Abstract
Objective: Hormone therapy (HT) increases the risk of venous thrombosis and stroke. Risk of venous thrombosis and stroke is higher in older, overweight, and obese women using HT. However, the impact of age and obesity on estrogen concentrations among HT users is not well defined.
Method: We measured serum levels of estrone, total and free estradiol, and SHBG in 180 postmenopausal women participating in the Estrogen in the Prevention of Atherosclerosis Trial (EPAT), 91 receiving estradiol therapy (ET) and 89 taking placebo, every 6 months over 2 yr. Mean on-trial levels of estrogens and SHBG were compared across age, body mass index (BMI), and waist to hip ratio categories among ET users and placebo separately.
Results: Among the ET users, total (P = 0.01) and free estradiol (P = 0.002) were significantly directly associated with BMI adjusted for age. SHBG was inversely related to waist to hip ratio adjusted for age (P = 0.005). Age was not associated with any of the estrogen or SHBG concentrations in ET or placebo groups. BMI was positively associated with estrone concentrations among older but not younger ET users (P for interaction = 0.03).
Conclusion: Overweight and obese women using ET attain greater concentrations of estrogen compared to women with normal BMI, whereas ET users with abdominal obesity attain lower SHBG levels. Obese older women using ET have the highest concentration of estrone. It may be useful to consider age and obesity when prescribing HT to minimize the risk of venous thrombosis or stroke in postmenopausal women. Further research regarding relationships among circulating hormone levels and risk for these conditions is required to substantiate this conclusion.
It may be useful to consider age and obesity when prescribing hormone therapy to minimize the risk of venous thrombosis or stroke in postmenopausal women.
Age and obesity, two important risk factors for thrombosis, have a substantial influence on physiological concentrations of sex hormones in women (1,2,3,4). However, the role of age and obesity in modulating the serum levels of estrogens in women using postmenopausal hormone therapy (HT) has not been fully studied. Because women tend to gain weight as they proceed beyond menopause, it is particularly important to understand this phenomenon because accumulating evidence suggests a protective to no effect of HT on cardiovascular disease in younger postmenopausal women who are in close proximity to menopause (5,6,7).
Age-associated differences in the pharmacokinetics of drugs may account for the differential effects of HT in younger and older postmenopausal women (8,9). Pharmacokinetic differences between older and younger postmenopausal women can be primarily attributed to decreased hepatic and renal function, reduced cardiac output, impaired pulmonary function, reduction in muscle mass, and changes in body composition with age (10,11). Oral bioavailability of many drugs is increased in the elderly due to decreased hepatic first pass metabolism, which is a consequence of decreased hepatic blood flow in conjunction with reduced hepatic drug-metabolizing capacity (12).
Although altered pharmacokinetics is recognized in the elderly, there is a scarcity of data regarding age-associated changes in circulating sex hormone levels after estrogen administration in postmenopausal women. If estrogen pharmacokinetics is altered in older relative to younger postmenopausal women, serum levels of estradiol (E2) and estrone (E1) in older women should be higher than in younger postmenopausal women. This could have implications for the thrombophilic effects of HT.
Another important factor that contributes to increased estrogenicity in postmenopausal women is obesity. Androstenedione and testosterone are converted to E1 and E2, respectively, in adipose tissue, which is the major source of circulating estrogens in women after menopause (13). Cross-sectional studies have shown that serum estrogen concentrations are directly associated with body mass index (BMI) in postmenopausal women (2,14). In contrast to estrogens, serum SHBG levels have been consistently reported to be inversely associated with BMI, and particularly with central obesity (2,15,16). Low serum SHBG levels result in higher concentrations of free E2 and free testosterone in overweight and obese women. However, little is known about the modifying effect of obesity on serum levels of estrogens and SHBG with postmenopausal HT.
Risk of stroke is greater in older and heavier women using postmenopausal HT (17). There is also evidence showing gradual elevation in the risk of venous thrombosis in older as well as overweight and obese women using postmenopausal HT compared with younger women with normal BMI taking placebo, suggesting an interaction between age, weight, and serum concentration of estrogens (18). Therefore, it is important to understand the interaction of aging and obesity with postmenopausal HT-induced serum estrogen concentrations. We hypothesized that older, heavier, postmenopausal women using E2 therapy (ET) will have higher concentrations of serum estrogen than younger, average body-sized women. To test this hypothesis, we measured serum estrogens and SHBG levels in 91 postmenopausal women randomized to ET and compared these levels in relation to age and obesity. To compare these associations with physiological concentrations of serum estrogens and SHBG, we also included an analysis of 89 postmenopausal women randomized to placebo.
