duct (CCD). J Gen Physiol 120: 603–615, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lu M, Leng Q, Egan ME, Caplan MJ, Boulpaep EL, Giebisch GH, Hebert SC. CFTR is required for PKA-regulated ATP sensitivity of Kir1.1 potassium channels in mouse kidney. J Clin Invest 116: 797–807, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu M, Wang T, Yan Q, Yang X, Dong K, Knepper MA, Wang W, Giebisch G, Shull GE, Hebert SC. Absence of small conductance K+ channel (SK) activity in apical membranes of thick ascending limb and cortical collecting duct in ROMK (Bartter's) knockout mice. J Biol Chem 277: 37881–37887, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lu Z, MacKinnon R. A conductance maximum observed in an inward-rectifier potassium channel. J Gen Physiol 104: 477–486, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lu Z, MacKinnon R. Electrostatic tuning of Mg2+ affinity in an inward-rectifier K+ channel. Nature 371: 243–246, 1994 [DOI] [PubMed] [Google Scholar]
- 85. Ma D, Jan LY. ER transport signals and trafficking of potassium channels and receptors. Curr Opin Neurobiol 12: 287–292, 2002 [DOI] [PubMed] [Google Scholar]
- 86. Ma D, Tang XD, Rogers TB, Welling PA. An Andersen-Tawil syndrome mutation in Kir2.1 (V302M) alters the G-loop cytoplasmic K+ conduction pathway. J Biol Chem 282: 5781–5789, 2007 [DOI] [PubMed] [Google Scholar]
- 87. MacGregor GG, Dong K, Vanoye CG, Tang L, Giebisch G, Hebert SC. Nucleotides and phospholipids compete for binding to the C terminus of KATP channels. Proc Natl Acad Sci USA 99: 2726–2731, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. MacGregor GG, Xu JZ, McNicholas CM, Giebisch G, Hebert SC. Partially active channels produced by PKA site mutation of the cloned renal K+ channel, ROMK2 (kir1.2). Am J Physiol Renal Physiol 275: F415–F422, 1998 [DOI] [PubMed] [Google Scholar]
- 89. MacKinnon R. Nobel Lecture. Potassium channels and the atomic basis of selective ion conduction. Biosci Rep 24: 75–100, 2004 [DOI] [PubMed] [Google Scholar]
- 90. McNicholas CM, Guggino WB, Schwiebert EM, Hebert SC, Giebisch G, Egan ME. Sensitivity of a renal K+ channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci USA 93: 8083–8088, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McNicholas CM, MacGregor GG, Islas LD, Yang Y, Hebert SC, Giebisch G. pH-dependent modulation of the cloned renal K+ channel, ROMK. Am J Physiol Renal Physiol 275: F972–F981, 1998 [DOI] [PubMed] [Google Scholar]
- 92. McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+ channel activity involves phosphorylation processes. Proc Natl Acad Sci USA 91: 8077–8081, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mennitt PA, Wade JB, Ecelbarger CA, Palmer LG, Frindt G. Localization of ROMK channels in the rat kidney. J Am Soc Nephrol 8: 1823–1830, 1997 [DOI] [PubMed] [Google Scholar]
- 94. Muto S, Giebisch G, Sansom S. An acute increase of peritubular K stimulates K transport through cell pathways of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 255: F108–F114, 1988 [DOI] [PubMed] [Google Scholar]
- 95. Nanazashvili M, Li H, Palmer LG, Walters DE, Sackin H. Moving the pH gate of the Kir1.1 inward rectifier channel. Channels (Austin) 1: 21–28, 2007 [PubMed] [Google Scholar]
- 96. Nichols CG, Ho K, Hebert S. Mg2+-dependent inward rectification of ROMK1 potassium channels expressed in Xenopus oocytes. J Physiol 476: 399–409, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J 26: 4005–4015, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nishida M, MacKinnon R. Structural basis of inward rectification: cytoplasmic pore of the G protein-gated inward rectifier GIRK1 at 1.8 Å resolution. Cell 111: 957–965, 2002 [DOI] [PubMed] [Google Scholar]
- 99. O'Connell AD, Leng Q, Dong K, MacGregor GG, Giebisch G, Hebert SC. Phosphorylation-regulated endoplasmic reticulum retention signal in the renal outer-medullary K+ channel (ROMK). Proc Natl Acad Sci USA 102: 9954–9959, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Palmada M, Embark HM, Yun C, Bohmer C, Lang F. Molecular requirements for the regulation of the renal outer medullary K+ channel ROMK1 by the serum- and glucocorticoid-inducible kinase SGK1. Biochem Biophys Res Commun 311: 629–634, 2003 [DOI] [PubMed] [Google Scholar]
