Abstract
Activation of renal interstitial fibroblasts is critically involved in the development of tubulointerstitial fibrosis in chronic kidney diseases. In this study, we investigated the effect of trichostatin A (TSA), a specific histone deacetylase (HDAC) inhibitor, on the activation of renal interstitial fibroblasts in a rat renal interstitial fibroblast line (NRK-49F) and the development of renal fibrosis in a murine model of unilateral ureteral obstruction (UUO). α-Smooth muscle actin (α-SMA) and fibronectin, two hallmarks of fibroblast activation, were highly expressed in cultured NRK-49F cells, and their expression was inhibited in the presence of TSA. Similarly, administration of TSA suppressed the expression of α-SMA and fibronectin and attenuated the accumulation of renal interstitial fibroblasts in the kidney after the obstructive injury. Activation of renal interstitial fibroblasts was accompanied by phosphorylation of signal transducer and activator of transcription 3 (STAT3), and TSA treatment also abolished these responses. Furthermore, inhibition of the STAT3 pathway with AG490 inhibited expression of α-SMA and fibronectin in NRK-49F cells. Finally, TSA treatment inhibited tubular cell apoptosis and caspase-3 activation in the obstructive kidney. Collectively, we suggest that pharmacological HDAC inhibition may induce antifibrotic activity by inactivation of renal interstitial fibroblasts and inhibition of renal tubular cell death. STAT3 may mediate those actions of HDACs.
Keywords: trichostatin A, histone deacetylase, renal interstitial fibroblasts, unilateral ureteral obstruction, STAT3
irrespective of the initial cause, tubulointerstitial fibrosis is a constant feature of chronic renal diseases (CKD) and contributes to the deterioration of renal function. Renal interstitial fibrosis is characterized by aberrant activation and growth of the renal fibroblasts (31, 37, 47). The activated fibroblast has been termed a myofibroblast that is defined by phenotypic changes such as the expression of α-smooth muscle actin (α-SMA) and increased production of extracellular matrix (ECM) components such as fibronectin (31, 37, 47). The presence of interstitial myofibroblasts correlates with the extent of tubulointerstitial scarring and functional outcome in clinical glomerulonephritis and IgA nephropathy (5, 12, 38). As such, understanding the molecular events responsible for activation of renal fibroblasts may lead to new approaches in the treatment of progressive renal diseases.
Recent studies show that activation of histone deacetylases (HDACs), a group of enzymes that catalyze the removal of acetyl groups from lysine residues in proteins, is associated with differentiation and proliferation of a variety of cell types and the pathogenesis of some chronic diseases. For example, inhibition of HDAC activity by trichostatin A (TSA) decreased platelet-derived growth factor (PDGF)-induced proliferation of NIH 3T3 fibroblasts (7) and prevented transforming growth factor-β (TGF-β1)-induced α-SMA expression and morphological changes in cultured human skin fibroblasts (40). TSA also blocked TGF-β1-induced epithelial-to-mesenchymal transition (EMT) in renal tubular epithelial cells (51). Furthermore, administration of TSA attenuated cardiac hypertrophy induced by pressure overload and angiotensin II (13, 21) and reversed atrial fibrosis and arrhythmia vulnerability in mice and/or rats (30). Despite the importance of HDACs in mediating cardiac hypertrophy, it remains unexplored whether HDAC activity is required for activation of renal interstitial fibroblasts and development of renal fibrosis.
HDAC-mediated biological events occur through targeting of both histones as well as nonhistone signal transduction proteins (17, 44). Among numerous nonhistone proteins that have been identified as HDAC substrates, STAT3 is activated and highly expressed in renal interstitial fibroblasts after obstructive injury (24) and mediates renal tubular cell apoptosis following oxidant and ischemia-reperfusion injuries (2, 49). STAT3 belongs to a family of latent cytoplasmic transcription factors. Activated STATs form homo- or heterodimers and then translocate to the nucleus, where they bind with DNA and regulate gene transcription (14). Increasing evidence suggests that the regulation of gene expression by STATs requires HDAC activity. For example, TSA inhibits STAT3-dependent transcriptional activity induced by PDGF in NIH 3T3 cells (7) and blocks STAT1 activation in IFN-γ-stimulated carcinoma cells (22). Also, the protective effects of suberoylanilide hydroxamic acid, another HDAC inhibitor, in a murine model of graft vs. host disease are associated with a block in the rapid accumulation of phosphorylated STAT1 in the liver and spleen (27).
HDACs consist of three general classes: class I (HDAC1, 2, 3, and 8); class II (HDAC4, 5, 6, 7, 9, and 10); and class III Sir-like proteins (17, 44). All members of class I HDACs and HDAC 10 are expressed in the kidney (10). Class I and II HDACs are specifically inhibited by TSA in vitro and in vivo (4, 34). In this study, we examined the effect of HDAC inhibition by TSA on the activation of renal interstitial fibroblasts in a rat renal interstitial fibroblast line (NRK-49F) and on the development of fibrosis in a mouse model of renal fibrosis induced by unilateral ureteral obstruction (UUO). Furthermore, we examined whether HDAC activity is required for the activation of STAT3 and the role of the STAT3 pathway in renal interstitial fibroblast activation.
MATERIALS AND METHODS
Materials.
TSA and AG490 were purchased from Biomol (Plymouth Meeting, PA). Antibodies to phospho-STAT3, STAT3, phospho-ERK1/2, ERK1/2, phospho-Akt, Akt, or acetyl-STAT3 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to α-SMA and fibronectin were obtained from Sigma (St. Louis, MO) and BD Biosciences (San Diego, CA), respectively. A fibroblast-specific protein-1 (FSP-1) antibody was purchased from Dako (Carpinteria, CA). Antibodies to acetyl-histone H4 and acetyl-histone H3 were purchased from Active Motif (Carlsbad, CA) . All other chemicals and reagents were purchased from Sigma.