Subjects and Methods
Study population and design
We analyzed data from the Estrogen in the Prevention of Atherosclerosis Trial (EPAT). EPAT was a randomized, double-blind, placebo-controlled trial that was designed to investigate whether unopposed ET slows the progression of subclinical atherosclerosis in healthy postmenopausal women. Details of eligibility criteria have been published previously (19). In brief, eligible women were at least 45 yr old, not current smokers, and postmenopausal with serum E2 less than 20 pg/ml. A total of 33 (32%) women in the placebo group and 43 (44%) women in the E2 group had a history of hysterectomy at enrollment. As per protocol, pelvic examination in all women and uterine ultrasonography in women with intact uterus were performed yearly. Uterine biopsy was done if the endometrial thickness was 5 mm or more. All women with 5 mm or more uterine thickness were advised to take medroxyprogesterone acetate by their gynecologist. Participants were randomly assigned to receive either 1 mg of oral micronized 17β-estradiol (n = 91) or placebo (n = 89) daily for 2 yr and were followed with a clinic visit every 2 months during the trial. Blood pressure and other vital signs were measured at each clinic visit. All study participants gave written informed consent, and the study was approved by the Institutional Review Board of the University of Southern California.
Anthropometric assessment
At each clinic visit, body weight and waist and hip circumference were measured by trained clinical staff. BMI was calculated as weight (in kilograms)/height (in meters)2. The ratio of waist to hip circumference [waist to hip ratio (WHR)] was calculated to assess visceral adiposity. Baseline BMI was categorized into the following three groups using Center for Disease Control cut points: <25, 25–29, and 30 kg/m2 or greater. Baseline WHR was categorized into three groups using the tertiles of distribution in the EPAT participants: below 0.80, 0.80–0.83, and above 0.83.
Blood sampling and laboratory assays
At study visits occurring every 6 months, blood samples were obtained after an 8-h fast, and serum was stored at −70 C until it was used to quantify analytes. Serum sex hormone and SHBG levels were measured at the end of the study in all stored samples. E2 and E1 levels were measured by RIA after their extraction with ethyl acetate: hexane (3:2) and subsequent separation by Celite column partition chromatography, as described previously (8,9). The sensitivities of the E2 and E1 assays were 3 and 5 pg/ml, respectively. Intraassay and interassay coefficients of variation were 7.0 and 11.0% for E2 and 7.4 and 10.0% for E1, respectively. SHBG was measured by direct chemiluminescent immunoassay on the Immulite analyzer (Siemens Medical Solutions, Los Angeles, CA). The sensitivity of the SHBG assay was 0.2 nmol/liter, with intraassay and interassay coefficients of variation of 5.9 and 9.4%, respectively. Free E2 was calculated using a validated algorithm (20,21,22).
Statistical analysis
Serum estrogen and SHBG levels were available in 180 study subjects, including 91 E2-treated and 89 placebo-treated women. Women were categorized into the following baseline age groups: younger than 60 yr, 60–65 yr, and older than 65 yr. For each woman, the average of each of the follow-up hormone and SHBG levels (levels measured during the trial after randomization) was computed and used in the analysis; a total of 348 samples from 89 placebo-treated women and 360 samples from 91 E2-treated women were collected during the trial period with a median (range) of four (one to five) samples per subject. To test the associations of age, BMI, and WHR with sex hormone levels in untreated and ET-treated women, analyses were conducted separately by randomized treatment group. In each treatment group, the mean estrogen and SHBG levels were compared across age, BMI, and WHR categories. Each sex hormone or SHBG level was modeled as the dependent variable in separate linear regression models. The independent variables included age, BMI, and WHR. Both univariate and multivariate models were tested. Models testing associations of sex hormones and SHBG levels with age were adjusted for BMI, and models testing WHR and BMI were adjusted for age. Because of high correlation, BMI and WHR were not included in the same model (Pearson’s correlation coefficient = 0.36; P < 0.0001). Among the ET-treated group, an interaction term between age and BMI was included in the model to test whether the association of BMI with estrogens or SHBG differed by age. Interaction between age and WHR was also tested using similar models. All statistical analyses used SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Baseline characteristics of study participants are summarized in Table 1. The women were on average 62 yr old, predominantly White (61%), and moderately overweight. By trial design, none of the women were currently smoking, although 48% were ex-smokers; 73% of the women had at least three drinks of alcohol each week. These characteristics were similar between women receiving E2 and placebo treatment (Table 1).