- 101. Palmer LG. Potassium secretion and the regulation of distal nephron K channels. Am J Physiol Renal Physiol 277: F821–F885, 1999 [DOI] [PubMed] [Google Scholar]
- 102. Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102a. Palmer LG, Choe H, Frindt G. Is the secretory K channel in rat CCT ROMK? Am J Physiol Renal Physiol 273: F404–410, 1997 [DOI] [PubMed] [Google Scholar]
- 103. Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int 57: 1324–1328, 2000 [DOI] [PubMed] [Google Scholar]
- 104. Palmer LG, Frindt G. Regulation of apical K channels in rat cortical collecting tubule during changes in dietary K intake. Am J Physiol Renal Physiol 277: F805–F812, 1999 [DOI] [PubMed] [Google Scholar]
- 105. Pegan S, Arrabit C, Slesinger PA, Choe S. Andersen's syndrome mutation effects on the structure and assembly of the cytoplasmic domains of Kir2.1. Biochemistry 45: 8599–8606, 2006 [DOI] [PubMed] [Google Scholar]
- 106. Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci 8: 279–287, 2005 [DOI] [PubMed] [Google Scholar]
- 107. Peters M, Ermert S, Jeck N, Derst C, Pechmann U, Weber S, Schlingmann KP, Seyberth HW, Waldegger S, Konrad M. Classification and rescue of ROMK mutations underlying hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int 64: 923–932, 2003 [DOI] [PubMed] [Google Scholar]
- 108. Peters M, Jeck N, Reinalter S, Leonhardt A, Tonshoff B, Klaus GG, Konrad M, Seyberth HW. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med 112: 183–190, 2002 [DOI] [PubMed] [Google Scholar]
- 109. Phillips LR, Enkvetchakul D, Nichols CG. Gating dependence of inner pore access in inward rectifier K+ channels. Neuron 37: 953–962, 2004 [DOI] [PubMed] [Google Scholar]
- 110. Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell 105: 511–519, 2001 [DOI] [PubMed] [Google Scholar]
- 111. Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-beta1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol 288: F846–F854, 2005 [DOI] [PubMed] [Google Scholar]
- 112. Proks P, Girard C, Haider S, Gloyn AL, Hattersley AT, Sansom MS, Ashcroft FM. A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome. EMBO Rep 6: 470–475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Rapedius M, Fowler PW, Shang L, Sansom MS, Tucker SJ, Baukrowitz T. H bonding at the helix-bundle crossing controls gating in Kir potassium channels. Neuron 55: 602–614, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rapedius M, Haider S, Browne KF, Shang L, Sansom MS, Baukrowitz T, Tucker SJ. Structural and functional analysis of the putative pH sensor in the Kir1.1 (ROMK) potassium channel. EMBO Rep 7: 611–616, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int 72: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 116. Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104: 4025–4029, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Robertson JL, Palmer LG, Roux B. Long-pore electrostatics in inward-rectifier potassium channels. J Gen Physiol 132: 613–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Roux B, MacKinnon R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science 285: 100–102, 1999 [DOI] [PubMed] [Google Scholar]
- 119. Ruknudin A, Schulze DH, Sullivan SK, Lederer WJ, Welling PA. Novel subunit composition in a renal Katp channel (Kir1.1a/CFTR). J Biol Chem 273: 14165–14171, 1998 [DOI] [PubMed] [Google Scholar]
- 120. Sackin H, Nanazashvili M, Palmer LG, Krambis M, Walters DE. Structural locus of the pH gate in the Kir1.1 inward rectifier channel. Biophys J 88: 2597–2606, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sackin H, Nanazashvili M, Palmer LG, Li H. Role of conserved glycines in pH gating of Kir1.1 (ROMK). Biophys J 90: 3582–3589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 272: F397–F404, 1997 [DOI] [PubMed] [Google Scholar]
- 123. Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci USA 96: 15298–15303, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Schulte U, Hahn H, Wiesinger H, Ruppersberg JP, Fakler B. pH-dependent gating of ROMK (Kir1.1) channels involves conformational changes in both N and C termini. J Biol Chem 273: 34575–34579, 1998 [DOI] [PubMed] [Google Scholar]
- 125. Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron 26: 155–167, 2000 [DOI] [PubMed] [Google Scholar]
- 126. Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev 79: S145–S166, 1999 [DOI] [PubMed] [Google Scholar]
