Abstract
Inflammatory cytokines are evoked by acute kidney injury (AKI) and may contribute to evolving renal disease. However, the impact of AKI-induced uremia on proinflammatory (e.g., TNF-α, MCP-1, TGF-β1) and anti-inflammatory (e.g., IL-10) cytokine gene expression remains unknown. This study was undertaken to gain some initial insights into this issue. CD-1 mice were subjected to left renal ischemia-reperfusion (I/R) in the absence or presence of uremia (± right ureteral transection). TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs, cytokine protein levels, and RNA polymerase II (Pol II) recruitment to these genes were assessed. Renal cytokine mRNA levels were also contrasted with unilateral vs. bilateral renal parenchymal damage (I/R or ureteral obstruction). Potential effects of uremia on cytokine mRNAs in the absence of parenchymal renal damage [bilateral ureteral transection (BUTx)] were sought. Finally, the impact of simulated in vitro uremia (HK-2 tubular cells exposed to peritoneal dialysate from uremic vs. normal mice) on cytokine mRNA and microRNA profiles was assessed. Uremia blunted TNF-α, MCP-1, and TGF-β1 mRNA increases in all three in vivo parenchymal acute renal failure models. These results were paralleled by reductions in cytokine protein levels and Pol II recruitment to their respective genes. Conversely, uremia increased IL-10 mRNA, both in the presence and absence (BUTx) of parenchymal renal damage. The uremic milieu also suppressed HK-2 cell proinflammatory cytokine mRNA levels and altered the expression of least 69 microRNAs (P < 0.0001). We conclude that both pro- and anti-inflammatory cytokine gene expressions are influenced by uremia, with a potential predilection toward an anti-inflammatory state. Changes in gene transcription (as reflected by Pol II recruitment), and possible posttranscriptional modifications (known to be induced by microRNAs), are likely involved.
Keywords: TNF-α, MCP-1, IL-10, TGF-β1, ischemia-reperfusion, urinary tract obstruction
a proinflammatory state is a well-recognized complication of chronic renal disease and it can adversely affect clinical outcomes (5, 28, 29). Over the past several years, it has become increasingly apparent that systemic, as well as intrarenal, inflammation can also occur with acute kidney injury (AKI)/acute renal failure (ARF). In this regard, ischemic and toxic injury stimulates proximal tubules to increase production of proinflammatory cytokines and chemokines (e.g., TNF-α, TGF-β1, and MCP-1) (6, 8, 10, 23–25, 30, 38, 39), and a recruitment of circulating inflammatory cells may also occur (1, 3, 14, 18, 19). In addition, the acutely damaged kidney is “primed” for exaggerated cytokine and chemokine production when confronted with proinflammatory stimuli, such as Toll receptor ligands (e.g., endotoxin) (Refs. 25, 33–36). Each of these reactions has the potential to exacerbate evolving renal injury and delay recovery. Furthermore, with renal cytokine efflux into the systemic circulation, extrarenal tissue injury may result (e.g., in heart, lung, and brain), potentially contributing to multi-organ failure (9, 11, 12, 14, 19, 37).
It is unknown whether uremia, the most notable consequence of ARF, impacts AKI-mediated cytokine and chemokine production. Specifically, we questioned whether acute uremia might modulate proinflammatory gene expression. This would seem to be an important issue to resolve if we are to understand an AKI-mediated proinflammatory state. The present study was undertaken to gain some initial insights into this issue. Toward this end, the expression of selected proinflammatory genes was assessed in acutely damaged left kidneys [ischemia-reperfusion (I/R)] in the presence and absence of contralateral right renal dysfunction (ureteral transection). By keeping the extent of tissue damage constant (unilateral renal injury), but by allowing this damage to exist in the presence or absence of uremia, the impact of the latter on tissue injury-induced cytokine/chemokine gene expression could be assessed. As a complementary approach, cytokine/chemokine gene expression was compared in the mice with either unilateral or bilateral parenchymal kidney damage (either I/R or ureteral obstruction). Finally, direct proximal tubule cytokine mRNA responses to an acute “uremic milieu” were assessed in cultured, human-derived, proximal tubule (HK-2) cells. The results obtained with these highly divergent experimental approaches were remarkably similar, and thus, form the basis of this report.
METHODS
Animal Models
All animal experiments were performed in male CD-1 mice (25–35 g; Charles River Laboratories, Wilmington, MA) using protocols that were approved by the Institutional Animal Care and Use Committee. They were maintained under routine laboratory conditions with free food and water access. All procedures were conducted under deep anesthesia (40–50 mg/kg ip pentobarbital sodium). Body temperature was maintained at 37°C with an external heating source. Four different surgical protocols were employed, as follows.
Unilateral Renal I/R Injury ± the Presence of Uremia
Twelve mice were anesthetized and subjected to a midline abdominal incision. The left renal vascular pedicle was visualized and occluded with an atraumatic vascular clamp. After 30 min, the clamp was removed and reperfusion was confirmed by the loss of renal cyanosis. Half of the mice (n = 6) were also subjected to right ureteral transection at its midpoint. By so doing, severe renal failure was induced without directly damaging the right renal parenchyma. In the remaining six mice, the right ureter was left intact. The abdominal cavities were then sutured in two layers and the mice were allowed to recover from anesthesia. Approximately 18 h later, the mice were reanesthetized and a blood sample was withdrawn from the inferior vena cava to assess the severity of azotemia [blood urea nitrogen (BUN) concentrations; the term “uremia” was arbitrarily used for animals that manifested a BUN of >100 mg/dl]. All left postischemic kidneys and all right kidneys that had not been subjected to ureteral transection (serving as uninjured controls) were resected and iced. The renal cortices were dissected and subjected to total protein (34) and RNA extraction (RNeasy Plus, Qiagen, Valenicia, CA). In addition, tissue aliquots were fixed in formalin for subsequent chromatin immunoprecipitation (ChIP) assay (22). The protein samples were used to assay TNF-α, MCP-1, TGF-β1, and IL-10 (ELISA; R&D Systems). Their cognate mRNAs were assessed by RT-PCR, with the values being expressed as a ratio with simultaneously determined GAPDH product (33–36). ChIP assay was used to assess the binding of RNA polymerase II (Pol II) to the start exon of each of these four test genes (22). Of note, the validity of using uninjured right kidneys as controls was confirmed by demonstrating that the values that were obtained from them were not significantly different from those values obtained in sham-operated animals.