Table 1.
Baseline characteristics of EPAT women
| Characteristics | Placebo | E2 | P value |
|---|---|---|---|
| n | 89 | 91 | |
| Age (yr) | 62.3 (7.1) | 60.8 (6.5) | 0.15 |
| Age categories (%) | |||
| <60 | 41 | 42 | 0.20 |
| 60–64 | 18 | 27 | |
| ≥65 | 42 | 31 | |
| Race (%) | |||
| Non-Hispanic white | 64 | 57 | 0.55 |
| African-American | 8 | 13 | |
| Hispanic | 18 | 21 | |
| Others | 10 | 9 | |
| BMI (kg/m2) | 29.0 (5.4) | 28.9 (5.6) | 0.93 |
| BMI categories (%) | |||
| <25 kg/m2 | 25 | 26 | 0.95 |
| 25–29 kg/m2 | 36 | 36 | |
| ≥30 kg/m2 | 39 | 37 | |
| WHR | 0.82 (0.05) | 0.82 (0.06) | 0.52 |
| WHR categories (%) | |||
| <0.80 | 35 | 29 | 0.82 |
| 0.80–0.83 | 33 | 28 | |
| >0.83 | 33 | 34 | |
| Ex-smoker (%) | 45 | 52 | 0.20 |
| Alcohol intake (at least 3 drinks per week) (%) | 33 | 21 | 0.08 |
Data are expressed as mean (sd) for continuous variables and percentage for categorical variables. Sample includes 180 women with baseline and at least one on-trial measure of sex hormone concentration.
Baseline levels of total E2, free E2, and SHBG also did not differ between treatment groups (Table 2). Baseline E1 levels were moderately higher in the group randomized to ET (P = 0.05). Average on-trial levels of E1, total E2, free E2, and SHBG were significantly higher in E2-treated compared with placebo-treated women (all P for difference <0.0001).
Table 2.
Serum levels of sex hormones and SHBG by treatment group
| Placebo | E2 | P valuea | |
|---|---|---|---|
| n | 89 | 91 | |
| E1 (pg/ml) | |||
| Baseline | 39.8 (13.3) | 47.0 (31.2) | 0.05 |
| Follow-up | 48.1 (42.0) | 310.3 (167.2) | <0.0001 |
| E2 (pg/ml) | |||
| Baseline | 19.1 (5.3) | 21.4 (19.7) | 0.27 |
| Follow-up | 14.7 (6.3) | 68.2 (16.2) | <0.0001 |
| Free E2 (pg/ml) | |||
| Baseline | 0.5 (0.2) | 0.6 (0.5) | 0.25 |
| Follow-up | 0.6 (0.2) | 1.6 (0.6) | <0.0001 |
| SHBG (nmol/liter) | |||
| Baseline | 35.2 (14.5) | 35.2 (18.5) | 0.90 |
| Follow-up | 36.5 (18.2) | 58.2 (23.6) | <0.0001 |
Data are expressed as mean (sd).
P values were from t test for independent samples.
Among placebo-treated women, the mean serum levels of total E2, free E2, E1, or SHBG were not associated with age either unadjusted or adjusted for BMI (Table 3). In the unadjusted analyses, serum total E2 (trend P = 0.05) and free E2 levels (trend P = 0.001) were positively associated with BMI, whereas serum SHBG levels were inversely associated with BMI in placebo-treated women (trend P = 0.002). In the unadjusted analysis, serum SHBG levels were inversely related (trend P = 0.02) and serum free E2 levels were borderline positively related (trend P = 0.06) with WHR in women taking placebo. Among placebo-treated women, serum E1 levels were not associated with BMI or WHR in either unadjusted or adjusted analyses. Associations of serum estrogens and SHBG levels with BMI and WHR remained with adjustment for age (Table 3).
Table 3.