- 127. Shuck ME, Piser TM, Bock JH, Slightom JL, Lee KS, Bienkowski MJ. Cloning and characterization of two K+ inward rectifier (Kir) 1.1 potassium channel homologs from human kidney (Kir1.2 and Kir1.3). J Biol Chem 272: 586–593, 1997 [DOI] [PubMed] [Google Scholar]
- 128. Simon D, Karet F, Rodriguez-Soriano J, Hamdan J, DiPietro A, Sanjad S, Lifton R. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1997 [DOI] [PubMed] [Google Scholar]
- 129. Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet 17: 171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 130. Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 131. Starremans PG, van der Kemp AW, Knoers NV, van den Heuvel LP, Bindels RJ. Functional implications of mutations in the human renal outer medullary potassium channel (ROMK2) identified in Bartter syndrome. Pflügers Arch 443: 466–472, 2002 [DOI] [PubMed] [Google Scholar]
- 132. Sterling H, Lin DH, Chen YJ, Wei Y, Wang ZJ, Lai J, Wang WH. PKC expression is regulated by dietary K intake and mediates internalization of SK channels in the CCD. Am J Physiol Renal Physiol 286: F1072–F1078, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. Inhibition of protein tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem 277: 4317–4323, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Stockklausner C, Klocker N. Surface expression of inward rectifier potassium channels is controlled by selective Golgi export. J Biol Chem 278: 17000–17005, 2003 [DOI] [PubMed] [Google Scholar]
- 135. Sun F, Hug MJ, Lewarchik CM, Yun CH, Bradbury NA, Frizzell RA. E3KARP mediates the association of ezrin and protein kinase A with the cystic fibrosis transmembrane conductance regulator in airway cells. J Biol Chem 275: 29539–29546, 2002 [DOI] [PubMed] [Google Scholar]
- 136. Tanemoto M, Vanoye CG, Dong K, Welch R, Abe T, Hebert SC, Xu JZ. Rat homolog of sulfonylurea receptor 2B determines glibenclamide sensitivity of ROMK2 in Xenopus laevis oocyte. Am J Physiol Renal Physiol 278: F659–F666, 2000 [DOI] [PubMed] [Google Scholar]
- 137. Tucker SJ, Gribble FM, Proks P, Trapp S, Ryder TJ, Haug T, Reimann F, Ashcroft FM. Molecular determinants of KATP channel inhibition by ATP. EMBO J 17: 3290–3296, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Vanoye CG, MacGregor GG, Dong K, Tang L, Buschmann AS, Hall AE, Lu M, Giebisch G, Hebert SC. The carboxyl termini of KATP channels bind nucleotides. J Biol Chem 277: 23260–23270, 2002 [DOI] [PubMed] [Google Scholar]
- 139. Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaitre X, Paillard M, Planelles G, Dechaux M, Miller RT, Antignac C. Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266, 2002 [DOI] [PubMed] [Google Scholar]
- 140. Vollmer M, Koehrer M, Topaloglu R, Strahm B, Omran H, Hildebrandt F. Two novel mutations of the gene for Kir 1.1 (ROMK) in neonatal Bartter syndrome. Pediatr Nephrol 12: 69–71, 1998 [DOI] [PubMed] [Google Scholar]
- 141. Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol 280: C192–C198, 2001 [DOI] [PubMed] [Google Scholar]
- 143. Wang T, Wang W, Klein-Robbenhaar G, Giebisch G. Effects of glyburide on renal tubule transport and potassium-channel activity. Renal Physiol Biochem 18: 169–182, 1995 [DOI] [PubMed] [Google Scholar]
- 144. Wang W, Cassola A, Giebisch G. Arachidonic acid inhibits the secretory K+ channel of cortical collecting duct of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 262: F554–F559, 1992 [DOI] [PubMed] [Google Scholar]
- 145. Wang W, Giebisch G. Dual effect of adenosine triphosphate on the apical small conductance K+ channel of the rat cortical collecting duct. J Gen Physiol 98: 35–61, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Wang W, Giebisch G. Dual modulation of renal ATP-sensitive K channel by protein kinases A and C. Proc Natl Acad Sci USA 88: 9722–9725, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annu Rev Physiol 59: 413–436, 1997 [DOI] [PubMed] [Google Scholar]
- 148. Wang W, Schwab A, Giebisch G. Regulation of small-conductance K channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990 [DOI] [PubMed] [Google Scholar]
- 149. Wang W, White S, Geibel J, Giebisch G. A potassium channel in the apical membrane of rabbit thick ascending limb of Henle's loop. Am J Physiol Renal Fluid Electrolyte Physiol 258: F244–F253, 1990 [DOI] [PubMed] [Google Scholar]