Unilateral vs. Bilateral I/R Injury (± Presence of Uremia)
As a second approach to assessing the impact of uremia on ischemic renal injury, the above protocol was repeated in 12 mice. However, rather than using right ureteral transection to induce uremia, in this experiment six of the mice underwent 30 min of bilateral renal I/R injury. The remaining half of the mice was subjected to only left renal I/R injury. Approximately 18 h later, the mice were reanesthetized, a blood sample was obtained for BUN measurement, and then the kidneys were resected and assayed for TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs, as noted above. (However, unlike the above unilateral ischemia ± ureteral transection experiments, Pol II binding and cytokine protein levels were not assessed.)
Unilateral vs. Bilateral Ureteral Obstruction (± Uremia)
To complement the above experiments, a third model of renal injury in the presence or absence of uremia was undertaken. Twelve mice were subjected to midline abdominal incisions. Each underwent left ureteral obstruction, induced by ligation of the ureter at its midpoint. Half of the mice were also subjected to right ureteral ligation. Approximately 18 h later, the mice were reanesthetized, a blood sample was obtained for BUN assessment, and then the kidneys were resected (unilateral obstructed kidneys + contralateral normal kidneys; left kidneys from mice with bilateral obstruction). The renal cortices were extracted for RNA and assayed for TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs.
Uremic Effects on Cytokine mRNA Expression in the Absence of Direct Renal Injury
The following experiment was undertaken to assess potential direct effects of uremia on renal cytokine mRNA levels. Twelve mice were subjected to either bilateral ureteral transection (BUTx; n = 6) or to sham surgery (n = 6). The former induces severe uremia in the absence of direct renal injury (31). Approximately 18 h later, a blood sample was obtained for BUN analysis, the kidneys were resected, and processed for TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs.
Effects of Uremia on LPS-Induced Cytokine Expression
Fourteen mice were subjected to 30 min of left I/R injury. Half of the mice also underwent right ureteral transection. Approximately 18 h later, the mice were reanesthetized and injected with Escherichia coli (0111:B4) LPS (2 mg/kg body wt, administered via the tail vein). Two hours later, bilateral nephrectomies were performed and the resected renal cortices were analyzed for TNF-α, TGF-β1, MCP-1, and IL-10 mRNAs. The results for the unilateral ischemic kidneys were contrasted to those obtained in the contralateral uninjured control kidneys, and to the left kidneys obtained from the mice that had been subjected to left I/R injury + right ureteral transection.
Uremia Effects on Cultured HK-2 Cell Cytokine mRNA Expression
Peritoneal dialysis with cell culture medium, as performed on uremic and control mice.
Ten mice were subjected to bilateral ureteral obstruction (BUO) to induce severe uremia. Twenty-four hours later, the mice were anesthetized and then 2 ml of keratinocyte serum free cell culture medium (K-SFM) (26) containing 5% glucose were injected into the peritoneal space. After 1 h (allowing for dialytic equilibrium with plasma), the peritoneal fluid was recovered from the abdominal cavity. The mice were then killed by aortic transection. BUN concentrations were determined on both terminal plasma samples and on the peritoneal dialysate (to confirm that dialytic equilibrium, at least as assessed by BUNs, had been achieved; BUNs ranged from 140–150 mg/dl in both plasma and dialysate). To obtain control peritoneal dialysis fluid, 10 normal or sham-operated mice were subjected to a 1-h peritoneal dialysis, as noted above.
Effect of uremic dialysate on HK-2 cell cytokine mRNAs.
HK-2 cells were maintained in K-SFM and passaged in T75 Costar flasks (26). For experimentation, they were trypsinized and seeded into six-well Costar plates. Approximately 8 h later, the normal K-SFM culture medium was removed and replaced with either the uremic (n = 4) or control dialysate (n = 4). The cells were then incubated overnight, RNA was extracted, and then the samples were analyzed for human TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs by competitive PCR (21, 22, 34–36). Results were expressed as a ratio to simultaneously obtained GAPDH mRNA.
Effects of uremic milieu on HK-2 cell microRNA expression.
Changes in microRNA (miRNA) expression can impact mRNA stability, mRNA translation, and possibly, gene transcription (2, 4, 13, 17). The following experiment tested the hypothesis that the uremic environment can alter miRNA profiles, and thus, potentially impact renal inflammation. Toward this end, the above HK-2 cell experiment was repeated (n of 3 with either peritoneal dialysate from control or uremic mice). After completing an overnight incubation, the cells were harvested for microRNAs, using a kit specifically designed to capture these low molecular weight species (Exiqon). The samples were frozen and sent for miRNA microarray analysis (Exiqon, Vedbaek, Denmark; mercury LNA). At the time of analysis, Exiqon was capable of probing for 1,355 separate miRNA species (see results). The microRNA data were analyzed as the ratio of the uremic/control cell signals. Statistical significance was only accepted at a P value of <0.0001 and with a Δ log median ratio between the samples of >0.5.