Levels of estrogens and SHBG by age, BMI, and WHR among placebo-treated women
| Age (yr)a
|
P for trend | BMI (kg/m2)b
|
P for trend | WHR (%)c
|
P for trend | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <60 | 60–64 | ≥65 | <25 | 25–29 | ≥30 | <0.80 | 0.80–0.83 | >0.83 | ||||
| Univariate analysis | ||||||||||||
| n | 36 | 16 | 37 | 22 | 32 | 35 | 31 | 29 | 29 | |||
| E2 | 22.2 (1.7) | 18.7 (2.6) | 21 (1.7) | 0.94 | 18.6 (2.2) | 19.9 (1.8) | 23.7 | 0.05 | 19.7 (1.8) | 20.2 (1.9) | 23.5 (1.9) | 0.16 |
| FE2 | 0.6 (0.04) | 0.5 (0.1) | 0.6 (0.04) | 0.35 | 0.5 (0.1) | 0.5 (0.04) | 0.7 (0.04) | 0.001 | 0.53 (0.04) | 0.57 (0.05) | 0.65 (0.05) | 0.06 |
| E1 | 50.1 (7.1) | 40.3 (10.6) | 49.6 (7.0) | 0.88 | 47.5 (9.1) | 47.2 (7.5) | 49.4 (7.2) | 0.85 | 46.4 (7.6) | 42.6 (7.8) | 55.5 (7.8) | 0.42 |
| SHBG | 33.0 (3.0) | 40.9 (4.5) | 38.0 (3.0) | 0.18 | 44.3 (3.7) | 38.4 (3.1) | 29.8 (2.9) | 0.002 | 42.4 (3.2) | 34.8 (3.3) | 31.9 (3.3) | 0.02 |
| Multivariate analysis | ||||||||||||
| n | 36 | 16 | 37 | 22 | 32 | 35 | 31 | 29 | 29 | |||
| E2 | 21.5 (1.7) | 18.8 (2.5) | 20.9 (1.7) | 0.94 | 18.4 (2.2) | 19.6 (1.8) | 23.2 (1.8) | 0.06 | 19.2 (1.9) | 20.02 (1.9) | 23.1 (2.03) | 0.13 |
| FE2 | 0.6 (0.04) | 0.5 (0.1) | 0.6 (0.04) | 0.35 | 0.5 (0.1) | 0.5 (0.04) | 0.7 (0.04) | 0.002 | 0.51 (0.01) | 0.56 (0.04) | 0.64 (0.05) | 0.03 |
| E1 | 49.9 (7.3) | 40.4 (10.7) | 49.6 (7.1) | 0.88 | 46.5 (9.2) | 45.9 (7.8) | 47.6 (7.6) | 0.85 | 45.2 (7.9) | 42.0 (8.0) | 53.9 (8.4) | 0.41 |
| SHBG | 34.9 (3.0) | 40.8 (4.4) | 38.5 (2.9) | 0.18 | 44.5 (3.8) | 38.9 (3.2) | 30.8 (3.1) | 0.003 | 43.8 (3.3) | 34.9 (3.3) | 32.3 (3.5) | 0.01 |
Data are expressed as mean (se). E2, Total estradiol; FE2, free estradiol.
Multivariate models with:
age adjusted for BMI;
BMI adjusted for age;
WHR adjusted for age.
Among the E2-treated women, univariate and multivariate analysis showed no association of age with any of the serum estrogen or SHBG levels (Table 4). In univariate analysis, BMI was directly associated with serum total E2 (trend P = 0.01) and free E2 levels (trend P = 0.002). None of the serum estrogens was associated with WHR, and serum E1 was not associated with BMI, but serum SHBG (trend P = 0.01) was significantly inversely associated with WHR in women receiving ET. Association of serum total E2, free E2, and SHBG with BMI or with WHR remained unchanged with adjustment for age (Table 4). Further adjustment for blood pressure or alcohol consumption did not alter the impact of age and obesity on serum estrogen and SHBG concentrations; therefore, blood pressure and alcohol consumption were not included in the final multivariate linear regression model.
Table 4.
