Abstract
Introduction: Thyroid cancer is the most common endocrine malignancy. The outcomes of patients with relapsed thyroid cancer treated on early-phase clinical trials have not been systematically analyzed.
Patients and Methods: We reviewed the records of consecutive patients with metastatic thyroid cancer referred to the Phase I Clinical Trials Program from March 2006 to April 2008. Best response was assessed by Response Evaluation Criteria in Solid Tumors.
Results: Fifty-six patients were identified. The median age was 55 yr (range 35–79 yr). Of 49 patients evaluable for response, nine (18.4%) had a partial response, and 16 (32.7%) had stable disease for 6 months or longer. The median progression-free survival was 1.12 yr. With a median follow-up of 15.6 months, the 1-yr survival rate was 81%. In univariate analysis, factors predicting shorter survival were anaplastic histology (P = 0.0002) and albumin levels less than 3.5 g/dl (P = 0.05). Among 26 patients with tumor decreases, none died (median follow-up 1.3 yr), whereas 52% of patients with any tumor increase died by 1 yr (P = 0.0001). The median time to failure in our phase I clinical trials was 11.5 months vs. 4.1 months for the previous treatment (P = 0.04).
Conclusion: Patients with advanced thyroid cancer treated on phase I clinical trials had high rates of partial response and prolonged stable disease. Time to failure was significantly longer on the first phase I trial compared with the prior conventional treatment. Patients with any tumor decrease had significantly longer survival than those with any tumor increase.
Patients with advanced thyroid cancer treated on phase I clinical trials had high rates of partial response and prolonged stable disease.
Thyroid cancer is the most frequent malignancy of the endocrine system (1). Overall, 37,340 new cases were diagnosed and 1,590 deaths due to thyroid cancer occurred in 2008 (2). The overall 5-yr survival rate is 97% (3), but the mortality rate increases with age, from 0.1% in patients under age 20 yr to 30.5% in patients 75–84 yr old (2).
Treatment of metastatic thyroid cancer includes surgical resection, radiotherapy, radioactive iodine, and chemotherapy (4). Chemotherapeutic regimens are inadequate for metastatic disease (5).
Although most patients with differentiated (i.e. papillary and follicular) thyroid carcinoma respond to initial treatment, 10–15% have a relapse, including 5% with distant metastases (6). Doxorubicin has been the traditional choice for metastatic differentiated thyroid carcinoma, with a partial response (PR) rate of 0–20%. Other chemotherapies have had equally disappointing results (6). In medullary thyroid carcinoma, distant metastases occur in 7–23% of patients (7), and the therapeutic options for anaplastic thyroid carcinoma are limited (8).
Loss of differentiation in thyroid cancer leads to loss of radioiodine uptake. DNA methylation inhibitors, histone deacetylase inhibitors, retinoic acid and retinoic X receptor activators, and peroxisomal proliferator-activated receptor-γ activators have been used to restore the expression of the sodium-iodide symporter (5). These interventions have been undertaken in an attempt to increase radioiodine uptake, but none has been generally effective.
Because of the low response rates and associated toxicities of traditional chemotherapy, the recommendation to participate in clinical trials has been incorporated in the National Comprehensive Cancer Network and American Thyroid Association guidelines (5).
In recent years, the discoveries of signaling pathways and mutational analysis (9,10) have introduced targeted therapies for treating thyroid carcinoma. Elucidating the molecular pathways involved in thyroid carcinogenesis has led to the development of novel targeted therapeutic approaches. To our knowledge, the outcomes of patients with metastatic thyroid cancer for whom standard therapy has failed or who have no standard treatment options available and who are referred for experimental therapy have not been systematically analyzed. The Phase I Clinic at The University of Texas M. D. Anderson Cancer Center focuses on treating patients in early clinical trials, predominantly with targeted agents. Here we report the presenting characteristics and outcomes of patients with metastatic thyroid cancer who were referred to this clinic.
Patients and Methods
We reviewed the records of consecutive patients who were first seen in the Phase I Clinical Trials Program at M. D. Anderson Cancer Center from March 2006 to April 2008 to determine the number of patients with primary thyroid cancer, their associated characteristics, and clinical outcomes. Data were collected from transcribed notes in the electronic database. Patient records were reviewed at the time of presentation in the Phase I Clinical Trials Program. Treatment was determined after clinical, laboratory, and pathological data were reviewed. Investigational regimens available for patient enrollment varied over time.
Eligible patients were older than 18 yr with metastatic or unresectable thyroid carcinoma requiring therapy and for whom approved curative therapies attempted in the past were no longer effective. All patients had evidence of measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (11), Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and a life expectancy longer than 3 months. Premenopausal women were required to have a negative pregnancy test, and patients of child-bearing potential were required to use contraception. A washout period of 4 wk preceding the initiation of each phase I therapy was required. Further eligibility criteria varied according to the particular study. All patients provided written informed consent before enrollment, and all trials were approved by the M. D. Anderson Institutional Review Board, which also granted waivers of informed consent and authorization for this retrospective study.
After initiation of an investigational therapy, patients were evaluated at 3- to 4-wk intervals. At each visit, a history review and physical examination were performed along with a comprehensive metabolic and hematologic panel. Patients were assessed for the onset of new symptoms and compliance to the investigational therapy. Computed tomography (CT) scans were obtained every two cycles of therapy. The sizes of the target lesions (lesions measurable in at least one dimension and having a diameter of at least twice the slice thickness of the scan) were compared with their sizes on the preenrollment CT scan, which was conducted no earlier than a month before enrollment (11).
End points and statistical methods
Descriptive statistics were used to summarize the patients’ characteristics. The χ2 test was used to examine the association between two categorical variables. The following covariates were analyzed: age; gender; race; history of smoking; ECOG performance status; histology; TSH level; calcitonin level; history of thyroidectomy; radioactive iodine; number of prior therapies; local recurrence; metastases in the liver, lung, lymph nodes, bone, or mediastinum; number of metastatic sites; leukocyte count; hemoglobin level; platelet count; and levels of albumin, lactate dehydrogenase, calcium, phosphorus, alkaline phosphatase, bilirubin, and serum creatinine.
