Abstract
Flow-stimulated net K secretion (JK) in the cortical collecting duct (CCD) is mediated by an iberiotoxin (IBX)-sensitive BK channel, and requires an increase in intracellular Ca2+ concentration ([Ca2+]i). The α-subunit of the reconstituted BK channel is phosphorylated by PKA and PKC. To test whether the BK channel in the native CCD is regulated by these kinases, JK and net Na absorption (JNa) were measured at slow (∼1) and fast (∼5 nl·min−1·mm−1) flow rates in rabbit CCDs microperfused in the presence of mPKI, an inhibitor of PKA; calphostin C, which inhibits diacylglycerol binding proteins, including PKC; or bisindolylmaleimide (BIM) and Gö6976, inhibitors of classic and novel PKC isoforms, added to luminal (L) and/or basolateral (B) solutions. L but not B mPKI increased JK in CCDs perfused at a slow flow rate; a subsequent increase in flow rate augmented JK modestly. B mPKI alone or with L inhibitor abolished flow stimulation of JK. Similarly, L calphostin C increased JK in CCDs perfused at slow flow rates, as did calphostin C in both L and B solutions. The observation that IBX inhibited the L mPKI- and calphostin C-mediated increases in JK at slow flow rates implicated the BK channel in this K flux, a notion suggested by patch-clamp analysis of principal cells. The kinase inhibited by calphostin C was not PKC as L and/or B BIM and Gö6976 failed to enhance JK at the slow flow rate. However, addition of these PKC inhibitors to the B solution alone or with L inhibitor blocked flow stimulation of JK. Interpretation of these results in light of the effects of these inhibitors on the flow-induced elevation of [Ca2+]i suggests that the principal cell apical BK channel is tonically inhibited by PKA and that flow stimulation of JK in the CCD is PKA and PKC dependent. The specific targets of the kinases remain to be identified.
Keywords: K secretion, ROMK, mechanoregulation, in vitro microperfusion, laminar shear
urinary k secretion in the distal nephron, including the late distal convoluted tubule (DCT), connecting tubule (CNT), and cortical collecting duct (CCD), is mediated by at least two apical K secretory channels: a low-conductance SK channel (14, 55, 80) and a high-conductance Ca2+- and stretch-activated BK (or maxi-K) channel (30, 42, 43, 65). Cumulative evidence now suggests that the SK channel, encoded by the ROMK gene, mediates baseline K secretion whereas the BK channel, composed of both a pore-forming α-subunit, encoded by the Drosophila slo gene, and a regulatory β-subunit (4, 35), allows for flow-stimulated K secretion. Whereas the ROMK channel is restricted to principal cells, conducting BK channels can be detected in both principal and intercalated cells, although their density in the latter cells exceeds that in the former (30, 42).
We have previously reported that an increase in luminal flow rate leads to a transient increase in intracellular Ca2+ concentration [Ca2+]i and a sustained increase in iberiotoxin (IBX)-sensitive and thus BK channel-mediated net K secretion (JK) in the rabbit CCD (33, 34, 54, 86). This BK channel-mediated flow-stimulated K secretion apparently requires an increase in [Ca2+]i, due to Ca2+ entry and internal store release (34). An unanswered question is how a transient increase in [Ca2+]i elicited by an acute increase in luminal flow rate leads to a sustained increase in net transepithelial K secretion. The persistent activation of channel-mediated ion currents in response to a transient stimulus has, in many other systems, been attributed to direct phosphorylation/dephosphorylation of the channel itself or an associated regulatory protein (54). In fact, the BK channel α-subunit is modulated by various protein kinases, including cAMP-dependent PKA (29, 50, 58, 68, 83, 91), PKC (6, 21, 22, 50, 58, 88, 91), cGMP-dependent PKG (6, 62), and cSrc (1, 32). Whereas PKC is generally inhibitory in native cells (7, 21, 58), PKG activates the channel (6, 62). cSrc can either activate or inhibit the channel (1, 32, 68, 71), as can PKA, depending on the cell type (29, 59, 83). The variability in functional response of the channel to distinct kinase and phosphatase signaling pathways has been proposed to be due to alternative splicing of the α-subunit (28, 68, 91), association of α-subunits with distinct regulatory β-subunits (11, 38) and/or associated proteins (35, 57, 89), as well as through differential assembly of BK channels with protein kinase/phosphatase signaling complexes (35, 79).
The purpose of the present study was to test whether the BK channel present in the mammalian distal nephron is regulated by endogenous PKA and PKC. To this end, the effects of cell-permeant specific inhibitors of these kinases on flow-stimulated net K and Na transport in isolated perfused tubules and BK channel activity in apical cell-attached patches of CCD cells were examined. Our results suggest that both PKA and PKC regulate BK channel activity. Whereas PKA appears to tonically inhibit the channel or a closely associated regulatory protein in principal cells in CCDs perfused at slow flow rates, PKC plays an indirect role in BK-mediated flow-stimulated JK, as activation of this kinase is necessary for mechanosensitive Ca2+ entry, internal store release, and/or trafficking of subapical/cytoplasmic vesicles containing preformed Ca2+ channels to the appropriate plasma membranes.
METHODS
Animals.
Adult (>6 wk) female New Zealand White rabbits (Covance, Denver, PA) and pathogen-free Sprague-Dawley (SD) rats of either sex (5–6 wk old; Taconic Farms, Germantown, NY) were housed in the animal care facility at the Mount Sinai School of Medicine (Center for Comparative Medicine) or New York Medical College. All animals were allowed free access to tap water and chow. Animals were euthanized in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the Institutional Animal Care and Use Committee at the Mount Sinai School of Medicine or New York Medical College, as appropriate.
Microperfusion of isolated rabbit CCDs.
Kidneys were removed via a midline incision, and single tubules were dissected freehand in cold (4°C) Ringer solution containing (in mM) 135 NaCl, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 lactate, 6.0 l-alanine, 5.0 HEPES, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O, as previously described (34). A single tubule was studied from each animal. Isolated collecting ducts were microperfused in vitro as previously described (34, 86). Briefly, each isolated tubule was immediately transferred to a temperature- and O2/CO2-controlled specimen chamber, mounted on concentric glass pipettes, and perfused and bathed at 37°C with Burg's perfusate containing (in mM) 120 NaCl, 25 NaHCO3, 2.5 K2HPO4, 2.0 CaCl2, 1.2 MgSO4, 4.0 Na lactate, 1.0 Na3 citrate, 6.0 l-alanine, and 5.5 d-glucose, pH 7.4, 290 ± 2 mosmol/kgH2O (34). During the 30-min equilibration period and thereafter, the perfusion chamber was continuously suffused with a gas mixture of 95% O2-5% CO2 to maintain pH of the Burg's solution at 7.4 at 37°C. The bathing solution was continuously exchanged at a rate of 10 ml/h using a syringe pump (Razel, Stamford, CT).
Transport measurements were performed in the absence of transepithelial osmotic gradients, and thus water transport was assumed to be zero. Three to four samples of tubular fluid were collected under water-saturated light mineral oil by timed filling of a calibrated 30-nl volumetric constriction pipette at each perfusion rate (slow and fast). To determine the concentrations of K and Na delivered to the tubular lumen, ouabain (200 μM) was added to the bath at the conclusion of each experiment to inhibit all active transport, and an additional three to four samples of tubular fluid were obtained for analysis. The cation concentrations of perfusate and collected tubular fluid were determined by helium glow photometry, and the rates of net transport (Jx; in pmol·min−1·mm−1 tubular length) were calculated using standard flux equations, as previously described (13). The calculated ion fluxes were averaged to obtain a single mean rate of ion transport for the CCD at each flow rate under each condition. The flow rate was varied by adjusting the height of the perfusate reservoir. The sequence of flow rates was randomized within each group of tubules to minimize any bias induced by time-dependent changes in ion transport.
As indicated, tubules were pretreated with myristoylated protein kinase A inhibitor 14–22 amide (mPKI; 5 μM), a cell-permeable peptide inhibitor of the free catalytic subunit of PKA (3), provided as a lyophilized solid which was diluted with water to generate a 10 mM stock solution; H89 (10 μM), a cell-permeant isoquinolinesulfonamide derivative that inhibits PKA (10); calphostin C (500 nM), which binds to the diacylglycerol (DAG) binding site, or C1 domain, present in not only PKC but also a number of other proteins including Munc13s (scaffolding proteins involved in exocytosis), chimaerins (family of Rac GTPase-activating proteins), protein kinase D (originally identified as PKCμ), and Ras guanyl nucleotide-releasing proteins (RasGRPs; exchange factors for Ras/Rap1) (24, 61, 73); bisindolylmaleimide 1 hydrochloride (BIM; 1 μM), a nonselective PKC inhibitor that blocks the ATP-binding site of PKCα, -β. -δ, -ε, and -ζ (37, 74); and Gö6976 (100 nM), a selective inhibitor of the classical Ca2+-dependent PKC isoforms (α, β, and γ) and PKD at the concentrations used (37). All agents were purchased from Calbiochem (La Jolla, CA) and, except for mPKI, were prepared as stock solutions in DMSO and diluted at least 1:1,000 to achieve the final concentration. Inhibitors were added to the luminal or basolateral solutions, as indicated, after the initial equilibration period and were present for at least 30 min before tubular fluid samples were first obtained. Samples of tubular fluid for measurement of net cation transport were collected in the continuous presence of the inhibitors.