Calculations and Statistics
All values are expressed as means ± 1 SE. mRNA and cytokine levels were analyzed by unpaired Student's t-test and were considered significant at a P value of <0.05. If multiple comparisons were made, the Bonferroni correction was applied.
RESULTS
Degrees of Azotemia Induced by the Unilateral vs. Bilateral Renal Injury
The degrees of azotemia induced by the employed experimental protocols are presented in Fig. 1. BUN concentrations in normal mice were 25 ± 1 mg/dl. As expected, unilateral I/R and unilateral ureteral obstruction (UUO) each induced only small BUN elevations (∼30–35 mg/dl). However, when bilateral renal dysfunction was induced by 1) unilateral I/R + unilateral ureteral transection (UTx), 2) bilateral I/R (Bil I/R), 3) BUO, or 4) BUTx, severe azotemia/uremia resulted (BUN range of 150–176 mg/dl).
Fig. 1.
Blood urea nitrogen (BUN) concentrations at ∼18 h following surgical protocols. The horizontal line depicts the mean BUN concentration for normal mice. As expected, unilateral ischemia-reperfusion (uni I/R) and unilateral ureteral obstruction (UUO) only slightly raised BUN levels. Conversely, unilateral I/R + contralateral ureteral transection (UTx), bilateral I/R (bil I/R), bilateral ureteral obstruction (BUO), and bilateral ureteral transection (BUTx) each evoked profound BUN elevations.
TNF-α mRNA Levels: Effects of Unilateral vs. Bilateral Renal Dysfunction
Both left unilateral I/R and left UUO caused an approximate four- to fivefold increase in TNF-α mRNA levels, compared with uninjured control (C) kidneys (Fig. 2). When severe uremia was induced by 1) unilateral I/R + contralateral UTx, 2) Bil I/R, or 3) BUO, the left kidney TNF-α mRNA increases were reduced by ∼75%.
Fig. 2.
Renal cortical TNF-α mRNA concentrations. A: comparison of values in control (right) kidneys (C) and in 18-h post left kidneys subjected to I/R ± contralateral UTx. B: comparison of values between control kidneys, postischemic left kidneys, and postischemic left kidneys in the presence of right kidney ischemic damage. C: comparison of values in control kidneys and in left kidneys from mice subjected to either unilateral (Unilat) or bilateral (bilat) ureteral obstruction (obstn). Unilateral I/R and UUO each increased TNF-α mRNA concentrations compared with values seen in control kidneys. When bilateral renal dysfunction was induced (by UTx, bil I/R, or bilateral obstruction), there were ∼75% reductions in TNF-α mRNA levels.
MCP-1 mRNA Levels: Effects of Unilateral vs. Bilateral Renal Dysfunction
As shown in Fig. 3, unilateral I/R and UUO each evoked massive increases in MCP-1 mRNA. When bilateral renal dysfunction was induced, as denoted above, the extent of these MCP-1 mRNA increases was markedly suppressed (e.g., by 80% with bilateral vs. UUO).
Fig. 3.
Renal cortical MCP-1 mRNA concentrations. The groupings are the same as shown in the Fig. 2 legend. In each case, unilateral renal injury evoked marked increases in MCP-1 mRNA levels. When bilateral renal dysfunction (and hence, uremia) was induced, these increases were markedly attenuated.
TGF-β1 mRNA Levels: Effects of Unilateral vs. Bilateral Renal Dysfunction
The pattern for TGF-β1 mRNA expression paralleled those described above for TNF-α and MCP-1, as follows: 1) both unilateral I/R and UUO significantly elevated TGF-β1 mRNA levels and 2) the extent of these increases was significantly attenuated when contralateral kidney injury was induced (Fig. 4).
Fig. 4.
Renal cortical TGF-β1 mRNA concentrations. The groupings are the same as shown in the Fig. 2 legend. In each case, unilateral renal injury evoked marked increases in TGF-β1 mRNA levels. When bilateral renal dysfunction (and hence, uremia) was induced, these increases were markedly attenuated.
IL-10 mRNA Levels: Effects of Unilateral vs. Bilateral Renal Dysfunction
The mRNA for the anti-inflammatory cytokine IL-10 manifested the opposite response to those noted above: 1) neither left I/R nor left ureteral obstruction significantly increased IL-10 mRNA levels and 2) when bilateral renal injury was induced, dramatic IL-10 mRNA increases (not decreases) in the left kidney values resulted (Fig. 5).
Fig. 5.
Renal cortical IL-10 mRNA concentrations. The groupings are the same as shown in Fig. 2. Neither Unilat I/R nor UUO caused a significant increase in IL-10 mRNA. However, when contralateral renal dysfunction was induced by either UTx, bil I/R, or BUO, striking increases in IL-10 mRNA resulted.
Uremia Renal Cytokine Profiles in the Absence of Parenchymal Renal Injury
BUTx induced severe azotemia (BUN ∼150 mg/dl, as shown in Fig. 1). However, it did not independently alter renal cortical TNF-α, MCP-1, or TGF-β1 mRNA levels. Conversely, the uremic environment induced a massive increase in renal cortical IL-10 mRNA levels (Fig. 6).
Fig. 6.
Effects of uremia on cytokine mRNA expression in the absence of direct renal injury. Mice were subjected to sham surgery [controls (C)] or to BUTx. The next day, renal cortical cytokine mRNA levels were assessed. BUTx did not significantly alter TNF-α, MCP-1, and TGF-β1 mRNA levels. Conversely, it induced a profound increase in IL-10 mRNA levels.
