Abstract
Bacterial infection of the kidney is associated with renal tubule dysfunction and dysregulation of systemic electrolyte balance. Whether bacterial molecules directly affect renal tubule transport is unknown. We examined the effects of LPS on HCO3− absorption in the isolated rat and mouse medullary thick ascending limb (MTAL). LPS decreased HCO3− absorption when added to bath or lumen. The MEK/ERK inhibitor U0126 eliminated inhibition by bath LPS but had no effect on inhibition by lumen LPS. Conversely, the mammalian target of rapamycin (mTOR) inhibitor rapamycin eliminated inhibition by lumen LPS but had no effect on inhibition by bath LPS. Inhibiting basolateral Na+/H+ exchange with amiloride eliminated inhibition of HCO3− absorption by lumen but not bath LPS. Confocal immunofluorescence showed expression of TLR4 in basolateral and apical membrane domains. Inhibition of HCO3− absorption by bath and lumen LPS was eliminated in MTALs from TLR4−/− mice. Thus LPS inhibits HCO3− absorption through distinct TLR4-dependent pathways in basolateral and apical membranes. These results establish that bacterial molecules can directly impair the transport function of renal tubules, identifying a new mechanism contributing to tubule dysfunction during bacterial infection. The LPS-induced reduction in luminal acidification may contribute to Gram-negative pathogenicity by promoting bacterial adherence and growth and impairing correction of infection-induced systemic acid-base disorders.
Keywords: bacterial kidney infection, medullary thick ascending limb, acid-base transport, Na+/H+ exchange, sepsis
kidney injury is a common complication and significant cause of morbidity and mortality for patients with sepsis or urinary tract infection (UTI) (10, 25, 66). Bacterial infection frequently is associated with renal tubule dysfunction and dysregulation of systemic electrolyte balance, including a urinary concentrating defect, impaired urinary acidification with reduced excretion of ammonium (despite systemic metabolic acidosis), increased fractional excretion of sodium, impaired absorption of glucose, and systemic hypotension (6, 8, 13, 14, 34, 53, 64–66, 73). Whether these effects involve a direct action of bacterial components to alter renal tubule transport is unknown. Lipopolysaccharide (LPS) is the dominant cell wall molecule of Gram-negative bacteria responsible for initiating innate immune and inflammatory responses (2, 14, 46). Recognition of LPS by target cells triggers intracellular signals and proinflammatory responses that are critical for host defense and the elimination of invading bacteria but also can lead to inflammatory kidney injury (2, 10, 14, 19, 46). Whether LPS acts directly to modify the transport functions of renal tubules has not been investigated.
The Toll-like receptors (TLR) are a family of transmembrane pattern-recognition receptors that recognize structural components unique to different microbial pathogens. At least 11 mammalian TLRs (TLR1–TLR11) have been identified (1, 2, 10). TLR4, the signaling receptor for Gram-negative LPS (1, 35, 54, 56), is constitutively expressed in proximal and distal nephron segments in vivo (19, 20, 39, 43, 60, 82, 83, 86) and mediates LPS-induced production of proinflammatory cytokines by renal epithelial cell lines (9, 11, 52, 60, 70). Mice with loss-of-function mutations in the TLR4 gene have impaired bacterial clearance during Escherichia coli pyelonephritis (35, 54, 56), and TLR4 on renal epithelial cells was required for control of ascending UTI in bone marrow chimeric mice (52). Beyond its role in defense against bacterial infection, increasing evidence suggests that TLR4 plays a role in mediating inflammatory kidney injury caused by ascending E. coli infection and LPS-induced sepsis (16, 19, 20, 52, 70). In addition, there is rapidly growing evidence at the whole kidney level that activation of TLR4 by endogenous ligands contributes to inflammatory kidney injury in a variety of noninfectious conditions, including ischemia-reperfusion injury, diabetes, nephrotoxic injury, and transplant rejection (5, 19, 39, 43, 62, 67, 72, 82, 83, 86, 88). To date, however, there have been no direct studies of the mechanisms or functional effects of TLR4 signaling in any native nephron segment. Whether activation of TLR4 alters renal epithelial transport and can contribute to infection-induced renal tubule dysfunction is unknown.
Ion transport processes in the renal thick ascending limb play a vital role in a number of important homeostatic functions, including maintenance of sodium and water balance and acid-base regulation (3, 29, 40). Recent studies suggest that the thick ascending limb also may play a role in the innate immune response of the kidney. TLR4 has been localized to the thick ascending limb by in situ hybridization and immunofluorescence microscopy (20, 39, 82). TLR4 expression in this segment is increased in response to sepsis and ischemia-reperfusion injury (20, 39, 82) and the thick ascending limb has been implicated in mediating inflammatory renal injury during these conditions (19, 21, 22, 39, 82, 83). Moreover, the medullary thick ascending limb (MTAL) has been identified as a site of cell damage and tubule dysfunction in response to microbial infection (84). These findings suggest that the thick ascending limb may be a potential target within the nephron for LPS-induced transport modulation.
Acid-base transport is relevant to the pathogenesis of Gram-negative bacterial infection on several levels. These include 1) changes in urine pH influence the virulence of E. coli through effects on bacterial adherence and growth (27, 37, 68); 2) the production of inflammatory mediators is affected by pH in several cell types (12, 23, 44, 48, 58); and 3) endotoxemia and Gram-negative sepsis are associated with systemic metabolic acidosis that contributes to the pathogenesis of organ dysfunction (8, 14, 38, 53, 66). The MTAL participates in urine acidification and acid-base regulation by reabsorbing most of the filtered HCO3− not reabsorbed by the proximal tubule (3, 29). Absorption of HCO3− by the MTAL depends on luminal H+ secretion mediated by the apical NHE3 Na+/H+ exchanger (4, 74, 75) and is regulated by a number of important physiological factors (28–30, 32, 74–76, 79). In the present study, we examined directly the effects of LPS on HCO3− absorption by the isolated, perfused MTAL. We show that LPS inhibits HCO3− absorption from either the basolateral or luminal cell surface. In addition, the results reveal a novel sidedness to LPS receptor signaling, whereby LPS alters HCO3− absorption through the activation of distinct TLR4-dependent signaling pathways in the basolateral and apical membranes. These studies provide the first evidence that bacterial molecules can act directly through innate immune receptors to modify the transport function of renal tubules, thereby identifying a new pathophysiological mechanism contributing to tubule dysfunction during bacterial infection. They also reveal a novel, pathoadaptive mechanism through which Gram-negative bacteria can adversely affect the progression and severity of urinary tract infection and endotoxemia, namely, through LPS-induced impairment of renal tubule acid secretion.
METHODS
Animals.
Male Sprague-Dawley rats (50–100 g body wt) were purchased from Taconic (Germantown, NY). Male C57BL/10SnJ (wild-type) and C57BL/10ScNJ (TLR4−/−) mice (7–8 wk old) were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/10ScNJ mice do not express TLR4 due to a Tlr4 gene deletion (54, 56). The animals were maintained under pathogen-free conditions in microisolator cages and received standard rodent chow (NIH 31 diet, Ziegler) and distilled water up to the time of the experiments. Body weight did not differ in wild-type and TLR4−/− mice (24 ± 1 g wild-type vs. 22 ± 1 g TLR4−/−). All protocols in this study were approved by the institutional animal care and use committee of The University of Texas Medical Branch.