A subset of CCDs, as indicated, was perfused with the scorpion venom toxin IBX (99% purity; Sigma, St. Louis, MO), an inhibitor of the high-conductance Ca2+-activated BK channel (5, 15). The final concentration of 50 nM was prepared by diluting the toxin in the luminal perfusate.
Measurement of [Ca2+]i.
Following equilibration, microperfused tubules were loaded with 10 μM of the acetoxymethyl ester of fura 2 (Calbiochem) added to the bath for 20 min. Using a Nikon Eclipse TE300 inverted epifluorescence microscope linked to a Cascade 512F camera (Photometrics) interfaced with a digital imaging system (MetaFluor, Universal Imaging, Westchester, PA), [Ca2+]i was measured in individually identified fura 2-loaded cells residing in the lateral wall of each perfused CCD (to capture the fluorescence signal from a single cell), visualized using a Nikon S Fluor ×40 objective (NA 0.9, WD 0.3), as previously described (34, 85). Autofluorescence was not detected at the camera gains utilized.
Collecting ducts were alternately excited at 340 and 380 nm, and images were acquired every 2–15 s (for the first minute following the increase in luminal flow rate) and were digitized for subsequent analysis. At the conclusion of each experiment, an intracellular calibration was performed, using 10 μM EGTA-AM in a Ca2+-free bath and then a 2 mM Ca2+ bath containing ionomycin (10 μM) (34). Standard equations were used to calculate experimental values of [Ca2+]i. At least four randomly chosen cells were analyzed in each collecting duct. The mean [Ca2+]i values for principal and intercalated cells, distinguished by their differing fluorescence intensities, as we have previously described (34), were calculated. As indicated, the effect of flow on [Ca2+]i was studied in some CCDs after pretreatment with the kinase inhibitors identified above added to the lumen or bath.
Patch-clamp recording.
SD rats were killed by cervical dislocation. Kidneys were immediately removed, and several thin coronal slices were cut with a razor blade and placed in ice-cold Ringer solution for microdissection of CCDs, as previously described (82). The Ringer solution contained (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 HEPES (pH 7.4). To immobilize tubules for patch clamp, CCDs were affixed to 5 × 5-mm cover glasses coated with poly-lysine D. The basic patch-clamp methods have been described previously (64, 82). In brief, each isolated CCD affixed to a cover glass was transferred to a chamber (1,000-μl total volume) mounted on the stage of a Nikon inverted microscope. The chamber was filled with Ringer solution. The CCD was cut open with a sharpened micropipette to expose the apical membrane. Patch pipettes were pulled using a Flaming/Brown micropipette puller (model P-87; Sutter Instrument) in two stages with borosilicate glass capillaries (Dagan, Minneapolis, MN). The pipettes were fire-polished and had resistances of 2–5 MΩ when filled with a solution containing (in mM) 140 KCl, 1.8 MgCl2, and 5 HEPES (pH 7.4). Experiments were performed at room temperature. Currents originating in each cell-attached patch were recorded using an Axon200 patch-clamp amplifier (Axon Instruments). The currents were low-pass filtered at 500 Hz (for Fig. 4)-1 kHz (Fig. 3) by an eight-pole Bessel filter (902LPF; Frequency Devices, Haverhill, MA). Data were digitized by using an Axon interface (Digidata 1200/1300), stored on the hard drive of a computer, and analyzed using pClamp software (Version 9; Axon, Sunnyvale, CA). Channel activity was defined as NPo, calculated as previously described (64, 82). Principal cells were recognized by their polygonal shape, while intercalated cells were generally round in shape and projected above the surface of the adjacent cells. After high-resistance seals were formed, BK channel activity in individually identified intercalated cells was determined by depolarizing the cell membrane to 100 mV, a maneuver that maximizes channel NPo in this cell type (42). Channel activity in principal cells is readily detected, when present, at a holding potential of 0 mV (30, 42) and was thus studied at this voltage.
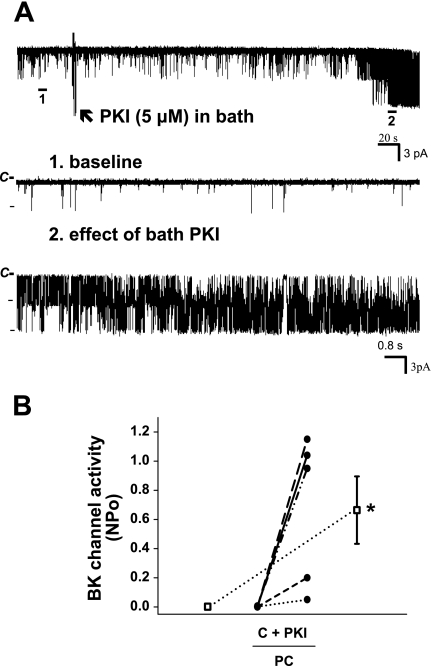
Fig. 4.
Effect of bath mPKI (5 μM) on BK channel activity in principal cells in the rat CCD. A: representative trace of the stimulatory effect of mPKI, added to the solution bathing a split-open CCD (arrow), on BK channel activity in a principal cell-attached patch studied at a holding potential of 0 mV. The top trace shows the time course of the entire experiment. Two parts of the trace are expanded (traces 1 and 2) to show detailed channel activity at faster time resolution. B: effect of mPKI, added to the solution bathing split-open CCDs, on NPo of the BK channel in individual cell-attached patches of PC (n = 5) studied at a holding potentials of 0 mV. PKI had no effect on BK channel activity in an additional 4 patches that did not respond to addition of ionomycin to the bath (which increases [Ca2+]i) with an increase in channel activity; this suggests that these nonresponsive cell-attached patches were devoid of BK channels. •, Paired data from individual cell-attached patches; □, means ± SE for the control and experimental data sets. *P < 0.05 compared with C.
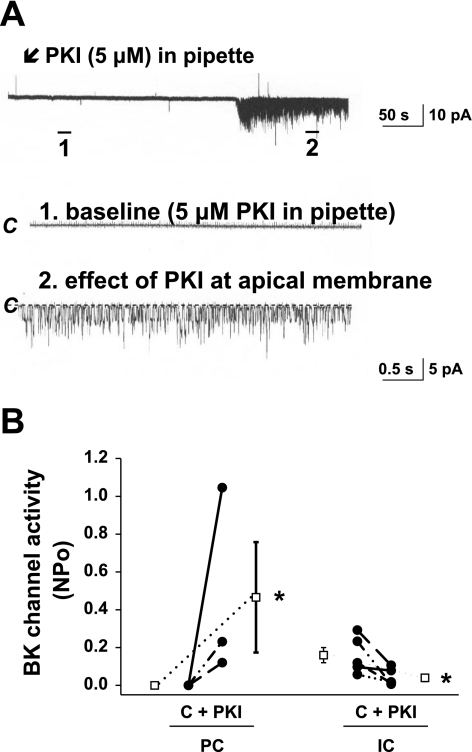
Fig. 3.
Effect of mPKI (5 μM) backfilled into the patch pipette and present in the pipette for the entire experiment, on BK channel activity in the rat CCD. A: representative traces of the stimulatory effect of apical mPKI, backfilled into the patch pipette and present in the pipette for the entire experiment, on BK channel activity in a principal cell-attached patch studied at a holding potential of 0 mV. The top trace shows the time course of the entire experiment. Two parts of the trace are expanded (traces 1 and 2) to show detailed channel activity at faster time resolution. The channel-closed state (“C”) is indicated by a dashed line. Channel openings are shown as downward deflections. The lag time between generation of the seal and appearance of channel activity is presumed to reflect the time necessary for diffusion of the inhibitor back-filled into the pipette to reach the membrane. B: effect of apical mPKI, backfilled into the patch pipette and present in the pipette for the entire experiment, on channel activity (NPo) of the BK channel in individual cell-attached patches of principal (PC; n = 10 in total, of which n = 7 did not respond) and intercalated (IC; n = 5) cells studied at holding potentials of 0 and 100 mV, respectively. C, baseline NPo, presumably in the absence of the inhibitor. •, Paired data from individual cell-attached patches; □, means ± SE for the control and experimental data sets. *P < 0.05 compared with C in the same cell type.
Statistics.
All results are expressed as means ± SE; n equals the number of animals used for in vitro microperfusion or cell-attached patches. Comparisons were made by paired or unpaired t-tests as appropriate, using commercially available statistical software for the calculations (SigmaStat; SPSS, Chicago, IL). Significance was asserted if P < 0.05.
RESULTS
Effect of mPKI on flow-stimulated JK and BK channel activity.
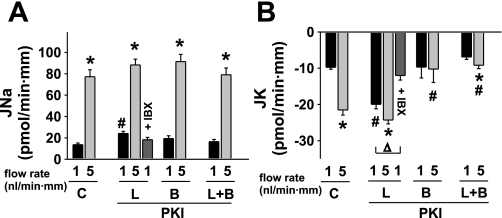
Rabbit CCDs were pretreated with the cell-permeable peptide inhibitor of the free catalytic subunit of PKA mPKI (5 μM), added to the luminal and/or basolateral solutions, and the rates of JK and JNa were measured at slow and fast flow rates. At a slow flow rate of 0.9 ± 0.1 nl·min−1·mm−1, JK and JNa in CCDs pretreated with luminal mPKI (n = 5) exceeded that measured in control tubules (n = 3) perfused at 1.0 ± 0.1 nl·min−1·mm−1 (P < 0.03) (Fig. 1, A and B). A fivefold increase in luminal flow rate to 4.9 ± 0.3 nl·min−1·mm−1 further stimulated JK in these same mPKI-treated CCDs (P < 0.01 vs. JK at a slow flow rate), albeit the increase in JK (ΔJK = 4.3 ± 0.7 pmol·min−1·mm−1) was less than that measured in untreated controls (Δ JK = 11.8 ± 1.0 pmol·min−1·mm−1; P < 0.001) (Fig. 1B). The increase in luminal flow rate also stimulated JNa in CCDs perfused with mPKI (P < 0.01 vs. JNa at a slow flow rate); the flow-stimulated increase was similar to that observed in untreated controls [P = not significant (NS)] (Fig. 1A).