Pol II Expression at Target Genes
As shown in (Fig. 7), unilateral renal I/R significantly increased Pol II levels at exon 1 of the TNF-α, MCP-1, and TGF-β1 genes (vs. control kidney values). These I/R- induced increases in Pol II binding were almost completely abrogated by contralateral UTx. Thus, the Pol II changes paralleled those observed in their cognate mRNA levels (suggesting that the mRNA increases reflected increased gene transcription). In contrast, I/R either without or with contralateral UTx had no effect on Pol II binding at exon 1 of the IL-10 gene.
Fig. 7.
RNA polymerase II (Pol II) binding at the start exons of the TNF-α, MCP-1, TGF-β1, and IL-10 genes. Uni I/R caused a significant increase in RNA Pol II binding to exon 1 of the TNF-α, MCP-1, and TGF-β1 genes. These increases were significantly blunted by the presence of contralateral UTx (which produced uremia), such that the mRNA levels no longer significantly differed from control values. In contrast, I/R injury caused no change in Pol II binding to the IL-10 gene, either in the presence or absence of uremia (I/R + UTx). Results are present as ng per mg chromatin protein applied in the assay.
Effects of Uremia on LPS-Induced Cytokine Expression
The presence of unilateral I/R greatly enhanced LPS-stimulated TNF-α, MCP-1, TGF-β1, and IL-10 mRNA increases, compared with those observed in LPS-stimulated control kidneys (Fig. 8). This is consistent with previous published data from this laboratory which indicate that renal injury can exacerbate LPS-mediated cytokine/chemokine responses (34–36). However, when unilateral I/R coexisted with severe azotemia (contralateral UTx), the unilateral I/R kidney had markedly blunted LPS-driven TNF-α, MCP-1, and TGF-β1 mRNA responses (NS vs. values seen in LPS-stimulated control kidneys). The opposite situation existed for IL-10 mRNA expression: the degree of LPS-mediated IL-10 mRNA increases in the postischemic kidney was enhanced by ∼15-fold by the presence of contralateral UTx.
Fig. 8.
Effects of uni I/R ± contralateral UTx on cytokine mRNA responses to LPS injection. Mice were subjected to uni I/R ± contralateral UTx. The following day, they were challenged with LPS injection and kidneys were harvested 2 h thereafter. The postischemic kidneys manifested greatly accentuated TNF-α, MCP-1, TGF-β1, and IL-10 mRNA increases, compared with control kidneys. These preferential TNF-α, MCP-1, and TGF-β1 mRNA responses to LPS were almost completely abrogated by the presence of uremia (uni I/R + contralateral UTx). Conversely, the extent of the LPS-induced IL-10 increases was greatly exaggerated by the presence of uremia.
HK-2 Cell mRNA and microRNA Assessments
Effect of uremic dialysate on cytokine mRNA levels.
TNF-α, MCP-1, and TGF-β1 mRNAs were all readily detectable in HK-2 cells exposed to peritoneal dialysate from control mice. As shown in Fig. 9, uremic peritoneal dialysate significantly reduced the levels of each of these mRNAs. This was particularly noteworthy in the case of TNF-α mRNA, where ∼75% reductions were observed. In contrast, IL-10 mRNA levels could not reliably be detected either in the presence or absence of either dialysate. This was despite using four different sets of primers designed to identify IL-10 message and detection of a known IL-10 mRNA standard.
Fig. 9.
HK-2 mRNA levels after exposure to control and uremic dialysates. TNF-α, MCP-1, and TGF-β1 mRNAs were each readily detected in HK-2 cells using human PCR primers, and each was relatively suppressed when the cells were maintained in uremic vs. control dialysate. In contrast, IL-10 mRNA could not be detected under either control or uremic conditions.
Effect of uremic dialysate on microRNA expression.
Out of the 1,355 miRNAs which were screened by Exiqon array, 69 were identified that met the selection criterion of P < 0.0001 and an LNR of >0.5 (Fig. 10). Of these 69, 21 were miRNAs whose identification remained unknown (miRNA − “Plus”; Exiqon). The 48 known miRNAs that were identified and that were differentially expressed in uremic vs. control dialysate-treated cells are presented in Table 1. Of these 48, 15 were overexpressed (up to 14-fold increases), and 33 were underexpressed (up to 5-fold decreases). By searching with miRBase Sequence Database, a large number of potential mRNA targets for these miRNAs were identified that can potentially impact cell injury and inflammation. One representative putative target for each of these miRNAs is presented in Table 1.
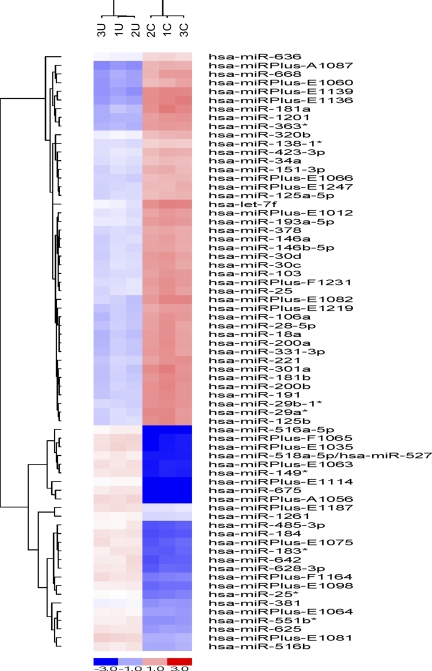
Fig. 10.
