Abstract
Liver ischemia-reperfusion injury (IRI) causes acute kidney injury (AKI) in mice characterized by renal endothelial cell apoptosis, renal tubular necrosis, inflammation, and filamentous (F)-actin disruption. Since heat shock protein 27 (HSP27) protects against apoptosis, necrosis, and stabilizes F-actin, we questioned whether overexpression of human HSP27 (huHSP27 OE) in mice would attenuate AKI after liver IRI. Twenty-four hours after hepatic IRI, HSP27 wild-type (WT) mice developed acute liver and kidney injury with elevated plasma alanine aminotransferase and creatinine, a reduced glomerular filtration rate, and histological evidence of renal endothelial cell apoptosis and tubular injury (necrosis, vacuolization, and F-actin disruption). The huHSP27 OE mice, however, were significantly protected against both liver and kidney injury after hepatic IRI. The huHSP27 OE mice also showed less induction of several proinflammatory mRNAs (TNF-α, MIP-2, and keratinocyte-derived cytokine), neutrophil infiltration, and reduction in apoptosis (terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling assay and DNA laddering) in the kidney compared with the HSP27 WT mice. Moreover, the huHSP27 OE mice showed significantly less disruption of F-actin in renal proximal tubules and better preserved vascular endothelial cell integrity compared with the huHSP27 OE mice. Finally, the kidney plays a major role in the hepatoprotective effects of huHSP27 overexpression as the hepatoprotection was reduced or abolished in mice subjected to unilateral or bilateral nephrectomy, respectively. Our results show that overexpression of huHSP27 protects against hepatic injury and AKI associated with liver IRI in vivo. Harnessing the mechanisms of cytoprotection with renal HSP27 may lead to new therapies for the perioperative AKI and liver injury associated with liver IRI.
Keywords: acute renal failure, apoptosis, endothelial cell, inflammation, necrosis
acute kidney injury (AKI) is one of the most challenging but unresolved clinical problems in medicine. In particular, AKI is extremely frequent in patients with either acute and chronic liver failure, and the mortality rate in patients with combined kidney and liver dysfunction is extremely high (11). Hepatic ischemia-reperfusion injury (IRI) is a major cause of liver failure and is frequently associated with major hepatic resection, liver transplantation, or septic shock (12). In addition, AKI is a common complication before or after orthotropic liver transplantation and exponentially increases patient mortality (11). Furthermore, even mild AKI can lead to drastic increases in mortality and morbidity in the intensive care unit, implying that kidneys can modulate and/or amplify extrarenal organ dysfunction (5).
We previously demonstrated that mice subjected to severe hepatic IRI develop AKI in <24 h characterized by early renal peritubular capillary endothelial cell apoptosis and rapid disruptions of the renal proximal tubule filamentous (F)-actin cytoskeletal architecture with subsequent proximal tubular necrosis and inflammation (24). Moreover, profound cortical vacuolization, tubular simplification, peritubular/interstitial neutrophil infiltration as well as significant juxtaglomerular hyperplasia were observed in mice that developed AKI after liver IRI (24). Therefore, we hypothesized that blocking renal endothelial cell apoptosis, tubular necrosis, and preserving the F-actin cytoskeleton would provide protection against AKI incurred after liver IRI.
Heat shock protein 27 (HSP27) is a member of a family of chaperone proteins that are upregulated in response to increases in temperature, as well as a wide range of cellular stresses including hypoxia, ischemia, and exposure to toxic drugs (2, 3, 6, 14). Furthermore, HSP27 is a potent antiapoptotic protein, reduces necrosis in many cell types, and is a key stabilizer of the F-actin cytoskeleton (18, 23, 34); all of these cellular effects could potentially lead to protection against AKI induced with liver IRI (29, 32, 36). Therefore, we utilized a murine model of liver IRI-induced AKI and tested the hypothesis that mice with global overexpression of human HSP27 (huHSP27) would show an increased resistance to AKI induced by liver IRI. We also tested whether huHSP27 overexpression in the kidney with subsequent reduction in AKI is at least partially responsible for reduced hepatic injury after liver IRI in huHSP27-overexpressing (OE) mice. We show that overexpression of huHSP27 in mice reduces renal injury, decreases renal inflammation and apoptosis, and preserves F-actin and vascular permeability compared with HSP27 wild-type (WT) mice after liver IRI. Moreover, removing kidneys from huHSP27 OE mice abolishes the hepatoprotective effects of huHSP27 overexpression.
MATERIALS AND METHODS
Mice.
Heterozygous breeder pairs of mice overexpressing human HSP27 were generously provided by Dr. Jacqueline de Belleroche (Dept. of Neuromuscular Diseases, Faculty of Medicine, Imperial College, London, UK). The generation and initial characterization of the huHSP27 OE and WT mice with a C57BL/10 and CBA/Ca background have been described previously (1). The huHSP27 OE transgenic mice overexpress human HSP27 tagged with hemaglutinin to track the expression of the transgene. The huHSP27 OE transgenic mice show widespread and robust expression of huHSP27 protein in the brain, kidney, liver, heart, spinal cord, muscle, lung, and pancreas (1). Because of the concerns for the potential genetic variability associated with a noncongenic strain of mice, we bred our heterozygous huHSP27 OE mice with C57BL/6 (Harlan Laboratories, Indianapolis, IN) mice for four generations. We performed PCR on genomic DNA extracted from tails and performed RT-PCR for human HSP27 from total RNA extracted from every mouse studied using primers that distinguish human from mouse HSP27. In preliminary studies, we also confirmed human HSP27 protein overexpression by immunoblotting for the mouse and human forms of HSP27 (Santa Cruz Biotechnology, Santa Cruz, CA) as described previously (7).
Murine model of hepatic IRI.