Best response was assessed by an M. D. Anderson radiologist every two cycles of therapy (cycle = 2–4 wk, depending on the protocol), using RECIST criteria (11). PR was defined as a 30% or greater decrease in the sum of the longest diameter of target lesions, excluding complete disappearance of disease. Progressive disease (PD) was defined as a 20% or greater increase in the sum of the longest diameter of target lesions. Stable disease (SD) was defined as smaller changes that did not meet the criteria for a PR or PD. For patients treated with more than one therapy, data for survival and progression-free survival (PFS) from the first therapy were used. Waterfall plot analysis was used to capture antitumor efficacy (12). Responses shown in the waterfall plot were grouped according to standard RECIST guidelines.
Survival was measured from date of presentation to the Phase I Clinical Trials Program until death from any cause or last follow-up. PFS was measured from date of presentation to the Phase I Clinical Trials Program until disease progression or death, whichever came first. Time to failure (TTF) was measured from the first day of treatment on a clinical trial in our Phase I Clinical Trials Program to the date the patient went off study because of toxicity, disease progression, or death, whichever came first. Toxicities were assessed using the National Cancer Institute common terminology criteria (NCI-CTC) for adverse events (version 3.0) (13). P < 0.05 was considered statistically significant. Statistical analyses were carried out using SAS 9.1 (SAS Institute, Cary, NC) and S-Plus software (version 7.0; Insightful Corp., Seattle, WA).
Results
Patient characteristics
Overall, 56 patients who participated in phase I clinical trials were identified. All patients had progressive disease at the time of enrollment. Histologic subtypes were anaplastic thyroid carcinoma (n = 6), follicular thyroid carcinoma (n = 4), medullary thyroid carcinoma (n = 27), and papillary thyroid carcinoma (n = 19) (Table 1). The median age was 55 yr (range 35–79 yr). There were 34 men and 22 women. The most common metastatic sites were lymph nodes (75% of patients), lung (68%), bone (41%), and liver (39%). Thirty- one patients (55%) had mediastinal involvement. Forty- one patients (73%) had one or more comorbidities. The most common comorbidities were hypertension (n = 19, 34%), hyperlipidemia (n = 14, 25%), and gastroesophageal reflux disease (n = 5, 9%). Three of 27 patients with medullary thyroid carcinoma (11%) had multiple endocrine neoplasia, type 2A. Five patients had a concurrent or preceding malignancy (breast cancer, n = 3; cancer of unknown primary site, n = 1; melanoma, n = 1). Fourteen patients (25%) had no reported comorbidities. The median number of prior nonsurgical therapies was one (range 0–7). Fifty-three patients (95%) had a history of thyroidectomy and 30 (54%) had received prior radioactive iodine. Patients with papillary and follicular thyroid carcinoma (n = 23) were refractory to radioactive iodine or had received the maximum dosage of this modality, with the exception of one patient who presented with extensive metastatic disease involving the cervical spine.
Table 1.
Presenting characteristics of patients with thyroid cancer referred to the Phase I Clinical Trials Program
| Covariate | Group | Patients, n | 1-yr survival, % | Pa | Patients evaluable for response, n (n = 49) | PR (% patients) | SD (% patients) | Pb | Increase in tumor measurement (% patients) | Decrease in tumor measurement (% patients) | Pc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, yr | <60 | 38 | 86 | 33 | 5 (15) | 21 (64) | 14 (42) | 19 (58) | |||
| ≥60 | 18 | 71 | 0.25 | 16 | 4 (25) | 6 (38) | 0.3 | 9 (56) | 7 (44) | 0.54 | |
| Gender | Female | 22 | 80 | 18 | 1 (6) | 12 (67) | 9 (50) | 9 (50) | |||
| Male | 34 | 82 | 0.58 | 31 | 8 (26) | 15 (48) | 0.99 | 14 (45) | 17 (55) | 0.78 | |
| History of smoking | Yes | 24 | 71 | 21 | 3 (14) | 13 (62) | 11 (52) | 10 (48) | |||
| No | 32 | 86 | 0.51 | 28 | 6 (21) | 14 (50) | 0.76 | 12 (43) | 16 (57) | 0.57 | |
| ECOG PS | 0 | 27 | 81 | 22 | 4 (18) | 13 (59) | 10 (45) | 12 (55) | |||
| 1–2 | 29 | 80 | 0.79 | 27 | 5 (19) | 14 (52) | 0.75 | 13 (48) | 14 (52) | 0.99 | |
| Histology | ATC | 6 | 21 | 6 | 1 (17) | 0 (0) | 5 (83) | 1 (17) | |||
| FTC | 4 | 100 | 3 | 0 (0) | 2 (67) | 3 (100) | 0 (0) | ||||
| MTC | 27 | 88 | 25 | 8 (32) | 14 (56) | 7 (28) | 18 (72) | ||||