Cell culture.
Rat renal interstitial fibroblasts (NRK-49F) were grown in DMEM (Sigma-Aldrich) containing 5% FBS, penicillin, and streptomycin in an atmosphere of 5% CO2-95% air at 37°C. For obtaining quiescent cells, cells were incubated in DMEM with 0.5% FBS for 24 h. Cells that were 60–70% confluent were used. When necessary, various inhibitors were directly added to the culture and then incubated for 24 or 48 h.
UUO model and TSA treatment.
UUO was performed in male C57 black mice that weighed 20–25 g (Jackson Laboratory, Bar Harbor, ME) as described previously (48). All animal experiments were performed according to national and institutional animal care and ethical guidelines and were approved by the Institutional Animal Care and Use Committee at Rhode Island Hospital. Briefly, after induction of general anesthesia by intraperitoneal injection of pentobarbital (50 mg/kg body wt), the abdominal cavity was exposed via a midline incision and the left ureter was ligated at two points with 4-0 silk. At 7 days after UUO, the mice were killed and the kidneys were removed for Western blot analysis or histological examination. UUO was confirmed by observation of dilation of the pelvis and proximal ureter and collapse of the distal ureter. The contralateral kidneys were used as controls. Immediately after ureter ligation, TSA (0.5 mg/kg) was administered to mice intraperitoneally and then daily injection for 6 days. Mice were randomized into four groups with four mice in each group as follows: 1) sham injury with vehicle (saline); 2) sham injury with TSA; 3) UUO injury with vehicle; and 4) UUO injury with TSA.
Nucleosome extraction.
NRK-49F cells (60–70% confluent) were starved in a serum-free medium for 24 h and then pretreated with 100 nM TSA or diluent (DMSO) for 1 h followed by 5% FBS stimulation for an additional 30 min. Cytoplasmic and nuclear fractions were isolated using a nuclear extract kit from Active Motif according to the manufacturer's instructions. Fifteen micrograms of cytoplasmic and nuclear proteins were subjected to immunoblot analysis of STAT3. GAPDH and lamin B were used as a cytoplasmic and nuclear protein control, respectively. Graphs were made with the ratios of cytoplasmic and nuclear STAT3 abundance relative to GAPDH or lamin B.
Western blot analysis.
Cell or tissue samples were prepared in a lysis buffer. After sonication and centrifugation, supernatants were collected, and the protein concentration was determined with the BCA protein assay kit (Pierce, Rockford, IL). Twenty micrograms of protein from each sample were separated by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). After treatment with 5% skim milk at 4°C overnight, membranes were incubated with various antibodies for 1 h and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibodies were visualized following chemiluminescence detection on autoradiographic film.
Immunochemical analysis.
For cell immunostaining, cells cultured on coverslips were washed with PBS, fixed with 4% paraformaldehyde, permeabilized with 0.1% (vol/vol) Triton X-100 and 0.1 mM glycine, and then incubated 30 min in PBS containing 5% serum. Cells were then treated with primary antibodies at room temperature for 1 h. After washing with PBS, cells were incubated with a mixture of FITC-labeled goat anti-rabbit IgG antibody for 1 h at room temperature. For immunohistochemistry, the tissue sections were deparaffinized and hydrated. Immunostaining of markers was performed using Vectastain ABC. For immunofluorescent staining, primary antibodies and fluorescent-conjugated secondary antibodies were applied to the sections after blocking with preimmune serum from the same species for the secondary antibody. Morphological analyses were performed by using light and fluorescent microscopy. Photographs of 10–15 random cortical fields (×400) from each animal were taken using a SPOT camera, and positive-staining cells were counted and positive-staining areas were measured using the National Institutes of Health (NIH) Image (1.62) program.
Interstitial fibrosis evaluated by sirius red staining.
Sirius red staining was performed for the histological evaluation of the interstitial area occupied by collagen fibrils as described previously (52). Kidney sections were examined by light microscopy. The positive tubulointerstitial areas were photographed and then measured using the NIH Image (1.62) program.
Statistical analysis.
Data are presented as means ± SD and were subjected to one-way ANOVA. Multiple means were compared by using Tukey's test. The differences between two groups were determined by Student's t-test. P < 0.05 was considered as statistically significant difference between mean values.
RESULTS
Inhibition of HDAC and STAT3 activation decreases expression of α-SMA and fibronectin.
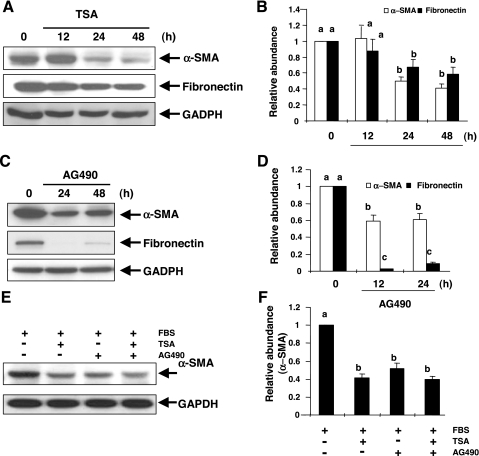
Myofibroblast is characterized by de novo expression of α-SMA and the overproduction of interstitial matrix components such as fibronectin (47, 53). It has been reported that HDAC activity is required for cardiac hypertrophy (13, 21) and STAT3 is activated in the kidney after UUO injury (24). Thus we assessed the effect of TSA, a specific inhibitor of HDACs (4, 34) and AG490, a selective inhibitor of the STAT3 pathway (33), on the expression of α-SMA and fibronectin in a rat renal interstitial fibroblast cell line (NRK-49F). α-SMA and fibronectin were expressed in NRK-49F cells under normal culture condition, indicating that cultured NRK-49F cells are phenotypically myofibroblasts (Fig. 1). Treatment of cells with TSA decreased the expression of α-SMA and fibronectin, which was observed at 24 h and sustained at least for 48 h (Fig. 1, A and B). Similarly, AG490 treatment resulted in suppression of α-SMA expression and fibronectin production at 24 and 48 h (Fig. 1, C and D). These data suggest that HDACs and STAT3 are critically involved in the activation of renal interstitial fibroblasts in vitro.
