Abstract
Oxygen consumption beyond the proximal tubule is mainly determined by active solute reabsorption, especially in the thick ascending limb of the Loop of Henle. Furosemide-induced suppression of oxygen consumption (FSOC) involves inhibition of sodium transport in this segment, which is normally accompanied by a marked decrease in the intrarenal deoxyhemoglobin detectable by blood oxygen level-dependent (BOLD)-magnetic resonance imaging (MRI). This study tested the hypothesis that the magnitude of BOLD-MRI signal change after furosemide is related to impaired renal function in renovascular hypertension. In 16 pigs with unilateral renal artery stenosis, renal hemodynamics, function, and tubular function (FSOC and fluid concentration capacity) were evaluated in both kidneys using MR and multidetector computerized tomography (MDCT) imaging. Animals with adequate FSOC (23.6 ± 2.2%, P > 0.05 vs. baseline) exhibited a mean arterial pressure (MAP) of 113 ± 7 mmHg, and relatively preserved glomerular filtration rate (GFR) of 60 ± 4.5 ml/min, comparable to their contralateral kidney (66 ± 4 ml/min, P > 0.05). In contrast, animals with low FSOC (3.1 ± 2.1%, P = NS vs. baseline) had MAP of 124 ± 9 mmHg and GFR (22 ± 6 ml/min) significantly lower than the contralateral kidneys (66 ± 4 ml/min, P < 0.05). The group with preserved GFR and FSOC showed an increase in intratubular fluid concentration as assessed by MDCT that was greater than that observed in the low GFR group, suggesting better preservation of tubular function in the former group. These results suggest that changes in BOLD-MRI after furosemide can differentiate between underperfused kidneys with preserved tubular function and those with tubular dysfunction. This approach may allow more detailed physiologic evaluation of poststenotic kidneys in renovascular hypertension than previously possible.
Keywords: renal artery stenosis, oxygenation
the evaluation and management of renovascular hypertension have been and continue to be a subject of intense debate (39, 43). The uncertainties are mostly due to difficulties in assessing individual renal function and viability in kidneys that are unequally perfused (15, 34). Large vessel occlusion of the renal circulation is known to affect blood flow and glomerular filtration in the poststenotic kidney. As filtration falls, fractional sodium reabsorption increases, leading to reduced sodium excretion in the affected kidney. Because sodium reabsorption in tubular segments beyond the proximal tubule requires energy, this process increases kidney work and oxygen consumption. In more severe stenoses, glomerular filtration rate (GFR) and sodium delivery eventually fall, while overall sodium reabsorption and oxygen consumption decline. In addition to tubular work, a decrease in oxygen delivery may lead to renal hypoxia, while subsequent replacement of viable renal tissue by fibrotic tissue decreases oxygen consumption. Therefore, basal renal tissue oxygenation, and in turn deoxyhemoglobin content, is determined by a balance among several determinants of oxygen delivery and consumption.
Blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) allows estimation of cortical and medullary deoxyhemoglobin, as we (16, 38) and others (18, 30) demonstrated. The inhibition of O2 consumption achieved by blocking sodium and chloride reabsorption in the thick ascending limb of Henle's Loop with furosemide is followed by a marked decrease in the intrarenal levels of deoxyhemoglobin. Administration of furosemide in conjunction with BOLD-MRI therefore allows detection and estimation of renal oxygen consumption dependent on tubular solute reabsorption. Hence, we hypothesized that the response to furosemide might be diminished in severe renovascular disease when tubular function is significantly impaired (25) and that this would be detectable by BOLD-MRI. In support of this hypothesis, Textor et al. (38) recently showed that in the stenotic kidney of patients with renovascular hypertension having a normal nephrogram, furosemide produced a significant reduction in medullary BOLD-MRI signal. On the other hand, no such reduction was observed in renovascular hypertensive patients with “nonfunctioning kidneys.” However, it remained unknown whether the blunted furosemide-induced suppression of oxygen consumption (FSOC) was due to decreased sodium delivery or to local dysfunction of the tubules.