Tubule perfusion and measurement of net HCO3− absorption.
MTALs were isolated and perfused in vitro as previously described (28, 33, 79). Tubules were dissected from the inner stripe of the outer medulla at 10°C in control bath solution (see below), transferred to a bath chamber on the stage of an inverted microscope, and mounted on concentric glass pipettes for perfusion at 37°C. The tubules were perfused and bathed in control solution that contained (in mM) 146 Na+, 4 K+, 122 Cl−, 25 HCO3−, 2.0 Ca2+, 1.5 Mg2+, 2.0 phosphate, 1.2 SO42−, 1.0 citrate, 2.0 lactate, and 5.5 glucose (equilibrated with 95% O2-5% CO2, pH 7.45, at 37°C). LPS and other experimental agents were added to the bath and lumen solutions as described in results. LPS from E. coli O111:B4 and lipid A, diphosphoryl from E. coli F583 were purchased from Sigma. Ultra-pure E. coli K12 LPS was from InvivoGen. Synthetic E. coli lipid A (compound 506) was from Peptides International. Heat-inactivated, low-endotoxin fetal bovine serum was from Gemini Bio-Products. The lipid A compounds were prepared as stock solutions in DMSO and diluted into bath and lumen solutions to final concentrations given in results. Solutions containing other experimental agents were prepared as described elsewhere (32, 33, 76). Equal concentrations of vehicle were added to control solutions in all protocols. The O111:B4 LPS serotype has been used extensively to define LPS-induced immune responses (11, 42, 59, 71, 84, 85). LPS was studied at 250 or 500 ng/ml because 1) these concentrations are similar to those used typically to induce innate immune responses in a variety of biological systems, including classic immune cells (7, 18, 63, 80) and renal epithelial cell lines (11, 52, 70, 84); and 2) they induce highly reproducible and reversible changes in MTAL transport (see results).
The protocol for study of transepithelial HCO3− absorption was as described (28, 33, 79). Tubules were equilibrated for 20–30 min at 37°C in the initial perfusion and bath solutions, and the luminal flow rate (normalized per unit tubule length) was adjusted to 1.5–1.9 nl·min−1·mm−1. One to three 10-min tubule fluid samples were then collected for each period (initial, experimental, and recovery). The tubules were allowed to reequilibrate for 5–10 min after an experimental agent was added to or removed from the bath solution. The absolute rate of HCO3− absorption (JHCO3−, pmol·min−1·mm−1) was calculated from the luminal flow rate and the difference between total CO2 concentrations measured in perfused and collected fluids (28). An average HCO3− absorption rate was calculated for each period studied in a given tubule. When repeat measurements were made at the beginning and end of an experiment (initial and recovery periods), the values were averaged. Single tubule values are presented in Figs. 1–6, 8, and 9. Mean values ± SE (n = number of tubules) are presented in the text.
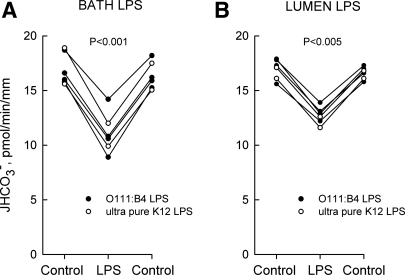
Fig. 1.
LPS inhibits HCO3− absorption from bath or lumen. Medullary thick ascending limbs (MTALs) from rats were isolated and perfused in vitro in control solution, and then LPS was added to and removed from the bath (500 ng/ml; A) or lumen (250 ng/ml; B) solution. The tubules were exposed to either Escherichia coli O111:B4 or ultra-pure E. coli K12 LPS. Absolute rates of HCO3− absorption (JHCO3−) were measured as described in methods. Data points are average values for single tubules. Lines connect paired measurements made in the same tubule. P values are for paired t-test. Mean values are given in results.
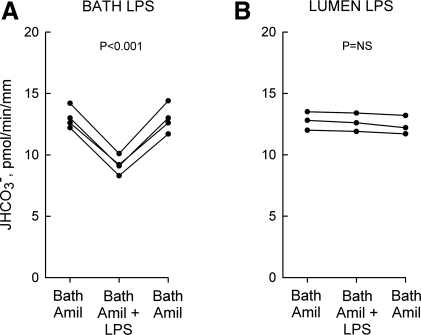
Fig. 6.
Bath amiloride blocks inhibition of HCO3− absorption by lumen but not bath LPS. MTALs from rats were bathed with 10 μM amiloride (Amil), and then LPS (ultra-pure K12) was added to and removed from the bath (500 ng/ml; A) or lumen (250 ng/ml; B) solution. JHCO3−, data points, lines, and P values are as in Fig. 1.
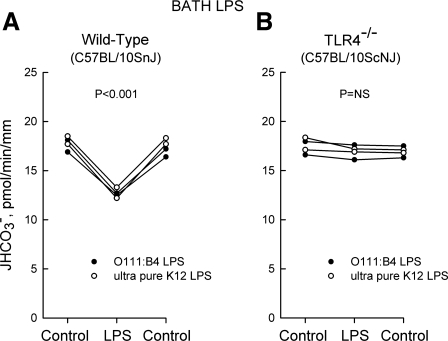
Fig. 8.
Inhibition of HCO3− absorption by bath LPS is eliminated in MTALs from TLR4−/− mice. MTALs from wild-type control (A) and TLR4−/− (B) mice were perfused in vitro in control solution, and then LPS (500 ng/ml) was added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 1.
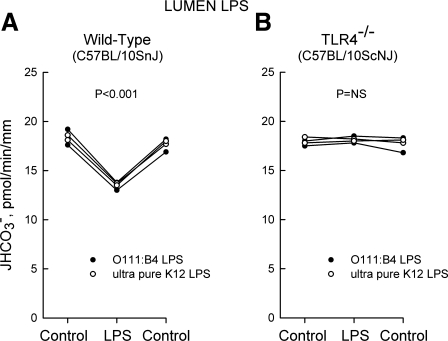
Fig. 9.
Inhibition of HCO3− absorption by lumen LPS is eliminated in MTALs from TLR4−/− mice. MTALs from wild-type control (A) and TLR4−/− (B) mice were perfused in vitro in control solution, and then LPS (250 ng/ml) was added to and removed from the lumen solution. JHCO3−, data points, lines, and P values are as in Fig. 1.
Confocal immunofluorescence microscopy.