HK-2 cell microRNA (miRNA) array. The figure demonstrates the distribution patterns of 69 miRNAs that were statistically different (P < 0.0001) between the cells maintained under control (1C, 2C, 3C) vs. the uremic (U1, U2, U3) dialysate conditions. The moieties labeled as “Plus” represent currently unidentified miRNAs. The known miRNAs, and their fold differences (uremic/control), between the 2 groups of cells are presented in Table 1. The clustering patterns of miRNAs are presented by the line graph “trees.”
Table 1.
MicroRNA changes induced in HK-2 cells by uremic dialysate
| miRNA | Fold- Change | Predicted Targets |
|---|---|---|
| hsa-miR-675 | 14.089 | Tumor necrosis factor receptor superfamily member |
| hsa-miR-516a-5p | 9.285 | Tumor necrosis factor ligand superfamily member |
| hsa-miR-149* | 8.435 | Transcription factor 15 |
| has-miR-518a-5p | 8.137 | Programmed cell death protein 6 |
| hsa-miR-184 | 5.195 | Rho-related BTB domain-containing protein 2 |
| hsa-miR-642 | 4.721 | GTP-binding protein Rit1 (Ras-like protein) |
| hsa-miR-183* | 4.482 | Programmed cell death protein 4 |
| hsa-miR-628-3p | 4.424 | TNF receptor-associated factor 3 |
| hsa-miR-485-3p | 4.337 | MAP kinase kinase 2 |
| hsa-miR-25* | 2.981 | Ras-related protein Rab-23 |
| hsa-miR-551b* | 2.747 | Histone binding protein RBBP7 |
| hsa-miR-625 | 2.681 | Putative MAPK-activating protein PM01 |
| hsa-miR-516b | 2.351 | Eukaryotic translation initiation factor 4E-binding protein 3 |
| hsa-miR-381 | 2.163 | NF-κB inhibitor α |
| hsa-miR-1261 | 1.465 | Insulin-like growth factor 1 |
| hsa-miR-636 | 0.666 | TNF receptor superfamily member 1A precursor |
| hsa-miR-138-1* | 0.515 | Angiotensin-converting enzyme |
| hsa-miR-320b | 0.509 | Ras-like without CAAX 1 |
| hsa-miR-34a | 0.463 | FK506-binding protein 1B |
| hsa-miR-423-3p | 0.444 | Microtubule-associated proteins |
| hsa-miR-125a-5p | 0.416 | Ras-related protein Rab-13 |
| hsa-miR-151-3p | 0.395 | Multidrug resistance-associated protein 8 |
| hsa-miR-378 | 0.371 | Interleukin-1 family member 6 |
| hsa-miR-193a-5p | 0.365 | TNF receptor-associated factor 3 interacting protein1 |
| hsa-miR-146b-5p | 0.356 | Ras-interacting protein 1 |
| hsa-miR-146a | 0.355 | Interleukin-1 receptor-associated kinase1 |
| hsa-miR-30c | 0.352 | Suppressor of cytokine signaling 1 |
| hsa-miR-25 | 0.347 | Kidney superoxide-producing NADPH oxidase |
| hsa-miR-103 | 0.330 | TGF-β receptor type III precursor |
| hsa-miR-30d | 0.330 | Mitogen-activated protein kinase kinase kinase |
| has let-7f | 0.328 | Geranylgeranyl transferase type-2 subunit β |
| hsa-miR-221 | 0.296 | Mitochondrial Fe transporter 1 |
| hsa-miR-125b | 0.296 | Mitochondrial Rho GTPase 2 |
| hsa-miR-29b-1* | 0.293 | Fe-responsive element-binding protein 2 |
| hsa-miR-29a* | 0.292 | Fe-responsive element-binding protein 2 |
| hsa-miR-28-5p | 0.292 | JAK3 |
| hsa-miR-331-3p | 0.277 | RNA polymerase II elongation factor |
| hsa-miR-200a | 0.276 | Angiotensin-converting enzyme 2 |
| hsa-miR-106a | 0.275 | Mitogen-activated protein kinase kinase kinase 9 |
| hsa-miR-191 | 0.275 | Cytokine receptor common γ chain precursor |
| hsa-miR-200b | 0.270 | Cytochrome c oxidase subunit 7C |
| hsa-miR-18a | 0.268 | TNF-α-induced protein |
| hsa-miR-181b | 0.266 | Cholesterol efflux regulatory protein |
| hsa-miR-301a | 0.251 | Hepatocyte growth factor receptor precursor |
| hsa-miR-363* | 0.246 | Serine/threonine protein kinase 6 |
| hsa-miR-1201 | 0.235 | SMAD family member 4 |
| hsa-miR-668 | 0.227 | Ferredoxin reductase |
| hsa-miR-181a | 0.217 | TGF-β receptor-associated protein |
The fold-changes in 48 known microRNAs are presented. The data are presented as the ratio of uremic/control samples. Examples of selected gene/mRNA targets that are potentially impacted by these microRNAs and which can impact renal injury and cytokine gene expression are presented.
Cytokine (Protein) Levels
Given that miRNA changes alter mRNA translation, the impact of uremia on the four test cytokines was assessed using the unilateral I/R ± contralateral UTx model. Unilateral I/R induced significant increases in TNF-α, MCP-1, and TGF-β1 protein levels, compared with the values observed in the control kidneys (P < 0.025-<0.001). When these cytokines were measured in kidneys obtained from mice with unilateral I/R + contralateral UTx, their concentrations no longer differed from control values. Thus, the presence of uremia appeared to suppress I/R-induced TNF-α, MCP-1, and TGF-β1 levels, thereby paralleling the corresponding reductions in their mRNA levels and Pol II binding. Conversely, IL-10 protein levels were slightly reduced by I/R, and this change was magnified in the presence of uremia (Fig. 11, right). This was despite the fact that I/R + UTx markedly increased renal cortical IL-10 mRNA levels (Fig. 5), suggesting a block in IL-10 mRNA translation.