In a swine model, we sought to create large vessel occlusion recognized as producing a wide range of poststenotic renal dysfunction. With the use of a multidetector computed tomography (MDCT) scanner, we can measure renal blood flow (RBF) in each kidney along with single-kidney GFR after injection of intravenous (IV) contrast. During the tubular phase of contrast transit, it is also possible indirectly to evaluate segmental tubular dynamics (19) independent of contrast media delivery via GFR. The use of these two powerful imaging techniques might thus shed light on tubular function in different nephron segments. Therefore, the present study was undertaken to test the hypothesis that BOLD-MRI can help differentiate adequate and impaired renal tubular responses to FSOC in renovascular hypertension. For this purpose, both the stenotic and contralateral kidneys of renovascular hypertensive pigs were studied with MRI-BOLD to determine FSOC. In addition, MDCT was used to determine concurrent renal function as well as segmental tubular function.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee. Domestic female pigs (body wt 30–40 kg) were used in this study.
Induction of renal artery stenosis.
Eight weeks before the imaging studies, unilateral renal artery stenosis (RAS) was induced. At baseline, under sterile conditions and fluoroscopic guidance, a metal wire coil was engaged in the proximal-middle section of the right renal artery as previously described (8, 9, 25, 33). This induces a variable reactive hypertrophy of the arterial intimal wall, resulting in different degrees of hypertension.
MRI acquisition was done at 8 wk after induction of RAS, followed by a 4-day recovery period, and finally CT scanning was performed. This allowed full recovery and minimized any possible interference between the two imaging procedures. For each imaging session, anesthesia was induced with intramuscular ketamine/xylazine (20 mg/kg, 2 mg/kg), and the pigs were intubated and mechanically ventilated with room air. Continuous IV infusion of ketamine (0.2 mg·kg−1·min−1) and xylazine (0.03 mg·kg−1·min−1) was used for the maintenance, with minimal effects on renal hemodynamics (2, 36, 40).
MRI-BOLD image acquisition.
Eight weeks after baseline intervention renal MR-BOLD imaging was performed at 1.5 Tesla (Signa Echo Speed; GE Medical Systems, Milwaukee, WI) to measure R2* levels in medullary and cortical regions of the kidney using customized abdominal organ protocols, as previously described (12, 13, 16, 29, 30). MRI examinations were performed during suspended respiration.
The principle of the BOLD method has been described in detail in previous publications (16, 29). Briefly, paramagnetic molecules induce magnetic field perturbations. In the blood, oxyhemoglobin is diamagnetic and its concentration has no effect on T2*, but deoxyhemoglobin is paramagnetic and decreases tissue T2*. Therefore, when the echo time of the gradient echo MRI acquisition increases, the MRI signal attenuation increases with increased concentration of deoxyhemoglobin. The slope of Ln (intensity) vs. echo time equals relaxation time rate R2* (=1/T2) (16, 29) and is directly proportional to the concentration of deoxyhemoglobin.
For data analysis, regions of interest were manually traced in the cortex and medulla on the 7-ms echo time image that gave the best anatomic details. For each echo time, the software automatically computed the average of MR signals within each region of interest. The BOLD signal, as characterized by the relaxivity R2*, was then measured (16). Following the initial BOLD acquisition, furosemide (20 mg) was administered intravenously into an ear vein catheter and flushed with 2 ml of saline. The BOLD measurements were repeated 15 min later. Finally, the change in R2* from prefurosemide to postfurosemide was determined as “delta-R2*.”
CT image acquisition.
Four days after BOLD-MRI, each animal was anesthetized again, intubated, and mechanically ventilated with room air. For MDCT preparation, vascular cut-downs were made in the left carotid artery and left jugular vein. Under sterile conditions, an 8F arterial guide was inserted in the carotid artery. A side arm of the arterial sheath served for monitoring of arterial pressure. A pigtail catheter was advanced through the left jugular vein sheath and positioned in the superior vena cava for contrast injections.
Scanning sequences.
After catheter placement, the animals were positioned in the MDCT (Somatom Sensation 64; Siemens Medical Solutions, Forchheim, Germany) scanning gantry. All tomographic levels containing both kidneys were identified with localization scans. For performance of a flow study, two mid-hilar tomographic levels in both kidneys were chosen, and a bolus of iopamidol (0.5 ml/kg over 2 s) was injected, as previously described (8–10, 19, 26, 33), for acquisition of 140 consecutive scans over 3 min. Renal volume study was performed after a 15-min rest in the helical mode (240 mAs, 120 kV, pitch of 1.2, and B40 medium kernel) to obtain contiguous 5-mm-thick levels for measurements of cortical, medullary, and total kidney volume. Blood pressure was monitored throughout the experiment.
MDCT data analysis.