| PTC | 19 | 84 | 0.002 | 15 | 0 (0) | 11 (73) | 0.004 | 8 (53) | 7 (47) | 0.01 | |
| TSH, μU/ml | Less than LLN | 0.52 | |||||||||
| Normal | 28 | 85 | 24 | 3 (12) | 15 (62) | 12 (48) | 12 (52) | ||||
| Greater than ULN | 18 | 72 | 17 | 4 (24) | 7 (41) | 9 (53) | 8 (47) | ||||
| 5 | 67 | 0.84 | 5 | 1 (20) | 3 (60) | 0.81 | 1 (20) | 4 (80) | |||
| MTC | |||||||||||
| Calcitonin, pg/ml | <100 | 2 | 2 | ||||||||
| ≥100 | 24 | 100 | 22 | 0 (0) | 2 (100) | 0 (0) | 2 (100) | ||||
| Missing | 1 | 91 | 0.67 | 3 | 8 (36) | 12 (55) | 0.99 | 6 (27) | 16 (73) | 0.99 | |
| Thyroidectomy | Yes | 53 | 82 | 46 | 8 (17) | 26 (57) | 22 (48) | 24 (52) | |||
| No | 3 | 67 | 0.49 | 3 | 1 (33) | 1 (33) | 0.99 | 1 (33) | 2 (67) | 0.99 | |
| Radioactive iodine | Yes | 30 | 79 | 0.41 | 23 | 0 (0) | 16 (70) | 15 (65) | 8 (35) | ||
| No | 26 | 84 | 0.38 | 26 | 9 (35) | 11 (42) | 0.75 | 8 (31) | 18 (69) | 0.02 | |
| No. of prior therapies | 0–1 | 29 | 74 | 28 | 6 (21) | 14 (48) | 13 (46) | 15 (54) | |||
| >1 | 27 | 89 | 21 | 3 (14) | 13 (62) | 0.76 | 10 (48) | 11 (52) | 0.99 | ||
| Local recurrence | Yes | 19 | 68 | 16 | 5 (31) | 4 (25) | 14 (42) | 19 (58) | |||
| No | 37 | 88 | 0.1 | 33 | 4 (12) | 23 (70) | 0.09 | 9 (56) | 7 (44) | 0.57 | |
| Metastases | |||||||||||
| Liver | Yes | 22 | 85 | 20 | 6 (30) | 11 (55) | 7 (35) | 13 (65) | |||
| No | 34 | 79 | 0.38 | 29 | 3 (10) | 16 (55) | 0.19 | 16 (55) | 13 (45) | 0.24 | |
| Lung | Yes | 38 | 78 | 33 | 4 (12) | 18 (55) | 18 (55) | 15 (45) | |||
| No | 18 | 88 | 0.58 | 16 | 5 (31) | 9 (56) | 0.17 | 5 (31) | 11 (69) | 0.14 | |
| Lymph node | Yes | 42 | 77 | 38 | 8 (21) | 19 (50) | 16 (42) | 22 (58) | |||
| No | 14 | 93 | 0.18 | 11 | 1 (9) | 8 (73) | 0.7 | 7 (64) | 4 (36) | 0.31 | |
| Bone | Yes | 23 | 82 | 20 | 4 (10) | 10 (50) | 9 (45) | 11 (55) | |||
| No | 33 | 81 | 0.84 | 29 | 5 (17) | 17 (59) | 0.75 | 14 (48) | 15 (52) | 0.99 | |
| Mediastinum | Yes | 31 | 76 | 28 | 7 (25) | 13 (46) | 12 (43) | 16 (57) | |||
| No | 25 | 87 | 0.21 | 21 | 2 (10) | 14 (67) | 0.76 | 11 (52) | 10 (48) | 0.54 | |
| Number of metastatic sites | 0–3 | 40 | 87 | 35 | 6 (17) | 22 (63) | 15 (43) | 20 (57) | |||
| >3 | 16 | 67 | 0.13 | 14 | 3 (21) | 5 (36) | 0.15 | 8 (57) | 6 (43) | 0.53 | |
| Leukocytes, × 109/liter | ≥10 | 53 | 82 | 46 | 9 (20) | 25 (54) | 22 (48) | 24 (52) | |||
| >10 | 3 | 67 | 0.49 | 3 | 0 (0) | 2 (67) | 0.99 | 1 (33) | 2 (67) | 0.99 | |
| Hemoglobin, g/dl | <11 | 7 | 57 | 7 | 0 (0) | 3 (43) | 5 (71) | 2 (29) | |||
| ≥11 | 49 | 85 | 0.08 | 42 | 9 (21) | 24 (57) | 0.07 | 18 (43) | 24 (57) | 0.23 | |
| Platelet count, × 109/liter | ≤350 | 51 | 83 | 45 | 8 (18) | 27 (60) | 20 (44) | 25 (56) | |||
| >350 | 5 | 60 | 0.2 | 4 | 1 (25) | 0 (0) | 0.05 | 3 (75) | 1 (25) | 0.33 | |
| Albumin, g/dl | <3.4 | 2 | 0 | 1 | 0 (0) | 1 (100) | 0 (0) | 1 (1) | |||
| ≥3.5 | 54 | 82 | 0.05 | 48 | 9 (19) | 26 (54) | 0.47 | 22 (46) | 26 (54) | 0.99 | |
| LDH, IU/liter | ≤618 | 46 | 79 | 39 | 8 (21) | 20 (51) | 19 (49) | 20 (51) | |||
| >618 | 10 | 90 | 0.41 | 10 | 1 (10) | 7 (70) | 0.71 | 4 (40) | 6 (60) | 0.73 | |
| Calcium | ≤LLN | 7 | 69 | 6 | 3 (50) | 2 (33) | 2 (33) | 4 (67) | |||
| >ULN | 49 | 83 | 0.56 | 43 | 6 (14) | 25 (58) | 0.99 | 21 (49) | 22 (51) | 0.67 | |
| Phosphorus, mg/dl | ≤3 | 10 | 67 | 8 | 0 (0) | 5 (62) | 6 (75) | 2 (25) | |||
| >3 | 46 | 84 | 0.33 | 41 | 9 (22) | 22 (54) | 0.42 | 17 (41) | 24 (59) | 0.13 | |
| Alkaline | ≤126 | 50 | 83 | 0.32 | 43 | 8 (19) | 24 (56) | 0.65 | 21 (49) | 22 (51) | 0.49 |
| Phosphorus, IU/liter | >126 | 6 | 67 | 6 | 1 (17) | 3 (50) | 2 (33) | 4 (67) | |||
| Bilirubin, mg/dl | ≤1 | 54 | 80 | 47 | 7 (15) | 27 (50) | 23 (49) | 24 (51) | |||
| >1 | 2 | 100 | 0.48 | 2 | 2 (100) | 0 (0) | 0.99 | 0 | 2 (100) | 0.49 | |
| Serum Creatinine greater than ULN | Yes | 3 | 82 | 3 | 0 (0) | 2 (67) | 1 (33) | 2 (67) | |||
| No | 53 | 67 | 0.51 | 46 | 9 (20) | 25 (54) | 0.99 | 22 (48) | 24 (52) | 0.99 |
Comparisons that reached statistical significance are shown in bold. ATC, Anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; LDH, lactic dehydrogenase; LLN, lower limit of normal; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma; ULN, upper limit of normal.
P value by log-rank test;
P value compares proportion of patients who had PR or SD with proportion of patients who had PD by covariate group (Fisher’s exact test);
P value compares proportion of patients with any decrease in tumor size with proportion of patients who had any increase in tumor size by covariate group (Fisher’s exact test).