Levels of estrogens and SHBG by age, BMI, and WHR among E2-treated women
| Age (yr)a
|
P for trend | BMI (kg/m2)b
|
P for trend | WHR (%)c
|
P for trend | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <60 | 60–65 | ≥65 | <25 | 25–29 | ≥30 | <0.80 | 0.80–0.83 | >0.83 | ||||
| Univariate analysis | ||||||||||||
| n | 38 | 25 | 28 | 26 | 31 | 34 | 29 | 28 | 34 | |||
| E2 | 65.6 (4.5) | 68.3 (5.3) | 72.2 (5.7) | 0.37 | 59.7 (5.5) | 64.5 (4.7) | 78.1 (4.7) | 0.01 | 70.4 (5.3) | 69.4 (5.3) | 65.4 (4.8) | 0.91 |
| FE2 | 1.6 (0.1) | 1.6 (0.1) | 1.6 (0.1) | 0.93 | 1.4 (0.1) | 1.5 (0.1) | 1.9 (0.1) | 0.002 | 1.59 (0.12) | 1.59 (0.12) | 1.60 (0.11) | 0.59 |
| E1 | 284.3 (27.2) | 322.7 (31.1) | 336.5 (34.2) | 0.21 | 308.0 (34.3) | 290.5 (29.3) | 331.2 (28.8) | 0.55 | 341.6 (31) | 319.2 (31.5) | 276.3 (28.6) | 0.90 |
| SHBG | 53.8 (3.9) | 61.4 (4.4) | 61.3 (4.8) | 0.19 | 65.7 (4.9) | 55.7 (4.1) | 55.6 (4.0) | 0.13 | 67.4 (4.9) | 58.1 (4.3) | 50.7 (3.9) | 0.01 |
| Multivariate analysis | ||||||||||||
| n | 38 | 29 | 24 | 26 | 31 | 34 | 29 | 28 | 34 | |||
| E2 | 65.0 (4.4) | 66.9 (5.6) | 71.2 (5.2) | 0.37 | 60.6 (5.6) | 64.4 (4.7) | 78.3 (4.8) | 0.01 | 70.7 (5.4) | 70 (5.4) | 65.1 (5.0) | 0.46 |
| FE2 | 1.6 (0.1) | 1.5 (0.1) | 1.6 (0.1) | 0.93 | 1.4 (0.1) | 1.5 (0.1) | 1.8 (0.1) | 0.002 | 1.59 (0.1) | 1.60 (0.1) | 1.59 (0.1) | 0.92 |
| E1 | 283.2 (27.3) | 320.9 (33.7) | 337.7 (32.4) | 0.21 | 316.3 (34.9) | 291.1 (29.3) | 334.0 (29.0) | 0.62 | 345.3 (31) | 322.2 (31.7) | 278.4 (29.6) | 0.11 |
| SHBG | 54.2 (3.8) | 62.5 (4.7) | 62.5 (4.5) | 0.19 | 66.9 (4.9) | 55.9 (4.1) | 56.3 (4.0) | 0.12 | 67.9 (4.3) | 58.4 (4.3) | 51.5 (4.1) | 0.005 |
Data are expressed as mean (se). E2, Total estradiol; FE2, free estradiol.
Multivariate models with:
age adjusted for BMI;
BMI adjusted for age;
WHR adjusted for age.
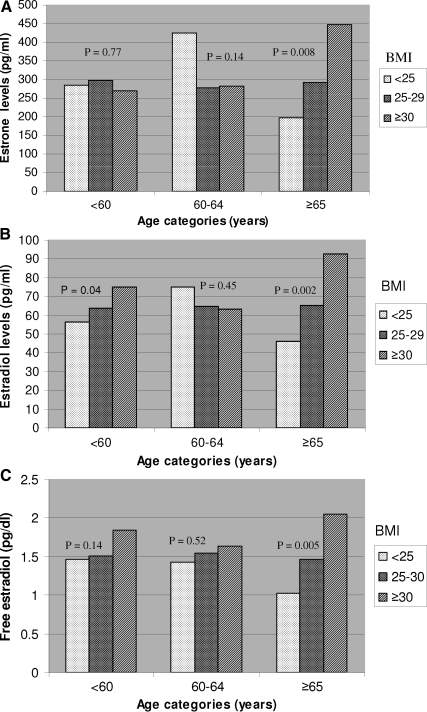
Among E2-treated women, a significant interaction between age and BMI categories was observed in relation to serum E1 levels (Fig. 1A). A significant positive association between serum E1 levels and BMI was only evident among women older than 65 yr (P for interaction = 0.03). Although a similar pattern was observed with serum total E2 and free E2 levels and BMI, the tests for interaction were not statistically significant (P for interaction 0.20 and 0.14, respectively; Fig. 1, B and C). No such interactions were observed in the placebo-treated women. There was no significant interaction between age and WHR in either placebo- or E2-treated women.
Figure 1.
A, Association of serum E1 with BMI stratified by age among women taking ET. Mean E1 levels for BMI categories are displayed within each age group. P value for interaction between age and BMI = 0.03. B, Association of serum total E2 with BMI stratified by age among women taking ET. Mean total E2 levels for BMI categories are displayed within each age group. P value for interaction between age and BMI = 0.20. C, Association of serum free E2 with BMI stratified by age among women taking ET. Mean free E2 levels for BMI categories are displayed within each age group. P value for interaction between age and BMI = 0.14.