The methodology used for MDCT data analysis has been previously described in detail (8–10, 19, 26, 33). Briefly, regions of interest were traced in the aorta and cortex and medulla of both kidneys and their densities were sampled. For each region, time-density curves were generated, fitted with extended gamma variate fits (19), and the area enclosed under the curve and its mean transit times were calculated from the curve-fitting parameters. The curves generated in the cortex provided cortical vascular (perfusion), proximal, and distal tubular transit, while medullary curves provided medullary vascular and tubular transit in the Loop of Henle. Renal regional perfusion (ml·min−1·ml tissue−1), single-kidney GFR (ml/min), cortical and medullary volumes, and RBF were subsequently calculated as previously described (7, 19, 21, 24). Intratubular fluid concentration (i.e., degree of concentration or dilution of tubular fluid) was calculated for each (cortical and medullary) nephron segment as the ratio of the area under each tubular curve to that of the cortical vascular curve and normalized (divided) by GFR. Once standardized, this parameter applies to broad physiological conditions regardless of independent changes in RBF or GFR and reflects concentration/dilution function of each tubular segment.
The method used to calculate volumes was based on a statistical point-counting volume estimation program (21–23) implemented with ANALYZE (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN).
Statistical analysis.
Results are expressed as means ± SE. Comparisons between experimental groups were performed using unpaired Student's t-test. Statistical significance was accepted if P < 0.05.
RESULTS
Effects of progressive constriction of the renal artery on RBF and GFR in the stenotic kidney.
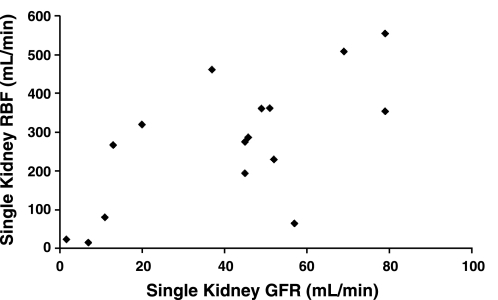
As might be expected, there was a significant correlation between the levels of RBF and GFR (Fig. 1, r = 0.65, P < 0.01).
Fig. 1.
Correlation between renal blood flow (RBF) and glomerular filtration rate (GFR) in animals with variable degrees of experimental renovascular hypertension. Data are presented for each experimental animal. r = 0.65, P < 0.005.
Effect of changes in GFR on FSOC in the medulla of the stenotic kidney.
The group of animals with lower GFR showed absolute medullary R2* values slightly above those in the group with higher GFR (19.1 ± 2.3 vs. 15.5 ± 0.8), although this did not reach statistical significance.
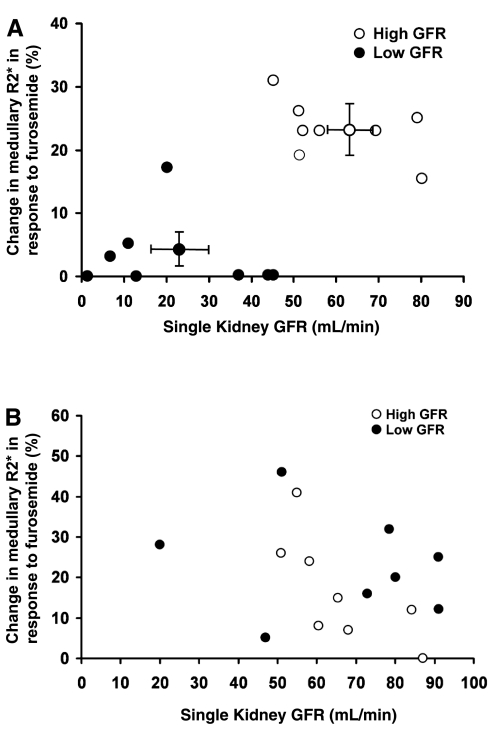
Figure 2A illustrates the relationship between different levels of GFR and changes in R2* induced by FSOC in pigs with experimentally induced renovascular disease. There was a significant relationship between the magnitude of poststenotic single-kidney GFR and the changes in medullary deoxyhemoglobin detected with MR-BOLD. The values of these parameters tended to aggregate into two distinctive groups. In eight kidneys (represented in Fig. 2A by ●), the levels of GFR did not exceed 45 ml/min and the change of deoxyhemoglobin signal (in 7/8 animals) was <5%. By contrast, eight other kidneys (represented in Fig. 2A by ○) with GFR values higher than 45 ml/min exhibited a change in deoxyhemoglobin signal of 23.6 ± 2.2% in response to furosemide. However, these limits were not absolute in defining the point at which the fall in GFR compromised sodium delivery. For example, two animals with the same levels of GFR (45 ml/min each) exhibited very different levels of deoxyhemoglobin signal responses to furosemide (0 and 31%). Similarly, two other animals with a comparable response of DHb-R2* signal (15 and 17%) to furosemide had different levels of GFR (80 and 20 ml/min, respectively). In general, the fall in sodium delivery was such that the response of the deoxyhemoglobin-R2* signal to furosemide was under 5% for a level of GFR below 45 ml/min. In contrast, GFR levels higher than 45 ml/min were associated with FSOC response greater that 18%.