MTALs were studied by confocal microscopy as previously described (32, 78). Rat and mouse MTALs were microdissected and mounted on Cell-Tak-coated coverslips at 10°C. The tubules were then incubated for 15 min at 37°C in a flowing bath using the same control solution as in HCO3− transport experiments. Following incubation, the tubules were washed with phosphate-buffered saline (PBS) and fixed and permeabilized in ice-cold acetone for 10 min. The tubules were incubated in Image-iT FX signal enhancer (Invitrogen) for 30 min at room temperature, washed, and blocked in 10% donkey serum in PBS for 1 h at room temperature. The tubules were then incubated overnight at 4°C with a 1:100 dilution of goat anti-mouse TLR4 polyclonal antibody (L-14, Santa Cruz Biotechnology), washed, and then incubated for 1 h at room temperature in Alexa 488-conjugated donkey anti-goat IgG antibody (1:100; Invitrogen) in blocking buffer. In some experiments, the anti-TLR4 antibody was incubated in the absence and presence of a five-fold excess by weight of blocking peptide (Santa Cruz) for 2 h at room temperature prior to tubule staining. Fluorescence staining was examined using a Zeiss laser-scanning confocal microscope (LSM510 UV META), as described (32, 78). Tubules were imaged longitudinally and z-axis optical sections (0.4 μm) were obtained through a plane at the center of the tubule, which provides a cross-sectional view of cells in the lateral tubule walls. For individual experiments, two to four tubules from the same kidney for each experimental condition, or from wild-type and TLR4−/− mice, were fixed and stained identically and imaged in a single session at identical settings of illumination, gain, and exposure time.
RESULTS
LPS inhibits HCO3− absorption from bath or lumen.
To determine whether LPS directly alters renal tubule transport, transepithelial HCO3− absorption was measured in rat MTALs isolated and perfused in vitro. Addition of LPS (500 ng/ml) to the bath decreased HCO3− absorption by 33%, from 16.7 ± 0.5 to 11.2 ± 0.5 pmol·min−1·mm−1 (Fig. 1A). Addition of LPS (250 ng/ml) to the tubule lumen decreased HCO3− absorption by 26%, from 16.9 ± 0.4 to 12.5 ± 0.4 pmol·min−1·mm−1 (Fig. 1B). Quantitatively similar inhibition was obtained in response to the addition of either E. coli O111:B4 or ultra-pure K12 LPS, indicating that the inhibitory effects are induced by LPS itself and not by contaminating products in the standard bacterial LPS preparation (11, 17, 36). Similar inhibition also was observed using purified R515 LPS (Alexis, data not shown). The inhibition by LPS is rapid (<15 min), sustained for up to 60 min, and reversible. These results demonstrate that LPS is able to modify the transport function of renal tubules directly from either the basolateral or luminal surface.
Lipid A inhibits HCO3− absorption from bath or lumen.
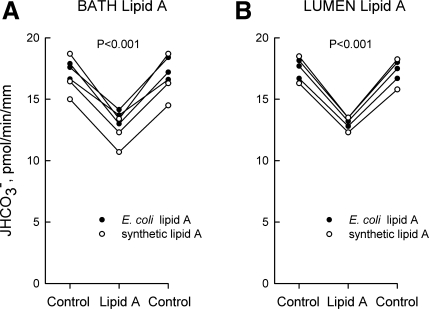
Lipid A is the bioactive component of LPS responsible for its immunoregulatory activity (2, 46). Addition of lipid A (500 ng/ml) to the bath decreased HCO3− absorption by 25%, from 17.0 ± 0.5 to 12.8 ± 0.5 pmol·min−1·mm−1 (Fig. 2A). Addition of lipid A (250 ng/ml) to the lumen decreased HCO3− absorption by 24%, from 17.3 ± 0.4 to 13.1 ± 0.2 pmol·min−1·mm−1 (Fig. 2B). The time course for inhibition by lipid A was similar to that of LPS. Similar inhibition was observed using lipid A from E. coli F583 or synthetic lipid A, indicating that the transport inhibition is triggered by lipid A itself and not by contaminants in the bacterial lipid A preparation (11, 17, 70). These results demonstrate that the inhibitory effects of LPS are reproduced by lipid A and support further the specificity of LPS-induced transport inhibition.
Fig. 2.
Lipid A inhibits HCO3− absorption from bath or lumen. MTALs from rats were perfused in vitro in control solution, and then lipid A was added to and removed from the bath (500 ng/ml; A) or lumen (250 ng/ml; B) solution. The tubules were exposed to either lipid A from E. coli F583 or synthetic lipid A. JHCO3−, data points, lines, and P values are as in Fig. 1. Mean values are given in results.
Serum enhances the sensitivity to LPS.
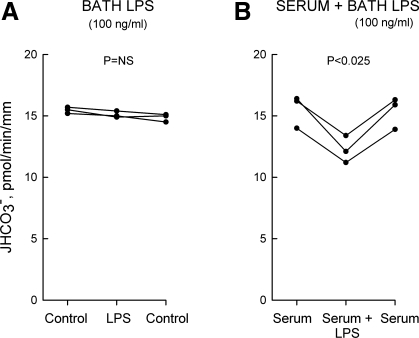
The sensitivity of target cells to LPS is enhanced by serum due to circulating proteins (LPS-binding proteins) that facilitate binding of LPS to its membrane receptors (24). To test this in the MTAL, tubules were studied with and without 10% heat-inactivated, low-endotoxin fetal bovine serum in the bath solution. In the absence of serum, adding 100 ng/ml LPS to the bath had no effect on HCO3− absorption (Fig. 3A). In tubules bathed with 10% serum, adding 100 ng/ml LPS to the bath decreased HCO3− absorption by 20%, from 15.4 ± 0.8 to 12.3 ± 0.6 pmol·min−1·mm−1 (Fig. 3B). The enhanced response in the presence of serum is consistent with interaction of LPS with the TLR4 receptor complex (24, 46; see below). All other experiments in this study were carried out in the absence of serum: 1) to study the effects of LPS on HCO3− absorption under the same conditions used previously for other regulatory factors (28–30, 76, 79); and 2) to avoid the potential confounding effects of serum on the activity of Na+/H+ exchangers and other transport proteins.
Fig. 3.
Serum enhances the sensitivity to LPS. MTALs from rats were studied in the absence (A) and presence (B) of 10% low-endotoxin fetal bovine serum in the bath solution. LPS (O111:B4, 100 ng/ml) was then added to and removed from the bath solution. JHCO3−, data points, lines, and P values are as in Fig. 1. NS, not significant. Mean values are given in results.
U0126 blocks inhibition by bath but not lumen LPS.
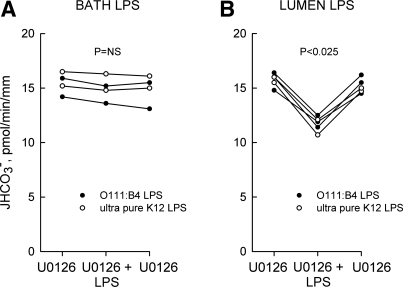
To determine whether bath and lumen LPS regulate MTAL transport through a common pathway, we examined the role of signaling pathways shown previously to inhibit HCO3− absorption in the MTAL. The MEK1/2 inhibitor U0126 selectively blocks ERK1/2 activation and ERK-mediated inhibition of Na+/H+ exchange and HCO3− absorption in the MTAL (32, 76, 79). U0126 (15 μM in the bath) completely eliminated inhibition of HCO3− absorption by bath LPS but had no effect on inhibition by lumen LPS (Fig. 4, A and B). These results support an essential role for the ERK pathway in mediating the basolateral LPS response.