Fig. 1.
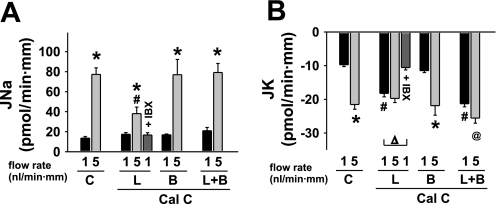
Effect of luminal (L) and/or basolateral (B) mPKI (5 μM) on basal and flow-stimulated net cation transport in isolated perfused rabbit cortical collecting ducts (CCDs). A: net Na absorption (JNa) increased in untreated control (C) tubules as the luminal flow rate was increased from 1 to 5 nl·min−1·mm−1. mPKI added to the luminal and/or basolateral solutions had no effect on flow-stimulated JNa, although the luminal inhibitor modestly increased JNa in tubules perfused at a slow flow rate. Luminal iberiotoxin (IBX), an inhibitor of BK but not ROMK channels, had no effect on baseline JNa in tubules perfused with mPKI. B: net K secretion (JK) increased ∼3-fold in response to a 5-fold increase in luminal flow rate in control CCDs. Addition of mPKI to the luminal but not basolateral solution increased JK in CCDs perfused at a flow rate of 1 nl·min−1·mm−1; a subsequent increase in flow rate augmented JK only slightly. Addition of mPKI to the basolateral solution alone or in the presence of luminal inhibitor inhibited flow stimulation of JK. Luminal IBX completely inhibited the mPKI-mediated increase in JK observed in CCDs perfused at a flow rate of 1 nl·min−1·mm−1. Values are means ± SE. For each protocol, n is indicated in results. *P < 0.05 compared with Jx at 1 nl·min−1·mm−1 in same tubules. #P < 0.05 compared with Jx in control tubules studied at same flow rate. Δ, P < 0.01 compared with JK at 1 nl·min−1·mm−1 in the same experimental group.
To determine whether the mPKI-induced stimulation of JK at the slow flow rate was mediated by the BK channel, the effect of the specific BK channel inhibitor IBX on JK in these CCDs was examined. As shown in Fig. 1B, JK in IBX-treated CCDs pretreated with luminal mPKI and perfused at a slow flow rate of 1.0 ± 0.1 nl·min−1·mm−1 (n = 3) was similar to that measured in control CCDs perfused at a similar flow rate (P = NS). IBX had no effect on JNa in the same CCDs pretreated with luminal mPKI (Fig. 1A).
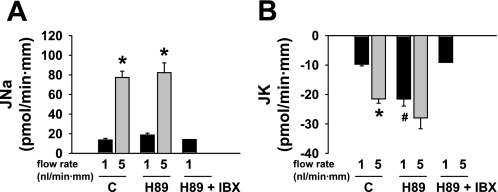
To rule out a nonspecific effect of luminal mPKI on JK, the effect of luminal H89 (10 μM), a cell-permeant isoquinolinesulfonamide derivative that inhibits PKA (10), on JNa and JK was examined at slow and fast flow rates. At a slow flow rate of 1.1 ± 0.2 nl·min−1·mm−1, JK in CCDs pretreated with luminal H89 (n = 6) was significantly greater than that measured in control tubules perfused at 1.0 ± 0.1 nl·min−1·mm−1 (n = 3; P < 0.01); in two CCDs perfused at a slow flow rate of 1 nl·min−1·mm−1, luminal IBX prevented the increase in JK elicited by luminal H89 (JK = −8.5 and −9.7 pmol·min−1·mm−1) (Fig. 2B). JNa was similar in H89-treated and control CCDs perfused at the slow flow rate (P = 0.14) (Fig. 2A). A fivefold increase in luminal flow rate to 4.8 ± 0.3 nl·min−1·mm−1 further stimulated JK in the H89-treated CCDs (P < 0.03 vs. JK at a slow flow rate) (Fig. 2B); the flow-stimulated increase in JNa in these same CCDs (P < 0.01 vs. JNa at a slow flow rate) was similar to that observed in untreated controls (P = NS) (Fig. 2A). Given that H89 has been shown to lack specificity for PKA, but also inhibits PKC isoforms (48), we elected to pursue further studies with mPKI, which specifically inhibits PKA activity and has no effect on other kinase families (78).
Fig. 2.
Effect of luminal (L) H89 (10 μM) on basal and flow-stimulated net cation transport in isolated perfused rabbit CCDs. A: JNa increased in untreated control (C) tubules as the luminal flow rate was increased from ∼1 to 5 nl·min−1·mm−1. H89 added to the luminal solution had no effect on flow-stimulated JNa. JNa in 2 tubules perfused with H89 and luminal IBX (10.5 and 17.8 pmol·min−1·mm−1) was similar to that measured in control CCDs and those perfused with H89 alone at a flow rate of ∼1 nl·min−1·mm−1. B: JK increased ∼3-fold in response to a 5-fold increase in luminal flow rate in control CCDs. Addition of H89 to the luminal solution increased JK in CCDs perfused at a flow rate of 1 nl·min−1·mm−1; a subsequent increase in flow rate augmented JK only slightly. JK in 2 tubules perfused with H89 and luminal IBX (−8.5 and −9.7 pmol·min−1·mm−1) was similar to that measured in control CCDs perfused at a flow rate of ∼1 nl·min−1·mm−1. Values are means ± SE. For each protocol, n is indicated in results. *P < 0.05 compared with Jx at 1 nl·min−1·mm−1 in same tubules. #P < 0.05 compared with Jx in control tubules studied at same flow rate.
To examine whether luminal mPKI alters BK channel activity, the effect of this inhibitor on channel activity (NPo) was studied in cell-attached patches of the apical membranes of both principal and intercalated cells in rat CCDs. We first sought to isolate the effect of the inhibitor on the apical patch of membrane within the pipette. Thus, in one set of experiments, patch pipettes were backfilled with mPKI (5 μM). BK channels were identified based on their large single-channel conductance and characteristic kinetics (30, 42). As shown in Fig. 3, an increase in activity of a high-conductance channel considered to be the apical BK channel was observed in 3 of 10 patches of principal cells (studied at a holding potential of 0 mV) after a delay of ∼300 s, presumably representing the time for mPKI to diffuse to the vicinity of the membrane patch. In eight principal cell-attached patches, mPKI backfilled in the pipette reduced NPo of the low-conductance ROMK channel from 2.6 ± 0.5 to 1.5 ± 0.6 (P < 0.05), consistent with diffusion of the inhibitor backfilled in the pipette solution to the vicinity of the membrane patch. Although it is possible that the PKI-induced increase in principal cell BK channel activity was due to stretch or cell injury (with a consequent increase in [Ca2+]i), we did not detect an increase in BK channel activity in an additional 15 principal cell-attached patches monitored for 12 min with “vehicle” (standard pipette solution) alone in the pipette.
A major limitation of the patch-clamp studies described above is that BK channels are present in low density in principal cells, thus making it likely that BK channels were not present in the seven cells that did not respond to mPKI added to the patch-clamp pipette and the principal cell-attached patches monitored in the absence of the inhibitor. Thus a second set of experiments similar to those described above was performed except that principal cells that failed to respond to PKI added to the bathing solution were subsequently exposed to bath ionomycin (1 μM) to increase [Ca2+]i and thereby activate silent BK channels in the cell-attached patch (30). As shown in Fig. 4, A (representative tracing) and B, PKI added to the bathing solution increased apical BK channel activity in five of a total of nine principal cell-attached patches (P < 0.05). Ionomycin had no effect on channel activity in the four patches that did not respond to mPKI, suggesting that these patches did not contain BK channels.
Apical mPKI (i.e., backfilled in the pipette) led to a reduction in NPo of the BK channel in intercalated cells, studied at a holding potential of 100 mV (n = 5; P < 0.05) (Fig. 3B).
In contrast to the effect of luminal mPKI, addition of the inhibitor to the basolateral solution alone (n = 4) did not stimulate JK at the slow flow rate of 1.1 ± 0.2 nl·min−1·mm−1 (P = NS vs. control at same flow rate) (Fig. 1B). Nor did an increase in flow rate in these tubules to 6.0 ± 0.6 nl·min−1·mm−1 elicit an increase in JK (P = NS vs. JK at the slow flow rate). Addition of mPKI to both luminal and basolateral solutions (n = 3) blocked the flow-stimulated increase in JK without any effect on JK at the slow flow rate (Fig. 1B), results similar to those observed in response to the basolateral inhibitor alone; JK was thus identical in CCDs pretreated with both luminal and basolateral mPKI and perfused at 1.0 ± 0.1 and 5.2 ± 0.3 nl·min−1·mm−1 (P = NS) (Fig. 1B). Neither basolateral alone or bilateral mPKI had a significant effect on baseline or flow-stimulated JNa (Fig. 1A).
These results suggest that apically localized PKA inhibits, either directly or indirectly, the BK channel in principal cells under low-flow conditions and that PKA activity is required to allow for a flow-stimulated increase in JK.
Effect of calphostin C on flow-stimulated JK and BK channel activity.