After Columbia University Institutional Animal Care and Use Committee approval, male huHSP27 OE or HSP27 WT mice (25–30 g) were subjected to partial liver IRI as described previously (24). In brief, left lateral and median lobes of the liver in anesthetized mice were subjected to varying period of ischemia (45, 60, or 75 min to induce mild, moderate, or severe hepatic IRI) with a microaneurysm clip occluding the hepatic triad above the bifurcation at 37°C. This method of partial, graded hepatic ischemia results in a segmental (∼70%) hepatic ischemia but spares the right lobe of the liver and prevents mesenteric venous congestion by allowing portal decompression throughout the right and caudate lobes of the liver. After hepatic ischemia, the liver was reperfused, and the wound was closed. Sham-operated mice were subjected to a laparotomy and identical liver manipulations without vascular occlusion. In some mice, we performed a unilateral or bilateral nephrectomy during liver ischemia (45 min.) to determine whether the kidneys participate in modulating liver IRI injury and whether renal huHSP27 plays a role in both hepatic and renal protection after liver IRI.
Assessment of hepatic and renal dysfunction after hepatic IRI.
Plasma alanine aminotransferase (ALT) activity 4 and 24 h after liver IRI was measured using the Infinity ALT assay kit according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham, MA). Renal function was assessed 1) by measuring plasma creatinine 4 and 24 h after liver IRI by a colorimetric method based on the Jaffé reaction (33) and 2) by estimating the glomerular filtration rate 24 h after liver IRI. The glomerular filtration rate was estimated with the FITC-inulin clearance technique as described by Qi et al. (30).
Histology and quantification of renal injury after hepatic IRI.
Twenty-four hours after reperfusion, both kidneys were collected and fixed in a 10% formalin solution overnight. After automated dehydration through a graded alcohol series, transverse liver slices were embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin-eosin (H&E). Renal H&E sections were evaluated for percent renal cortical vacuolization, peritubular/proximal tubule leukocyte infiltration (score: 0–3+), and the degree of proximal tubule hypereosinophilia (indicating proximal tubular necrosis, score: 0–3+) by an experienced renal pathologist (V. D. D'Agati) who was blinded to the treatment each animal had received.
Assessment of renal inflammation after hepatic IRI.
Kidney inflammation after hepatic ischemia was determined with detection of neutrophil infiltration by immunohistochemistry 24 h after hepatic IRI and by measuring mRNA-encoding markers of inflammation in the kidney isolated 4 and 24 h after liver IRI, including keratinocyte-derived cytokine (KC), ICAM-1, monocyte chemoattractive protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2), and TNF-α 4 and 24 h after liver IRI as described previously (24).
Detection of renal apoptosis after hepatic IRI.
We utilized two independent assays to assess the degree of renal apoptosis after IR: with in situ terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) assay and detection of DNA laddering. For DNA laddering, kidneys were removed 24 h after liver IR and apoptotic DNA fragments were extracted according to the methods of Herrmann et al. (16) and electrophoresed at 70 V in a 2.0% agarose gel in Tris-acetate-EDTA buffer. This method of DNA extraction selectively isolates apoptotic, fragmented DNA and leaves behind the intact chromatin. The gel was stained with ethidium bromide and photographed under UV illumination. DNA ladder markers (100 bp) were added to a lane of each gel as a reference for the analysis of internucleosomal DNA fragmentation. For the TUNEL assay, fixed kidney sections obtained at 24 h after hepatic IRI were deparaffinized in xylene and rehydrated through graded ethanols to water. In situ TUNEL staining was used for detecting DNA fragmentation in apoptosis using a commercially available in situ cell death detection kit (Roche, Nutley, NJ) according to the manufacturer's instructions.
F-actin staining of kidney sections after hepatic IRI.
As breakdown of renal tubule F-actin contributes to the development of AKI, we visualized the F-actin cytoskeleton by staining with phalloidin as an early index of renal injury (24). Twenty-four hours after liver IRI, kidney tissues were embedded in Tissue-Tek oxytetracycline compound (Fisher Scientific, Pittsburgh, PA) and cut into 5-μm sections. To reduce background staining, the sections were incubated in 1% FBS dissolved in PBS for 10 min at room temperature. The sections were then stained with 1:40 dilution of Alexa Fluor (Red)-conjugated Phalloidin in PBS from ∼6.6 μM (200 U/ml) stock solution (Invitrogen, Carlsbad, CA) for 30 min at 37°C in a humidified chamber in the dark. Sections were then washed twice in PBS and mounted with Vectashield (Vector Laboratories, Burlingame, CA). F-actin images were visualized with an Olympus IX81 epifluorescence microscope (Tokyo, Japan) and captured and stored using SlideBook 4.2 software (Intelligent Imaging Innovations, Denver, CO) on a personal computer. The mean fluorescent intensity after background correction was calculated from five random blinded sections with identical surface areas per slide to quantify F-actin degradation after liver IR. To minimize the variations in fluorescent intensity, slides from sham-operated animals and animals subjected to liver IRI were processed together.
Analysis of kidney vascular permeability after hepatic IRI.
Changes in kidney vascular permeability were assessed by quantitating extravasations of Evans blue dye (EBD) into the tissue as described (24). Briefly, 2% EBD (Sigma Biosciences, St. Louis, MO) was administered at a dose of 20 mg/kg (iv) 24 h after liver IRI. One hour later, mice were killed and perfused through the heart with PBS and EDTA with 10 ml cold saline with heparin (100 U/ml). Both kidneys were then removed, allowed to dry overnight at 60°C, and the dry weights were determined. EBD was extracted in formamide (20 ml/g dry tissue; Sigma Biosciences), homogenized, and incubated at 60°C overnight. Homogenized samples were centrifuged at 5,000 g for 30 min, and the supernatants were measured at 620 and 740 nm in a spectrophotometer. The extravasated EBD concentration was calculated against a standard curve, and the data are expressed as micrograms of EBD per gram of dry tissue weight.
Protein determination.
Protein contents were determined with a bicinchoninic acid protein assay kit (Pierce Chemical, Rockford, IL), using BSA as a standard.
Reagents.
Unless otherwise specified, all reagents were purchased from Sigma.
Statistical analyses.
All data are reported as means ± SE. The overall significance of the results was examined using one-way analysis of variance, and the significant differences between the groups were considered at a P < 0.05 with the appropriate Tukey's post hoc test made for multiple comparisons. The ordinal values of the kidney injury scores were analyzed by the Mann-Whitney nonparametric test. Twenty-four-hour survival rates between HSP27 WT and huHSP27 OE hepatic IRI groups were compared with χ2 test.