Fig. 4.
U0126 blocks inhibition of HCO3− absorption by bath but not lumen LPS. MTALs from rats were bathed with 15 μM U0126, and then LPS was added to and removed from the bath (500 ng/ml; A) or lumen (250 ng/ml; B) solution. JHCO3−, data points, lines, and P values are as in Fig. 1.
Rapamycin blocks inhibition by lumen but not bath LPS.
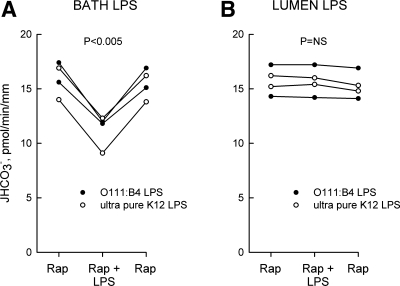
Inhibition of Na+/H+ exchange and HCO3− absorption via PI3K-mammalian target of rapamycin (mTOR) in the MTAL is blocked by the specific mTOR inhibitor rapamycin (32). In direct contrast to the preceding results with U0126, rapamycin (20 nM in the bath) had no effect on inhibition of HCO3− absorption by bath LPS but completely eliminated inhibition by lumen LPS (Fig. 5, A and B). These results suggest that PI3K-mTOR plays an important role in mediating the luminal response to LPS and, together with the experiments in Fig. 4, establish that basolateral and luminal LPS inhibit HCO3− absorption through different signal transduction pathways.
Fig. 5.
Rapamycin blocks inhibition of HCO3− absorption by lumen but not bath LPS. MTALs from rats were bathed with 20 nM rapamycin (Rap), and then LPS was added to and removed from the bath (500 ng/ml; A) or lumen (250 ng/ml; B) solution. JHCO3−, data points, lines, and P values are as in Fig. 1.
Bath amiloride blocks inhibition by lumen but not bath LPS.
In previous studies, we demonstrated that both the ERK and mTOR signaling pathways inhibit HCO3− absorption in the MTAL through inhibition of the basolateral NHE1 Na+/H+ exchanger (32, 33, 76). To test whether NHE1 may be involved in mediating inhibition by LPS, we examined the effects of LPS on HCO3− absorption in tubules bathed with 10 μM amiloride, which selectively prevents inhibition of HCO3− absorption mediated through NHE1 (31, 33, 77, 78). Bath amiloride had no effect on inhibition of HCO3− absorption by bath LPS but eliminated inhibition by lumen LPS (Fig. 6, A and B). These results indicate that bath and lumen LPS inhibit HCO3− absorption through different transport mechanisms and suggest that NHE1 plays a key role in mediating the inhibition by lumen LPS.
TLR4 is expressed in basolateral and apical membrane domains.
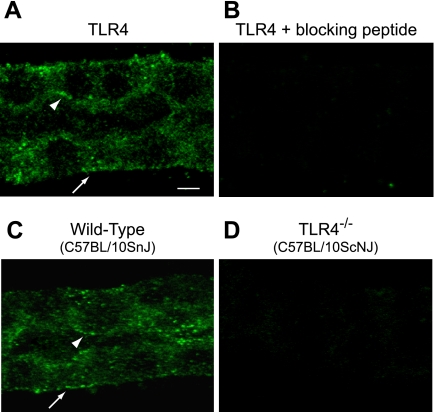
TLR4 is the signaling receptor for Gram-negative LPS in mammalian cells (1, 35, 54, 56). To determine its role in the MTAL, tubules microdissected from normal rats (Fig. 7, A and B) and from wild-type control (C57BL/10SnJ) and TLR4−/− (C57BL/10ScNJ) mice (54, 56) (Fig. 7, C and D) were stained with anti-TLR4 antibody and analyzed by confocal immunofluorescence. In both rat and wild-type mouse MTALs, staining for TLR4 was present in the basolateral and apical membrane domains as well as in the cytoplasm (Fig. 7, A and C). The TLR4 staining was absent in the presence of specific blocking peptide (Fig. 7B) and in MTALs from the TLR4−/− mice (Fig. 7D). TLR4 staining in basolateral and apical membrane domains was confirmed using a second anti-TLR4 antibody raised against a different epitope (Abcam; data not shown). These results are consistent with the view that TLR4 is the receptor responsible for mediating MTAL responses to basolateral and luminal LPS.
Fig. 7.
Toll-like receptor (TLR) 4 is expressed in basolateral and apical membrane domains of the MTAL. MTALs dissected from rats (A and B) and from wild-type (C) and TLR4−/− (D) mice were stained with anti-TLR4 antibody and analyzed by confocal immunofluorescence as described in methods. Images are Z-axis sections (0.4 μm) taken through a plane at the center of the tubule, which shows a cross-sectional view of cells in the lateral tubule walls (32, 78). TLR4 staining was seen in the basolateral (arrows) and apical (arrowheads) membrane domains of rat and wild-type mouse MTALs. The TLR4 staining was absent in the presence of blocking peptide and in MTALs from TLR4−/− mice. Images are representative of at least 5 tubules of each type. Scale bar = 5 μm.
Inhibition of HCO3− absorption by LPS is eliminated in MTALs from TLR4−/− mice.
To determine the functional significance of TLR4 for ion transport regulation, we examined the effects of LPS on HCO3− absorption in tubules from wild-type control and TLR4−/− mice. The results of these experiments are shown in Figs. 8 and 9. The basal (control) rate of HCO3− absorption did not differ in MTALs from wild-type and TLR4−/− mice. Similar to results obtained in the rat, addition of LPS to the bath or lumen decreased HCO3− absorption in MTALs from wild-type mice (Figs. 8A and 9A). In contrast, addition of LPS to the bath or lumen had no effect on HCO3− absorption in MTALs from TLR4−/− mice (Figs. 8B and 9B). These results support the conclusion that TLR4 is the signaling receptor that mediates inhibition of HCO3− absorption by both basolateral and luminal LPS.
DISCUSSION
Gram-negative bacterial infection of the kidney due to ascending infection or via the bloodstream is frequently associated with tubule dysfunction that includes reduced urinary concentrating ability, increased fractional excretion of sodium and glucose, and impaired urinary acidification with reduced excretion of ammonium (6, 8, 13, 14, 34, 53, 64–66, 73). The pathophysiological mechanisms responsible for these functional defects are not understood. Evaluation of the effects of endotoxin on the kidney in vivo is complicated by the presence of factors such as changes in local or circulating cytokine and hormone levels and changes in renal perfusion that may indirectly influence tubule function (14, 16, 34, 64–66). Increased cytokine levels have been implicated in several studies in mediating reduced expression of renal transport proteins following systemic endotoxin injection (34, 64, 65). In the present study, we demonstrate that LPS directly inhibits HCO3− absorption in the isolated, perfused MTAL from either the basolateral or luminal surface and that these effects are mediated through activation of TLR4. These results establish that bacterial molecules act directly to modify the transport function of renal tubules and demonstrate a direct role for TLRs in the regulation of renal epithelial ion transport. In addition, our study reveals a novel sidedness to TLR4 signaling, in which LPS induces different intracellular signaling pathways through activation of TLR4 in the basolateral and apical membranes.