Calphostin C (500 nM) binds to the DAG binding site present in not only PKC but a number of other proteins (24, 61). This inhibitor was added to the luminal and/or basolateral solutions of microperfused rabbit CCDs, and the rates of JK and JNa were measured at slow and fast flow rates. At a slow flow rate of 1.0 ± 0.1 nl·min−1·mm−1 (n = 9), JK in CCDs pretreated with luminal calphostin C exceeded that measured in untreated tubules (n = 3) perfused at 1.0 ± 0.1 nl·min−1·mm−1 (P < 0.05) (Fig. 5B). A fivefold increase in luminal flow rate to 5.6 ± 0.4 nl·min−1·mm−1 failed to further stimulate JK in these CCDs (P = NS vs. JK at the slow flow rate). Luminal calphostin C had no effect on JNa in CCDs perfused at 1 nl·min−1·mm−1 (P = NS vs. control at the same flow rate) (Fig. 5A). Although an increase in flow rate significantly increased JNa (P < 0.05 vs. JNa at the slow flow rate) (Fig. 5A), the flow-induced increase in JNa in CCDs pretreated with luminal calphostin C was blunted compared with that measured in untreated CCDs (ΔJNa = 21.6 ± 6.8 vs. 63.5 ± 7.1 pmol·min−1·mm−1, respectively; P < 0.01). As the focus of this study was on the BK channel, we did not further explore the molecular mechanism underlying this observation.
Fig. 5.
Effect of luminal (L) and/or basolateral (B) calphostin C (500 nM) on flow-stimulated net cation transport in isolated perfused rabbit CCDs. A: JNa increased in untreated control (C) tubules as the luminal flow rate was increased from ∼1 to 5 nl·min−1·mm−1. Calphostin C added to the luminal but not bilateral solutions modestly inhibited the magnitude of flow-stimulated JNa. Luminal IBX had no effect on baseline JNa in tubules perfused with calphostin C. B: JK increased ∼3-fold in response to a 5-fold increase in luminal flow rate in control CCDs. Addition of calphostin C to the luminal but not basolateral solution increased JK in CCDs perfused at a flow rate of 1 nl·min−1·mm−1; a subsequent increase in flow rate failed to augment JK further. Addition of calphostin C to both luminal and basolateral solutions had the same effect as addition of the inhibitor to the luminal solution alone. Luminal IBX completely inhibited the increase in JK observed in CCDs perfused with calphostin C and studied at a flow rate of 1 nl·min−1·mm−1. Values are means ± SE. For each protocol, n is indicated in results. *P < 0.05 and @P = 0.05 compared with Jx at 1 nl·min−1·mm−1 in same tubules. #P < 0.05 compared with Jx in control tubules studied at same flow rate. Δ, P < 0.01 compared with JK at 1 nl·min−1·mm−1 in the same experimental group.
To determine whether the calphostin C-induced stimulation of JK at slow flow rates was mediated by the BK channel, the effect of IBX on JK in CCDs pretreated with luminal calphostin C was examined. In six CCDs perfused at a slow flow rate of 0.8 ± 0.1 nl·min−1·mm−1, luminal IBX prevented the increase in JK elicited by luminal calphostin C (Fig. 5B); an increase in luminal flow rate in these tubules failed to stimulate JK further. IBX had no significant effect on JNa in CCDs pretreated with calphostin C at either flow rate (Fig. 5A).
In contrast to the effect of luminal calphostin C, addition of the inhibitor to the basolateral solution alone (n = 3) failed to increase JK at a slow flow rate of 1.0 ± 0.1 nl·min−1·mm−1 (Fig. 5B). In response to an increase in flow rate to 4.6 ± 0.7 nl·min−1·mm−1, this group of CCDs exhibited a significant increase in JK (P < 0.05) (Fig. 5B). Addition of calphostin C to both luminal and basolateral solutions (n = 3) led to similar effects to those observed in CCDs exposed to luminal calphostin C alone (Fig. 5B). Specifically, the inhibitor applied to both apical and basolateral membranes stimulated JK in CCDs perfused at a slow flow rate of 0.9 ± 0.1 nl·min−1·mm−1 to a rate greater than that measured in control tubules perfused at this flow rate (P < 0.05) (Fig. 5B). A fivefold increase in luminal flow rate to 5.2 ± 0.2 nl·min−1·mm−1 in CCDs pretreated with luminal and basolateral calphostin C elicited only a modest increase in JK (ΔJK = 4.2 ± 0.6 pmol·min−1·mm−1) compared with that observed in untreated control CCDs (ΔJK = 11.8 ± 1.0 pmol·min−1·mm−1; P < 0.03). Neither basolateral alone nor bilateral calphostin C had a significant effect on baseline or flow-stimulated JNa (Fig. 5A).
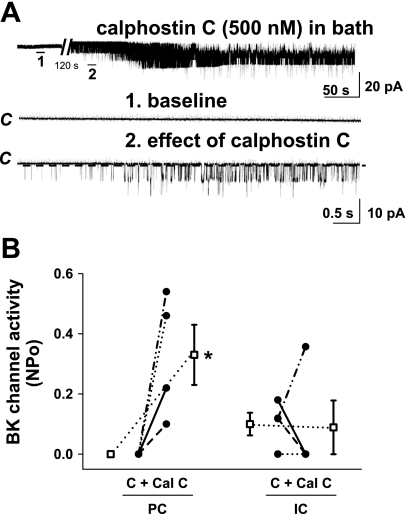
To determine whether calphostin C altered apical BK channel activity, the effect of this inhibitor on channel NPo was studied in cell-attached patches of the apical membranes of both intercalated and principal cells in rat CCDs. Addition of calphostin C to the bathing solution of split-open CCDs (i.e., access to both apical and basolateral membranes) increased activity of apical BK channels in principal cells studied at a holding potential of 0 mV (n = 4; P < 0.05) but had no significant effect on channel activity in intercalated cells (n = 4; P = NS) (Fig. 6). Addition of calphostin C to the pipette solution alone, as described above, had no effect on BK channel activity in principal cells (0 to 0.11 ± 0.11; n = 10; P = NS).
Fig. 6.
Effect of calphostin C (500 μM), added to the solution bathing split-open rat CCDs, on BK channel activity. A: representative traces of the stimulatory effect of calphostin C on silent apical BK channels in a principal cell-attached patch. The top trace shows the time course of the entire experiment performed at a holding potential of 0 mV. The expanded traces (1–3) below show detailed channel activity at a faster time resolution. The channel-closed state (“C”) is indicated by a dashed line. B: effect of calphostin C on NPo of the BK channel in individual cell-attached patches of PC (n = 4) and IC (n = 4) studied at holding potentials of 0 and 100 mV, respectively. •, Paired data from individual cell-attached patches; □, means ± SE for the control and experimental data sets. *P < 0.05 compared with C in the same cell type.
These results suggest that a C1 domain, DAG-binding protein inhibits, either directly or indirectly, the BK channel in principal cells under low-flow conditions and that PKC is required for flow-stimulated JK.
Effect of BIM and Gö6976 on flow-stimulated JK and BK channel activity.
Rabbit CCDs were pretreated with the PKC inhibitors BIM (1 μM) and Gö6976 (100 nM), added to the luminal and/or basolateral solutions, and the rates of JK and JNa were measured at slow and fast flow rates. Luminal BIM and Gö6976 had no discernable effect on the rate of JK (P = NS vs. JK in controls) in three CCDs perfused at a slow flow rate of 0.9 ± 0.1 nl·min−1·mm−1 (Fig. 7B) ; an increase in tubular fluid flow rate to 5.5 ± 0.5 nl·min−1·mm−1 in these tubules led to a typical increase in JK (P < 0.05 vs. JK at a slow flow rate) (Fig. 7B). Both inhibitors added to the pipette (n = 4) or solution bathing (n = 5) split-open rat CCDs failed to stimulate BK channel activity in principal cells (data not shown).
Fig. 7.
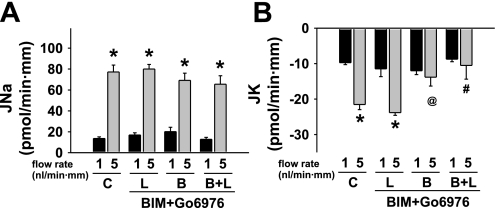
Effect of luminal (L) and/or basolateral (B) bisindolylmaleimide 1 hydrochloride (BIM;1 μM) and Gö6976 (100 nM) on flow-stimulated net cation transport in isolated perfused rabbit CCDs. A: JNa increased in untreated control (C) tubules as the luminal flow rate was increased from ∼1 to 5 nl·min−1·mm−1. BIM and Gö6976 added to the luminal and/or basolateral solutions had no effect on flow-stimulated JNa. B: JK increased ∼3-fold in response to a 5-fold increase in luminal flow rate in control CCDs. Addition of BIM and Gö6976 to the basolateral solution alone or in the presence of luminal inhibitor inhibited flow stimulation of JK. Values are means ± SE. For each protocol, n is indicated in results. *P < 0.05 compared with transport rate at 1 nl·min−1·mm−1 in same tubules. #P < 0.05 and @P = 0.05 compared with transport rate in control tubules studied at same flow rate.
In contrast to the effect of luminal BIM and Gö6976, addition of the inhibitor to the basolateral solution alone (n = 3) prevented flow-stimulation of JK as the tubular fluid flow rate was increased from 1.0 ± 0.1 to 4.9 ± 0.2 nl·min−1·mm−1 (P = NS) (Fig. 7B). Similarly, in the presence of both luminal and basolateral inhibitors (n = 3), an increase in flow rate from 0.9 ± 0.1 to 5.1 ± 0.4 nl·min−1·mm−1 failed to elicit an increase in JK (P = NS) (Fig. 7B). BIM and Gö6976 had no significant effect on baseline or flow-stimulated JNa whether added to the luminal and/or basolateral solutions (Fig. 7A).