RESULTS
Reduced AKI and hepatic injury after liver IRI in huHSP27 OE mice compared with HSP27 WT mice.
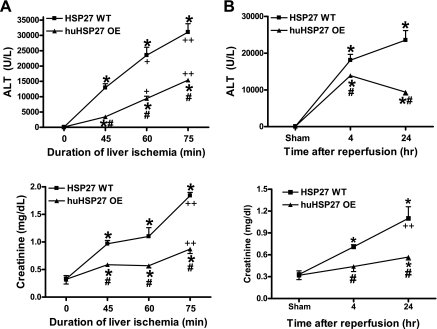
The plasma levels of ALT and creatinine in sham-operated HSP27 WT or huHSP27 OE mice were similar (Fig. 1). Increasing the duration of hepatic ischemia (45, 60, or 75 min) resulted in increased hepatic as well as renal injury after IRI in both HSP27 WT and huHSP27 OE mice (Fig. 1A). In HSP27 WT mice subjected to 60-min liver IRI, plasma ALT significantly increased at 4 and 24 h after 60-min liver IRI (Fig. 1B). Moreover, the HSP27 WT mice developed AKI 4 and 24 h after 60-min hepatic IRI (Fig. 1B). However, the huHSP27 OE mice showed significantly less elevation in plasma ALT and creatinine compared with the HSP27 WT mice at 4 and at 24 h (Fig. 1B). The survival rates for the HSP27 WT mice (76%, n = 21) and the huHSP27 OE mice (91%, n = 20) were not statistically different at 24 h after 60-min liver IRI (P = 0.19).
Fig. 1.
A: plasma alanine aminotransferase (ALT) and creatinine (Cr) levels in heat shock protein 27 wild-type (HSP27 WT) and human HSP27-overexpressing (huHSP27 OE) mice subjected to sham operation (sham) and 45-, 60-, or 75-min liver ischemia and 24 h of reperfusion injury (IRI). *P < 0.01 vs. sham group. #P < 0.01 vs. IRI group. +P < 0.05 vs. 45-min liver ischemia group. ++P < 0.05 vs. 60-min liver ischemia group. B: plasma ALT and Cr levels in HSP27 WT and huHSP27 OE mice subjected to sham operation (sham) and 60-min liver IRI. The blood samples were collected at 4 and 24 h after reperfusion. Values are means ± SE. *P < 0.01 vs. sham group. #P < 0.01 vs. IRI group. ++P < 0.05 vs. 4-h reperfusion group.
In addition to plasma creatinine, we measured the glomerular filtration rate in mice with the FITC-inulin clearance technique as described by Qi et al. (30). The glomerular filtration rate in sham-operated HSP27 WT and huHSP27 OE was similar (0.96 ± 0.11, n = 4 vs. 1.08 ± 0.18 ml·min−1·100 g body wt−1, n = 4). Sixty minutes of liver IRI significantly reduced the glomerular filtration rate in HSP27 WT mice within 24 h (0.11 ± 0.05 ml·min−1·100 g body wt−1, n = 4). The glomerular filtration rate was significantly better preserved in huHSP27 OE mice (0.59 ± 0.2 ml·min−1·100 g body wt−1, n = 4, P < 0.01 vs. HSP27 WT mice subjected to liver IR) 24 h after liver IRI.
HSP27 WT mice subjected to 45-min liver IRI also developed liver and kidney injury. Furthermore, the huHSP27 OE mice subjected to 45-min liver IRI demonstrated reduced liver and kidney injury compared with the WT mice at 24 h. Increasing hepatic ischemia time to 75 min increased both liver and kidney injury in HSP27 WT mice, and the huHSP27 OE mice continued to demonstrate reduced hepatic injury and improved renal function after 75-min liver ischemia and 24 h of reperfusion (Fig. 1A).
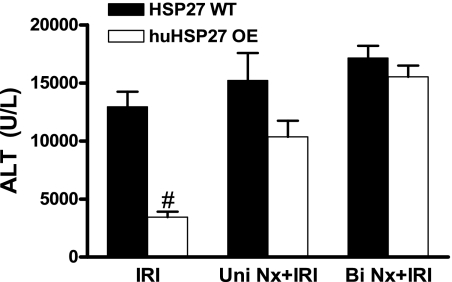
Unilateral or bilateral nephrectomy abolishes hepatic protection with huHSP27 overexpression.
Twenty-four hours after unilateral nephrectomy without hepatic ischemia, mouse plasma creatinine did not change significantly (0.46 ± 0.06 mg/dl, n = 5) compared with the sham-operated mice. However, unilateral nephrectomy significantly reduced the hepatic protection provided by huHSP27 overexpression in huHSP27 OE mice subjected to liver IRI (P = 0.13 vs. HSP27 WT mice subjected to liver IRI, Fig. 2). Bilateral nephrectomy abolished the hepatic protection provided by huHSP27 overexpression as HSP27 WT and huHSP27 OE mice developed similar degree of liver injury after 45 min of liver ischemia and 24 h of reperfusion (P = 0.29 vs. HSP27 WT mice, Fig. 2), demonstrating that kidneys are required for huHSP27 overexpression-mediated liver protection after liver IRI. The HSP27 WT mice did not show increased liver injury after unilateral or bilateral nephrectomy.
Fig. 2.
Plasma ALT levels in HSP27 WT and huHSP27 OE mice subjected to sham operation (sham) and 45-min liver ischemia and 24 h of reperfusion (IRI). Some mice were subjected to concomitant unilateral (Uni Nx) or bilateral nephrectomy (Bi Nx) during liver ischemia. The blood samples were collected at 24 h after reperfusion. Values are means ± SE. *P < 0.01 vs. sham group. #P < 0.01 vs. HSP27 WT IRI group.
huHSP27 OE mice show reduced renal cortical vacuolization, proximal tubular simplification, hypereosinophilia, and leukocytosis after liver IRI.