- 150. Wang WH. Two types of K+ channel in thick ascending limb of rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 267: F599–F605, 1994 [DOI] [PubMed] [Google Scholar]
- 151. Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet 360: 692–694, 2002 [DOI] [PubMed] [Google Scholar]
- 152. Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem 275: 20502–20507, 2000 [DOI] [PubMed] [Google Scholar]
- 153. Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001 [DOI] [PubMed] [Google Scholar]
- 154. Weinman EJ. New functions for the NHERF family of proteins. J Clin Invest 108: 185–186, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with G protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol 68: 491–505, 2006 [DOI] [PubMed] [Google Scholar]
- 156. Welling PA. Cross-talk and the role of KATP channels in the proximal tubule. Kidney Int 48: 1017–1023, 1995 [DOI] [PubMed] [Google Scholar]
- 157. Wible BA, Taglialatela M, Ficker E, Brown AM. Gating of inwardly rectifying K+ channels localized to a single negatively charged residue. Nature 371: 246–249, 1994 [DOI] [PubMed] [Google Scholar]
- 158. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 159. Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001 [DOI] [PubMed] [Google Scholar]
- 160. Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 161. Xiao J, Zhen XG, Yang J. Localization of PIP2 activation gate in inward rectifier K+ channels. Nat Neurosci 6: 811–818, 2003 [DOI] [PubMed] [Google Scholar]
- 162. Xu JZ, Hall AE, Peterson LN, Bienkowski MJ, Eessalu TE, Hebert SC. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am J Physiol Renal Physiol 273: F739–F748, 1997 [DOI] [PubMed] [Google Scholar]
- 163. Xu ZC, Yang Y, Hebert SC. Phosphorylation of the ATP-sensitive, inwardly rectifying K+ channel, ROMK, by cyclic AMP-dependent protein kinase. J Biol Chem 271: 9313–9319, 1996 [DOI] [PubMed] [Google Scholar]
- 164. Yang J, Yu M, Jan YN, Jan LY. Stabilization of ion selectivity filter by pore loop ion pairs in an inwardly rectifying potassium channel. Proc Natl Acad Sci USA 94: 1568–1572, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Yoo D, Fang L, Mason A, Kim BY, Welling PA. A phosphorylation-dependent export structure in ROMK (Kir 1.1) channel overrides an endoplasmic reticulum localization signal. J Biol Chem 280: 35281–35289, 2005 [DOI] [PubMed] [Google Scholar]
- 166. Yoo D, Flagg TP, Olsen O, Raghuram V, Foskett JK, Welling PA. Assembly and trafficking of a multiprotein ROMK (Kir 1.1) channel complex by PDZ interactions. J Biol Chem 279: 6863–6873, 2004 [DOI] [PubMed] [Google Scholar]
- 167. Yoo D, Kim BY, Campo C, Nance L, King A, Maouyo D, Welling PA. Cell surface expression of the ROMK (Kir 1.1) channel is regulated by the aldosterone-induced kinase, SGK-1, and protein kinase A. J Biol Chem 278: 23066–23075, 2003 [DOI] [PubMed] [Google Scholar]
- 168. Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid-inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol 13: 2823–2830, 2002 [DOI] [PubMed] [Google Scholar]
- 169. Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F669, 2002 [DOI] [PubMed] [Google Scholar]
- 170. Zeng WZ, Li XJ, Hilgemann DW, Huang CL. Protein kinase C inhibits ROMK1 channel activity via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. J Biol Chem 278: 16852–16856, 2003 [DOI] [PubMed] [Google Scholar]
- 171. Zeng WZ, Liou HH, Krishna UM, Falck JR, Huang CL. Structural determinants and specificities for ROMK1-phosphoinositide interaction. Am J Physiol Renal Physiol 282: F826–F834, 2002 [DOI] [PubMed] [Google Scholar]
- 172. Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22: 537–548, 1999 [DOI] [PubMed] [Google Scholar]
- 173. Zhang YY, Robertson JL, Gray DA, Palmer LG. Carboxy-terminal determinants of conductance in inward-rectifier K channels. J Gen Physiol 124: 729–739, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Zhou H, Chepilko S, Schutt W, Choe H, Palmer LG, Sackin H. Mutations in the pore region of ROMK enhance Ba2+ block. Am J Physiol Cell Physiol 271: C1949–C1956, 1996 [DOI] [PubMed] [Google Scholar]
- 175. Zhou H, Tate SS, Palmer LG. Primary structure and functional properties of an epithelial K channel. Am J Physiol Cell Physiol 266: C809–C824, 1994 [DOI] [PubMed] [Google Scholar]