Fig. 1.
Effect of trichostatin A (TSA) and AG490 on expression of α-smooth muscle actin (SMA) and fibronectin. NRK-49F cells were incubated with 100 nM TSA (A) or 40 μM AG490 (C) alone for the indicated time or together for 24 h (E) and harvested for immunoblot analysis with antibodies against α-SMA, fibronectin, or GADPH. Representative immunoblots from 3 experiments are shown. Expression levels of α-SMA or fibronectin were quantified by densitometry and are expressed as fold-induction over controls (B, D, and F). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
Since HDACs and STAT3 are both involved in regulating the activation of renal interstitial fibroblasts, we further determined whether combined inhibition of these two pathways produces a synergetic effect. As shown in Fig. 1, E and F, incubation of NRK-49F cells with TSA and AG490 did not result in a greater inhibition on expression of α-SMA than TSA or AG490 alone, suggesting that HDACs and STAT3 may act in the same pathway to activate renal interstitial fibroblasts.
Inhibition of HDACs blocks serum-induced STAT3 tyrosine phosphorylation and increases its acetylation.
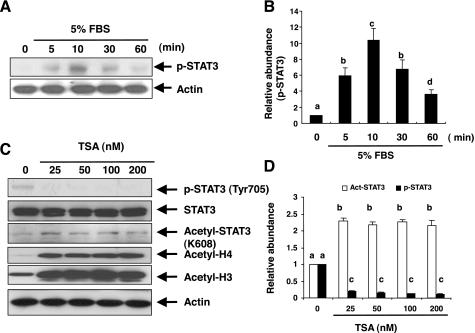
A recent study showed that HDAC inhibitors impede IFN-γ-induced activation of STAT1 by dephosphorylation of STAT1 on tyrosine 701 (22). To assess the effect of HDAC inhibition on the activation of STAT3 signaling pathways in cultured NRK-49F cells, we first examined STAT3 activation by measuring its phosphorylation levels using immunoblot analysis and an antibody that recognizes phosphorylated STAT3 at tyrosine 705 (p-STAT3). Total STAT3 contents were measured using immunoblot analysis and an antibody that recognizes STAT3 independently of its phosphorylation state. p-STAT3 was not detected in serum-starved NRK-49F cells, and treatment with 5% FBS resulted in an increase in its phosphorylation levels, which occurred within 5 min and was maximal at 10 min (Fig. 2, A and B).
Fig. 2.
Effect of TSA on serum-induced phosphorylation and acetylation of signal transducer and activator of transcription 3 (STAT3). Serum-starved NRK-49F cells were exposed to 5% FBS for 0–60 min (A) or pretreated with TSA for 1 h at the indicated concentrations and then exposed to 5% FBS for an additional 1 h (C). Cell lysates were subjected to immunoblot analysis with antibodies to phospho-STAT3 (Tyr705), acetyl-STAT3 (K685), STAT3, or actin. Representative immunoblots from 3 experiments are shown. Expression levels of phospho-STAT3 or acetyl-STAT3 were quantified by densitometry and are expressed as fold-induction over controls (B and D). Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
Next, we assessed the effect of TSA on STAT3 phosphorylation and acetylation in cultured NRK-49F cells with 5% FBS. As shown in Fig. 2, C and D, treatment with TSA abolished the STAT3 tyrosine phosphorylation at Tyr 705. STAT3 acetylation was detectable under normal culture condition and enhanced in the presence of TSA. HDAC suppression by TSA would be expected to induce histone hyperacetylation due to unopposed histone acetyltransferase. TSA at 25 nM induced a significant increase in acetylation of histone H3 and H4, which was sustained through 50–200 nM (Fig. 2C), confirming the efficacy of HDAC suppression. These data suggest that the HDAC activity is essential for regulation of the STAT3 pathway.
HDAC activity is required for serum-stimulated STAT3 nuclear translocation.