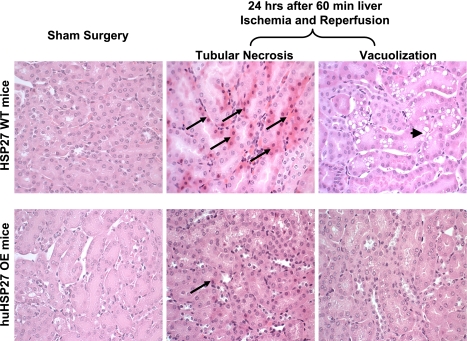
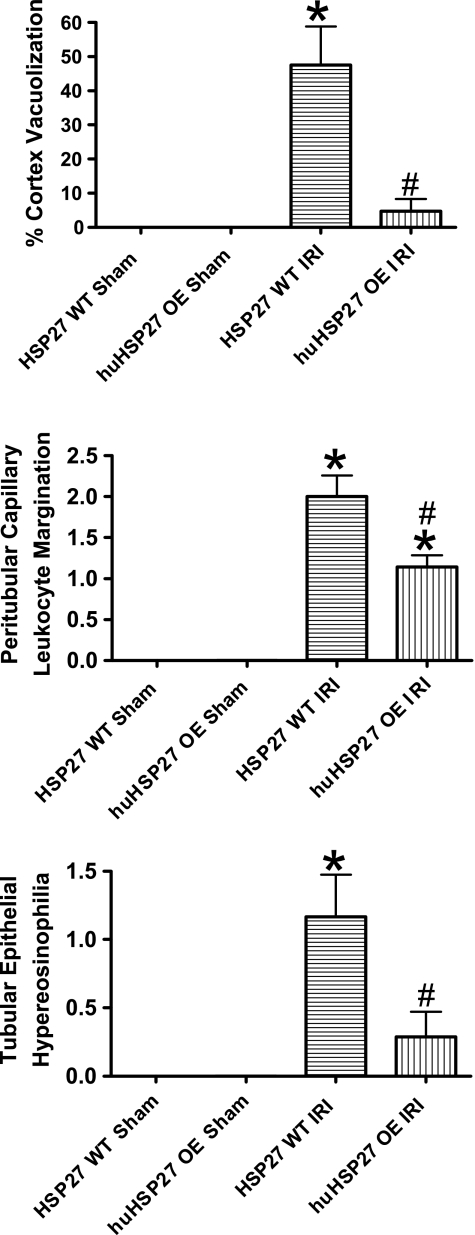
We demonstrated previously that our model of partial hepatic ischemia produced graded liver necrosis with increasing duration of ischemia in mice after IRI (21). Correlating with significantly improved function, reduced liver necrosis was observed in huHSP27 OE mice subjected to hepatic IRI compared with the HSP27 WT mice (data not shown). When we examined the kidneys from the HSP27 WT mice subjected to liver IRI, we observed multifocal acute tubular injury including S3 segment proximal tubule necrosis, cortical tubular simplification, cytoplasmic vacuolization, and dilated lumina as well as focal granular bile/heme casts. Moreover, endothelial cell apoptosis of peritubular capillaries was also visible in H&E sections as linear arrays of apoptotic bodies corresponding to interstitial capillary-lining endothelium. The number of glomeruli with detectable juxtaglomerular apparatus in H&E sections and the number of cells per juxtaglomerular apparatus were both increased after liver IRI, indicating hyperplasia of the juxtaglomerular apparatus. Representative H&E slides from kidneys of HSP27 WT mice and huHSP27 OE mice subjected to 60-min liver ischemia and 24 h of reperfusion or to sham operation are shown in Fig. 3. Correlating with significantly improved renal function, the huHSP27 OE mice showed reduced renal tubular hypereosinophilia and cortical vacuolization (Fig. 3). The quantifications of percent renal cortical vacuolization, the degree of peritubular/proximal tubule leukocyte infiltration, and the degree of proximal tubule hypereosinophilia are shown in Fig. 4. These indices of renal tubular injury were significantly less in the huHSP27 OE mice subjected to liver IRI (60-min liver ischemia and 24 h of reperfusion).
Fig. 3.
Representative (of 6 slides) hematoxylin- and eosin-stained photomicrographs in kidney sections for HSP27 WT and huHSP27 OE mice subjected to sham operation or to liver IRI (magnification ×200). Hypereosinophilic (long arrow) and vacuolization (short arrow) of proximal tubules were prominent in HSP27 WT but not in huHSP27 OE mice subjected to liver IRI.
Fig. 4.
Renal histological assessment after liver IRI in HSP27 WT and huHSP27 OE mice. Mice were subjected to sham operation (n = 4) or to liver IRI (n = 6 for HSP27 WT and n = 7 for huHSP27 OE). Twenty-four hours later, HSP27 WT mice subjected to liver IRI showed an increased percentage of cortical vacuolization, peritubular capillary leukocyte margination, and proximal tubule hypereosinophilia. Values are means ± SE.*P < 0.01 vs. sham-operated mice. #P < 0.05 vs. HSP27 WT IRI group.
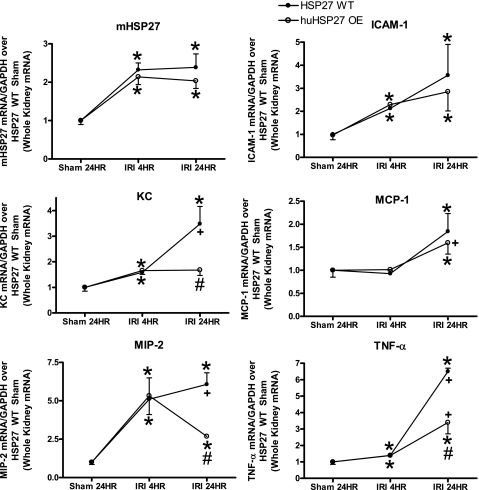
huHSP27 OE mice showed reduced TNF-α, KC, and MIP-2 mRNA expression in the kidney 24 h after liver IRI.
We measured the expression of proinflammatory cytokine mRNAs in the kidneys 4 and 24 h after 60-min liver IRI with RT-PCR. Hepatic IRI was associated with significantly increased proinflammatory ICAM-1, KC, MIP-2, and TNF-α mRNA expression at 4 h and ICAM-1, TNF-α, KC, MCP-1, and MIP-2 mRNA expression at 24 h in the kidneys of HSP27 WT mice (Fig. 5). However, the huHSP27 OE mice showed significantly reduced increases in TNF-α, KC, and MIP-2 mRNA 24 h after liver IRI compared with the HSP27 WT mice (Fig. 5). There were no significant differences in induction of MCP-1 or ICAM-1 mRNAs at 24 h after liver IRI. Both HSP27 WT and huHSP27 OE mice showed similar induction of proinflammatory mRNAs at 4 h after liver IRI (Fig. 5). Induction of mouse HSP27 mRNA in the kidneys was equivalent in both groups of mice after liver IRI.