The direct action of LPS to impair HCO3− absorption in the MTAL may contribute to the pathogenesis of Gram-negative infection on several levels. First, the relative alkalinization of the luminal fluid may influence a number of infective processes. Recent studies show that physiological increases in urine pH over the range 5.0–7.0 increase the transcription of fimbrae genes in E. coli (68). This effect is observed within 20 min and results in increased expression of type 1 pilli on the bacterial surface that are necessary for bacterial adherence. Consequently, the LPS-induced increase in lumen pH would promote attachment of bacteria to tubule cells that is critical for bacterial colonization and induction of the inflammatory response (15, 47, 68). LPS-induced alkalinization of the luminal fluid would promote other pathogenic processes, including bacterial cell growth (27, 37, 68), the formation of Gram-negative-induced phosphate and calcium stones (57), and resistance to certain antibiotics (87). Second, absorption of HCO3− by the MTAL depends on H+ secretion mediated by the apical membrane NHE3 Na+/H+ exchanger (4, 74, 75). Thus it is virtually certain that LPS decreases Na+/H+ exchange activity in the MTAL (see below). Inhibition of Na+/H+ exchange and the associated intracellular acidification impairs the induction of proinflammatory cytokines in several cell types, indicating that inflammatory processes may require a fully functional NHE (12, 23, 44, 48, 58). Inhibition of Na+/H+ exchange activity and H+ secretion by LPS could thus impair the renal tubule innate immune response. Third, endotoxemia and sepsis are associated clinically with systemic metabolic acidosis, which contributes to the pathogenesis of multiorgan dysfunction (8, 14, 38, 53, 66). The direct action of LPS to inhibit renal tubule H+ secretion would exacerbate and prevent the correction of sepsis-induced acidosis. LPS-induced impairment of luminal acidification also could contribute to the urine acidifying defect and failure to conserve HCO3− reported during urinary tract infections (13) and to reduced urinary ammonium excretion in endotoxemia (6). Thus our study identifies a novel, pathoadaptive mechanism through which Gram-negative bacteria can adversely affect the progression and severity of urinary tract infections and endotoxemia at the cellular, epithelial, and systemic levels.
TLR4, the signaling receptor for LPS in mammalian cells, mediates LPS-induced production of proinflammatory cytokines by renal epithelial cell lines (9, 11, 52, 60, 70) and plays a critical role in the renal defense against ascending bacterial infection (35, 52, 54, 56). In addition, there is rapidly growing evidence that TLR4 plays a key role in mediating inflammatory kidney injury in a variety of conditions, including sepsis (16, 19, 20), ischemia-reperfusion injury (39, 82, 83), diabetes (62, 72), and nephrotoxic injury (43, 86, 88). Our study provides the first evidence that TLR4 signaling is directly involved in mediating alterations in renal tubule transport. TLR4 is expressed constitutively throughout the nephron, including segments of the proximal tubule, thick ascending limb, and collecting duct (9, 19, 20, 39, 43, 60, 82, 83, 86, 88). TLR4 has been localized to the apical membrane of the rat cortical thick ascending limb (20), and TLR4 expression in this segment is increased in response to sepsis and ischemia-reperfusion injury (20, 39, 82). In the present study, we found that TLR4 is present in both the basolateral and apical membrane domains of rat and mouse MTAL. The inhibition of HCO3− absorption by basolateral or luminal LPS is eliminated in MTALs from TLR4−/− mice, indicating that TLR4 is the receptor responsible for mediating transport inhibition. These results establish that TLRs play a direct role in regulating ion transport in renal tubules and identify a previously unrecognized mechanism that can contribute to endotoxemia-induced defects in renal tubule function. In addition, our study provides the first direct evidence that renal tubules are able to recognize and respond to bacterial molecules through basolateral TLRs. Constitutive expression of basolateral TLR4 in the MTAL would enable this segment to monitor the composition of the interstitial fluid and initiate rapid innate immune defense mechanisms in response to the spread of bloodborne bacteria from the vascular compartment into the interstitial space (5, 14, 22, 61). In addition, endogenous TLR4 ligands such as heat shock proteins and the extracellular matrix components hyaluronin, fibronectin, and heparin sulfate are released into the interstitium by damaged cells during ischemic or toxin-induced kidney injury (5, 19, 67, 69, 83, 88). Interaction of these ligands with basolateral TLR4 in the MTAL would promote inflammatory responses that lead to tubule dysfunction and renal damage in these noninfectious conditions. Recent studies show that TLR2, which recognizes Gram-positive bacterial molecules, also is constitutively expressed at the basolateral surface of renal tubule cells in the outer medulla of mouse and human kidney (69). TLR2, in addition to TLR4, has been implicated in mediating ischemic renal injury (39, 41, 69, 82). Whether TLR2 plays a role in modulating renal tubule transport remains to be determined.
A surprising finding in this study is that LPS modifies MTAL function through the activation of distinct TLR4-dependent signaling pathways at the basolateral and apical membranes. The results of experiments using highly selective inhibitors suggest that basolateral LPS inhibits HCO3− absorption through activation of the ERK pathway, whereas lumen LPS inhibits HCO3− absorption through activation of the PI3K-mTOR pathway. Both pathways have been shown previously to inhibit HCO3− absorption in the MTAL (32, 76, 79), and there is precedent for activation of these pathways by LPS in other systems. ERK is activated by LPS in inflammatory cells (1, 7, 18, 50, 59) and epithelial cell lines (9, 70) and plays a role in activating transcription factors that promote the expression of proinflammatory cytokines (1, 7, 9, 18, 59). The PI3K-mTOR pathway is activated by LPS in classic immune cells, where it is involved in controlling cell migration and survival and serves as a negative feedback system to prevent excessive and prolonged activation of innate immune responses (26, 42, 50, 80, 81). Our studies provide the first evidence that activation of mTOR is a component of TLR4-induced signaling in renal cells. Of potential clinical significance, we found that the signaling response of the MTAL to lumen LPS is blocked by the immunosuppressive drug rapamycin. This provides new insight into a mechanism that may contribute to the increased sensitivity of transplanted kidneys to ascending bacterial infection (45). Further detailed studies to examine the effects of LPS on the biochemical activities of proteins in the ERK and PI3K-mTOR pathways are required to confirm directly the importance of these pathways in the MTAL innate immune response. Our results suggest strongly that the ERK and PI3K-mTOR pathways play major roles in coupling innate immune receptors to alterations in renal tubule transport.