These data suggest flow-stimulated JK, but not basal JK or JNa under low- or high-flow conditions, is regulated at least in part by PKC, possibly by a signaling pathway localized to the basolateral aspect of the CCD.
Effect of protein kinase inhibitors on basal and flow-stimulated [Ca2+]i.
We have previously reported that flow-stimulated JK requires an increase in [Ca2+]i. To test whether the inhibitor-associated changes in JK were due to alterations in [Ca2+]i, rabbit CCDs were pretreated with mPKI (5 μM), calphostin C (500 nM), or BIM (1 μM) and Gö6976 (100 nM), and the effects on [Ca2+]i in principal and intercalated cells were examined in CCDs perfused under low flow and then in response to an acute increase in luminal flow rate. Inhibitors were added to the luminal and/or basolateral solutions based on the transport data presented in Figs. 1, 5, and 7.
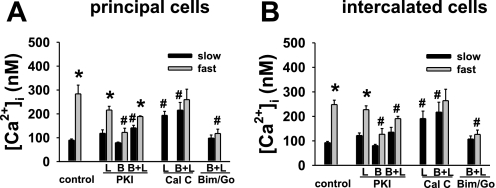
In CCDs perfused at a slow flow rate in the presence of mPKI added to the lumen (n = 3) or bath (n = 3) alone, [Ca2+]i in principal and intercalated cells did not differ (P = NS) from that measured in untreated CCD (n = 3) principal and intercalated cells. When mPKI was added to the luminal and basolateral solutions of CCDs perfused at a slow flow rate (n = 3), [Ca2+]i in intercalated cells was also similar to that observed in untreated cells (P = NS), although [Ca2+]i in principal cells exceeded that detected in controls (P < 0.03).
An acute increase in luminal flow rate led to a rapid, albeit brief rise in [Ca2+]i, a response we have previously described in detail and attributed to release of Ca2+ from internal stores and/or mechanosensitive Ca2+ influx (33, 34), in principal and intercalated cells exposed to luminal or bilateral mPKI (Fig. 8). The magnitude of the flow-induced increase in [Ca2+]i in principal cells in untreated control CCDs (Δ[Ca2+]i = 194.0 ± 41.3 nM) was not statistically different from that measured in CCDs perfused with luminal mPKI (Δ[Ca2+]i = 97.6 ± 19.0 nM; P = 0.10). However, the flow-induced increase in [Ca2+]i in principal cells exposed to bilateral mPKI (Δ[Ca2+]i = 47.8 ± 9.0 nM) was less than that measured in controls (P < 0.03). Addition of mPKI to the bath alone inhibited the flow-induced increase in [Ca2+]i in both principal (P = 0.03 vs. control) and intercalated (P = 0.03 vs. control) cells (Fig. 8).
Fig. 8.
Effect of luminal (L) and/or basolateral (B) kinase inhibitors on baseline (black) and peak (gray) [Ca2+]i in CCDs perfused at, respectively, slow and fast flow rates in PC and IC. Values are means ± SE. For each protocol, n is indicated in results. *P < 0.05 compared with slow flow in the same group of tubules. #P < 0.05 vs. control at same flow rate.
Addition of calphostin C to the luminal solution alone (n = 3) or both luminal and basolateral solutions (n = 3) increased [Ca2+]i in CCDs perfused at a slow flow rate in both principal and intercalated cells to values significantly greater (P < 0.03) than those measured in untreated CCDs, and to Ca2+ concentrations proposed to be adequate to stimulate BK channel activity in the rat CCD (43). An acute increase in luminal flow rate in the CCDs pretreated with lumen and basolateral calphostin C failed to further augment [Ca2+]i; the magnitude of the flow-induced increases in [Ca2+]i were less than those measured in untreated principal and intercalated cells (P < 0.03 vs. control for individual cell type).
As expected, based on the transport data, [Ca2+]i in principal and intercalated cells in CCDs (n = 3) perfused at a slow flow rate in the presence of BIM and Gö6976 added to the lumen and bath solutions did not differ (P = NS) from the values measured in untreated cells. An acute increase in luminal flow rate in these tubules failed to elicit an increase in [Ca2+]i in either cell type (P = NS vs. peak [Ca2+]i in same cell type at a slow flow rate).
DISCUSSION
We have previously reported that an increase in luminal flow leads to a transient increase in [Ca2+]i and a sustained increase in BK channel-mediated (IBX-sensitive) JK in the CCD (33, 34, 86). The question as to how a transient flow-induced increase in [Ca2+]i leads to a sustained increase in JK can be most easily explained by a Ca2+-associated or mechano-induced stimulation of specific target molecules or transducer proteins (54). Elevations in [Ca2+]i trigger multiple signaling pathways, many of which depend on protein phosphorylation/dephosphorylation. Abundant electrophysiological and biochemical evidence has led to the notion that BK channels are associated with a variety of protein kinases and phosphatases localized in a regulatory complex at the plasma membrane (22, 35, 50, 79, 83). Furthermore, mechanical stimuli, including shear and stretch, activate kinases including PKC (9) and PKA (81). The focus of the present study was to test the hypothesis that endogenous PKA and PKC participate in the regulation of BK channel-mediated flow-stimulated JK in the mammalian CCD.
The results of the present study provide novel insight into the regulation of cation transport in the distal nephron. A major finding, widely relevant to the study of polarized transport in absorptive and secretory epithelia, is the clear sidedness of the response of the cells therein to “cell-permeant” kinase inhibitors. Specifically, addition of mPKI, calphostin C, or BIM and Gö6976 to the luminal fluid had different effects on cation transport than did addition of these agents to the bathing solution. These results are consistent with a functional asymmetry of regulatory pathways in the CCD, as has been described in other polarized renal epithelial cells (2, 51, 75), and the localization of discrete signaling microenvironments, which include multiprotein complexes containing receptors, second messengers, kinases, phosphatases, and substrates to the apical and/or basolateral membranes. Furthermore, our data suggest that the BK channel localized to the apical membrane is closely associated with signaling and other accessory proteins, as has been described for the channel in smooth muscle and brain (reviewed in Ref. 35).
Under low-flow conditions, apical BK channels in principal, but not intercalated cells, appear to be tonically inhibited by PKA. The observation that luminal but not basolateral mPKI activates IBX-sensitive JK in CCDs perfused at a slow flow rate (Fig. 1B), in the absence of an increase in [Ca2+]i (Fig. 8), provides compelling evidence for the presence of a compartmentalized cAMP-dependent signaling system at the apical membrane that constitutively suppresses activity of the apical BK channel. One possibility is that the apical BK channel is closely associated with PKA anchored via its regulatory subunits to that membrane via an A kinase anchoring protein (AKAP), as has been proposed in the brain (26, 35). The modest flow-induced increase in JK in CCDs perfused with luminal mPKI (Fig. 1B) likely represents the incremental activation of already almost maximally activated channels by a localized flow-induced increase in [Ca2+]i (Fig. 8).
The very disparate effects detected between luminal and basolateral exposure to mPKI (Fig. 1) might be interpreted to represent a nonspecific effect of the agent on one or the other membrane. However, luminal H89 (Fig. 2) had similar effects to those observed with luminal mPKI, data providing additional evidence for a regulatory role of apically localized PKA in BK channel function. We propose that the variable inhibitory effects of luminal vs. basolateral mPKI may reflect anchoring of the inhibitor to the cell membrane, which limits its access to the cell interior and thus hinders its interaction with the entire cytoplasmic pool of soluble PKA catalytic subunits when applied at the unique membranes, as has been proposed by others to account for incomplete inhibition of PKA-mediated effects by this reagent (39). As others have reported effects of mPKI on vectorial transport in polarized transporting epithelia when the inhibitor is added solely to the luminal (45) or basolateral (18) solutions, we believe that mPKI does gain access into the interior of the cell. Finally, it should be noted that other transport proteins in kidney (i.e., type IIa Na-Pi cotransporters) are differentially affected by apical vs. basolateral kinase activators and inhibitors, consistent with the notion that signaling cascades may be polarized to unique membranes (75).
Basolateral (in the absence or presence of luminal) mPKI did not increase JK in tubules perfused at a slow flow rate but inhibited flow-stimulated JK, an observation we attribute to the blunted [Ca2+]i response of these CCDs to flow (Fig. 8) and indicative that PKA activity is required to allow for a flow-stimulated increase in [Ca2+]i and JK. The extent to which PKA regulates the ionic conductances mediating mechanosensitive Ca2+ entry, release of Ca2+ from intracellular stores, and/or trafficking of subapical/cytoplasmic vesicles containing preformed BK and/or Ca2+ channels into the appropriate plasma membranes has yet to be explored.