Fig. 2.
A: relationship between changes in medullary blood oxygen level-dependent magnetic resonance imaging (BOLD-MRI) R2* induced by furosemide suppression of oxygen consumption with the level of GFR in the stenotic kidney of animals with experimentally induced renovascular hypertension. GFR levels below 45 ml/min are represented with ●. GFR levels above 45 ml/min are represented with ○. Average levels for both groups with high and low GFR are also represented with their respective SE. B: relationship between changes in medullary BOLD-MRI R2* induced by furosemide suppression of oxygen consumption with the level of GFR in the contralateral kidney of animals with experimental renovascular hypertension. ● Represent levels in the contralateral kidney that correspond to low GFR in the stenotic kidney, while ○ represent levels in he contralateral kidney that correspond to high levels of GFR in the stenotic kidney.
The average values of GFR that corresponded to their respective changes of BOLD-R2* signals for the two groups of animals mentioned above are also represented in Fig. 2A. It can be seen that a low level of GFR (22.5 ± 6.2 ml/min) corresponds to an average change of deoxyhemoglobin-R2* signal of 3.1 ± 2%; in contrast, the group of animals with higher levels of GFR (60.1 ± 4.54 ml/min), and likely a less severe degree of arterial stenosis, showed an average change in deoxyhemoglobin-R2* signal of 23.6 ± 2.1%.
Effects of changes in GFR on FSOC in the medulla of the contralateral kidney.
In contrast to the stenotic kidney, the contralateral kidney did not exhibit any correlation between the level of GFR and the change in the deoxyhemoglobin signal induced by furosemide in the renal medulla (Fig. 2B). To facilitate comparisons, the levels of GFR (plotted against the % change in R2*) from the contralateral kidney have been labeled with open or closed circles depending on whether they originated from animals exhibiting high or low GFR in their respective stenotic kidney. In almost all the animals, the values of GFR in the contralateral kidney were higher than 45 ml/min. However, the values of GFR did not exhibit any correlation with the corresponding values of percent change in R2*.
Correlation between FSOC with renal function in the contralateral and stenotic kidney.
As shown in Table 1, animals that exhibited deoxyhemoglobin-R2* response to furosemide above >18% had similar RBF, GFR, renal volume, and tissue perfusion between the contralateral and stenotic kidneys. In these animals, the increase from baseline in mean arterial pressure (MAP) was 18 ± 1 mmHg. In contrast, the animals that exhibited a weak deoxyhemoglobin-R2* response to furosemide (below 5%) were characterized by significantly lower levels of RBF, GFR, renal volume, and tissue perfusion in the stenotic vs. contralateral kidney (P < 0.01 for all). Furthermore, in these animals the increase of MAP from baseline was 28 ± 0.2 mmHg, which was slightly, albeit not significantly (P < 0.18), greater than in animals with preserved GFR. Note that the levels of MAP in both groups of animals before the constriction of the renal artery were virtually the same (Table 1).
Table 1.
Changes in renal function in renovascular hypertensive animals according to responses to furosemide
| MAP, mmHg |
RBF, ml/min |
GFR, ml/min |
Renal Volume, ml |
Tissue Perfusion, ml/min |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Hypertension | ST | CONT | ST | CONT | ST | CONT | ST | CONT | |
| Responders (R2*>18%) | 95±5 | 113±7 | 337±51 | 408±30 | 60±4 | 66±4 | 92±7 | 97±5 | 4.03±0.4 | 4.6±0.1 |
| Nonresponders (R2*<5%) | 96±5 | 124±9 | 205±55 | 476±53* | 22±6 | 66±4* | 55±12 | 116±9* | 3.4±0.4 | 4.6±0.5* |
Values are means ± SE. MAP, mean arterial pressure; RBF, renal blood flow; GFR, glomerular filtration rate; CONT, contralateral kidney.