Fig. 5.
Densitometric quantifications of relative band intensities from RT-PCR reactions (normalized to GAPDH). Values are means ± SE. *P < 0.05 vs. HSP27 WT sham group. #P < 0.05 vs. HSP27 WT IRI group. ++P < 0.05 vs. 4-h reperfusion group.
huHSP27 OE mice showed reduced renal neutrophil infiltration 24 h after IRI.
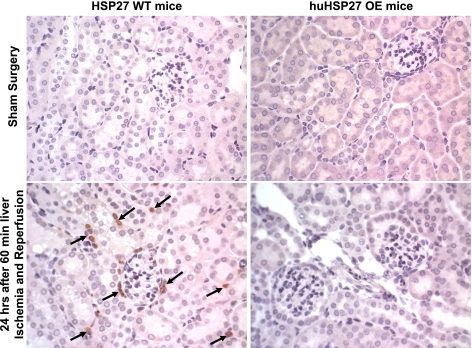
Sixty minutes of hepatic ischemia resulted in recruitment of neutrophils into the kidney (Fig. 6). Neutrophil infiltration 24 h after hepatic ischemia was significantly less in huHSP27 OE mice compared with the HSP27 WT mice. Figure 6 shows representative images of neutrophil immunohistochemistry of kidney sections from HSP27 WT or huHSP27 OE mice subjected to sham operation or to 60 min of liver ischemia and 24 h of reperfusion. In sham-operated HSP27 WT or huHSP27 OE mice, we were unable to detect any neutrophils. Twenty-four hours after liver IRI, we detected 27 ± 8 neutrophils/field (×200 magnification, n = 6) in the kidneys of HSP27 WT mice. The huHSP27 OE mice had significantly reduced renal neutrophil infiltration 24 h after liver IRI (6 ± 2 neutrophils/field, ×200 magnification, n = 7, P < 0.05).
Fig. 6.
Representative photomicrographs of neutrophil accumulation (dark brown punctate stain, arrows) in kidney sections (magnification ×400). HSP27 WT mice subjected to 60 min of liver ischemia and 24 h of reperfusion injury exhibited neutrophil accumulation whereas the huHSP27 OE mice showed very scant neutrophil infiltration after liver IRI (representative of 6–7 experiments).
huHSP27 OE mice show decreased renal apoptosis after IRI.
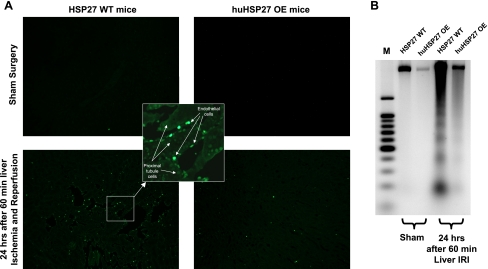
We utilized two techniques to detect apoptosis: TUNEL staining and DNA laddering. Twenty-four hours after 60 min of hepatic IRI, huHSP27 OE mice showed significantly reduced apoptosis of the kidney compared with the HSP27 WT mice with reduced TUNEL staining (Fig. 7A, representative of 5 experiments) and DNA laddering (Fig. 7B, representative of 4 experiments). The TUNEL staining showed that endothelial cell apoptosis was predominant in the kidney (inset).
Fig. 7.
A: representative fluorescence photomicrographs of kidney sections illustrating apoptotic nuclei [terminal deoxynucleotidyl transferase biotin-dUTP nick end-labeling (TUNEL) fluorescence staining, ×100] at 24 h after 60 min of liver IRI. TUNEL-positive cells were mainly peritubular capillary endothelial cells. Photographs are representative of 5 independent experiments. Inset: higher magnification of image showing TUNEL-positive cells. In the kidney, endothelial cells not proximal tubule cells underwent apoptosis. B: representative DNA ladder (of 4 experiments). Apoptotic DNA fragments were separated from intact chromatin and loaded onto the agarose gel.
huHSP27 OE mice show decreased degradation of renal tubule F-actin architecture after liver IRI.
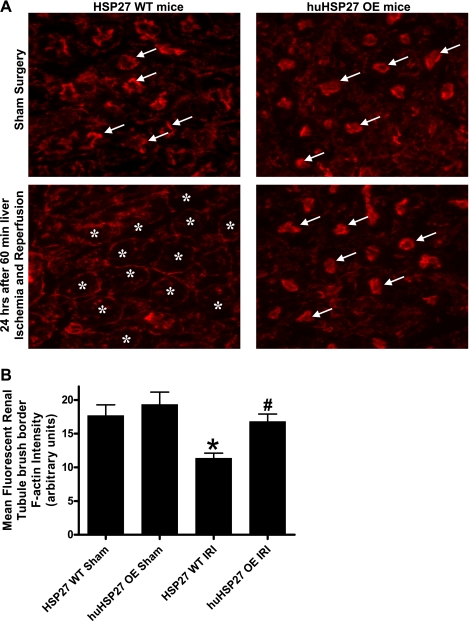
Sixty minutes of liver IRI resulted in a severe loss of F-actin in renal proximal tubules in 24 h. Twenty-four-hour post-hepatic IRI-induced disruptions of the F-actin cytoskeleton in renal proximal tubular epithelial cells are shown in Fig. 8A. HSP27 WT or huHSP27 OE mice subjected to sham surgery showed intense staining in the tubular epithelial cells and in the basal plasma membrane. In contrast, kidneys from HSP27 WT mice subjected to liver IRI showed loss of F-actin staining in the tubular epithelial cells. However, the huHSP27 OE mice show significantly better preserved F-actin structure after liver IRI as the staining is quite similar to that in sham-operated mice. Mean fluorescent F-actin intensity also showed reduced renal proximal tubule F-actin degradation in huHSP27 OE mice after liver IRI (Fig. 8B).