Our results demonstrate further that the basolateral and apical LPS signaling pathways inhibit HCO3− absorption through effects on different ion transport proteins. Previously, we demonstrated that both the ERK and PI3K-mTOR pathways decrease HCO3− absorption in the MTAL through inhibition of the basolateral NHE1 Na+/H+ exchanger, which results secondarily in inhibition of apical NHE3 and luminal H+ secretion (32, 33, 76). In the present study, we found that inhibiting NHE1 with bath amiloride (31, 33, 77, 78) eliminates the inhibition of HCO3− absorption by lumen LPS but does not prevent inhibition by bath LPS. These results suggest that NHE1 plays a critical role in mediating mTOR-dependent inhibition of HCO3− absorption by lumen LPS but is not involved in mediating ERK-dependent inhibition by basolateral LPS. A potential alternative mechanism for the inhibition by bath LPS is direct coupling of the ERK pathway to inhibition of NHE3, as observed previously with aldosterone (79). These findings raise the possibility that the different TLR4 pathways activated by LPS at the basolateral and apical membranes are targeted to inhibit different Na+/H+ exchanger isoforms. Further studies examining NHE1 and NHE3 activities are needed to establish directly that these exchangers are targets for innate immune regulation through TLR4 in the kidney and to define the roles of these exchangers in mediating LPS-induced inhibition of HCO3− absorption. Effects of LPS on additional transport proteins involved in HCO3− absorption, including basolateral HCO3− efflux pathways, also remain to be investigated.
In a recent study using a renal tubule epithelial cell line, stimulation of the cells at either the basolateral or apical surface with LPS resulted in increased production of inflammatory cytokines (11). As presented above, our study reveals a previously unrecognized complexity in TLR4 signaling in the MTAL in which stimulation of basolateral or apical receptors with LPS leads to the production of different intracellular signals. It is not known whether the sidedness of TLR4 signaling we observed is applicable to other epithelial cells or represents a selective property of the MTAL. A key question is what the molecular mechanisms are that underlie the TLR4 signal specificity. Possible contributing factors could include the preferential recruitment of different adaptor proteins to TLR4 signaling complexes in the basolateral and apical membranes or the direct or indirect interaction of TLR4 with other membrane-specific cell-surface receptors (1, 2, 49, 51). It will also be important to define the significance of the sidedness of TLR4 signaling for renal inflammation and disease. Our results show that the basolateral and apical TLR4 pathways are coupled to the modulation of different transport proteins in the MTAL. Selective inhibition of NHE1 through apical TLR4 signaling could interfere with a variety of cell functions in addition to HCO3− absorption that depend on NHE1, including cell pH and cell volume regulation and cell survival (55). It is also possible that the basolateral and apical pathways are coupled to the production of different proinflammatory mediators in a way that enhances the efficiency of the renal innate immune response. For example, activation of ERK through basolateral TLR4 could lead to increased production of chemokines, consistent with the ERK-dependent production of MIP-2 induced through TLR4 in cultured collecting duct cells (9). In this way, infiltration of bacteria from the vasculature to the interstitial fluid could rapidly increase the production of chemotactic molecules through activation of basolateral TLR4 in the MTAL, resulting in recruitment of neutrophils from the vascular space to the interstitium for antibacterial defense. Conversely, activation of PI3K-mTOR through apical TLR4 could promote epithelial cell survival or prevent excessive inflammation in response to ascending bacterial infection. The coupling of basolateral and apical TLR4 pathways to the production of specific proinflammatory molecules, along with an ability of tubule cells to preferentially secrete cytokines across specific membrane domains (11), would provide a highly efficient system for selective targeting of innate immune responses by renal epithelial cells during kidney infection. Last, it will be essential to determine whether disease states in which TLR4 plays a role in kidney injury (sepsis, ischemia-reperfusion, diabetes, nephrotoxic drugs) may differentially modulate the basolateral and apical TLR4 pathways, and how this may contribute to inflammatory responses and dysregulation of tubule transport in these disorders.
In summary, we demonstrate that LPS inhibits HCO3− absorption directly in the MTAL from either the basolateral or luminal surface. The inhibition of lumen acidification by LPS could contribute to bacterial pathogenicity through a variety of mechanisms, including promoting bacterial adherence and growth and impairing the correction of systemic metabolic acidosis in sepsis. The inhibition by LPS is mediated through distinct TLR4-dependent signaling pathways in the basolateral and apical membranes. These results provide the first evidence that bacterial molecules can directly modify the transport function of renal tubules through TLRs, thereby identifying a new pathophysiological mechanism contributing to renal tubule dysfunction during bacterial infection. Our results reveal a novel complexity of TLR4 signaling in MTAL cells, whereby activation of TLR4 in the basolateral and apical membranes by LPS leads to the induction of different intracellular signaling pathways. Further studies aimed at understanding the mechanisms and functional significance of this differential TLR4 signaling may lead to new therapeutic strategies that target specific TLR4-induced pathways to enhance host defense or prevent maladaptive inflammatory responses that lead to kidney injury in a variety of infectious and noninfectious conditions.
GRANTS
This work was supported by American Heart Association, South Central Affiliate, Grant-in-Aid 0855057F and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-038217.
REFERENCES
- 1. Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol 4: 499–511, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Alpern RJ. Renal acidification mechanisms. In: The Kidney, edited by Brenner BM, Rector FC., Jr Philadelphia, PA: Saunders, 2000, vol. I, p. 455–519 [Google Scholar]
- 4. Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int 48: 1206–1215, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Anders HJ, Banas B, Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 15: 854–867, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Austgen TR, Chen MK, Moore W, Souba WW. Endotoxin and renal glutamine metabolism. Arch Surg 126: 23–27, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Nat Acad Sci USA 103: 3274–3279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baumgart K, Radermacher P, Calzia E, Hauser B. Pathophysiology of tissue acidosis in septic shock: blocked microcirculation or impaired cellular respiration. Crit Care Med 36: 640–642, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Chassin C, Goujon JM, Darche S, duMerle L, Bens M, Cluzeaud F, Werts C, Ogier-Denis E, LeBouguenec C, Buzoni-Gatel D, Vandewalle A. Renal collecting duct epithelial cells react to pyelonephritis-associated Escherichia coli by activating distinct TLR4-dependent and -independent inflammatory pathways. J Immunol 177: 4773–4784, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Chowdhury P, Sacks SH, Sheerin NS. Minireview: functions of the renal tract epithelium in coordinating the innate immune response to infection. Kidney Int 66: 1334–1344, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chowdhury P, Sacks SH, Sheerin NS. Toll-like receptors TLR2 and TLR4 initiate the innate immune response of the renal tubular epithelium to bacterial products. Clin Exp Immunol 145: 346–356, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coakley RJ, Taggart C, Greene C, McElvaney NG, O'Neill SJ. Ambient Pco2 modulates intracellular pH, intracellular oxidant generation and interleukin-8 secretion in human neutrophils. J Leukocyte Biol 74: 603–610, 2002 [PubMed] [Google Scholar]