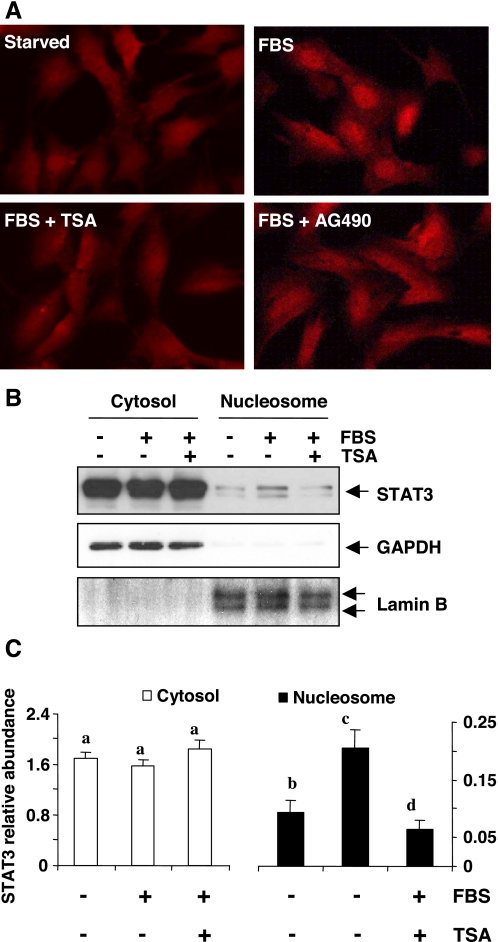
Since activated STAT3 forms homo- or heterodimers and then translocates into the nucleus where it binds to specific DNA-response elements and regulates the expression of target genes (8), we further examined the effect of HDAC inhibition on STAT3 nuclear translocation by immunofluorescent microscopy and immunoblot analysis. The results from immunofluorescent microscopy clearly demonstrated that STAT3 was mainly localized in the cytosol of serum-starved cells. Stimulation with 5% FBS for 1 h induced STAT3 nuclear translocation (Fig. 3A), and this was inhibited by TSA pretreatment. Immunoblot analysis also showed that most of the STAT3 was distributed in the cytosol of serum-starved NRK-49F cells. Treatment with 5% FBS for 30 min resulted in an increase in the nuclear content of STAT3, which was accompanied by a slight decrease in cytosolic STAT3. Pretreatment with TSA blocked serum-induced STAT3 nuclear translocation (Fig. 3, B and C). Notably, both STAT3α (86 kDa) and STAT3β (79 kDa) were detected in NRK-49 cells. These data, together with the inhibitory effect of TSA on serum-induced STAT3 phosphorylation, suggest that HDAC activity is required for STAT3 activation.
Fig. 3.
Effect of TSA on serum-induced translocation of STAT3. NRK-49F cells were serum-starved for 24 h and then treated with 5% FBS in the presence or absence of 100 nM TSA for 1 h (A) or 30 min (B). A: cells were stained with anti-STAT3 antibody and then subjected to microscopic analysis (×600). B: cytosolic and nuclear proteins were separated and subjected to immunoblot analysis of STAT3, GADPH, or lamin B. C: expression levels of cytosolic and nuclear STAT3 were quantified by densitometry and normalized to GADPH or lamin B levels, respectively. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
TSA inhibits α-SMA expression and STAT3 phosphorylation in a mouse model of UUO.
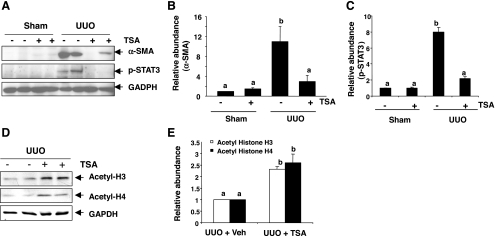
Previous studies showed that phosphorylated STAT3 was expressed in interstitial fibroblasts in the fibrotic kidney (24, 47). We thus examined the effect of TSA on α-SMA expression and STAT3 phosphorylation in the fibrotic kidney induced by UUO injury. Western blot analyses revealed the presence of α-SMA and phosphorylated STAT3 in the kidney tissue after UUO. In contrast, α-SMA was barely detected and phosphorylated STAT3 was not detectable in the kidney tissue of sham-operated animals. TSA treatment totally blocked UUO injury-induced expression of α-SMA and phosphorylation of STAT3 (Fig. 4, A–C). Quantitative determination exhibited an ∼10-fold induction of the relative abundance of α-SMA protein in the obstructed kidney after UUO compared with sham controls (Fig. 4B). To determine the efficacy of TSA in vivo, we also examined the expression level of acetylated histone H3 and H4 in mice subjected to UUO injury alone or UUO with TSA. As shown in Fig. 4, D and E, the basal level of acetylated histone H3 and H4 was detected in the kidney after UUO injury and further increased when TSA was administered, indicating an effective inhibition of TSA on the HDAC activity in the mouse kidney following UUO injury.
Fig. 4.
Effect of TSA on expression of α-SMA and phosphorylation of STAT3 in the kidney after unilateral ureteral obstruction (UUO). Kidney tissue lysates prepared from the obstructed kidneys were subjected to immunoblot analysis with specific antibodies against α-SMA, phospho-STAT3, GAPDH, acetyl-H3, or acetyl-H4 (A and D). Expression levels of the indicated proteins in different groups were quantified by densitometry and are expressed as fold-induction over sham controls (value = 1.0) after normalization with GADPH (B, C, and E). Values are means ± SD (n = 4). Bars with different letters (a–c) are significantly different from one another (P < 0.05).
These data are in line with our observations in vitro that HDACs are critically involved in regulation of renal fibroblast activation and STAT3 phosphorylation. As STAT3 activation is associated with α-SMA expression in renal fibroblasts in vitro (Fig. 1C), an increase in expression of phosphorylated STAT3 in the obstructive kidney suggests that STAT3 activation may also be involved in the regulation of α-SMA expression in vivo.
TSA decreases the accumulation of renal interstitial fibroblasts in a mouse model of UUO.