Fig. 8.
A: representative (of 5 experiments) fluorescent photomicrographs of FITC-phalloidin labeling to visualize filamentous (F)-actin in the kidneys from HSP27 WT or huHSP27 OE mice subjected to sham operation or to 60-min liver ischemia and 24 h of reperfusion. The F-actin stains of proximal tubular epithelial cells are prominent in the brush border from sham-operated mice (arrows), which is severely degraded in the kidneys of HSP27 WT mice subjected to liver IRI (*). B: quantification of mean fluorescent proximal tubule F-actin intensity as a measure of F-actin preservation in liver sections (n = 5). *P < 0.05 vs. HSP27 WT sham group. #P < 0.01 vs. HSP27 WT IRI group.
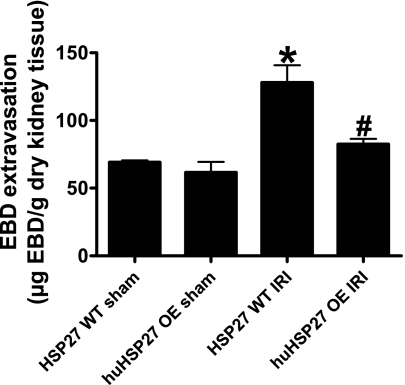
huHSP27 OE mice show decreased renal vascular permeability after IRI.
We measured renal vascular permeability after 60 min of liver ischemia and 24 h of reperfusion with EBD injection. EBD binds to plasma proteins, and its appearance in extravascular tissues reflects an increase in vascular permeability. Analysis of EBD extravasations in the kidneys from HSP27 WT and huHSP27 OE mice subjected to sham operation and liver IRI is shown in Fig. 9. Liver IRI increased the EBD content in the kidney for both groups; however, the increase in EBD content was significantly higher for the HSP27 WT mice compared with the huHSP27 OE mice.
Fig. 9.
Evans blue dye (EBD) extravasation as an index of renal vascular permeability. HSP27 WT or huHSP27 OE mice were subjected to sham operation (n = 4) or to 60-min liver ischemia and 24 h of reperfusion (n = 6 for HSP27 WT mice and n = 7 for huHSP27 OE mice). Kidney EBD was extracted in formamide, and the amount of extravasated EBD concentration in the kidney was calculated against a standard curve. *P < 0.05 vs. HSP27 WT sham group. #P < 0.01 vs. HSP27 WT IRI group.
DISCUSSION
The major findings of this study are that mice overexpressing human HSP27 (huHSP27 OE mice) demonstrate reduced renal and hepatic injury after liver IRI. The reduction in AKI was characterized by 1) reduced renal proximal tubule necrosis, 2) less cortical vacuolization and simplification, 3) decreased peritubular capillary endothelial cell apoptosis, and 4) reduced neutrophil infiltration coupled with less induction of several proinflammatory cytokines (TNF-α, KC, and MIP-2). Furthermore, we demonstrate that the huHSP27 OE mice showed reduced renal tubular F-actin breakdown and improved preservation of renal vascular integrity after hepatic IRI. Finally, HSP27 OE-mediated hepatic protection requires intact renal function as binephrectomized HSP27 OE mice are no longer protected against liver injury after hepatic ischemia and reperfusion.
We recently reported a murine model of liver IRI-induced AKI in which mice subjected to 60 min of warm liver ischemia developed severe and reproducible AKI in <24 h (24). We demonstrated in our previous study that plasma ALT and creatinine have a direct relationship: mice with higher ALT showed increased renal dysfunction after liver IR (24). However, we also noted that the degree of renal dysfunction was mild when the plasma ALT levels were <10,000 U/l, especially for the huHSP27 OE mice subjected to liver IRI. The interactions between kidney and liver injury after hepatic IRI in vivo are extremely complex. We speculate that the propagation of liver and kidney injury after hepatic IRI is interrelated, with liver IRI causing renal injury which further potentiates liver injury.
Reduced kidney injury after liver IRI in huHSP27 OE mice can be due to either 1) reduced hepatic injury resulting in subsequently less AKI and/or 2) direct renal-protective effects of huHSP27 overexpression. Reduced AKI after hepatic IRI may also subsequently reduce hepatic injury in huHSP27 OE mice. We very recently demonstrated that huHSP27 OE mice demonstrate increased resistance against hepatic necrosis, inflammation, and apoptosis after hepatic IRI in vivo (8). We showed that depletion of Kupffer cells with gadolinium chloride provided significant protection against liver IRI in HSP27 WT mice but not in huHSP27 OE mice, suggesting that the overexpression of huHSP27 in the Kupffer cells may be responsible for the hepatic protection observed in huHSP27 OE mice. We hypothesize that overexpression of huHSP27 in Kupffer cells negates the deleterious effects of Kupffer cells in producing liver injury after IR; therefore, depletion of Kupffer cells failed to provide further liver protection in huHSP27 OE mice. Furthermore, we postulate that huHSP27 overexpression in hepatocytes failed to protect against the direct hepatocyte necrotic injury as huHSP27 OE mice are not further protected against liver IR after Kupffer cell depletion. We now show in this study that loss of renal function reduces hepatoprotective effects of huHSP27 overexpression in mice as the huHSP27 OE mice subjected to unilateral or bilateral nephrectomy demonstrate significant or near total loss of hepatic protection, respectively, after IRI (Fig. 2). Our current and previous studies suggest that both mechanisms play important roles in huHSP27-mediated hepatoprotection: 1) huHSP27 overexpression in Kupffer cells within the liver produces direct hepatic protection and 2) the direct renal-protective effects of huHSP27 overexpression play crucial roles in reducing hepatic injury liver IRI.