Fig. 11.
Renal cortical TNF-α, MCP-1, TGF-β1, and IL-10 cytokine levels. TNF-α, MCP-1, and TGF-β1 cytokine protein levels were each elevated in postischemic kidneys. These elevations were reduced in the presence of contralateral UTx (i.e., uremia). Conversely, IL-10 levels were suppressed by I/R, particularly in the presence of uremia.
DISCUSSION
In 1954, Koletsky (15) demonstrated that uninephrectomy enhances recovery from postischemic renal injury, imposed on the contralateral kidney. A generation later, Finn (7) further explored this phenomenon using renal hemodynamic and micropuncture techniques. They found that an initial reduction in functional renal mass (uninephrectomy) enhanced blood flow to the contralateral postischemic kidney. This hemodynamic change led to the “recruitment” of otherwise “nonfunctional” nephrons, thereby helping to maintain postischemic glomerular filtration rate. In 1994, we undertook additional experiments to further explore this phenomenon (32). Our goal was to determine whether an initial reduction in renal mass might evoke adaptive changes within residual nephrons and directly protect them from superimposed ischemic damage. Toward this end, rats were subjected to one and a half nephrectomy. The following day, proximal tubules were harvested from the remaining renal tissue, and their susceptibility to in vitro hypoxic injury was assessed. Indeed, the initial reduction in functional renal mass, and/or the resulting azotemia, conferred cytoprotective effects (32). Recent observations from our laboratory further support the view. When uremia was induced in mice by BUTx, proximal tubules harvested from these mice manifested in vitro protection against hypoxic cell death (31). Thus, in composite, the above described studies indicated that reduced functional renal mass and/or uremia can confer protection against superimposed renal damage. However, the subcellular pathways through which this protection is operative have not been well-defined.
Because of the emerging recognition of inflammation as a secondary determinant of diverse forms of AKI, we hypothesized that this inflammatory response might be blunted by a uremic milieu. If so, this could potentially contribute to the above described phenomena. Hence, the present study sought some experimental support for this hypothesis. By inducing unilateral ischemic renal injury in the presence or absence of contralateral UTx, the impact of uremia on inflammatory gene expression in the postischemic kidney could be assessed. The results obtained using this injury model support this hypothesis, and the major findings can be summarized as follows: first, uremia suppressed TNF-α mRNA, TGF-β1 mRNA, and MCP-1 mRNA in postischemic kidneys; second, reduced gene transcription likely contributed to this result, given that Pol II, the key enzyme that drives gene transcription (16, 20), was reduced at the start exon of each of these three genes; third, corresponding reductions in TNF-α, TGF-β1, and MCP-1 proinflammatory/profibrotic cytokines were observed; and fourth, in striking contrast to these findings, the mRNA for the anti-inflammatory cytokine IL-10 was markedly increased by the presence of uremia without a corresponding change in IL-10 cytokine levels or Pol II binding. Potential reasons for the different directional changes in IL-10 mRNA vs. TNF-α/TGF-β1/MCP-1 mRNAs will be speculated upon later in this discussion.
A limitation of the above described unilateral I/R ± contralateral UTx model is that it lacks direct clinical relevance. Hence, we wished to determine whether comparable findings might be observed in the setting of bilateral parenchymal renal disease. Hence, mRNA assessments were contrasted between left postischemic kidneys in mice with absent or present right kidney ischemic damage. This protocol produced the same mRNA profiles that were noted above, i.e., uremia (Bil I/R) reduced TNF-α, TGF-β1, and MCP-1 mRNA levels, and increased IL-10 mRNA levels, compared with unilateral I/R injury. To further explore the impact of uremia on kidney injury-induced cytokine expression, a second clinically relevant AKI model was explored: unilateral vs. BUO. The results obtained with this model recapitulated those noted above: uremia suppressed TNF-α, TGF-β1, and MCP-1 mRNAs and increased IL-10 mRNA levels. Thus, all three of these in vivo models indicated that uremia can, indeed, impact cytokine gene expression in an acutely damaged kidney.
These findings next led us to assess whether uremia, per se, might directly impact cytokine mRNA levels, i.e., independent of any structural renal damage. To make this assessment, cytokine mRNA levels were measured in kidneys obtained from mice ∼18 h after BUTx (which induces no histologic renal injury) (31) and compared with levels observed in kidneys from sham-operated controls. No differences in TNF-α, TGF-β1, or MCP-1 mRNAs were observed. However, a marked stimulation of IL-10 mRNA resulted. As discussed above, in each of our three experimental AKI models, discordant directional changes were noted between TNF-α, TGF-β1, MCP-1 mRNAs vs. IL-10 mRNA (uremia- induced suppressions vs. stimulation, respectively). Thus, the results obtained with the BUTx model imply the following: 1) that the above noted uremia-induced suppressions of TNF-α/TGF-β1/MCP-1 mRNAs appear to be dependent on the presence of concomitant cellular injury (since BUTx did not affect these cytokine mRNAs) and 2) the uremia-induced increase in IL-10 mRNA likely reflects an injury-independent change that is directly evoked by a uremic milieu. In data not presented, we observed that BUTx also selectively increases IL-10 mRNA levels within the spleen. Hence, the presently documented uremic effect on renal IL-10 mRNA levels may reflect a systemic, rather than a renal specific, event.