Alternative splicing of the pore-forming α-subunit of BK channels modifies its regulation by protein phosphorylation, as summarized in the beginning of this paper. Studies of excised patches of HEK cells reveal that endogenous PKA activity activates heterologously expressed BK channels that lack splice inserts (ZERO), yet inhibits channels expressing the STREX 59 amino acid insert, which introduces a new PKA consensus site into the channel sequence (68, 69). PKA-dependent inhibition of BK channels requires that only a single subunit of the four is phosphorylated at the STREX-specific PKA consensus site (35, 67). Thus STREX has been proposed to act as a molecular switch converting the phenotype of BK channels from one activated by PKA to one inhibited by this kinase (67). We have recently reported that the rabbit CCD expresses message encoding the STREX variant (13). The composition of BK channels in principal and intercalated cells has yet to be defined, but the differing effects of apical mPKI on BK channel NPo in these two cell types (Fig. 3) provide strong evidence for the notion that channels in the former cells include the STREX exon whereas those in the latter cells do not. It should also be noted that the BK channel β1-subunit appears to be localized exclusively to principal and connecting tubules cells and is absent in intercalated cells (19), whereas β4 is expressed exclusively in intercalated cells (as unpublished observations in Ref. 47). Differences in the β-subunit isoform comprising the BK channel between intercalated and principal cells may also account for the variability in sensitivity to kinase inhibitors demonstrated in the present study.
Numerous studies using pharmacological activators and inhibitors assumed to target PKC have suggested that this kinase, either directly or indirectly, regulates Na and K channel activity in the rat CCD and transepithelial cation transport in the rabbit CCD (23, 31, 80). Phorbol 12-myristate, 13-acetate (PMA) or l-α-1,2-dioctanoylglycerol (l-a-1,2-DOG), agents which had historically been presumed to activate PKC, inhibit the apical Na conductance in A6 cells (31) and inhibit JNa and JK in rabbit CCDs microperfused at rates of 0.4–2.6 nl·min−1·mm−1 (23) in a dose-dependent manner. However, the specificity of these drugs has since been questioned. It is now known, for example, that phorbol esters activate chimaerins, protein kinase D, RasGRPs, Munc13s, and DAG kinase γ (25).
In the rabbit CCD perfused at a slow flow rate of ∼1 nl·min−1·mm−1, calphostin C added to either the luminal perfusate alone or both luminal and basolateral solutions uncovered an IBX-sensitive K secretory flux that could not be further augmented by an increase in luminal flow rate (Fig. 5B). Calphostin C also increased NPo of BK channels in principal cells in the rat CCD (Fig. 6). While these results suggest that apical BK channels in principal cells of tubules perfused at a slow flow rate are tonically inhibited by one or more DAG-binding proteins, the observation that this inhibitor elevated [Ca2+]i to >200 nM (Fig. 8), a threshold above which BK channels are activated in the intact K-secreting tubule (43), suggests it likely that the effect of luminal calphostin on the BK channel reflects channel activation by the increase in [Ca2+]i. While we do not understand how calphostin C increases [Ca2+]I, we speculate that this may be due to direct and/or indirect effects of the inhibitor on mechanosensitive Ca2+ entry, internal store release, and/or trafficking of Ca2+ channels to the membrane. An alternative explanation for the calphostin C-induced effect on IBX-sensitive JK, as has been described for the PKCδ inhibitor rottlerin (20), is that the drug may directly stimulate BK channels in an interaction not associated with PKC or the level of [Ca2+]i (88). Specifically, rottlerin applied to the extracellular membrane of cell-attached patches of pituitary tumor GH3 cells (i.e., added to the patch pipette), increases BK channel Po in a concentration-dependent manner, a response not affected by addition of 10 μM BAPTA-AM to the bath and attributed to a direct interaction of the compound with the α-subunit (88).
It is worth noting that although calphostin C increased [Ca2+]i in both principal and intercalated cells in the CCD (Fig. 8), this agent inhibited BK channel NPo in intercalated cells (Fig. 6), an observation that suggests that intercalated cell BK channels are less sensitive to increases in [Ca2+]i than are the channels in principal cells. Indeed, Pacha et al. (42) reported stretch activation of BK channels in rabbit intercalated cells studied under conditions designed to minimize changes in [Ca2+]i (excision of patches into low-Ca2+ solutions and chelation of Ca2+ in the pipette or bath) and concluded that mechanoactivation of rabbit intercalated cell channels is due to membrane deformation and not changes in [Ca2+]i. These data are again consistent with fundamental differences in the composition of the BK channel in principal and intercalated cells.
The absence of an effect of luminal BIM and Gö6976 on JK at slow flow rates (Fig. 7) implies that the target of luminal calphostin C is not a classic or novel isoform of PKC. Although the identity of the calphostin C-sensitive DAG-binding protein possibly involved in regulation of BK channel activity remains to be clarified, a role for RasGRP1, a Ras guanyl nucleotide-releasing protein with Ca2+ and DAG-binding motifs, present in the mammalian distal tubule (90), in downstream activation of the Ras/Raf/MEK/ERK cascade, is intriguing as MAPK has been reported constitutively to inhibit the BK channel in rat principal cells (30). Notably, RasGRP1 plays a critical role in mediating the phorbol ester-induced suppression of activity of the NaCl cotransporter in the DCT through stimulation of the MAPK pathway (27), presumably via alterations in cell surface expression.
The inhibition of flow-stimulated JK and an increase in [Ca2+]i in CCDs perfused and bathed with BIM and Gö6976 provides support for the premise that flow-activated mechanosensitive Ca2+ channels, internal store release, and/or or trafficking of BK and Ca2+ channels to the membrane are regulated by PKC in the CCD. The absence of an effect of Bim and Gö6976 on single-channel activity in split-open CCDs suggests it unlikely that PKC regulates the BK channel itself or recruitment of BK channels to the apical membrane. However, we acknowledge that it may be difficult to assess recruitment of channels to the plasma membrane in a cell-attached patch, given the distortions of the apical membrane that occur when the patch is created.
Our results do not allow us to identify which of the >10 mammalian PKC isoform(s) participates in flow stimulation of JK in vivo. Immunolocalization studies in the mouse kidney (49) reveal that CCD intercalated but not principal cells express PKCα, -βI, and -δ. In the rat kidney (46), immunodetectable PKCα was identified in principal cells as well as intercalated cells. It is possible that several different PKC isozymes are activated in the rabbit CCD by flow, including both Ca2+-dependent and Ca2+-independent isozymes, each of which may have specific downstream substrates.
Among the candidate mechanosensitive entry Ca2+ channels identified in the CCD, which include the temperature-sensitive TRPV4 (33, 66, 72, 87), TRPC6 (17), and the complex of TRPP1 (polycystin 1) and TRPP2 (polycystin 2)(41), members of the transient receptor potential (TRP) superfamily of ion channels, TRPV4 (16) and TRPC6 (60), are activated by DAG and/or PKC. TRPP-1 has been reported to be phosphorylated by PKA (44, 84). While the data summarized in Fig. 8 are compatible with a requirement for direct phosphorylation of the mechanosensitive Ca2+ conductance to elicit a flow-induced increase in [Ca2+]i in the CCD, it is also possible that PKC and PKA are necessary for internal Ca2+ store release and/or trafficking of channels to the membrane in response to mechanical forces. Thus, for example, PKC, in an additive manner to Ca2+, may enhance the recruitment of vesicles to a readily releasable pool, as proposed in neurosecretory cells (40), where the process has been ascribed to remodeling of cortical F-actin (76, 77).
An increase in luminal flow rate in CCDs perfused with luminal PKI H89 or calphostin only minimally increased JK. Indeed, flow-stimulated JK never exceeded ∼25 pmol·min−1·mm−1 in any tubule studied. The physiological basis for this apparent saturation of JK is uncertain. One might expect that at high flow rates, the fall in luminal K concentration (Table 1) should maximize the concentration gradient for continued K secretion across the apical membrane (36). However, studies by ourselves (53) and others (12, 63) demonstrate that at luminal flow rates exceeding 5 nl·min−1·mm−1, the rate of JK appears to saturate. Engbretson and Stoner (12) further demonstrated in the rabbit CCD that flow-stimulated JK was not influenced by the transepithelial electrical gradient nor the chemical gradient and concluded that changes in axial flow rate may affect other determinants of K secretion, such as the apical membrane K permeability or other K secretory pathways, or indirectly via its effect on Na absorption. Subsequently, Malnic et al. (36) suggested, based on studies performed in the rat distal nephron microperfused in vivo, that K secretion is limited at high flow rates due to a limited capacity for Na absorption, which saturates at high flow rates in rabbit CCD (56), and/or the basolateral pump to respond to increased Na delivery with enhanced absorption (36).
Table 1.
Effect of kinase inhibitors on mean K concentrations of tubular fluid collected from cortical collecting ducts
| Condition | [K]L at Slow Flow Rate, meq/l | [K]L at Fast Flow Rate, meq/l |
|---|---|---|
| Control | 13.9±0.5 | 8.3±0.4 |
| PKI | ||
| L | 23.4±1.9 | 10.0±0.6 |
| B | 14.3±2.6 | 6.6±0.8 |
| L+B | 12.0±1.3 | 6.8±0.6 |
| H89, L | 26.4±1.2 | 10.3±1.1 |
| Calphostin C | ||
| L | 23.2±1.9 | 8.6±0.7 |
| B | 15.9±2.2 | 8.9±0.6 |
| L+B | 27.0±1.5 | 9.5±0.4 |
| BIM+Gö6976 | ||
| L | 15.0±1.2 | 8.8±0.6 |
| B | 16.7±0.6 | 10.2±2.7 |
| L+B | 15.1±1.0 | 6.6±0.5 |
Values are means ± SE. [K]L, mean K concentration of tubular fluid; L, luminal; B, basolateral; BIM, bisindolylmaleimide.