Although we saw global apoptotic changes in the liver after IR, the pattern of apoptosis in the kidney after liver IRI was unique (and different compared with the pattern observed after renal IR) in that endothelial cell apoptosis predominated (Fig. 7A). We propose that protection of renal endothelial cell apoptosis is a crucial mechanism of renal protection with huHSP27 overexpression after hepatic IR. Our experimental results directly demonstrate renal peritubular endothelial cell protection with huHSP27 overexpression after liver IRI. We saw dramatic increases in TUNEL-positive kidney peritubular capillary endothelial cells (Fig. 7A) after liver IRI (with almost complete sparing of renal proximal tubule apoptosis), and huHSP27 overexpression produced a drastic reduction in the apoptosis of these pericapillary endothelial cells. In addition, significant impairment of liver and kidney vascular integrities was observed after liver IRI as these organs appear enlarged with significantly increased EBD extravasation (Fig. 9). Reduction in renal endothelial cell apoptosis would lead to better preserved vascular permeability and reduced neutrophil infiltration with subsequently reduced proximal tubule cell death. All of these changes were observed in the kidneys of huHSP27 OE mice subjected to liver IR.
HSP27 is a molecular chaperone protein with diverse cytoprotective effects (25) and is known to attenuate apoptosis, stabilize cytoskeletal architecture, and decrease necrotic cell death (9, 18, 29). It is well established that HSP27 phosphorylation or upregulation improves cell survival against stress or injury (6, 9). Phosphorylation of HSP27 enhances resistance of actin to breakdown and maintains cytoskeletal architecture after ATP depletion and IRI (15, 17, 18). This may be due in part to preservation of cytoskeletal actin in a filamentous state over a globular state. Furthermore, overexpression of HSP27 has been shown to confer significant cytoresistance against heat shock, simulated ischemia, proapoptotic agents (i.e., TNF-α), oxidant stress, and a number of cytotoxic drugs (26). These principles may explain reduced liver and kidney injury and less apoptosis and necrosis after IRI in huHSP27 OE mice. Overexpression of phosphorylation-deficient HSP27 has also been shown to confer significant cytoresistance against proapoptotic agents (i.e., TNF-α) by counteracting apoptosis via interacting with caspases and cytochrome c (9, 10). Our data further extend these previous in vitro findings in that in vivo overexpression of human HSP27 in mice protects against liver IRI-induced renal apoptosis. One of the limitations of this study, however, is that we did not directly study the interactions between HSP27 and cytoprotective processes (actin stabilization, caspase 3 inhibition, reduction in cytochrome c release). These more detailed signaling questions are topics of ongoing studies with our model of liver IR.
We have previously demonstrated that hepatic IR causes induction of several proinflammatory cytokines and chemokines (TNF-α, ICAM-1, MIP-2, MCP-1, and KC) in the liver tissues that peaked at ∼4–5 h after reperfusion (24). Moreover, plasma TNF-α and IL-6 levels increased significantly after liver IR (Park SW, Chen SWC, Kim M, D'Agati VD, Lee HT, unpublished observations). Acute kidney injury following liver IR is characterized by early renal endothelial apoptosis with subsequent proximal tubular necrosis and inflammation (24). Hepatic IR also caused significant upregulation of several proinflammatory chemokine and cytokine mRNAs in the kidney. However, unlike in the liver where the peak proinflammatory mRNA expression occurred early (∼4–5 h after reperfusion), the proinflammatory mRNAs in the kidneys continued to increase and peaked ∼24 h after liver IR (Fig. 5). Of many cell types in the kidney, endothelial cells are first exposed to the circulating cytokines and chemokines cells released from the liver. We postulate that the massive cytokine upregulation and release from the liver travels to the kidney, causing early renal endothelial dysfunction and death, with subsequent vascular impairment and neutrophil infiltration leading to proximal tubule necrosis and inflammatory changes in the kidney. Neutralization of TNF-α or IL-6 produced significant protection against both liver as well as kidney injury after liver IR (Park SW, Chen SWC, Kim M, D'Agati VD, Lee HT, unpublished observations), supporting the hypothesis that remote renal injury after liver IR is initiated by the chemokines and cytokines released from the liver.
Several proinflammatory mRNAs (TNF-α, KC, and MIP-2) examined were significantly attenuated in huHSP27 OE mice kidney after hepatic IRI. The chemokines MIP-2 and KC and cytokine TNF-α are important mediators in the pathogenesis of AKI (13). Upregulation of these proinflammatory chemokines and cytokines leads to neutrophil recruitment during AKI induced by renal IRI or nephrotoxic agent (27). Overexpression of huHSP27 may have directly but selectively attenuated the proinflammatory mRNA expression in the kidney. More likely, however, reduction in renal TNF-α expression with huHSP27 overexpression resulted in secondary reduction in MIP-2 and ICAM-1 expression in the kidney. TNF-α is known to promote the migration of inflammatory cells into the renal parenchyma via the upregulation of KC and MIP-2 in the kidney (31).
The role of neutrophils in the pathophysiology of organ injury (including the liver and the kidney) is well recognized (19). Our study demonstrated that liver IRI caused neutrophil infiltration into the kidney within 24 h. Neutrophils are activated during and after liver ischemia, and activated neutrophils attach to and then transverse the hepatic capillary endothelium into the subendothelial space, where they release enzymes and cytokines, causing direct hepatocyte injury and recruit other injurious cells, such as monocytes and macrophages (22, 28). Activated neutrophils release substances to produce further tissue injury, such as products of arachidonic acid metabolism, oxygen free radicals, and neutrophil elastase (19). huHSP27 overexpression resulted in improved kidney vascular endothelial cell integrity (indicated by reduced EBD infiltration) and most likely limited the polymorphonuclear neutrophil infiltration into the kidney.
IRI in vivo results in F-actin cytoskeleton degradation, which further compromises organ function (4, 20). For example, F-actin disruption is a well-known stimulus of apoptosis in several cell lines (35). We saw severe disruption of F-actin in renal proximal tubules after liver IRI in HSP27 WT mice. We demonstrated in this study that huHSP27 overexpression resulted in markedly better preserved F-actin of the liver as well as the kidney. An intact F-actin cytoskeleton in kidneys of mice subjected to liver IRI may have contributed to reduced AKI observed in these mice.