Treatment
Thirty-four patients were treated with single-agent targeted therapy; 19 with a two-drug targeted therapy combination; two with a cytotoxic agent combined with targeted therapy; and one with chemotherapy (Table 2). Twenty-four patients had received other antitumor medical therapy before enrollment (supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org). Twenty-three had measurable disease according to RECIST criteria and had achieved PR (two patients with medullary thyroid carcinoma), SD (n = 6 papillary thyroid carcinoma, n = 6 medullary thyroid carcinoma, n = 1 anaplastic thyroid carcinoma), and PD (n = 3 papillary thyroid carcinoma, n = 2 medullary thyroid carcinoma, n = 2 anaplastic thyroid carcinoma) as best response with the prior therapy. The response to prior therapy was unknown in one patient.
Table 2.
First phase I clinical trials in 56 patients
| Treatment | Mechanism of action | Patients | % | Investigator, year |
|---|---|---|---|---|
| XL-184 | VEGFR2 and Met TKI | 18 | 32 | Salgia et al., 2008 (28) |
| Tipifarnib + sorafenib | FTI + VEGFR2, VEGFR3, PDGFR-β, Raf-1, FLT3 TKI | 13 | 23 | Chintala et al., 2008 (29) |
| RTA 402 CDDO-Me | NF-κB and STAT3 inhibition | 6 | 11 | Hong et al., 2008 (30) |
| Azacitidine + valproic acid | DNA hypomethylation | 4 | 7 | Braiteh et al., 2008 (31) |
| Patupilone | Microtubule stabilization | 2 | 4 | Kurzrock et al., 2007 (32) |
| MK-2461 | c-Met TKI | 2 | 4 | Camacho et al., 2008 (33) |
| Other | 11a | 20 |
FLT3, fms-like tyrosine kinase 3; FTI, farnesyltransferase inhibitor; Met, hepatocyte growth factor/scatter factor receptor; NF-κB, nuclear factor κβ; PDGFR-β, platelet-derived growth factor receptor-β; STAT3, signal transducer and activator of transcription-3; TKI, tyrosine kinase inhibitor; VEGFR2, vascular endothelial growth factor receptor 2; VEGFR3, vascular endothelial growth factor receptor 3.
One patient each was treated with salirasib (Ras inhibitor); sunitinib, paclitaxel, and carboplatin; AZD 4877 (kinesin spindle protein inhibitor); PXD 101 (histone deacetylase inhibitor); bevacizumab plus sorafenib; nab-rapamycin (albumin-bound mammalian target of rapamycin kinase inhibitor); E7107 (spliceosome inhibitor); valproic acid plus sunitinib; liposomal doxorubicin, bortezomib (proteasome inhibitor), and gemcitabine; hepatic arterial infusion of paclitaxel; and KX2-391 (Src kinase inhibitor).
Response
Of 56 patients, tumor measurements were available for 45 patients. Of the 11 patients for whom tumor measurements were not available, four had rapid PD after the first cycle of therapy (anaplastic thyroid cancer, n = 2; papillary thyroid carcinoma with anaplastic features, n = 1; and papillary thyroid carcinoma, n = 1). These patients were not restaged by CT scans but are included in the waterfall plot (Fig. 1) as having PD. Four patients discontinued therapy before repeat assessment because they experienced NCI-CTC grade 3–4 toxicities (grade 3 lipase elevation, n = 2; grade 3 skin rash/infusion reaction, n = 2); and one patient died from pneumonia on cycle 1, d 11, an event considered not attributable to the study drug. Two patients had nonmeasurable disease (lytic bone lesions, n = 1; ill defined hepatic lesions, n = 1), and both had no onset of new lesions on repeat imaging assessments while on investigational treatment.
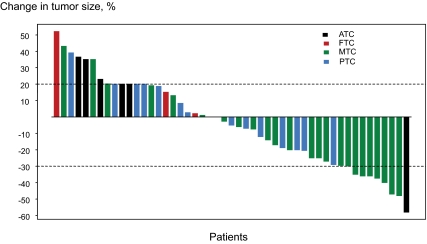
Figure 1.
Best response by RECIST to first clinical trial: changes from baseline in tumor measurements. The mean change of best response was −3.9%, with sd 27%. The different histological types are illustrated with different colors [anaplastic thyroid carcinoma (ATC): black (PR: one patient, PD: five patients); papillary thyroid carcinoma (PTC): blue (SD: ten patients, PD: four patients); follicular thyroid carcinoma (FTC): red (SD: three patients, PD: one patient); and medullary thyroid carcinoma (MTC): green (PR: eight patients, SD: fourteen patients, PD: three patients)].
Of 49 patients (45 with tumor measurements and four with clinical PD) evaluable for response, nine patients (18.4%) had a PR, and 27 patients (55.1%) had SD (Fig. 1). PR and SD lasted for 6 months or longer in nine of nine patients and 16 of 27 patients, respectively.
Factors predicting higher rates of combined PR and SD were histology other than anaplastic thyroid cancer (P = 0.004) and platelet count less than 350 × 109/liter (P = 0.05). When patients who had any increase in tumor measurement were compared with those who had any decrease, factors predicting decrease in tumor measurements were histology other than anaplastic thyroid cancer (P = 0.01) and prior radioactive iodine (P = 0.02).
Of 56 patients, 19 were treated on two or more protocols, 16 of whom were evaluable for response. The three nonevaluable patients had no follow-up imaging studies. Of the 16 evaluable patients, four had PR, 10 had SD, and two had PD. In addition, four patients were treated on three protocols. Of these four patients, two had SD (changes in tumor measurement, −23 and 1%), one had PD, and the fourth patient did not have imaging studies.