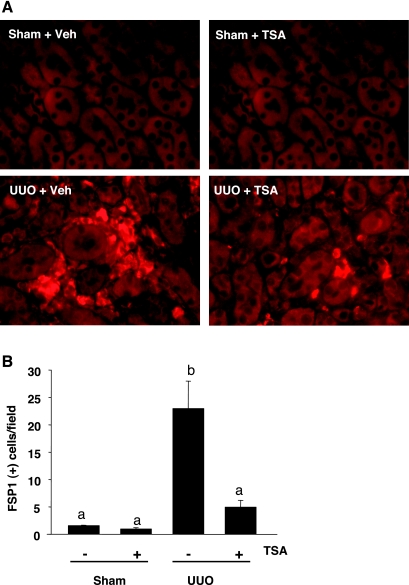
It has been reported that FSP-1 is a specific marker of myofibroblasts (20), we examined whether TSA treatment affects the accumulation of myofibroblasts in the kidney after UUO injury. Figure 5A shows that the UUO-injured kidney displayed a large number of FSP-positive cells, which were localized in the interstitial area surrounding the tubules and significantly inhibited by TSA administration (Fig. 5B). Therefore, HDAC activity is required for the accumulation of myofibroblasts in the kidney after UUO injury.
Fig. 5.
Effect of TSA on expression of fibroblast-specific protein-1 (FSP-1) in the kidney after UUO. A: immunofluorescence staining of kidney sections exhibited FSP-1-positive cells localizing in the peritubular areas. B: FSP-1-positive cells/high-power field (×600) were counted. Values are means ± SD of 4 animals/group. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
TSA inhibits the deposition of fibronectin in a mouse model of UUO.
As a consequence of myofibroblast activation, excess ECM components including fibronectin are produced to cause massive tissue fibrosis, as seen in diseased kidneys (28, 29). To examine the effect of TSA on production of fibronectin, the kidney tissue was stained with an anti-fibronectin antibody and examined by microscopy. Figure 6A shows that a large amount of fibronectin was expressed in the fibrotic kidney, which was in the peritubular area, and TSA administration attenuated its expression. Quantitative analysis revealed that TSA treatment resulted in a decrease in fibronectin expression by threefold relative to that in the kidney injured by UUO alone (Fig. 6B).
Fig. 6.
Effect of TSA on expression of fibronectin in the kidney after UUO. A: representative micrographs demonstrating the expression and localization of fibronectin in the mouse kidney as indicated. B: graph showing the percentage of fibronectin-positive area (arrows) relative to the whole area from 10 random cortical fields (×200). Values are means ± SD. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
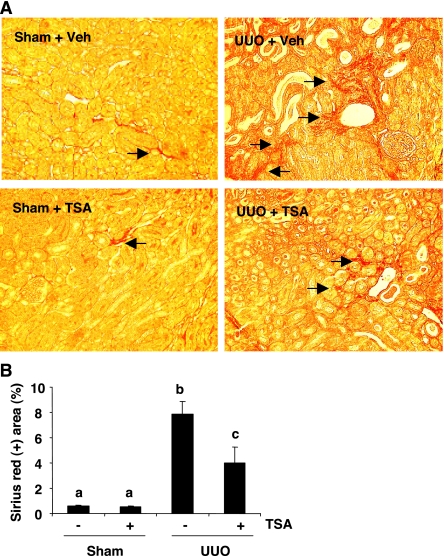
TSA decreases renal interstitial fibrosis.
Sirius red staining has been extensively used for analysis of interstitial fibrosis (9). As shown in Fig. 7, sirius red staining showed an increase in interstitial collagen fibrils after UUO compared with sham-operated control. The sirius red-positive interstitial area was significantly less in UUO-injured mice with TSA treatment (Fig. 7), indicating that TSA is an effective antifibrotic agent in vivo.
Fig. 7.
Effect of TSA on renal fibrosis after UUO. A: photomicrographs illustrating sirius red staining of sham, UUO-injured kidneys with/without TSA administration. B: graph showing the percentage of sirius red-positive tubulointerstitial area (red) relative to the whole area from 10 random cortical fields (×200). Values are means ± SD. Bars with different letters (a–c) are significantly different from one another (P < 0.05).
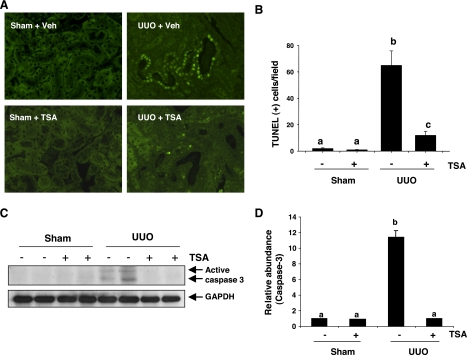
TSA inhibits tubular apoptotic cell death and caspase-3 activation in a mouse model of UUO.
Recent studies showed that inhibition of HDACs by TSA protected mouse renal tubular cells from apoptosis at a low dose (50 nM) (1) and also induced apoptosis of rat renal tubular cells at a higher dose (5 μM) in cell culture systems (11). To assess the effect of TSA on tubular cell viability in vivo, terminal transferase-dUTP-nick-end labeling (TUNEL) staining was conducted to monitor the apoptotic cell death in sham-operated and UUO-injured kidneys after TSA treatment. As shown in Fig. 8, A and B, a large number of TUNEL-positive cells were observed in renal tubules after UUO injury. Administration of TSA dramatically decreased the number of apoptotic tubule cells. Apoptotic cell death was not detected in the kidney subjected to sham operation with TSA treatment.
Fig. 8.