P < 0.05 vs. stenotic kidney (ST); n = 8 each group.
Changes in density of X-ray contrast medium along the proximal, Henle's Loop, and distal nephron segments.
The changes in X-ray density along the cortical proximal tubule, renal medullary Loop of Henle, and cortical distal nephrons in animals with high GFR and low GFR recorded in the stenotic and contralateral kidney using MDCT are presented in Table 2. In both groups and for both kidneys, X-ray density was somewhat higher in the Loop of Henle than in proximal or distal tubules. However, in the stenotic kidney of animals with high GFR the X-ray density in the Loop of Henle was higher when compared with the low GFR group, suggesting higher fluid reabsorption, although this difference did not achieve statistical significance (P = 0.14) due to large variability.
Table 2.
Tubular contrast concentration in the stenotic and contralateral kidneys of renovascular hypertensive pigs
| Kidney Area | Stenotic |
Contralateral |
||
|---|---|---|---|---|
| High GFR | Low GFR | High GFR | Low GFR | |
| Proximal | 1.12±0.07 | 1.09±0.16 | 1.16±0.07 | 1.21±0.14 |
| Henle | 3.88±1.68 | 1.98±0.26 | 2.90±0.75 | 1.91±0.27 |
| Distal | 1.72±0.12 | 1.18±0.22 | 1.67±0.18 | 1.24±0.26 |
Values are means ± SE.
DISCUSSION
The results of this study indicate that changes in medullary tubular function after furosemide in poststenotic kidneys as measured by BOLD-MRI correlate with the degree of residual GFR of that kidney. Therefore, this technique might be useful to evaluate both kidneys in renovascular hypertension.
Renal oxygen consumption.
The renal parenchyma has two major components that determine O2 consumption. These include a basal O2 consumption (20), which is inherent to the existence of a minimal metabolic activity (∼1 mM/g tissue), and a much higher level of O2 consumption (6–7 mM/g tissue) related to tubular solute reabsorption. The proximal tubules reabsorb in an isosmotic fashion 67% of filtered sodium and consume 27% of total O2 (4, 11, 18). In contrast, the Loop of Henle reabsorbs 30% of the filtered sodium and consumes 65% of O2 (4, 11, 18). This high-energy expenditure is attributed to the fact that the Loop of Henle is impermeable to water, and sodium chloride is reabsorbed against an osmotic gradient (14, 17, 18). O2 is also consumed by more distally located tubules but total sodium reabsorption in these segments constitutes only a small fraction of sodium filtered in the glomeruli (6%). Specific blockade of sodium reabsorption in the thick ascending Loop of Henle, which can be achieved by inhibiting the 2 Cl, sodium, K cotransporter (1, 5, 32) with furosemide, produces a decrease of O2 consumption with a proportional decrease in the intrarenal levels of deoxyhemoglobin. Since the intrarenal oxygenation can be evaluated noninvasively with BOLD-MRI (29), FSOC provides an index of active tubular solute reabsorption.
Changes in BOLD-MRI induced by FSOC in the stenotic kidney.
Previous findings showed that the coil method used to produce a unilateral renal artery constriction yielded variable degrees of obstruction, the severity of which correlated with the increase in MAP as well as with the magnitude of the fall in RBF and GFR (25). Animals with mild stenosis develop an increase in MAP that overcomes the impedance of the arterial constriction, thereby restoring RBF, GFR, and tubular fluid delivery. We then hypothesized that such compensation could maintain the response to furosemide comparable to that of the contralateral kidney (Table 1). However, as the stenosis becomes more severe, a pronounced decline in RBF may lead to ischemia and decrease basal renal oxygenation and increase R2* values (a nonstatistically significant trend observed in animals with low GFR), while the fall of GFR significantly limits fluid delivery and therefore tubular sodium reabsorption. We suspected it would decrease proportionally the FSOC response as measured with BOLD-MRI. In addition, tubular injury might decrease tubular fluid concentration capacity and result in tubular dysfunction (8), which would also contribute to blunt FSOC responses. The results of our study demonstrated a rather abrupt transition in BOLD-MR responses to furosemide, since below a GFR level of 45 ml/min there was a very limited FSOC response by BOLD-MRI, whereas FSOC responses were virtually normalized above a GFR level of 45 ml/min. However, a few animals exhibited “transitional values” characterized by comparable levels of GFR and different responses of BOLD-MRI to FSOC, or vice versa, similar responses to FSOC and different levels of GFR. We propose that these disparities may be related to the fact that the stenotic kidney develops variable interstitial fibrosis and tubular atrophy (6), which can alter renal capillary circulation and renal interstitial pressure. This, in turn, affects tubular fluid flow and tubular fluid reabsorption in a heterogeneous manner, which tend to become independent of the level of GFR.