Survival
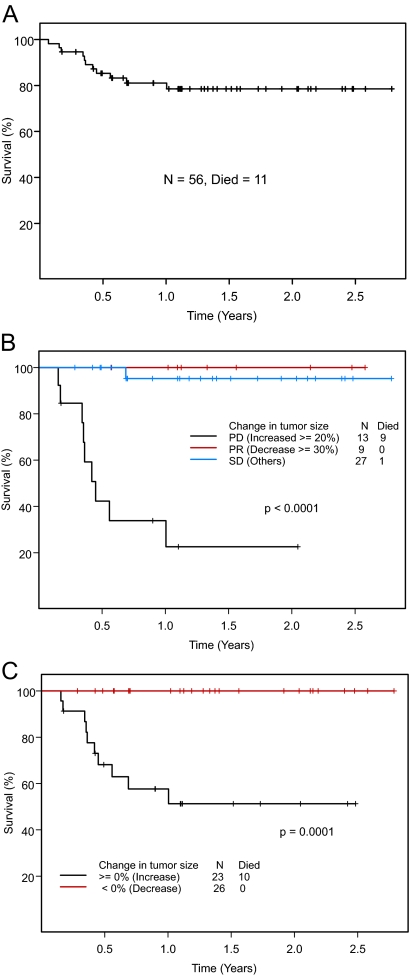
The median follow-up duration for 56 patients was 15.6 months [95% confidence interval (CI) 13.2–19.1 months] (Fig. 2A). The median survival was not reached at 33 months. The 1-yr survival rate was 81% (95% CI 71–92%). A plateau in the survival curve was noted after 1 yr. Overall, 11 patients died. Eight patients died from PD, two from infectious complications likely related to PD, and one from preexisting progressive cancer of unknown origin. All but one patient were off study at the time of death. The characteristics of patients who died are shown in Table 3.
Figure 2.
A, Survival in 56 patients with thyroid cancer seen in the Phase I Clinic. B, Survival in 49 patients evaluable for response by RECIST. One patient who died from pneumonia unrelated to study drug was not evaluable for response and therefore is not included in this analysis. C, Survival in 49 evaluable patients by greater than 0% decrease vs. any increase in tumor size. One patient who died from pneumonia unrelated to study drug was not evaluable for response and therefore is not included in this analysis.
Table 3.
Characteristics of patients with thyroid cancer who were referred to Phase I Clinical Trials Program and died
| Age | Sex | Histology | Albumin, g/dl | Hgb, g/dL | Local recurrence | Change in tumor measurement, % | Survival, months | Cause of death | |
|---|---|---|---|---|---|---|---|---|---|
| 729836 | 77 | M | Anaplastic | 3.6 | 10.4 | Yes | ≥20a | 5.4 | PD |
| 737622 | 79 | F | Anaplastic | 3.9 | 9.6 | Yes | ≥20a | 1.8 | PD |
| 738655 | 54 | M | Anaplastic | 4 | 12.9 | Yes | +23 | 5.0 | Pneumonia |
| 667923 | 74 | M | Anaplastic | 3.5 | 12.7 | No | +35 | 6.7 | PD |
| 420240 | 43 | F | Medullary | 4.5 | 13.3 | No | +19 | 8.2 | PD |
| 689320 | 41 | M | Medullary | 4.1 | 14.1 | No | +20 | 4.1 | PD |
| 742026 | 35 | M | Medullary | 4.8 | 14.2 | Yes | +35 | 4.2 | PD |
| 256707 | 45 | F | Papillary | 3.2 | 15 | Yes | Early death | .8 | Pneumonia |
| 707527 | 69 | M | Papillary | 4.4 | 13.6 | Yes | ≥20a | 2.0 | PD |
| 268002 | 59 | F | Papillary | 4.7 | 11 | No | +39 | 12.1 | PD |
| 714488 | 73 | F | Papillary | 3.9 | 9.9 | No | ≥20a | 4.3 | PD |
Hgb, Hemoglobin.
Four patients had clinically rapid PD, and they were not restaged with imaging studies. By default, their percent change in tumor measurement was 20% or greater.
Survival by response assessed by RECIST is shown in Fig. 2B. At 1 yr, the survival rates of patients who had maximum responses of PR, SD, or PD were 100, 95, and 34% (P < 0.0001), respectively. The differences in survival may, in part, reflect differences in the responses to treatment of the various histological types. Patients with medullary thyroid carcinoma were more likely to achieve a PR or SD (88%); patients with differentiated thyroid carcinoma were more likely to achieve SD (72%), and patients with anaplastic thyroid carcinoma, a rare and traditionally aggressive disease, were more likely to have PD as their best response (83%). Among 26 patients with any decrease in tumor measurements compared with baseline, no deaths were noted, whereas 10 patients among the 23 who had any increase in tumor measurements died (P = 0.0001) (Fig. 2C).
The 1-yr survival rates in patients with anaplastic, papillary, medullary, and follicular thyroid cancer were 21, 84, 88, and 100%, respectively (P = 0.002) (Table 1). Patients with normal albumin levels (>3.4 g/dl) had higher rates of survival compared with patients with albumin levels below the lower limit of normal (univariate analysis): at 1 yr, the survival rates were 82 and 0%, respectively (P = 0.05). Hemoglobin level of 11 g/dl or greater and lack of local recurrence of thyroid cancer were marginally associated with a longer duration of survival (P = 0.08 and P = 0.10, respectively) (Table 1). Other factors were not predictive of survival.
Progression-free survival
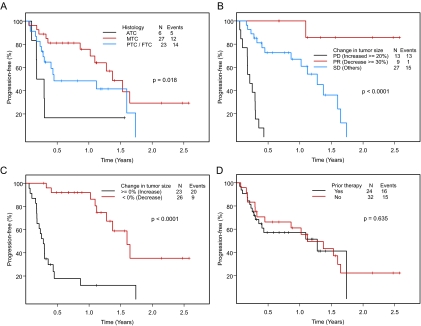
The median PFS was 1.12 yr. The 1-yr PFS rate was 58% (95% CI 46–74%) (Fig. 3A). To date, 31 patients have had disease progression. PFS by response assessed by RECIST is shown in Fig. 3B. The median PFS of patients who had maximum responses of PR, SD, or PD was not reached at 30, 15.3, and 2.5 months (P < 0.0001), respectively. At 1 yr, the respective rates of PFS were 100, 67, and 0%. Among 26 patients with any decrease in tumor measurements compared with baseline, nine patients had PD, compared with 10 of 23 patients who had any increase in tumor measurements (P < 0.0001) (Fig. 3C). The median PFS of patients who had any decrease in tumor measurements compared with baseline was 19.2 months compared with 3.3 months in patients who had any increase in tumor measurements. At 1 yr, the respective rates of PFS were 92% and 12%.
Figure 3.
A, PFS in 56 patients with thyroid cancer seen in a phase I clinic, according to histological type (ATC, anaplastic thyroid carcinoma; MTC, medullary thyroid carcinoma; and PTC/FTC, papillary thyroid carcinoma/follicular thyroid carcinoma grouped together as differentiated thyroid carcinoma). B, PFS in 49 patients evaluable for response by RECIST. C, PFS in 49 evaluable patients by greater than 0% decrease vs. any increase in tumor size. D, Comparison of PFS among patients who received at least one prior medical therapy and those who did not.