Also not understood is the mechanism underlying the dissociation of JK and JNa when BK channels are activated or suppressed by the various kinase antagonists. Of note is that we have demonstrated a similar dissociation of the two transport processes, ostensibly intimately linked via the basolateral Na-K-ATPase, in the rabbit CCD in a number of other situations, including in the immediate postnatal period (53) and in animals subject to dietary Na manipulation sufficient to alter circulating aldosterone concentrations in the physiological range (13). Net transepithelial fluxes and transepithelial potential in the CCD perfused in its native geometry reflect the composite function of the full repertoire of polarized membrane channels and transporters, including ENaC, ROMK, and the BK channel, H+ and H-K-ATPases, Cl− and Ca2+ channels at the apical membrane, and the Na-K pump and channels and transporters at the basolateral membrane. Furthermore, each of these transport proteins may be uniquely modified by cellular kinases, phosphatases, and other regulatory proteins. While this complexity raises the question as to the suitability of the isolated, perfused tubule as a model with which to study transport, one can argue that the function and regulation of single transport molecules examined by, for example, single-channel recordings of split-open tubules by patch-clamp analysis, can best be interpreted in the context of their role in transepithelial transport in the corresponding native tubule.
Uncertainty remains as to the identity of the cell type in the CCD that mediates flow-stimulated JK. The results of the present study suggest that BK channels in principal cells may be constitutively inhibited by PKA at slow flow rates. As such, quiescent BK channels in these cells may represent a potential pool of channels that can be activated and recruited to secrete K under high-flow conditions. Li et al. (30) have also speculated that BK channels in principal cells, constitutively inhibited by ERK and P38 MAPK in rats ingesting a normal K diet, may participate in net K secretion under conditions associated with inhibition of the MAPK pathway. However, our detection of an increase in BK channel NPo in only 3 of 10 principal cells exposed to apical mPKI in the patch pipette (Fig. 3) suggests it unlikely that flow stimulation of JK in this segment can be attributed solely to “disinhibition” of channel activity in this cell population. Yet, intercalated cells exposed to mPKI in the pipette exhibited a reduction in BK channel NPo. While the differing responses of the channels in the two cell types to apical mPKI likely reflect differing compositions of the channel between the cells in terms of the α-splice variants and β-isoforms, and thus their kinase sensitivity, as discussed above, splitting a tubule open for patch-clamp studies likely alters the cytoskeleton organization at the apical membrane as well as local Ca2+ concentrations in the vicinity of the channel. Thus, as suggested above, one must exercise caution in extrapolating results generated from studies in split-open tubules to those obtained from analysis of intact segments perfused in their native geometry.
The focus of the present study was on the role of protein kinases in regulation of the BK channel. However, protein phosphatases 1 (PP1) and 2 (PP2A) have been reported to play a critical role in BK channel regulation in colonic smooth muscle cells (8), neuroendocrine anterior pituitary corticotroph cells (69, 70), and renal mesangial cells (52). To the extent that the BK channel in other tissues is intimately associated with both kinases and phosphatases, future efforts must expand on the current study to examine the contribution of phosphatases to channel modulation.
In sum, the results of the present study indicate that PKA and PKC regulate BK channel activity in the mammalian distal nephron. PKA appears to tonically inhibit the channel and/or an associated regulatory protein in principal but not intercalated cells in CCDs perfused at slow flow rates, suggesting that the apical principal cell channel is closely associated with a compartmentalized cAMP-dependent signaling system. The differing responses of principal and intercalated cell BK channels to the PKA inhibitor likely reflects variability in the α-splice variants and β-isoforms expressed in these unique cell types. Flow-induced activation of PKA and PKC appears to be necessary for mechanosensitive Ca2+ entry, internal store release, and/or trafficking of subapical/cytoplasmic vesicles containing preformed BK and/or Ca2+ channels into the appropriate plasma membrane. Finally, our data provide additional support for functional asymmetry of regulatory/signaling pathways in the CCD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-038470 (to L. M. Satlin), DK-051391 (to T. R. Kleyman), P30 DK-079307 (The Pittsburgh Center for Kidney Research), and RO1DK-54983 (to W. H. Wang). Y. Wei was supported by an American Heart Association Scientist Development Grant (0530092N).
ACKNOWLEDGMENTS
The authors gratefully acknowledge Beth Zavilowitz (Mount Sinai School of Medicine) for technical support. Abstracts of this work were presented at the Annual Meetings of the American Society of Nephrology in 2007 (San Francisco, CA) and 2008 (Philadelphia, PA).
REFERENCES
- 1. Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA 99: 14560–14565, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amsler K, Kuwada SK. Membrane receptor location defines receptor interaction with signaling proteins in a polarized epithelium. Am J Physiol Cell Physiol 276: C91–C101, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Ashby CD, Walsh DA. Characterization of the interaction of a protein inhibitor with adenosine 3′,5′-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase J. Biol Chem 247: 6637–6642, 1972 [PubMed] [Google Scholar]
- 4. Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science 253: 551–555, 1991 [DOI] [PubMed] [Google Scholar]
- 5. Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Barman SA, Zhu S, White RE. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 286: L1275–L1281, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Barman SA, Zhu S, White RE. Protein kinase C inhibits BKCa channel activity in pulmonary arterial smooth muscle. Am J Physiol Lung Cell Mol Physiol 286: L149–L155, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Carl A, Kenyon JL, Uemura D, Fusetani N, Sanders KM. Regulation of Ca2+-activated K+ channels by protein kinase A and phosphatase inhibitors. Am J Physiol Cell Physiol 261: C387–C392, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Cheng JJ, Wung BS, Chao YJ, Wang DL. Sequential activation of protein kinase C (PKC)-alpha and PKC-epsilon contributes to sustained Raf/ERK1/2 activation in endothelial cells under mechanical strain. J Biol Chem 276: 31368–31375, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990 [PubMed] [Google Scholar]
- 11. Dworetzky SI, Boissard CG, Lum-Ragan JT, McKay MC, Post-Munson DJ, Trojnacki JT, Chang CP, Gribkoff VK. Phenotypic alteration of a human BK (hSlo) channel by hSlobeta subunit coexpression: changes in blocker sensitivity, activation/relaxation and inactivation kinetics, and protein kinase A modulation. J Neurosci 16: 4543–4550, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Engbretson BG, Stoner LC. Flow-dependent potassium secretion by rabbit cortical collecting tubule in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 253: F896–F903, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Estilo G, Liu W, Pastor-Soler N, Mitchell P, Carattino MD, Kleyman TR, Satlin LM. Effect of aldosterone on BK channel expression in mammalian cortical collecting duct. Am J Physiol Renal Physiol 295: F780–F788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frindt G, Palmer LG. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F143–F151, 1989 [DOI] [PubMed] [Google Scholar]
- 15. Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, Garcia ML. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090, 1990 [PubMed] [Google Scholar]
- 16. Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 278: 27129–27137, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Goel M, Sinkins WG, Zuo CD, Hopfer U, Schilling WP. Vasopressin-induced membrane trafficking of TRPC3 and AQP2 channels in cells of the rat renal collecting duct. Am J Physiol Renal Physiol 293: F1476–F1488, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Good DW, George T. Neurotrophin-3 inhibits HCO absorption via a cAMP-dependent pathway in renal thick ascending limb. Am J Physiol Cell Physiol 281: C1804–C1811, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Grimm PR, Foutz RM, Brenner R, Sansom SC. Identification and localization of BK-β subunits in the distal nephron of the mouse kidney. Am J Physiol Renal Physiol 293: F350–F359, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199: 93–98, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Hagen BM, Bayguinov O, Sanders KM. β1-Subunits are required for regulation of coupling between Ca2+ transients and Ca2+-activated K+ (BK) channels by protein kinase C. Am J Physiol Cell Physiol 285: C1270–C1280, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hall SK, Armstrong DL. Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem 275: 3749–3754, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Hays SR, Baum M, Kokko JP. Effects of protein kinase C activation on sodium, potassium, chloride, and total CO2 transport in the rabbit cortical collecting tubule. J Clin Invest 80: 1561–1570, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hurley JH, Newton AC, Parker PJ, Blumberg PM, Nishizuka Y. Taxonomy and function of C1 protein kinase C homology domains. Protein Sci 6: 477–480, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kazanietz MG. Novel “nonkinase” phorbol ester receptors: the C1 domain connection. Mol Pharmacol 61: 759–767, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem 274: 4934–4938, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Ko B, Joshi LM, Cooke LL, Vazquez N, Musch MW, Hebert SC, Gamba G, Hoover RS. Phorbol ester stimulation of RasGRP1 regulates the sodium-chloride cotransporter by a PKC-independent pathway. Proc Natl Acad Sci USA 104: 20120–20125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lagrutta A, Shen KZ, North RA, Adelman JP. Functional differences among alternatively spliced variants of Slowpoke, a Drosophila calcium-activated potassium channel. J Biol Chem 269: 20347–20351, 1994 [PubMed] [Google Scholar]