Effect of TSA on renal tubular cell apoptosis after UUO injury. A: representative micrographs demonstrating the expression and localization of apoptosis in different groups as indicated (×400). B: the number of apoptotic tubular cells was counted and expressed as means ± SD of 4 animals/group. Bars with different letters (a–c) are significantly different from one another (P < 0.05). C: whole kidney tissue lysates were subjected to immunoblot analysis with antibodies against caspase-3 or GAPDH. D: expression levels of active caspase-3 were quantified by densitometry and are expressed as fold-induction over controls. Values are means ± SD of 3 independent experiments. Bars with different letters (a–d) are significantly different from one another (P < 0.05).
Caspase-3 is the major caspase that mediates tubular cell apoptosis following ureteral obstruction (45). We also examined the effect of TSA on the expression of active caspase-3 after UUO injury by immunoblot analysis. As shown in Fig. 8, C and D, active caspase-3 was not detected in the kidney sections of sham-operated and TSA-treated mice without UUO injury. In contrast, UUO injury induced activation of capase-3, as shown by expression of two active forms of it. TSA administration completely blocked caspase-3 activation. On this basis, we suggest that inhibition of HDAC activity by TSA protects renal tubular cells from apoptosis after UUO injury.
DISCUSSION
HDACs are a family of enzymes that are responsible for the removal of acetyl groups from histones and other nonhistone proteins (10, 18, 36). Recent studies point to the importance of these enzymes in controlling proliferation and differentiation of various cells (7). However, the molecular and functional consequences of HDAC inhibition in renal interstitial fibroblasts have not been previously described. In this study, we report that inhibition of HDAC activity by TSA decreased the expression of α-SMA and fibronectin in cultured rat renal fibroblasts. Furthermore, TSA administration attenuated the interstitial fibrosis and expression of α-SMA and fibronectin in the kidney after UUO injury. As progressive renal interstitial fibrosis is not only the predominant pathological feature of obstructive nephropathy but is also considered as a common final pathway of nearly all forms of CKD, our findings suggest that HDACs may play an essential role in mediating the development of renal interstitial fibrosis in CKD caused by different renal diseases.
HDAC-mediated activation of renal fibroblasts is involved in the activation of the STAT3 signaling pathway. This claim was supported by our observations as follows. First, activation of fibroblasts was accompanied by phosphorylation of STAT3 at tyrosine 705 in both cultured renal interstitial fibroblasts and in the fibrotic kidney induced by UUO. Second, inhibition of the STAT3 pathway with AG490 inhibited the expression of α-SMA and fibronectin. Third, inactivation of HDACs with TSA abolished STAT3 phosphorylation induced by serum in vitro and by UUO injury in vivo. Since it has been reported that active STAT3 is primarily expressed in myofibroblasts in the obstructive kidney, a complete blockade of STAT3 phosphorylation after UUO injury suggests that HDAC is the key enzyme responsible for the activation of STAT3 in renal interstitial fibroblasts. In support of this idea, we also found that inhibition of HDAC activity by TSA blocked serum-induced STAT3 nuclear translocation. The requirement of the deacetylase activity of HDAC is not limited to STAT3 activation. Activation of STAT1 and STAT6 has also been reported to require HDAC activity (6, 22). Thus the STAT pathway may be important in transducing HDAC activation to its biological actions.
The mechanism(s) underlying HDAC regulates STAT3 tyrosine phosphorylation and activation remains elusive. Since TSA-induced suppression of STAT3 phosphorylation at tyrosine 705 is accompanied by an increase in STAT3 acetylation at lysine 685, one possibility is that, in the setting of nonphysiological inhibition of HDAC by TSA, lysine acetylation prevents tyrosine phosphorylation, thereby shutting down STAT3 activation. The crystal structure of STAT3 shows that acetylated lysine 685 is situated on the external surface of the SH2 domain where tyrosine residue 705 is located (39). As such, these two posttranslational modifications may be mutually exclusive. STAT3 acetylation on lysine 685 may cause a conformational change that interferes with substrates and ATP to access the catalytic pocket. A very recent study showed that acetylation of STAT1 counteracts IFN-induced STAT1 phosphorylation (23), favoring the hypothesis that STAT3 acetylation regulates its phosphorylation. Another possibility is that cross talk between phosphorylation and acetylation occurs at the JAKs and/or various growth factor/cytokine receptors that regulate STAT3 activation. If this is the case, STAT3 tyrosine phosphorylation would be also disrupted indirectly upon HDAC inhibition. In this regard, it has been reported that many cytoplasmic and membrane proteins including cytokine receptors also undergo acetylation (16, 43, 50), and this acetylation is associated with inhibition of enzymatic activities. For example, acetylation of acetyl-CoA synthase (42) and nitric oxide synthase (32) leads to inactivation of these enzymes. Additional studies are required to elucidate the mechanisms by which HDACs regulate the activation of STAT3.
HDACs may also indirectly contribute to renal fibrosis by inducing apoptosis and subsequent release of fibrogenic cytokines. It is well known that ureteral obstruction leads to renal damage, with tubular cell apoptosis occurring as an early event followed by the release of cytokines and chemokines from the injured tubular cell, resulting in inflammatory reaction and interstitial fibrosis (3). Thus the inhibition of initial apoptosis is likely to limit the generation of signals from dying cells that would subsequently attract and promote the inflammatory response. This, in turn, would prevent the renal accumulation of a number of proapoptotic and profibrotic cytokines. Recently, Arany et al. (1) reported that treatment with 50 nM TSA increased the survival of cultured renal epithelial cells following cisplatin exposure. In the present study, we examined the effect of TSA on renal tubular apoptosis in a mouse model of renal fibrosis induced by UUO and found that TSA administration at 0.5 mg/kg attenuated renal epithelial cell apoptosis and blocked caspase-3 activation, indicating the prosurvival effect of TSA in renal tubular cells in this model. However, it should be noted that TSA may have a toxic effect on renal epithelial cells when large doses are used. Dong et al. (11) have recently reported that exposure of rat renal epithelial cells to TSA at 5 μM, a dose 50-fold higher than that used in our in vitro studies, induced apoptotic cell death. As such, TSA may have both prosurvival or proapoptotic effects, depending on the concentration used.