In recent years, this laboratory documented that both unilateral renal ischemic injury (21, 34) and UO (35) sensitize the involved kidney to LPS-mediated cytokine and cytokine mRNA generation. Given the current findings that uremia can suppress proinflammatory cytokine expression, we questioned whether it might also modify this LPS hyperresponsive state. To gain insights into this issue, mice with unilateral I/R were challenged with LPS in the presence or absence of uremia (± contralateral UTx). Mice with unilateral ischemia manifested the expected exaggerated increases in LPS-stimulated TNF-α, MCP-1, TGF-β1, and IL-10 mRNAs, as previously noted (21, 34). However, when mice with unilateral ischemic injury + contralateral UTx were challenged with LPS, only trivial TNF-α, MCP-1, and TGF-β1 mRNA increases were observed. Conversely, the presence of uremia enhanced, by fivefold, the LPS-mediated IL-10 mRNA response. Thus, the uremia-induced effect on cytokine gene expression appears to extend to a Toll ligand receptor/LPS-stimulated state.
It remains unknown as to what cell type(s) within renal cortex expressed the above noted uremia-induced changes in cytokine gene expression. Because the proximal tubule is the prime site of ischemic renal damage, we sought to determine whether uremia impacts cytokine gene expression in this nephron segment. Toward this end, experiments were undertaken in cultured HK-2 cells. The most direct approach for exploring the impact of a uremic milieu on HK-2 cytokine gene expression would have been to culture the HK-2 cells in serum obtained from normal vs. uremic mice. However, a technical issue precluded this approach: use of plasma would have required mixing it with cell culture medium, thereby substantially diluting out (e.g., 1:4) uremic compounds, and hence, reducing their potential biologic effects. To obviate this problem, we used cell culture medium as peritoneal dialysate in control and uremic mice. Within 1 h, dialytic equilibrium with plasma was achieved, at least as assessed by equal urea concentrations in plasma and dialysate. By recovering these dialysates, sufficient quantities of “conditioned” culture media were obtained to permit their being added undiluted to HK-2 cells. Compared with control dialysate, the uremic dialysate suppressed TNF-α mRNA by ∼75%, with lesser, albeit statistically significant, reductions in TGF-β1 mRNA and MCP-1 mRNA being observed. Hence, these cell culture results further suggest that uremia can, indeed, alter cytokine gene expression, in general, and specifically within proximal tubule cells. In contrast, we were not able to document IL-10 mRNA in these HK-2 cell experiments either under control or uremic conditions. This was despite testing four different sets of IL-10 primers, and despite the fact that IL-10 mRNA standards were readily detected. Thus, it may be that proximal tubules express very low levels of IL-10 mRNA and that its ready detection in whole renal cortex may have reflected events in nonproximal tubular cortical cells (e.g., within glomeruli, or infiltrating inflammatory cells).
Over the past 10 years, it has become increasingly apparent that mRNA levels, and subsequent mRNA translation into protein, can be impacted by the generation of miRNAs (2, 13, 17). These are single-strand short (21–23 nucleotides) RNAs that are encoded by the genome, but are not translated into protein (i.e., noncoding RNAs). Each primary transcript (pri-miRNA) is processed into a pre-miRNA and ultimately into a functionally significant miRNA by Dicer enzyme. Most miRNAs are partially complementary to multiple mRNAs, and it is via this interaction that they impact mRNA expression, either by enhancing mRNA destruction or via induction of a translation block (2, 13, 17). They may also induce DNA methylation, thereby altering gene transcription (27). In the current experiments, we were struck by the fact that the observed IL-10 mRNA increases were accompanied by reductions in IL-10 protein levels, implying a translation block. Hence, we questioned whether the uremic milieu might impact proximal tubule miRNA profiles. Of note, this is a question which, to our knowledge, has never been previously addressed. As an initial test of this hypothesis, Exiqon performed miRNA array analysis on HK-2 cells that were exposed to control vs. uremic dialysate. Dramatic differences were observed, affecting at least 69 miRNAs, 48 of which have previously been described. Within these 48, both up- and downregulation were observed (n = 15 and 33, respectively). Obviously, it is impossible to conclude that these marked alterations in miRNA profiles actually contributed to the observed cytokine changes. However, the results do indicate, for the first time, that the uremic environment can directly impact miRNA expression, and possibly, mRNA translation as well as gene transcription. This new insight could potentially have broad ranging implications for the pathogenesis of both acute as well as chronic renal failure.
In conclusion, the results of the present study indicate that the presence of uremia can alter cytokine gene expression following acute renal injury. The balance of the changes observed, i.e., a decrease in proinflammatory/profibrotic cytokines and a potential increase in anti-inflammatory IL-10 gene expression, suggests a predilection toward an anti-inflammatory state. These findings are particularly noteworthy given that uremia is generally viewed as systemic proinflammatory state (5, 8, 19, 28, 29). The results observed in whole renal cortex likely reflect, at least in part, proximal tubular events, based on observations that experimental uremia can suppress TNF-α, MCP-1, and TGF-β1 gene expression in cultured proximal tubule cells. Both altered gene transcription, as reflected by changes in Pol II binding, and potential alterations in mRNA translation, as can be induced by altered miRNA profiles, may be involved. The functional impact of these changes on renal disease progression remains to be defined.
GRANTS
This work was supported by National Institutes of Health Research Grants R37–38432 and RO-1 DK-68520.