The median PFS durations in patients with papillary, follicular, medullary, and anaplastic thyroid cancer were 13.4, 3.2, 16.5, and 2.8 months, respectively (P = 0.03, Fig. 3A). Hemoglobin level greater than 11 g/dl was marginally associated with a longer duration of PFS (P = 0.08). Other covariates, including albumin levels and local recurrence, were not predictive for duration of PFS. There was a trend for a longer PFS in patients with medullary thyroid carcinoma compared with patients with differentiated thyroid carcinoma (P = 0.066) (supplemental Fig. 1A). When the PFS rates in papillary, follicular, and medullary thyroid carcinoma were compared, no statistical difference was seen (P = 0.102) (supplemental Fig. 1B). There was no statistically significant difference in the PFS between patients who received prior medical therapies and those who did not (median PFS in the prior therapy group 1.12 yr vs. 1.28 yr in the nonprior therapy group, P = 0.635; Fig. 3D).
Time to failure
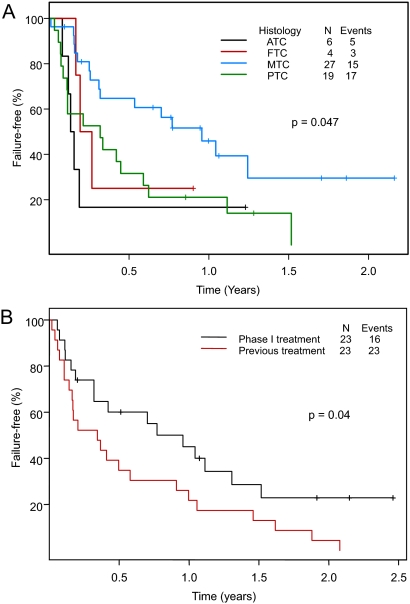
The median TTF in patients with papillary, follicular, medullary, and anaplastic thyroid cancer was 0.32, 0.15, 0.96, and 0.15 yr, respectively (Fig. 4A). At the time of the analysis, 27 and six patients had PD and adverse events, respectively, that were possibly or clearly related to the investigational therapy and were taken off study. The patients with medullary thyroid carcinoma had a longer TTF compared with the patients with differentiated thyroid carcinoma (P = 0.015) (supplemental Fig. 2A). When TTF was compared among patients with medullary, papillary, and follicular thyroid carcinoma, there was a trend for a longer TTF in patients with medullary thyroid carcinoma (P = 0.051) (supplemental Fig. 2B). Twenty-three patients with measurable disease were previously treated with systemic antitumor therapy. The median TTF for the previous treatment was 4.1 months (95% CI 2.0–12.0 months) compared with a TTF of 11.5 months (95% CI 3.8–30 months) for the phase I investigational treatment (P = 0.04) (Fig. 4B).
Figure 4.
A, TTF in 56 patients with thyroid cancer seen in the Phase I Clinic, according to histologic type (ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; PTC, papillary thyroid carcinoma). B, TTF in 23 (of 56) patients with measurable disease (one patient who received prior therapy did not have measurable disease according to RECIST criteria) who were previously treated with another therapy. The median TTF to previous treatment was 4.1 months (95% CI 2.0–12.0 months) compared with 11.5 months (95% CI 3.8–30 months) to treatment in our Phase I Clinical Trials Program.
Toxicity
Four patients discontinued therapy because they experienced NCI-CTC grade 3–4 toxicities (grade 3, lipase elevation, n = 2; grade 3, skin rash/infusion reaction, n = 2); and one patient died from pneumonia on cycle 1, d 11, an event considered not attributable to the study drug. Thirty-seven patients experienced adverse events no greater than grade 2. Other grade 3 adverse events not directly leading to drug discontinuation included neutropenia (n = 2), hypertension (n = 1), elevations in lipase and liver function tests (n = 6), diarrhea (n = 2), hemoptysis and gastrointestinal hemorrhage (n = 2), precancerous cutaneous lesion (n = 1), and thrombosis probably related to study drug (n = 1).
Discussion
This is the first study to summarize the clinical outcomes of patients with metastatic thyroid cancer referred to a phase I clinic. All patients but one were treated with targeted agent-containing therapies. The rates of PR and SD by RECIST lasting 6 months or longer were 18.4 and 32.7%, respectively. The median PFS of patients who had a maximum response of PR was not reached at 30 months. Interestingly, 53.1% of patients had any degree of decrease in tumor measurements compared with baseline. Considering that, with a median follow-up of 15.6 months, only 11 of 56 patients have died, an intriguing finding in our study is a 1-yr survival rate of 81% and a plateau in the survival curve after 1 yr. In addition, among 49 patients evaluable for response, no death was noted among those who had a decrease in tumor measurements, whereas 10 deaths were noted in the patient group who had increases in tumor measurements. The respective 1-yr survival rates were 100% and 52% (P = 0.0001). In contrast, using the RECIST guidelines, one of 27 patients with SD died, compared with no deaths among the nine patients with PR. Response by RECIST was associated less accurately with survival, suggesting that decrease in tumor measurement is a more accurate predictor of survival than response by RECIST guidelines. In univariate analysis, factors predicting lower survival rates were anaplastic (vs. other) histology (P = 0.0002) and albumin levels less than 3.5 g/dl (P = 0.05). The small number of deaths in our study population precluded a multivariate analysis, which would have allowed us to determine independent factors predicting survival.
The median PFS in the study was 1.12 yr. Given that several targeted agents were used in our phase I clinical trials, the PFS was at least comparable with that published in phase II clinical trials of single-agent sorafenib and motesanib diphosphate in advanced thyroid cancer (14,15,16).
An intriguing observation was that among 23 of 56 patients who were previously treated with another systemic therapy, treatment on a clinical trial in our Phase I Clinical Trials Program was associated with a significantly longer TTF (11.5 months) compared with the previous treatment (4.1 months) (P = 0.04). This suggests that phase I clinical trials with targeted agents may be preferable to treatment with standard therapies in this population.