- 29. Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol 56: 193–212, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Li D, Wang Z, Sun P, Jin Y, Lin DH, Hebert SC, Giebisch G, Wang WH. Inhibition of MAPK stimulates the Ca2+-dependent big-conductance K channels in cortical collecting duct. Proc Natl Acad Sci USA 103: 19569–19574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ling BN, Eaton DC. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol Renal Fluid Electrolyte Physiol 256: F1094–F1103, 1989 [DOI] [PubMed] [Google Scholar]
- 32. Ling S, Woronuk G, Sy L, Lev S, Braun AP. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem 275: 30683–30689, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Liu W, Morimoto T, Woda C, Kleyman TR, Satlin LM. Ca2+ dependence of flow-stimulated K secretion in the mammalian cortical collecting duct. Am J Physiol Renal Physiol 293: F227–F235, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol 570: 65–72, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 256: F932–F941, 1989 [DOI] [PubMed] [Google Scholar]
- 37. Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 268: 9194–9197, 1993 [PubMed] [Google Scholar]
- 38. McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 14: 645–650, 1995 [DOI] [PubMed] [Google Scholar]
- 39. Muniz M, Martin ME, Hidalgo J, Velasco A. Protein kinase A activity is required for the budding of constitutive transport vesicles from the trans-Golgi network. Proc Natl Acad Sci USA 94: 14461–14466, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagy G, Matti U, Nehring RB, Binz T, Rettig J, Neher E, Sorensen JB. Protein kinase C-dependent phosphorylation of synaptosome-associated protein of 25 kDa at Ser187 potentiates vesicle recruitment. J Neurosci 22: 9278–9286, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33: 129–137, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Pacha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Parnell SC, Magenheimer BS, Maser RL, Calvet JP. Identification of the major site of in vitro PKA phosphorylation in the polycystin-1 C-terminal cytosolic domain. Biochem Biophys Res Commun 259: 539–543, 1999 [DOI] [PubMed] [Google Scholar]
- 45. Pastor-Soler NM, Hallows KR, Smolak C, Gong F, Brown D, Breton S. Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294: C488–C494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pfaff IL, Wagner HJ, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol 10: 1861–1873, 1999 [DOI] [PubMed] [Google Scholar]
- 47. Pluznick JL, Sansom SC. BK channels in the kidney: role in K+ secretion and localization of molecular components. Am J Physiol Renal Physiol 291: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Prestle J, Pfizenmaier K, Brenner J, Johannes FJ. Protein kinase C mu is located at the Golgi compartment. J Cell Biol 134: 1401–1410, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Redling S, Pfaff IL, Leitges M, Vallon V. Immunolocalization of protein kinase C isoenzymes α, βI, βII, δ, and ε in mouse kidney. Am J Physiol Renal Physiol 287: F289–F298, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Reinhart PH, Levitan IB. Kinase and phosphatase activities intimately associated with a reconstituted calcium-dependent potassium channel. J Neurosci 15: 4572–4579, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reshkin SJ, Forgo J, Murer H. Apical and basolateral effects of PTH in OK cells: transport inhibition, messenger production, effects of pertussis toxin, and interaction with a PTH analog. J Membr Biol 124: 227–237, 1991 [DOI] [PubMed] [Google Scholar]
- 52. Sansom SC, Stockand JD, Hall D, Williams B. Regulation of large calcium-activated potassium channels by protein phosphatase 2A. J Biol Chem 272: 9902–9906, 1997 [DOI] [PubMed] [Google Scholar]
- 53. Satlin LM. Postnatal maturation of potassium transport in rabbit cortical collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 266: F57–F65, 1994 [DOI] [PubMed] [Google Scholar]
- 54. Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol 291: F923–F931, 2006 [DOI] [PubMed] [Google Scholar]
- 55. Satlin LM, Palmer LG. Apical K+ conductance in maturing rabbit principal cell. Am J Physiol Renal Physiol 272: F397–F404, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol 280: F1010–F1018, 2001 [DOI] [PubMed] [Google Scholar]
- 57. Schopperle WM, Holmqvist MH, Zhou Y, Wang J, Wang Z, Griffith LC, Keselman I, Kusinitz F, Dagan D, Levitan IB. Slob, a novel protein that interacts with the Slowpoke calcium-dependent potassium channel. Neuron 20: 565–573, 1998 [DOI] [PubMed] [Google Scholar]
- 58. Shipston MJ, Armstrong DL. Activation of protein kinase C inhibits calcium-activated potassium channels in rat pituitary tumour cells. J Physiol 493: 665–672, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem 271: 9197–9200, 1996 [DOI] [PubMed] [Google Scholar]
- 60. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586–16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays 26: 730–738, 2004 [DOI] [PubMed] [Google Scholar]
- 62. Stockand JD, Sansom SC. Mechanism of activation by cGMP-dependent protein kinase of large Ca2+-activated K+ channels in mesangial cells. Am J Physiol Cell Physiol 271: C1669–C1677, 1996 [DOI] [PubMed] [Google Scholar]
- 63. Stokes JB. Potassium secretion by cortical collecting tubule: relation to sodium absorption, luminal sodium concentration, and transepithelial voltage. Am J Physiol Renal Fluid Electrolyte Physiol 241: F395–F402, 1981 [DOI] [PubMed] [Google Scholar]
- 64. Sun P, Liu W, Lin DH, Yue P, Kemp R, Satlin LM, Wang WH. Epoxyeicosatrienoic acid activates BK channels in the cortical collecting duct. J Am Soc Nephrol 20: 513–523, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998 [DOI] [PubMed] [Google Scholar]
- 66. Taniguchi J, Tsuruoka S, Mizuno A, Sato JI, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol 292: F667–F673, 2007 [DOI] [PubMed] [Google Scholar]
- 67. Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci USA 101: 11897–11902, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Tian L, Hammond MS, Florance H, Antoni FA, Shipston MJ. Alternative splicing determines sensitivity of murine calcium-activated potassium channels to glucocorticoids. J Physiol 537: 57–68, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tian L, Knaus HG, Shipston MJ. Glucocorticoid regulation of calcium-activated potassium channels mediated by serine/threonine protein phosphatase. J Biol Chem 273: 13531–13536, 1998 [DOI] [PubMed] [Google Scholar]
- 71. Tian L, McClafferty H, Chen L, Shipston MJ. Reversible tyrosine protein phosphorylation regulates large conductance voltage- and calcium-activated potassium channels via cortactin. J Biol Chem 283: 3067–3076, 2008 [DOI] [PubMed] [Google Scholar]
- 72. Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol 287: F17–F24, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Tognon CE, Kirk HE, Passmore LA, Whitehead IP, Der CJ, Kay RJ. Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol Cell Biol 18: 6995–7008, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Krilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266: 15771–15781, 1991 [PubMed] [Google Scholar]
- 75. Traebert M, Volkl H, Biber J, Murer H, Kaissling B. Luminal and contraluminal action of 1–34 and 3–34 PTH peptides on renal type IIa Na-Pi cotransporter. Am J Physiol Renal Physiol 278: F792–F798, 2000 [DOI] [PubMed] [Google Scholar]
- 76. Trifaro JM, Lejen T, Rose SD, Pene TD, Barkar ND, Seward EP. Pathways that control cortical F-actin dynamics during secretion. Neurochem Res 27: 1371–1385, 2002 [DOI] [PubMed] [Google Scholar]
- 77. Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron 14: 353–363, 1995 [DOI] [PubMed] [Google Scholar]
- 78. Walsh DA, Angelos KL, Van Patten SM, Glass DB, Garetto LP. (Editors). Peptides and Protein Phosphorylation. Boca Raton, FL: CRC, 1990, p. 43–84 [Google Scholar]
- 79. Wang J, Zhou Y, Wen H, Levitan IB. Simultaneous binding of two protein kinases to a calcium-dependent potassium channel. J Neurosci 19: RC4, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang WH, Giebisch G. Dual modulation of renal ATP-sensitive K+ channel by protein kinases A and C. Proc Natl Acad Sci USA 88: 9722–9725, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang Y, Maciejewski BS, Lee N, Silbert O, McKnight NL, Frangos JA, Sanchez-Esteban J. Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am J Physiol Lung Cell Mol Physiol 291: L820–L827, 2006 [DOI] [PubMed] [Google Scholar]
- 82. Wei Y, Zavilowitz B, Satlin LM, Wang WH. Angiotensin II inhibits the ROMK-like small conductance K channel in renal cortical collecting duct during dietary potassium restriction. J Biol Chem 282: 6455–6462, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. White RE, Schonbrunn A, Armstrong DL. Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature 351: 570–573, 1991 [DOI] [PubMed] [Google Scholar]
- 84. Wilson PD, Geng L, Li X, Burrow CR. The PKD1 gene product, “polycystin-1,” is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest 79: 1311–1323, 1999 [PubMed] [Google Scholar]
- 85. Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol 283: F437–F446, 2002 [DOI] [PubMed] [Google Scholar]
- 86. Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Ontogeny of flow-stimulated potassium secretion in rabbit cortical collecting duct: functional and molecular aspects. Am J Physiol Renal Physiol 285: F629–F639, 2003 [DOI] [PubMed] [Google Scholar]
- 87. Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol 293: F1699–F1713, 2007 [DOI] [PubMed] [Google Scholar]
- 88. Wu SN, Wang YJ, Lin MW. Potent stimulation of large-conductance Ca2+-activated K+ channels by rottlerin, an inhibitor of protein kinase C-delta, in pituitary tumor (GH3) cells and in cortical neuronal (HCN-1A) cells. J Cell Physiol 210: 655–666, 2007 [DOI] [PubMed] [Google Scholar]
- 89. Xia X, Hirschberg B, Smolik S, Forte M, Adelman JP. dSLo interacting protein 1, a novel protein that interacts with large-conductance calcium-activated potassium channels. J Neurosci 18: 2360–2369, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yamashita S, Mochizuki N, Ohba Y, Tobiume M, Okada Y, Sawa H, Nagashima K, Matsuda M. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J Biol Chem 275: 25488–25493, 2000 [DOI] [PubMed] [Google Scholar]
- 91. Zhou XB, Arntz C, Kamm S, Motejlek K, Sausbier U, Wang GX, Ruth P, Korth M. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 276: 43239–43245, 2001 [DOI] [PubMed] [Google Scholar]