Notably, even in kidneys with relatively preserved GFR, FSOC is slightly (albeit not markedly) lower than the ∼30% decrease in R2* observed in normal human and pig renal medullas in response to furosemide (27, 28). This might be due to redistribution of sodium reabsorption to other tubular segments that do not respond to furosemide, tubular damage, or changes in sodium reabsorption efficiency (42).
Contralateral kidneys.
We observed no correlation between high values of GFR recorded in the contralateral kidney of both groups and the marked changes in BOLD-MRI induced by FSOC. These changes are compatible with a natriuretic compensatory response of the contralateral kidney, which counteracts the decrease of sodium delivery in the stenotic kidney. It should be noted that such a natriuretic response in the contralateral kidney is most likely mediated by a proximal sodium rejection, since sodium reabsorption in the Loop of Henle remains dependent on sodium delivery as it occurs during abundant sodium delivery.
Reduced FSOC as a marker for the stage of renal impairment in renovascular hypertension.
The results of this study also revealed that indirect estimations of sodium reabsorption, through the response of BOLD-MRI to furosemide, might provide a useful index to disclose the stage of development of renovascular hypertension and its effects on the kidneys. In fact, significant FSOC in the stenotic kidney suggests that the increase in MAP in this group has been capable of overcoming the impedance of renal arterial constriction maintaining renal function. This has important clinical implications because it might be speculated that under conditions of preserved function renal revascularization may restore blood pressure and renal function. In contrast, the absence of a change in BOLD-MRI signal after furosemide challenge in the renal medulla of animals with low GFR is compatible with limited sodium reabsorption in the Loop of Henle. These findings were also suggested by MDCT-determined tubular fluid concentration and by the marked decrease in the other dependent parameters such as RBF, GFR, and renal volume. Decreased renal function and reduced oxygen consumption in the stenotic kidney appear to reflect a more advanced state of deterioration that may not respond to restoration of blood flow by angioplasty.
Changes in tubular fluid reabsorption in the stenotic and contralateral kidneys of animals with severe and mild disease.
Changes in the density (which reflects tissue concentration) of X-ray contrast medium along the proximal and distal tubules and in the renal medulla are illustrated in Table 2. The contrast media concentration is related inversely to and therefore serves as an index of tubular fluid reabsorption. These density measures are indexed to the concentration of contrast media in the cortical vessels and normalized for GFR. Interestingly, medullary density of contrast in stenotic kidneys with normal GFR appeared to be higher compared with stenotic kidneys with low GFR, although the difference has not reached statistical significance. Because these parameters are normalized for GFR, this slight difference can be attributed at least partly to an increase in sodium reabsorption (rather than decreased delivery) in the proximal segments, which characterizes kidneys with low perfusion pressure (3, 8, 35, 37) and can extend to the Loop. These differences of densities also explain the differences in the FSOC responses in the Loop of Henle. The lower tubular fluid concentration in the Loop of Henle of animals with low GFR may reflect failure to transport solute and decreased capacity to concentrate tubular fluid in this segment, consistent with the BOLD-MRI finding of medullary tubular dysfunction in this group. Nevertheless, it is likely that additional factors that modulate FSOC reduced sodium delivery also contributed to the observed decrease, such as redistribution or changes in efficiency of sodium reabsorption (41).
Conclusions.
The findings reported in this study provide further insight into application of BOLD-MRI as a noninvasive method of assessing renal tubular dysfunction in poststenotic kidneys. They underscore the complex means by which a reduction in FSOC may demonstrate functional tubulointerstitial injury that may potentially help delineate the response to renal revascularization. The MDCT-derived measure of tubular fluid concentration suggests that the impaired FSOC observed in low-functioning stenotic kidneys is not caused only by a decrease in sodium and fluid delivery, but also by local tubular dysfunction. However, while these data are suggestive, future studies will be needed to verify and establish the mechanisms of decreased FSOC in dysfunctional kidneys.