REFERENCES
- 1. Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H. Renal ischemia-reperfusion leads to long-term infiltration of activated and effector memory T lymphocytes. Kidney Int 75: 526–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asirvatham AJ, Gregoried CJ, Zihua H, Magner WJ, Tomasi TB. MicroRNA targets in immune genes and the Dicer/Argonaute and ARE machinery components. Mol Immunol 45: 1995–2006, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Gene 8: 93–103, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Cirillo O, Sautin YY, Kanellis J, Kang DH, Gesualdo L, Nakagawa T, Johnson RJ. Systemic inflammation, metabolic syndrome, and progressive renal disease. Nephrol Dial Transplant 24: 1384–1387, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Donnahoo KK, Meldrum DR, Shenkar R, Chung SC, Abraham E, Harken AH. Early renal ischemia with and without reperfusion activates NF-κB and increases TNF-α bioactivity in the kidney. J Urol 63: 1328–1332, 2000 [PubMed] [Google Scholar]
- 7. Finn WF. Enhanced recovery from postischemic acute renal failure: micropuncture studies in the rat. Circ Res 46: 440–448, 1980 [DOI] [PubMed] [Google Scholar]
- 8. Gormley SM, McBride WT, Armstrong MA, McClean E, MacGowan SW, Campalani G, McMurray TJ. Plasma and urinary cytokine homeostasis and renal function during cardiac surgery without cardiopulmonary bypass. Cytokine 17: 61–65, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanakiriya SK, Croatt AJ, Haggard JJ, Ingelfinger JR, Tang SS, Alam J, Nath KA. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am J Physiol Renal Physiol 284: F546–F554, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol 26: 105–113, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Kim VN, Nam JW. Genomics of microRNA. Trends Genet 22: 165–173, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ko GJ, Rabb H, Hassoun HT. Kidney-lung crosstalk in the critically ill patient. Blood Purif 28: 75–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koletsky S. Effects of temporary interruption of renal circulation in rats. Arch Pathol 58: 592–603, 1954 [PubMed] [Google Scholar]
- 16. Kornberg RD. The molecular basis of eukaryotic transcription. Proc Natl Acad Sci USA 104: 12955–12961, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin H, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Gene 5: 522–531, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Trans Rev 23: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 19: 1360–1370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marx J. Transcription enzyme structure solved. Science 292: 411–414, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Naito M, Bomsztyk K, Zager RA. Endotoxin mediated polymerase II RNA recruitment to target genes in acute renal failure. J Am Soc Nephrol 19: 1321–1330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naito M, Bomsztyk K, Zager RA. Renal ischemia-induced cholesterol loading: transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol 174: 54–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int 59: 106–117, 2001 [DOI] [PubMed] [Google Scholar]
- 25. Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-α production by renal epithelial cells. Am J Physiol Renal Physiol 292: F812–F819, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Sinkkonen L, Hugenschmidt T, Berninger P, Gaidatzis D, Mohn F, Artus-Revel CG, Zavolan M, Svoboda P, Filipowicz W. MicroRNAs control de novo DNA methylation through regulation of transcriptional repressors in mouse embryonic stem cells. Nat Struct Molec Biol 15: 259–267, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Snaedal S, Heimbürger O, Qureshi AR, Danielsson A, Wikström B, Fellström B, Fehrman-Ekholm I, Carrero JJ, Alvestrand A, Stenvinkel P, Bárány P. Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: implications for patient survival. Am J Kidney Dis 53: 1024–1033, 2009 [DOI] [PubMed] [Google Scholar]
- 29. Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M. IL-10, IL-6, TNF-α central factors in the altered cytokine network of uremia–the good the bad and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Yang N, Luo M, Li R, Huang Y, Zhang R, Wu Q, Wang F, Li Y, Yu X. Blockage of JAK/STAT signaling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant 23: 91–100, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Zager RA. Uremia induces proximal tubular cytoresistance and heme oxygenase-1 expression in the absence of acute kidney injury. Am J Physiol Renal Physiol 296: F362–F368, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zager RA, Iwata M, Burkhart KM, Schimpf BA. Postischemic acute renal failure protects proximal tubules from O2 deprivation injury, possibly by inducing uremia. Kidney Int 45: 1760–1768, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Zager RA, Johnson AC, Geballe A. Gentamicin suppresses endotoxin driven TNF-α production in human and mouse proximal tubule cells. Am J Physiol Renal Physiol 293: F1373–F1380, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Zager RA, Johnson ACM, Hanson SY, Lund BS. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and release. Am J Physiol Renal Physiol 289: F289–F297, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Zager RA, Johnson AC, Hanson SY, Lund S. Acute nephrotoxic and obstructive injury primes the kidney to endotoxin-driven cytokine/chemokine production. Kidney Int 69: 1181–1188, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Zager RA, Johnson ACM, Lund S, Hanson S. Acute renal failure: determinants and characteristics of the injury-induced hyperinflammatory response. Am J Physiol Renal Physiol 291: F546–F556, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Zarbock A, Schmolke M, Spieker T, Jurk K, Van Aken H. Acute uremia but not renal inflammation attenuates aseptic occult lung injury. A. Critical role for uremic neutrophils. J Am Soc Nephrol 17: 3124–3131, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Zhang B, Ramesh G, Norbury CC, Reeves WB. Cisplatin-induced nephrotoxicity is mediated by tumor necrosis factor-α produced by renal parenchymal cells. Kidney Int 72: 37–44, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Zhang B, Ramesh G, Uematsu S, Akira S, Reeves WB. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J Am Soc Nephrol 19: 923–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]