Discussion
A number of studies have reported the impact of obesity on endogenous sex hormone levels in postmenopausal women (1,4). Our results on circulating steroid hormone concentrations in non-ET users in relation to body size and composition are consistent with those reports. Specifically, we documented that BMI was positively associated with serum estrogen concentrations and inversely associated with SHBG levels in postmenopausal women not using ET. After menopause, when ovarian estrogen production ceases, adipose tissue serves as the primary source of estrogen production via aromatization that converts androstenedione to E2 and E1 (23). Therefore, the positive association between BMI and circulating total E2 and free E2 in postmenopausal women can be explained by the increased fat mass. Consistent with other reports, our results show a strong inverse association between WHR and serum SHBG in women not using HT (1). WHR is a measure of central obesity or visceral fat; visceral adiposity has been described as more androgenic and associated with decreased SHBG synthesis (15). The positive association between serum free E2 concentrations and WHR can be explained by the reduction in SHBG synthesis in this group of women.
Age was not associated with serum E1 or E2 levels in a cross-sectional study of 438 postmenopausal women not taking HT, whereas SHBG concentration increased significantly with age (24). In this current study, we also did not find any association between age and endogenous estrogen concentrations in our 89 postmenopausal women not taking ET. Our data suggested a slight increase in serum SHBG levels with increasing age, but this positive association did not reach statistical significance, possibly because of the small sample size.
Although numerous studies have examined the impact of obesity on endogenous sex hormone concentrations among postmenopausal women, this is the first study to document serum estrogen and SHBG concentrations according to obesity status among women using postmenopausal ET. Among women receiving oral E2 for up to 2 yr, age was not associated with either serum estrogen or SHBG concentrations. However, we observed a significant positive association between BMI and serum E2 concentrations in these women; overweight and obese women had significantly higher levels of serum total and free E2 levels compared with women with BMI below 25 kg/m2. This is an indication of continued contribution of total fat mass in E2 production among ET users.
Among ET users, our data showed a significant inverse association between WHR and serum SHBG concentrations. SHBG is synthesized in the liver and oral ET increases serum SHBG concentrations through hepatic first pass metabolism. Oral ET significantly increased the serum SHBG concentrations in the overall sample (Table 2). However, the oral ET-induced elevation of serum SHBG concentration was lowest among women with the highest WHR. Because the pretreatment level of serum SHBG was lower in the women with high WHR as observed in the placebo group, ET did not enhance SHBG production as much as in women with less visceral fat. In EPAT, the mean (sd) pretreatment to posttreatment increase in serum SHBG levels in ET users was 25.8 (18.7), 23.9 (19.6), and 20.4 (14.9) among women with WHR less than 0.80, 0.80 to 0.83, and greater than 0.83, respectively (data not displayed in the tables). Alternatively, it is theoretically possible that the first pass effect occurs to a lesser degree in women with increased WHR because visceral fat is highly vascularized. Both BMI and WHR are associated with increased risk of stroke in women (25), and there is a growing body of evidence that central obesity is even a stronger risk factor for stroke than BMI (26). Although higher serum SHBG concentrations have been shown to be protective against atherosclerosis and cardiovascular disease (27), it is largely unknown if and how SHBG relates to stroke risk.
Our finding of an interaction between age and BMI on serum E1 levels among ET users is important. HT increases the risk of stroke and venous thromboembolism (VTE) in postmenopausal women (28,29), especially with increasing dosages. The Nurses’ Health Study has shown a gradual increase in stroke risk with higher dosages of HT (30). In a large observational study, age and obesity significantly influenced the risk of stroke among HT users (17). The risk of first-ever stroke increased by 71% with age (per 6 yr), 37% with BMI (per sd of 3.58 kg/m2), and 40% with waist circumference (per sd of 9 cm). Consistent with the stroke findings, data from the Women’s Health Initiative trial documented an increased risk of venous thrombosis among estrogen plus progestin users; this HT-associated risk gradually increased with older age and higher BMI among women using HT compared with younger and low BMI women taking placebo (18). Although obesity can be associated with thromboembolic events by several mechanisms, including increased levels of inflammation, dyslipidemia, and adipokines, it seems plausible that higher serum estrogen and lower SHBG levels in overweight and obese women may contribute to the increased risk of VTE and stroke. However, this hypothesis has not been tested. The impact of serum concentrations of estrogens and SHBG on VTE and stroke in postmenopausal women needs to be evaluated in large-scale epidemiological studies.
It should also be noted that circulating estrogen levels can be influenced by polymorphisms of multiple genes involved in steroid hormone metabolism (31). However, the association of these genetic determinants with obesity is not clear. Polymorphisms in estrogen receptor-α were not a major correlate of obesity (32); however, it has been reported to be associated with coronary artery disease but not with estrogen levels (33). Further research is required to understand the role of these genetic variations in the association between obesity, sex hormone concentrations, and thromboembolic events.