Although our data suggest that suppression of renal fibroblast activation and inhibition of tubular cell apoptosis serve as the important antifibrotic mechanisms of HDAC inhibitors, other actions of HDAC inhibition may also contribute to this process. Yoshikawa et al. (51) examined the effect of TSA on the EMT in cultured tubular epithelial cells and found that TSA can prevent TGF-β1-induced EMT. Mechanistic studies revealed that TSA induced expression of two inhibitory factors of TGF-β1 signals: inhibitors of DNA binding/differentiation 2 (Id2) and BMP-7 (51). Recently, they further demonstrated that treatment with TSA attenuated the progression of proteinuria and glomerulosclerosis in a mouse model of nephrotoxic serum nephritis (NTN) by promoting the expression of BMP-7 in multipotent kidney side population cells (19). Therefore, HDACs may contribute to renal fibrosis through multiple mechanisms.
Currently, individual HDAC family members in mediating fibroblast activation and tubular cell apoptosis remain to be identified. As TSA is a specific inhibitor for class I and II HDACs, it is anticipated that certain isozymes of HDACs in these two classes mediate these responses. Among eight enzymes in class I and II HDACs, HDAC1, HDAC2, and HDAC3 are ubiquitously expressed and have been reported to mediate proliferation and differentiation in tumor cells (25, 41, 46). Experiments are underway to examine whether any of those HDACs is responsible for renal interstitial fibroblast activation.
In summary, we demonstrated for the first time that blockade of HDACs inhibits the activation of renal interstitial fibroblasts in vitro and in vivo. HDAC activity is required for the activation of STAT3, and inhibition of the STAT3 pathway also inhibited renal fibroblast activation. These findings suggest that HDACs may mediate activation of renal interstitial fibroblasts through a mechanism involved in STAT3 activation. Currently, more than a dozen of HDAC inhibitors are in clinical trials for treatment of malignancies of almost all organ origins (26, 35), and a HDAC inhibitor, suberoylanilide hydroxamic acid, is already in clinical use for the treatment of cutaneous lymphoma (15). Thus it will be interesting to further assess the clinical value of HDAC inhibition as therapeutics for renal diseases associated with fibroblast activation, especially, for CKD in the future.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-071997.
REFERENCES
- 1. Arany I, Herbert J, Herbert Z, Safirstein RL. Restoration of CREB function ameliorates cisplatin cytotoxicity in renal tubular cells. Am J Physiol Renal Physiol 294: F577–F581, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Arany I, Megyesi JK, Nelkin BD, Safirstein RL. STAT3 attenuates EGFR-mediated ERK activation and cell survival during oxidant stress in mouse proximal tubular cells. Kidney Int 70: 669–674, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int 68: 925–937, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bieliauskas AV, Pflum MK. Isoform-selective histone deacetylase inhibitors. Chem Soc Rev 37: 1402–1413, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bob FR, Gluhovschi G, Herman D, Potencz E, Gluhovschi C, Trandafirescu V, Schiller A, Petrica L, Velciov S, Bozdog G, Vernic C. Histological, immunohistochemical and biological data in assessing interstitial fibrosis in patients with chronic glomerulonephritis. Acta Histochem 110: 196–203, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ, Younes A. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood 112: 1424–1433, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catania A, Iavarone C, Carlomagno SM, Chiariello M. Selective transcription and cellular proliferation induced by PDGF require histone deacetylase activity. Biochem Biophys Res Commun 343: 544–554, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev 28: 185–200, 2008 [DOI] [PubMed] [Google Scholar]
- 9. De Heer E, Sijpkens YW, Verkade M, den Dulk M, Langers A, Schutrups J, Bruijn JA, van Es LA. Morphometry of interstitial fibrosis. Nephrol Dial Transplant 15, Suppl 6: 72–73, 2000 [DOI] [PubMed] [Google Scholar]
- 10. de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J 370: 737–749, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong G, Wang L, Wang CY, Yang T, Kumar MV, Dong Z. Induction of apoptosis in renal tubular cells by histone deacetylase inhibitors, a family of anticancer agents. J Pharmacol Exp Ther 325: 978–984, 2008 [DOI] [PubMed] [Google Scholar]
- 12. el Nahas M, Muchaneta-Kubara EC, Tamimi N, Goumenos D. Glomerulosclerosis: the role of interstitial myofibroblasts in its progression. Curr Top Pathol 93: 167–171, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, Jones P, Gallinari P, De Francesco R, Ciliberto G, Steinkuhler C, Esposito G, Condorelli G. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res 80: 416–424, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE 2006: pe30, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, Faderl S, Koller C, Morris G, Rosner G, Loboda A, Fantin VR, Randolph SS, Hardwick JS, Reilly JF, Chen C, Ricker JL, Secrist JP, Richon VM, Frankel SR, Kantarjian HM. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood 111: 1060–1066, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol 20: 71–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hildmann C, Riester D, Schwienhorst A. Histone deacetylases–an important class of cellular regulators with a variety of functions. Appl Microbiol Biotechnol 75: 487–497, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Huang L. Targeting histone deacetylases for the treatment of cancer and inflammatory diseases. J Cell Physiol 209: 611–616, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Imai N, Hishikawa K, Marumo T, Hirahashi J, Inowa T, Matsuzaki Y, Okano H, Kitamura T, Salant D, Fujita T. Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells 25: 2469–2475, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Klampfer L, Huang J, Swaby LA, Augenlicht L. Requirement of histone deacetylase activity for signaling by STAT1. J Biol Chem 279: 30358–30368, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kramer OH, Knauer SK, Greiner G, Jandt E, Reichardt S, Guhrs KH, Stauber RH, Bohmer FD, Heinzel T. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev 23: 223–235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuratsune M, Masaki T, Hirai T, Kiribayashi K, Yokoyama Y, Arakawa T, Yorioka N, Kohno N. Signal transducer and activator of transcription 3 involvement in the development of renal interstitial fibrosis after unilateral ureteral obstruction. Nephrology (Carlton) 12: 565–571, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Lagger G, O'Carroll D, Rembold M, Khier H, Tischler J, Weitzer G, Schuettengruber B, Hauser C, Brunmeir R, Jenuwein T, Seiser C. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J 21: 2672–2681, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee MJ, Kim YS, Kummar S, Giaccone G, Trepel JB. Histone deacetylase inhibitors in cancer therapy. Curr Opin Oncol 20: 639–649, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leng C, Gries M, Ziegler J, Lokshin A, Mascagni P, Lentzsch S, Mapara MY. Reduction of graft-versus-host disease by histone deacetylase inhibitor suberonylanilide hydroxamic acid is associated with modulation of inflammatory cytokine milieu and involves inhibition of STAT1. Exp Hematol 34: 776–787, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Li R, Yang N, Zhang L, Huang Y, Zhang R, Wang F, Luo M, Liang Y, Yu X. Inhibition of Jak/STAT signaling ameliorates mice experimental nephrotic syndrome. Am J Nephrol 27: 580–589, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Liapis H, Barent B, Steinhardt GF. Extracellular matrix in fetal kidney after experimental obstruction. J Urol 166: 1433–1438, 2001 [PubMed] [Google Scholar]
- 30. Liu F, Levin MD, Petrenko NB, Lu MM, Wang T, Yuan LJ, Stout AL, Epstein JA, Patel VV. Histone-deacetylase inhibition reverses atrial arrhythmia inducibility and fibrosis in cardiac hypertrophy independent of angiotensin. J Mol Cell Cardiol 45: 715–723, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 15: 1–12, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104: 14855–14860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A, Levitzki A, Roifman CM. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature 379: 645–648, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Monneret C. Histone deacetylase inhibitors. Eur J Med Chem 40: 1–13, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Moradei O, Vaisburg A, Martell RE. Histone deacetylase inhibitors in cancer therapy: new compounds and clinical update of benzamide-type agents. Curr Top Med Chem 8: 841–858, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Mottet D, Castronovo V. Histone deacetylases: target enzymes for cancer therapy. Clin Exp Metastasis 25: 183–189, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Neilson EG. Mechanisms of disease: fibroblasts—a new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Nishitani Y, Iwano M, Yamaguchi Y, Harada K, Nakatani K, Akai Y, Nishino T, Shiiki H, Kanauchi M, Saito Y, Neilson EG. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int 68: 1078–1085, 2005 [DOI] [PubMed] [Google Scholar]
- 39. O'Shea JJ, Kanno Y, Chen X, Levy DE. Cell signaling: Stat acetylation—a key facet of cytokine signaling? Science 307: 217–218, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Rombouts K, Niki T, Greenwel P, Vandermonde A, Wielant A, Hellemans K, De Bleser P, Yoshida M, Schuppan D, Rojkind M, Geerts A. Trichostatin A, a histone deacetylase inhibitor, suppresses collagen synthesis and prevents TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp Cell Res 278: 184–197, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Senese S, Zaragoza K, Minardi S, Muradore I, Ronzoni S, Passafaro A, Bernard L, Draetta GF, Alcalay M, Seiser C, Chiocca S. Role for histone deacetylase 1 in human tumor cell proliferation. Mol Cell Biol 27: 4784–4795, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298: 2390–2392, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Tang X, Gao JS, Guan YJ, McLane KE, Yuan ZL, Ramratnam B, Chin YE. Acetylation-dependent signal transduction for type I interferon receptor. Cell 131: 93–105, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tong JK. Dissecting histone deacetylase function. Chem Biol 9: 668–670, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Truong LD, Choi YJ, Tsao CC, Ayala G, Sheikh-Hamad D, Nassar G, Suki WN. Renal cell apoptosis in chronic obstructive uropathy: the roles of caspases. Kidney Int 60: 924–934, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Wilson AJ, Byun DS, Popova N, Murray LB, L'Italien K, Sowa Y, Arango D, Velcich A, Augenlicht LH, Mariadason JM. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281: 13548–13558, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol 13: 96–107, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Yang N, Luo M, Li R, Huang Y, Zhang R, Wu Q, Wang F, Li Y, Yu X. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant 23: 91–100, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell 31: 449–461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yoshikawa M, Hishikawa K, Marumo T, Fujita T. Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol 18: 58–65, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Zhang G, Kim H, Cai X, Lopez-Guisa JM, Carmeliet P, Eddy AA. Urokinase receptor modulates cellular and angiogenic responses in obstructive nephropathy. J Am Soc Nephrol 14: 1234–1253, 2003 [DOI] [PubMed] [Google Scholar]
- 53. Zoja C, Abbate M, Remuzzi G. Progression of chronic kidney disease: insights from animal models. Curr Opin Nephrol Hypertens 15: 250–257, 2006 [DOI] [PubMed] [Google Scholar]