The BOLD-MRI method has several advantages. While CT can also evaluate renal tubular function (19, 23), the use of IV contrast in CT studies mandates restrictions in patients with renal disease. Similarly, conventional angiogram provides information only about the anatomic degree of stenosis, also through the use of contrast media. Other techniques to assess distal nephron function, such as micropuncture, are too invasive and used only experimentally. Instead, BOLD-MRI constitutes a new noninvasive tool that does not require the use of contrast media and is clinically applicable. Compared with Doppler-resistive index (31), our method also provides detailed anatomical and functional information on the renal parenchyma, differentiates cortex and medulla, and studies tubular function. This might provide higher-resolution information than currently available about changes in specific renal regions and functional features, which might potentially have predictive power regarding the choice of treatment or renal response to therapy. Therefore, MR in conjunction with BOLD can become a very useful tool to assess both kidneys in renovascular disease and hypertension.
GRANTS
This study was supported by National Institutes of Health Grants RO1-HL-16496, DK-73608, DK-77013, HL-77131, and P01-HL-085307.
ACKNOWLEDGMENTS
We express gratitude to K. Zodrow for typing and editing this manuscript. We acknowledge the work of our friend and colleague, the late Dr. J. Carlos Romero.
REFERENCES
- 1. Aukland K, Johannesen J, Kiil F. In vivo measurements of local metabolic rate in the dog kidney. Effect of mersalyl, chlorothiazide, ethacrynic acid and furosemide. Scand J Clin Lab Invest 23: 317–330, 1969 [DOI] [PubMed] [Google Scholar]
- 2. Bailie MD, Alward CT, Sawyer DC, Hook JB. Effect of anesthesia on cardiovascular and renal function in the newborn piglet. J Pharmacol Exp Ther 208: 298–302, 1979 [PubMed] [Google Scholar]
- 3. Bianchi G, Baldoli E, Lucca R, Barbin P. Pathogenesis of arterial hypertension after the constriction of the renal artery leaving the opposite kidney intact both in the anaesthetized and in the conscious dog. Clin Sci 42: 651–664, 1972 [DOI] [PubMed] [Google Scholar]
- 4. Brezis M, Agmon Y, Epstein FH. Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1059–F1062, 1994 [DOI] [PubMed] [Google Scholar]
- 5. Burnham C, Karlish SJ, Jorgensen PL. Identification and reconstitution of a Na+/K+/Cl− cotransporter and K+ channel from luminal membranes of renal red outer medulla. Biochim Biophys Acta 821: 461–469, 1985 [DOI] [PubMed] [Google Scholar]
- 6. Cantin M, Solymoss B, Benchimol S, Desormeaux Y, Langlais J, Ballak M. Metaplastic and mitotic activity of the ischemic (endocrine) kidney in experimental renal hypertension. Am J Pathol 96: 545–565, 1979 [PMC free article] [PubMed] [Google Scholar]
- 7. Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension 45: 1042–1049, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Chade AR, Rodriguez-Porcel M, Grande JP, Krier JD, Lerman A, Romero JC, Napoli C, Lerman LO. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 106: 1165–1171, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol 23: 1295–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 10. Daghini E, Primak AN, Chade AR, Krier JD, Zhu XY, Ritman EL, McCollough CH, Lerman LO. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 243: 405–412, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Epstein FH. Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997 [DOI] [PubMed] [Google Scholar]
- 12. Epstein FH, Prasad P. Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int 57: 2080–2083, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Epstein FH, Veves A, Prasad PV. Effect of diabetes on renal medullary oxygenation during water diuresis. Diabetes Care 25: 575–578, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Glynn IM, Karlish SJ. The sodium pump. Annu Rev Physiol 37: 13–55, 1975 [DOI] [PubMed] [Google Scholar]
- 15. Gomez SI, Haas JA, Romero JC. Renovascular hypertension: pathophysiology and evaluation of renal function. In: Comprehensive Hypertension, edited by Kip GY, Hall JE. Philadelphia, PA: Mosby Elsevier, 2007, p. 101–111 [Google Scholar]
- 16. Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC. Blood oxygen level-dependent measurement of acute intrarenal ischemia. Kidney Int 65: 944–950, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kiil F. Na-K-ATP-ases as regulators of sodium and water excretion. Scand J Clin Lab Invest 28: 375–378, 1971 [DOI] [PubMed] [Google Scholar]