Strengths of this study include repeated measures of sex hormones and use of reliable hormone assays. In addition to the paucity of studies on this topic, the reliability of existing estrogen data is often questionable due to insufficient purification of steroids before their quantification by RIA, lack of thorough assay validation, and lack of long-term quality control samples to monitor assay changes over time. In the present study, we used well-established, highly reliable assays. Extraction/chromatography RIAs were used to quantify serum E1 and E2, and a highly specific direct chemiluminescent immunoassay was used to quantify SHBG in serum samples obtained at baseline and every 6 months over 2 yr of oral E2 or placebo treatment. In addition, all samples from each subject were included in the same assay to reduce intrasubject measurement variability. One limitation of the study is the relatively small sample of women on ET. These results need to be reproduced in larger groups of women.
Despite the beneficial effects of estrogen on inflammation (34), serum lipids (35), carotid atherosclerosis (35), coronary artery calcium (36), and fracture (8), thrombophilic effects of estrogen can lead to VTE or stroke. Results of this analysis demonstrate that age and obesity are important determinants of the serum levels of estrogens and SHBG among HT users, suggesting possible clinical usefulness of considering age and obesity in guiding the administration of HT. Current guidelines do not recommend HT use for prevention of chronic conditions such as CHD, and HT use has sharply declined since 2002; however, according to a recent report, 10 to 15% of postmenopausal women used HT in 2005 (37). Further research is warranted to determine whether adjustment of HT dose based on serum estrogen levels may be useful when prescribing HT in older and heavier women, who are at greater risk of venous thrombosis or stroke.
Footnotes
This work was supported by National Institutes of Health Grant RO1 AG-18798.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 6, 2009
Abbreviations: BMI, Body mass index; E1, estrone; E2, estradiol; ET, E2 therapy; HT, hormone therapy; VTE, venous thromboembolism; WHR, waist to hip ratio.
References
- Baglietto L, English DR, Hopper JL, MacInnis RJ, Morris HA, Tilley WD, Krishnan K, Giles GG 2009 Circulating steroid hormone concentrations in postmenopausal women in relation to body size and composition. Breast Cancer Res Treat 115:171–179 [DOI] [PubMed] [Google Scholar]
- Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R 2004 Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol 150:161–171 [DOI] [PubMed] [Google Scholar]
- Mahabir S, Baer DJ, Johnson LL, Hartman TJ, Dorgan JF, Campbell WS, Clevidence BA, Taylor PR 2006 Usefulness of body mass index as a sufficient adiposity measurement for sex hormone concentration associations in postmenopausal women. Cancer Epidemiol Biomarkers Prev 15:2502–2507 [DOI] [PubMed] [Google Scholar]
- McTiernan A, Wu L, Chen C, Chlebowski R, Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van Horn L, Wang CY 2006 Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity (Silver Spring) 14:1662–1677 [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ 2007 Postmenopausal hormone therapy in clinical perspective. Menopause 14:944–957 [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ 2008 Postmenopausal hormone therapy and cardiovascular disease in perspective. Clin Obstet Gynecol 51:564–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE 2006 Implications of recent clinical trials of postmenopausal hormone therapy for management of cardiovascular disease. Ann NY Acad Sci 1089:444–453 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ, Chaikittisilpa S, Roy S 2003 Pharmacologic deficiency in the Women’s Health Initiative Study. J Reprod Med 48:485–486 [PubMed] [Google Scholar]
- Greenblatt DJ, Abernethy DR, Shader RI 1986 Pharmacokinetic aspects of drug therapy in the elderly. Ther Drug Monit 8:249–255 [DOI] [PubMed] [Google Scholar]
- Montamat SC, Cusack BJ, Vestal RE 1989 Management of drug therapy in the elderly. N Engl J Med 321:303–309 [DOI] [PubMed] [Google Scholar]
- Durnas C, Loi CM, Cusack BJ 1990 Hepatic drug metabolism and aging. Clin Pharmacokinet 19:359–389 [DOI] [PubMed] [Google Scholar]