Global overexpression of HSP27 may not always lead to organ protection against IRI. We were surprised to discover that systemic overexpression of HSP27 increased renal injury after IRI with significantly increased KC upregulation and activation in vivo (7). Our findings after liver IRI contrast with our previous finds in the kidney where huHSL27 OE mice demonstrated increased renal inflammation and neutrophil infiltration after IRI (7). The reason for this discrepancy remains to be elucidated. It appears that renal IRI leads to heightened systemic inflammation (and enhanced plasma KC activation) that overcomes the protective effects of huHSP27 overexpression in renal proximal tubule cells. In contrast, liver IRI appears to produce a less systemic inflammatory response compared with renal IRI, leading to less KC activation and upregulation.
In summary, we demonstrate in this study that mice overexpressing huHSP27 are protected against AKI after liver IRI with reduced endothelial cell apoptosis, proximal tubule necrosis, and inflammation. The huHSP27-mediated renal protection plays a major role in hepatic protection after liver IRI in these mice. Given the protective benefit of HSP27 against hepatic IRI and that hepatic IRI is common in patients after liver surgery, liver transplantation or sepsis, our findings may have important future therapeutic implications.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1 DK-58547.
REFERENCES
- 1. Akbar MT, Lundberg AM, Liu K, Vidyadaran S, Wells KE, Dolatshad H, Wynn S, Wells DJ, Latchman DS, de Belleroche J. The neuroprotective effects of heat shock protein 27 overexpression in transgenic animals against kainate-induced seizures and hippocampal cell death. J Biol Chem 278: 19956–19965, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Arrigo AP. Hsp27: novel regulator of intracellular redox state. IUBMB Life 52: 303–307, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Arrigo AP, Firdaus WJ, Mellier G, Moulin M, Paul C, Diaz-Latoud C, Kretz-Remy C. Cytotoxic effects induced by oxidative stress in cultured mammalian cells and protection provided by Hsp27 expression. Methods 35: 126–138, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Benkoel L, Dodero F, Hardwigsen J, Campan P, Botta-Fridlund D, Lombardo D, Le Treut YP, Chamlian A. Effect of ischemia-reperfusion on bile canalicular F-actin microfilaments in hepatocytes of human liver allograft: image analysis by confocal laser scanning microscopy. Dig Dis Sci 46: 1663–1667, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 18: 442–445, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, Garrido C. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2: 645–652, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Chen SW, Kim M, Kim M, Song JH, Park SW, Wells D, Brown K, Belleroche JD, D'Agati VD, Lee HT. Mice that overexpress human heat shock protein 27 have increased renal injury following ischemia reperfusion. Kidney Int 75: 499–510, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen SW, Park SW, Kim M, Brown KM, D'Agati VD, Lee HT. Human heat shock protein 27 overexpressing mice are protected against hepatic ischemia and reperfusion injury. Transplantation 87: 1478–1487, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Concannon CG, Gorman AM, Samali A. On the role of Hsp27 in regulating apoptosis. Apoptosis 8: 61–70, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expr 9: 195–201, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Liver Transpl 8: 91–109, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury—a fresh look. Exp Mol Pathol 74: 86–93, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14: 2503–2515, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Garrido C, Bruey JM, Fromentin A, Hammann A, Arrigo AP, Solary E. HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J 13: 2061–2070, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J. Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 110: 357–368, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Herrmann M, Lorenz HM, Voll R, Grunke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res 22: 5506–5507, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res 80: 383–392, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Huot J, Houle F, Spitz DR, Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res 56: 273–279, 1996 [PubMed] [Google Scholar]
- 19. Jaeschke H. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 290: G1083–G1088, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Kellerman PS, Norenberg SL, Jones GM. Early recovery of the actin cytoskeleton during renal ischemic injury in vivo. Am J Kidney Dis 27: 709–714, 1996 [DOI] [PubMed] [Google Scholar]
- 21. Kim J, Kim M, Song JH, Lee HT. Endogenous A1 adenosine receptors protect against hepatic ischemia reperfusion injury in mice. Liver Transpl 14: 845–854, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol Renal Fluid Electrolyte Physiol 256: F794–F802, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Landry J, Huot J. Regulation of actin dynamics by stress-activated protein kinase 2 (SAPK2)-dependent phosphorylation of heat-shock protein of 27 kDa (Hsp27). Biochem Soc Symp 64: 79–89, 1999 [PubMed] [Google Scholar]
- 24. Lee HT, Park SW, Kim M, D'Agati VD. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest 89: 196–208, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin JL, Hickey E, Weber LA, Dillmann WH, Mestril R. Influence of phosphorylation and oligomerization on the protective role of the small heat shock protein 27 in rat adult cardiomyocytes. Gene Expr 7: 349–355, 1999 [PMC free article] [PubMed] [Google Scholar]
- 26. Mearow KM, Dodge ME, Rahimtula M, Yegappan C. Stress-mediated signaling in PC12 cells - the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem 83: 452–462, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Miura M, Fu X, Zhang QW, Remick DG, Fairchild RL. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol 159: 2137–2145, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron 90: 133–138, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome C release. Mol Cell Biol 22: 816–834, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qi Z, Whitt I, Mehta A, Jin J, Zhao M, Harris RC, Fogo AB, Breyer MD. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol 286: F590–F596, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Ramesh G, Reeves WB. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest 110: 835–842, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samali A, Robertson JD, Peterson E, Manero F, van Zeijl L, Paul C, Cotgreave IA, Arrigo AP, Orrenius S. Hsp27 protects mitochondria of thermotolerant cells against apoptotic stimuli. Cell Stress Chaperones 6: 49–58, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Slot C. Plasma creatinine determination. A new and specific Jaffé reaction method. Scand J Clin Lab Invest 17: 381–387, 1965 [DOI] [PubMed] [Google Scholar]
- 34. Weber NC, Toma O, Wolter JI, Wirthle NM, Schlack W, Preckel B. Mechanisms of xenon- and isoflurane-induced preconditioning—a potential link to the cytoskeleton via the MAPKAPK-2/HSP27 pathway. Br J Pharmacol 146: 445–455, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White SR, Williams P, Wojcik KR, Sun S, Hiemstra PS, Rabe KF, Dorscheid DR. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am J Respir Cell Mol Biol 24: 282–294, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Wyttenbach A, Sauvageot O, Carmichael J, Diaz-Latoud C, Arrigo AP, Rubinsztein DC. Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum Mol Genet 11: 1137–1151, 2002 [DOI] [PubMed] [Google Scholar]