The optimal therapy for patients with metastatic thyroid cancer has not been defined. Although surgical resection is indicated for recurrence of locoregional thyroid carcinoma, and even for symptomatic distant metastases, ablation using radiofrequency, embolization, or other regional therapies are often used. Therapies for thyroid cancer also include cytotoxic chemotherapeutic regimens. The efficacy of doxorubicin was shown in a small, uncontrolled study (17), and historically, it has been the drug of choice in advanced metastatic thyroid cancer. The addition of cisplatin yielded higher response rates at the cost of additional toxicities (18). Bleomycin, adriamycin, and cisplatin combination therapy induced a response rate of 43% and was associated with a median survival of 11 months (19). Overall, however, clinicians are reluctant to administer cytotoxic chemotherapy in thyroid malignancies unless the disease is advanced or rapidly progressive (6). The prospective clinical trials with cytotoxic chemotherapy in thyroid cancer have enrolled a small number of patients; the antitumor activity (if any) was limited, and the toxicities have been considerable (6).
The discovery of drugs targeting aberrant molecular pathways spawned several clinical trials (Table 4). A phase I study with E7080 (20) and phase II clinical trials with axitinib (21), sorafenib (14), thalidomide (22), and yttrium-labeled somatostatin analogs (23) have demonstrated encouraging results. Phase II clinical trials have shown that the Ras-Raf-MAPK-ERK signaling pathway and the vascular endothelial growth factor receptor inhibitor sorafenib induce a PR in 15–23% of patients with advanced thyroid cancer (14,15). Additionally, 53–56% of patients had SD and the median PFS was 15–18 months (14,15). Motesanib diphosphate, an inhibitor of vascular endothelial growth factor receptors, platelet-derived growth factor receptor, and KIT (CD117 or Stem Cell Factor Receptor), induced an objective response in 14% of patients; 67% achieved SD and median PFS was 10 months (16) (Table 4). Clinical trials with motesanib diphosphate (24), vandetanib (25), sunitinib (26), sorafenib plus tipifarnib (27), XL 184 (28), and pazopanib are ongoing.
Table 4.
Selected clinical trials in thyroid cancer
| Drug | Investigator, year | No. of patients | CR+PR (%) | SD (%) | Median PFS (wk) | Survival | Comments |
|---|---|---|---|---|---|---|---|
| Cytotoxic therapies | |||||||
| BAP (19)a | De Besi et al., 1991 | 21 | 43 | 19 | NR | 11 months | All histologic types; best responses seen in MTC and ATC |
| Targeted therapies | |||||||
| Imatinib (34) | de Groot et al., 2007 | 15 | None | 27 | NR | NR | Phase II; MTC |
| Vandetanib (35) | Wells et al., 2007 | 30 | 17 | 73 | NR | NR | Phase II; hereditary MTC |
| Motesanib (16) | Sherman et al., 2008 | 93 | 14 | 67 | 40 | 1 yr, 73% | Phase II; PTC, FTC, Hürthle cell variant |
| Sorafenib (14) | Gupta-Abramson et al., 2008 | 30 | 23 | 53 | 79 | NR | Phase II; all histological types |
| Axitinib (21) | Cohen et al., 2008 | 60 | 30 | 38 | 73 | Phase II; all histological types | |
| Gefitinib (36) | Pennell et al., 2008 | 27 | None | 48 | 15 | 17.5 months | Phase II; all histological types |
| Sorafenib (15) | Kloos et al., 2009 | 41 | 15 | 56 | 80 | NR | Phase II; metastatic PTC |
| Therapies that restore loss of differentiation | |||||||
| Vorinostat (37) | Woyach et al., 2009 | 19 | None | 48b | NR | NR | Phase II; PTC, FTC, Hürthle cell variant, MTC |
ATC, Anaplastic thyroid carcinoma; CR, complete response; FTC, follicular thyroid carcinoma; MTC, medullary thyroid carcinoma; NR, not reported, PTC, papillary thyroid carcinoma.
Bleomycin + adriamycin + cisplatin.
SD was seen only in differentiated thyroid carcinoma. No patient with MTC achieved SD.
In conclusion, our results demonstrate that patients with advanced thyroid cancer treated on phase I clinical trials of predominantly targeted agents had high rates of durable PR and prolonged SD. Of interest, TTF on their first phase I trial was significantly longer than that of their last systemic medical treatment. These observations suggest that these patients benefit from enrolling on early clinical trials and refute the belief that any apparent benefit may be due to a favorable natural history of the subpopulation treated. Furthermore, for these patients, a cutoff criterion of any decrease in tumor size (vs. any increase) was predictive of both overall and progression-free survival. Molecular profiling of patients with advanced thyroid cancer and selection of personalized targeted therapies are likely to further optimize the clinical outcomes of these patients.
Supplementary Material
Footnotes
This work was supported by Grant RR024148 from the National Center for Research Resources (NCRR), National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Reengineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: CI, Confidence interval; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; NCI-CTC, National Cancer Institute common terminology criteria; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; TTF, time to failure.