- 13. Cochran M, Peacock M, Smith DA, Nordin BEC. Renal tubular acidosis of pyelonephritis with renal stone disease. Br Med J 2: 721–729, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Connell H, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA 93: 9827–9832, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cunningham PN, Wang Y, Guo R, He G, Quigg RJ. Role of Toll-like receptor 4 in endotoxin-induced acute renal failure. J Immunol 172: 2629–2635, 2004 [DOI] [PubMed] [Google Scholar]
- 17. David MD, Cochrane CL, Duncan SK, Schrader JW. Pure lipopolysaccharide or synthetic lipid A induces activation of p21Ras in primary macrophages through a pathway dependent on Src family kinases and PI3K. J Immunol 175: 8236–8241, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-α induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083, 2000 [DOI] [PubMed] [Google Scholar]
- 19. El-Achkar TM, Dagher PC. Renal Toll-like receptors: recent advances and implications for disease. Nat Clin Pract Nephrol 2: 568–581, 2006 [DOI] [PubMed] [Google Scholar]
- 20. El-Achkar TM, Huang X, Plotkin Z, Sandoval RM, Rhodes G, Dagher PC. Sepsis induces changes in the expression and distribution of Toll-like receptor 4 in the rat kidney. Am J Physiol Renal Physiol 290: F1034–F1043, 2006 [DOI] [PubMed] [Google Scholar]
- 21. El-Achkar TM, Plotkin Z, Marcic B, Dagher PC. Sepsis induces an increase in thick ascending limb Cox-2 that is TLR4 dependent. Am J Physiol Renal Physiol 293: F1187–F1196, 2007 [DOI] [PubMed] [Google Scholar]
- 22. El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol 295: F534–F544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer B, Muller B, Fischer KG, Baur N, Kreutz W. Acidic pH inhibits non-MHC-restricted killer cell functions. Clin Immunol 96: 252–263, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD-2 complex. Microbes Infect 6: 1361–1367, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Foxman B, Barlow R, D'Arcy H, Gillespi B, Sobel JD. Urinary tract infection. Self-reported incidence and associated costs. Ann Epidemiol 10: 509–515, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol 24: 358–363, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Geerlings SE, Brouwer EC, Gaastra W, Verhoef J, Hoepelman AI. Effect of glucose and pH on uropathogenic and non-uropathogenic Escherichia coli: studies with urine from diabetic and non-diabetic individuals. J Med Microbiol 48: 535–539, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Good DW. Inhibition of bicarbonate absorption by peptide hormones and cyclic adenosine monophosphate in rat medullary thick ascending limb. J Clin Invest 85: 1006–1013, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Good DW. The thick ascending limb as a site of renal bicarbonate reabsorption. Semin Nephrol 13: 225–235, 1993 [PubMed] [Google Scholar]
- 30. Good DW, George T, Wang DW. Angiotensin II inhibits HCO3− absorption via a cytochrome P-450-dependent signaling pathway in rat medullary thick ascending limb. Am J Physiol Renal Physiol 276: F726–F736, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Good DW, George T, Watts BA., III Basolateral membrane Na+/H+ exchange enhances HCO3− absorption in rat medullary thick ascending limb: evidence for functional coupling between basolateral and apical membrane Na+/H+ exchangers. Proc Nat Acad Sci USA 92: 12525–12529, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Good DW, George T, Watts BA., III Nerve growth factor inhibits Na+/H+ exchange and HCO3− absorption through parallel phosphatidylinositol 3-kinase-mTOR and ERK pathways in thick ascending limb. J Biol Chem 283: 26602–26611, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Good DW, Watts BA, III, George T, Meyer J, Shull GE. Transepithelial HCO3− absorption is defective in renal thick ascending limbs from NHE1 Na+/H+ exchanger null mutant mice. Am J Physiol Renal Physiol 287: F1244–F1249, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Grinevich V, Knepper M, Verbalis J, Reyes I, Aguilera G. Acute endotoxemia in rats induces down-regulation of V2 vasopressin receptors and aquaporin-2 content in the kidney medulla. Kidney Int 65: 54–62, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Hagberg L, Hull R, Hull S, McGhee JR, Michalek SM, Eden CS. Difference in susceptibility to Gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun 46: 839–844, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J Immunol 165: 618–622, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Kaye D. Antibacterial activity of human urine. J Clin Invest 47: 2374–2390, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kidani Y, Tanijuchi T, Kanakura H, Takemoto Y, Tsuda K, Yamamoto K. Sevoflurane pretreatment inhibits endotoxin-induced shock in rats. Anesth Analg 101: 1152–1156, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Knepper MA, Gamba G. Urine concentration and dilution. In The Kidney, edited by Brenner BM. Philadelphia, PA: Saunders, 2004, vol. 1, p. 599–636 [Google Scholar]
- 41. Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJD, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest 115: 2894–2903, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li X, Tupper JC, Bannerman DD, Winn RK, Rhodes CJ, Harlan JM. Phosphoinositide 3 kinase mediates Toll-like receptor 4-induced activation of NF-κB in endothelial cells. Infect Immun 71: 4414–4420, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lim SW, Li C, Ahn KO, Moon IS, Ahn C, Lee JR, Yang CW. Cyclosporine-induced renal injury induces Toll-like receptor and maturation of dendritic cells. Transplantation 80: 691–699, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Mastronarde JG, Monick MM, Gross TJ, Hunninghake GW. Amiloride inhibits cytokine production in epithelium infected with respiratory syncytial virus. Am J Physiol Lung Cell Mol Physiol 271: L201–L207, 1996 [DOI] [PubMed] [Google Scholar]
- 45. Memikoglu KO, Kevin K, Sengul S, Soypacaci Z, Erturk S, Erbay B. Urinary tract infections following renal transplantation: A single-center experience. Transplant Proc 39: 3131–3134, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Miller S, Ernst R, Bader M. LPS, TLR4 and infectious disease diversity. Nature 3: 36–46, 2005 [DOI] [PubMed] [Google Scholar]
- 47. Mulvey MA, Lopez-Boado YS, Wilson CL, Roth R, Parks WC, Heuser J, Hultgren SJ. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282: 1494–1497, 1998 [DOI] [PubMed] [Google Scholar]
- 48. Nemeth ZH, Deitch EA, Lu Q, Szabo C, Hasko G. NHE blockade inhibits chemokine production and NF-kappa B activation in immunostimulated endothelial cells. Am J Physiol Cell Physiol 283: C396–C403, 2002 [DOI] [PubMed] [Google Scholar]
- 49. O'Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adapters in Toll-like receptor signaling. Nat Rev Immunol 7: 353–364, 2007 [DOI] [PubMed] [Google Scholar]