- 18. Kiil F. Renal energy metabolism and regulation of sodium reabsorption. Kidney Int 11: 153–160, 1977 [DOI] [PubMed] [Google Scholar]
- 19. Krier JD, Ritman EL, Bajzer Z, Romero JC, Lerman A, Lerman LO. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 281: F630–F638, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Lassen NA, Munck O, Thaysen JH. Oxygen consumption and sodium reabsorption in the kidney. Acta Physiol Scand 51: 371–384, 1961 [DOI] [PubMed] [Google Scholar]
- 21. Lerman LO, Bell MR, Lahera V, Rumberger JA, Sheedy PF, 2nd, Sanchez Fueyo A, Romero JC. Quantification of global and regional renal blood flow with electron beam computed tomography. Am J Hypertens 7: 829–837, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Lerman LO, Bentley MD, Bell MR, Rumberger JA, Romero JC. Quantitation of the in vivo kidney volume with cine computed tomography. Invest Radiol 25: 1206–1211, 1990 [DOI] [PubMed] [Google Scholar]
- 23. Lerman LO, Flickinger AL, Sheedy PF, 2nd, Turner ST. Reproducibility of human kidney perfusion and volume determinations with electron beam computed tomography. Invest Radiol 31: 204–210, 1996 [DOI] [PubMed] [Google Scholar]
- 24. Lerman LO, Rodriguez-Porcel M, Sheedy PF, 2nd, Romero JC. Renal tubular dynamics in the intact canine kidney. Kidney Int 50: 1358–1362, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Lerman LO, Schwartz RS, Grande JP, Sheedy PF, Romero JC. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol 10: 1455–1465, 1999 [DOI] [PubMed] [Google Scholar]
- 26. Lerman LO, Taler SJ, Textor SC, Sheedy PF, 2nd, Stanson AW, Romero JC. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int 49: 846–854, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Li LP, Storey P, Pierchala L, Li W, Polzin J, Prasad P. Evaluation of the reproducibility of intrarenal R2* and DeltaR2* measurements following administration of furosemide and during waterload. J Magn Reson Imaging 19: 610–616, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen M, Dissing TH, Morkenborg J, Stodkilde-Jorgensen H, Hansen LH, Pedersen LB, Grenier N, Frokiær J. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int 67: 2305–2312, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation 94: 3271–3275, 1996 [DOI] [PubMed] [Google Scholar]
- 30. Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int 55: 294–298, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Radermacher J. Resistive index: an ideal test for renovascular disease or ischemic nephropathy? Nat Clin Pract Nephrol 2: 232–233, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Reeves WB, Molony DA. The physiology of loop diuretic action. Semin Nephrol 8: 225–233, 1988 [PubMed] [Google Scholar]
- 33. Rodriguez-Porcel M, Krier JD, Lerman A, Sheedy PF 2nd, Romero JC, Napoli C, Lerman LO. Combination of hypercholesterolemia and hypertension augments renal function abnormalities. Hypertension 37: 774–780, 2001 [DOI] [PubMed] [Google Scholar]
- 34. Romero JC, Feldstein AE, Rodriguez-Porcel MG, Cases-Amenos A. New insights into the pathophysiology of renovascular hypertension. Mayo Clin Proc 72: 251–260, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Selkurt EE, Hall PW, Spencer MP. Influence of graded arterial pressure decrement on renal clearance of creatinine, p-amino-hippurate and sodium. Am J Physiol 159: 369–378, 1949 [DOI] [PubMed] [Google Scholar]
- 36. Shiga Y, Minami K, Uezono Y, Segawa K, Nagaoka E, Shiraishi M, Noguchi T, Shigematsu A. Effects of the intravenously administered anaesthetics ketamine, propofol, and thiamylal on the cortical renal blood flow in rats. Pharmacology 68: 17–23, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Shipley RE, Study RS. Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. Am J Physiol 167: 676–688, 1951 [DOI] [PubMed] [Google Scholar]
- 38. Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC. Blood oxygen level dependent (BOLD) magnetic resonance in renal artery stenosis. J Am Soc Nephrol 19: 780–788, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med 52: 421–442, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Tranquilli WJ, Thurmon JC, Benson GJ. Organ blood flow and distribution of cardiac output in hypocapnic ketamine-anesthetized swine. Am J Vet Res 44: 1578–1582, 1983 [PubMed] [Google Scholar]
- 41. Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, Romero JC. Regional decreases in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol 296: R67–R71, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol 288: H22–H28, 2005 [DOI] [PubMed] [Google Scholar]
- 43. Wilcox CS. Ischemic nephropathy: noninvasive testing. Semin Nephrol 16: 43–52, 1996 [PubMed] [Google Scholar]