- Siiteri PK 1987 Adipose tissue as a source of hormones. Am J Clin Nutr 45:277–282 [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Key TJ, Peeters PH, Lahmann PH, Lukanova A, Dossus L, Biessy C, Vineis P, Sacerdote C, Berrino F, Panico S, Tumino R, Palli D, Nagel G, Linseisen J, Boeing H, Roddam A, Bingham S, Khaw KT, Chloptios J, Trichopoulou A, Trichopoulos D, Tehard B, Clavel-Chapelon F, Gonzalez CA, Larrañaga N, Barricarte A, Quirós JR, Chirlaque MD, Martinez C, Monninkhof E, Grobbee DE, Bueno-de-Mesquita HB, Ferrari P, Slimani N, Riboli E, Kaaks R 2006 Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int J Cancer 118:2832–2839 [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R 2004 Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579–591 [DOI] [PubMed] [Google Scholar]
- Hajamor S, Després JP, Couillard C, Lemieux S, Tremblay A, Prud'homme D, Tchernof A 2003 Relationship between sex hormone-binding globulin levels and features of the metabolic syndrome. Metabolism 52:724–730 [DOI] [PubMed] [Google Scholar]
- Li C, Engström G, Hedblad B, Berglund G, Janzon L 2006 Risk of stroke and hormone replacement therapy. A prospective cohort study. Maturitas 54:11–18 [DOI] [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR 2004 Estrogen plus progestin and risk of venous thrombosis. JAMA 292:1573–1580 [DOI] [PubMed] [Google Scholar]
- Hodis HN, Mack WJ, Lobo RA, Shoupe D, Sevanian A, Mahrer PR, Selzer RH, Liu Cr CR, Liu Ch CH, Azen SP 2001 Estrogen in the prevention of atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 135:939–953 [DOI] [PubMed] [Google Scholar]
- Rinaldi S, Geay A, Déchaud H, Biessy C, Zeleniuch-Jacquotte A, Akhmedkhanov A, Shore RE, Riboli E, Toniolo P, Kaaks R 2002 Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 11:1065–1071 [PubMed] [Google Scholar]
- Södergård R, Bäckström T, Shanbhag V, Carstensen H 1982 Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem 16:801–810 [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck L, Kaufman JM 1999 A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84:3666–3672 [DOI] [PubMed] [Google Scholar]
- Gruber CJ, Tschugguel W, Schneeberger C, Huber JC 2002 Production and actions of estrogens. N Engl J Med 346:340–352 [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D 2000 Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab 85:645–651 [DOI] [PubMed] [Google Scholar]
- Hu G, Tuomilehto J, Silventoinen K, Sarti C, Männistö S, Jousilahti P 2007 Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med 167:1420–1427 [DOI] [PubMed] [Google Scholar]
- Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T 2008 Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke 39:3145–3151 [DOI] [PubMed] [Google Scholar]
- Reinecke H, Bogdanski J, Woltering A, Breithardt G, Assmann G, Kerber S, von Eckardstein A 2002 Relation of serum levels of sex hormone binding globulin to coronary heart disease in postmenopausal women. Am J Cardiol 90:364–368 [DOI] [PubMed] [Google Scholar]
- Bath PM, Gray LJ 2005 Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ 330:342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico M, Plu-Bureau G, Lowe GD, Scarabin PY 2008 Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ 336:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ 2000 A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med 133:933–941 [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Chubak J, Aiello EJ, Ulrich CM, Atkinson C, Potter JD, Yasui Y, Stapleton PL, Lampe JW, Farin FM, Stanczyk FZ, McTiernan A 2004 Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev 13:94–101 [DOI] [PubMed] [Google Scholar]
- Nilsson M, Dahlman I, Jiao H, Gustafsson JA, Arner P, Dahlman-Wright K 2007 Impact of estrogen receptor gene polymorphisms and mRNA levels on obesity and lipolysis—a cohort study. BMC Med Genet 8:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alevizaki M, Saltiki K, Cimponeriu A, Kanakakis I, Xita N, Alevizaki CC, Georgiou I, Sarika HL 2007 Severity of cardiovascular disease in postmenopausal women: associations with common estrogen receptor α polymorphic variants. Eur J Endocrinol 156:489–496 [DOI] [PubMed] [Google Scholar]
- Zanger D, Yang BK, Ardans J, Waclawiw MA, Csako G, Wahl LM, Cannon 3rd RO 2000 Divergent effects of hormone therapy on serum markers of inflammation in postmenopausal women with coronary artery disease on appropriate medical management. J Am Coll Cardiol 36:1797–1802 [DOI] [PubMed] [Google Scholar]
- Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ 2008 Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab 93:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML 2007 Estrogen therapy and coronary-artery calcification. N Engl J Med 356:2591–2602 [DOI] [PubMed] [Google Scholar]
- Shetty KD, Vogt WB, Bhattacharya J 2009 Hormone replacement therapy and cardiovascular health in the United States. Med Care 47:600–606 [DOI] [PubMed] [Google Scholar]