- 50. Ojaniemi M, Glumoff V, Harju K, Lijeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol 33: 597–605, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Nat Acad Sci USA 97: 13766–13771, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Patole PS, Schubert S, Hildinger K, Khandoga S, Khandoga A, Segerer S, Henger A, Kretzler M, Werner M, Krombach F, Schlondorff D, Anders HJ. Toll-like receptor-4: Renal cells and bone marrow cells signal for neutrophil recruitment during pyelonephritis. Kidney Int 68: 2582–2587, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest 94: 663–671, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Ann Rev Pharmacol Toxicol 42: 527–552, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4). J Exp Med 189: 615–625, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rahman N, Meng M, Stoller M. Infections and urinary stone disease. Curr Pharm Des 9: 975–981, 2003 [DOI] [PubMed] [Google Scholar]
- 58. Rolfe MW, Kunkel SL, Rowens B, Standiford TJ, Cragoe EJ, Jr, Strieter RM. Suppression of human alveolar macrophage-derived cytokines by amiloride. Am J Respir Cell Mol Biol 6: 576–582, 1992 [DOI] [PubMed] [Google Scholar]
- 59. Saemann MD, Weichart T, Zeyda M, Staffler G, Schunn M, Stuhlmeier KM, Sobanov Y, Stulnig TM, Akira S, von Gabain A, von Ahse U, Horl WH, Zlabinger GJ. Tamm-Horsfall glycoprotein links innate immune cell activation with adaptive immunity via a Toll-like receptor-4 dependent mechanism. J Clin Invest 115: 468–475, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Samuelson P, Hang L, Wult B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun 72: 3179–3186, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sanford JP, Hunter BW, Donaldson P. Localization and fate of Escherichia coli in hematogenous pyelonephritis. J Exp Med 116: 285–294, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sataranatarajan K, Feliers D, Mariappan MM, Lee MJ, Jimenez F, Chen Y, Gosh-Choudhury G, Ahuja S, Kasinath BS. Toll-like receptor 4 knockout mice are resistant to diabetic kidney injury (Abstract). J Am Soc Nephrol 19: 107A, 2008 [Google Scholar]
- 63. Sato S, Nomura F, Kawai T, Takeuchi O, Muhlradt PF, Takeda K, Akira S. Synergy and cross-tolerance between toll-like receptor (TLR)2- and TLR4-mediated signaling pathways. J Immunol 165: 7096–7101, 2000 [DOI] [PubMed] [Google Scholar]
- 64. Schmidt C, Hocherl K, Bucher M. Regulation of renal glucose transporters during severe inflammation. Am J Physiol Renal Physiol 292: F804–F811, 2007 [DOI] [PubMed] [Google Scholar]
- 65. Schmidt C, Hocherl K, Schweda F, Kurtz A, Bucher M. Regulation of renal sodium transporters during severe inflammation. J Am Soc Nephrol 18: 1072–1083, 2007 [DOI] [PubMed] [Google Scholar]
- 66. Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 67. Schroppel B, He JC. Expression of Toll-like receptors in the kidney: their potential role beyond infection. Kidney Int 69: 785–787, 2006 [DOI] [PubMed] [Google Scholar]
- 68. Schwan W, Lee J, Lenard F, Mathews B, Beck M. Osmolarity and pH growth conditions regulate fim gene transcription and Type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun 70: 1391–1402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shigeoka AA, Holscher TD, King AJ, Hall FW, Kiosses WK, Tobias PS, Mackman N, McKay DB. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J Immunol 178: 6252–6258, 2007 [DOI] [PubMed] [Google Scholar]
- 70. Tsuboi N, Yoshikai Y, Matsuo S, Kikuchi T, Iwami KI, Nagai Y, Takeuchi O, Akira S, Matsuguchi T. Roles of Toll-like receptors in C-C chemokine production by renal tubular epithelial cells. J Immunol 169: 2026–2033, 2002 [DOI] [PubMed] [Google Scholar]
- 71. Visintin A, Halmen K, Khan N, Monks B, Golenbock D, Lien E. MD-2 expression is not required for cell surface targeting of Toll-like receptor 4 (TLR4). J Leukoc Biol 80: 1584–1592, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Wang JJ, Zhang SX, Mott R, Chen Y, Knapp RR, Cao W, Ma JX. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol 294: F1166–F1173, 2008 [DOI] [PubMed] [Google Scholar]
- 73. Wang W, Li C, Summer SN, Falk S, Wang W, Ljubanovic D, Schrier RJ. Role of AQP1 in endotoxemia-induced acute kidney injury. Am J Physiol Renal Physiol 294: F1473–F1480, 2008 [DOI] [PubMed] [Google Scholar]
- 74. Watts BA, III, Good DW. Apical membrane Na+/H+ exchange in rat medullary thick ascending limb: pHi-dependence and inhibition by hyperosmolality. J Biol Chem 269: 20250–20255, 1994 [PubMed] [Google Scholar]
- 75. Watts BA, III, Good DW. Hyposmolality stimulates apical membrane Na+/H+ exchange and HCO3− absorption in renal thick ascending limb. J Clin Invest 104: 1593–1602, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Watts BA, III, Good DW. Extracellular signal-regulated kinase mediates inhibition of Na+/H+ exchange and HCO3− absorption by nerve growth factor in MTAL. Am J Physiol Renal Physiol 282: F1056–F1063, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Watts BA, III, George T, Good DW. Nerve growth factor inhibits HCO3− absorption in renal thick ascending limb through inhibition of basolateral membrane Na+/H+ exchange. J Biol Chem 274: 7841–7847, 1999 [DOI] [PubMed] [Google Scholar]
- 78. Watts BA, III, George T, Good DW. The basolateral NHE1 Na+/H+ exchanger regulates transepithelial HCO3− absorption through actin cytoskeleton remodeling in renal thick ascending limb. J Biol Chem 280: 11439–11447, 2005 [DOI] [PubMed] [Google Scholar]
- 79. Watts BA, III, George T, Good DW. Aldosterone inhibits apical NHE3 and HCO3− absorption via a nongenomic, ERK-dependent pathway in medullary thick ascending limb. Am J Physiol Renal Physiol 291: F1005–F1013, 2006 [DOI] [PubMed] [Google Scholar]
- 80. Weinstein SL, Finn AJ, Dave SH, Meng F, Lowell CA, Sanghera JS, DeFranco AL. Phosphatidylinositol 3-kinase and mTOR mediate lipopolysaccharide-stimulated nitric oxide production in macrophages via interferon-β. J Leukoc Biol 67: 405–414, 2000 [DOI] [PubMed] [Google Scholar]
- 81. Williams DL, Ozment-Skelton T, Li C. Modulation of the phosphoinositide 3-kinase signaling pathway alters host response to sepsis, inflammation, and ischemia/reperfusion injury. Shock 25: 432–439, 2006 [DOI] [PubMed] [Google Scholar]
- 82. Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, van't Veer C. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol 168: 1286–1293, 2002 [DOI] [PubMed] [Google Scholar]
- 83. Wu H, Chen G, Wybern KR, Yin J, Bertolio P, Eris JM, Alexander SI, Sharland AF, Chadban SJ. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Yang CW, Wu MS, Pan MJ, Hong JJ, Yu CC, Vandewalle A, Huang CC. Leptospira outer membrane protein activates NF-κB and downstream genes expressed in medullary thick ascending limb cells. J Am Soc Nephrol 11: 2017–2026, 2000 [DOI] [PubMed] [Google Scholar]
- 85. Zager R, Johnson A, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006 [DOI] [PubMed] [Google Scholar]
- 86. Zager RA, Johnson ACM, Lund S, Randolph-Habecker J. Toll-like receptor (TLR4) shedding and depletion: acute proximal tubular cell responses to hypoxic and toxic injury. Am J Physiol Renal Physiol 292: F304–F312, 2007 [DOI] [PubMed] [Google Scholar]
- 87. Zhanel GG, Karlowsky JA, Davidson RJ, Hoban DJ. Influence of human urine on the in vitro activity and postantibiotic effect of ciprofloxacin against Escherichia coli. Chemotherapy 37: 218–223, 1991 [DOI] [PubMed] [Google Scholar]
- 88. Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]