References
- Nikiforov YE 2008 Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 21(Suppl 2):S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surveillance Epidemiology and End Results Program of the National Cancer Institute. http://seer.cancer.gov/statfacts/html/thyro.html [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ 2008 Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed] [Google Scholar]
- Sherman S, Gillenwater AM 2003 Cancer medicine 6. 6th ed. Hamilton, Ontario, Canada: BC Becker Inc. [Google Scholar]
- Tuttle RM, Leboeuf R 2007 Investigational therapies for metastatic thyroid carcinoma. J Natl Compr Canc Netw 5:641–646 [DOI] [PubMed] [Google Scholar]
- Baudin E, Schlumberger M 2007 New therapeutic approaches for metastatic thyroid carcinoma. Lancet Oncol 8:148–156 [DOI] [PubMed] [Google Scholar]
- Schlumberger M, Carlomagno F, Baudin E, Bidart JM, Santoro M 2008 New therapeutic approaches to treat medullary thyroid carcinoma. Nat Clin Pract Endocrinol Metab 4:22–32 [DOI] [PubMed] [Google Scholar]
- Neff RL, Farrar WB, Kloos RT, Burman KD 2008 Anaplastic thyroid cancer. Endocrinol Metab Clin North Am 37:525–538, xi [DOI] [PubMed] [Google Scholar]
- Taccaliti A, Boscaro M 2009 Genetic mutations in thyroid carcinoma. Minerva Endocrinol 34:11–28 [PubMed] [Google Scholar]
- Jo YS, Li S, Song JH, Kwon KH, Lee JC, Rha SY, Lee HJ, Sul JY, Kweon GR, Ro HK, Kim JM, Shong M 2006 Influence of the BRAF V600E mutation on expression of vascular endothelial growth factor in papillary thyroid cancer. J Clin Endocrinol Metab 91:3667–3670 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG 2000 New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 [DOI] [PubMed] [Google Scholar]
- Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O'Dwyer PJ 2006 Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 24:2505–2512 [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program of the National Cancer Institute. http://ctep.cancer.gov/ [Google Scholar]
- Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS 2008 Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely Jr PE, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH 2009 Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol 27:1675–1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SI, Wirth LJ, Droz JP, Hofmann M, Bastholt L, Martins RG, Licitra L, Eschenberg MJ, Sun YN, Juan T, Stepan DE, Schlumberger MJ 2008 Motesanib diphosphate in progressive differentiated thyroid cancer. N Engl J M 359:31–42 [DOI] [PubMed] [Google Scholar]
- Gottlieb JA, Hill Jr CS 1974 Chemotherapy of thyroid cancer with adriamycin. Experience with 30 patients. N Engl J Med 290:193–197 [DOI] [PubMed] [Google Scholar]
- Shimaoka K, Schoenfeld DA, DeWys WD, Creech RH, DeConti R 1985 A randomized trial of doxorubicin versus doxorubicin plus cisplatin in patients with advanced thyroid carcinoma. Cancer 56:2155–2160 [DOI] [PubMed] [Google Scholar]
- De Besi P, Busnardo B, Toso S, Girelli ME, Nacamulli D, Simioni N, Casara D, Zorat P, Fiorentino MV 1991 Combined chemotherapy with bleomycin, adriamycin, and platinum in advanced thyroid cancer. J Endocrinol Invest 14:475–480 [DOI] [PubMed] [Google Scholar]
- Nemunaitis JJ, Senzer NN, Kurzrock R, Ng CS, Das A, Atienza RS, Zang EA, Jansen M, Ashworth S, Hong DS 2008 Phase I dose-escalation study of E7080, a multikinase inhibitor, in patients with advanced solid tumors. ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 26 (Abstract 14583, p. 634s) [Google Scholar]
- Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, Kane MA, Sherman E, Kim S, Bycott P, Tortorici M, Shalinsky DR, Liau KF, Cohen RB 2008 Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26:4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ain KB, Lee C, Williams KD 2007 Phase II trial of thalidomide for therapy of radioiodine-unresponsive and rapidly progressive thyroid carcinomas. Thyroid 17:663–670 [DOI] [PubMed] [Google Scholar]
- Iten F, Müller B, Schindler C, Rochlitz C, Oertli D, Mäcke HR, Muller-Brand J, Walter MA 2007 Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res 13:6696–6702 [DOI] [PubMed] [Google Scholar]
- Rosen LS, Kurzrock R, Mulay M, Van Vugt A, Purdom M, Ng C, Silverman J, Koutsoukos A, Sun YN, Bass MB, Xu RY, Polverino A, Wiezorek JS, Chang DD, Benjamin R, Herbst RS 2007 Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:2369–2376 [DOI] [PubMed] [Google Scholar]
- Haddad RI, Krebs AD, Vasselli J, Paz-Ares LG, Robinson B 2008 A phase II open-label study of vandetanib in patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol 26:6024 (Abstract, p. 322s) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulart B, Carr L, Martins RG, Eaton K, Kell E, Wallace S, Capell P, Mankoff D 2008 Phase II study of sunitinib in iodine refractory, well-differentiated thyroid cancer (WDTC) and metastatic medullary thyroid carcinoma (MTC). J Clin Oncol 26(Suppl); Abstract 6062, p. 331s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D, Ye L, Gagel R, Chintala L, El Naggar AK, Wright J, Kurzrock R 2008 Medullary thyroid cancer: targeting the RET kinase pathway with sorafenib/tipifarnib. Mol Cancer Ther 7:1001–1006 [DOI] [PubMed] [Google Scholar]
- Salgia R, Sherman S, Hong DS, Ng CS, Frye J, Janisch L, Ratain MJ, Kuzrock R 2008 A phase I study of XL184, a RET, VEGFR2, and MET kinase inhibitor, in patients (pts) with advanced malignancies, including pts with medullary thyroid cancer (MTC). 2008 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 26(Suppl): Abstract 3522, p. 158s [Google Scholar]
- Chintala L, Kurzrock R, Fu S, Naing A, Wheler JJ, Moulder SL, Newman R, Gagel R, Sebti S, Wright JJ, Hong DS 2008 Phase I study of tipifarnib and sorafenib in patients with biopsiable advanced cancer (NCI protocol 7156). 2008 ASCO Annual Meeting Proceedings (Post-Meeting Edition). J Clin Oncol 26(Suppl): Abstract 3593, p. 176s [Google Scholar]
- Hong DS, Kurzrock R, Supko JG, Lawrence DP, Wheler JJ, Meyer CJ, Mier JW, Andreeff M, Shapiro GI, Dezube BJ 2008 Phase I trial with a novel oral NF-κB/STAT3 inhibitor RTA 402 in patients with solid tumors and lymphoid malignancies. J Clin Oncol 26(Suppl): Abstract 3517, p. 157s [Google Scholar]
- Braiteh F, Soriano AO, Garcia-Manero G, Hong D, Johnson MM, Silva Lde P, Yang H, Alexander S, Wolff J, Kuzrock R 2008 Phase I study of epigenetic modulation with 5-azacytidine and valproic acid in patients with advanced cancers. Clin Cancer Res 14:6296–6301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzrock R 2007 Evaluate the effects of patupilone on the pharmacokinetics of midazolam and omeprazole in patients with advanced malignancies. http://clinicaltrialsgov/ct2/show/NCT00420615 [Google Scholar]
- Camacho LH, Moulder SL, LoRusso PM, Blumenschein GR, Bristow PJ, Kurzrock R, Fu S, Schlienger K, Bergstrom DA 2008 First in human phase I study of MK-2461, a small molecule inhibitor of c-Met, for patients with advanced solid tumors. J Clin Oncol 26:(Suppl): Abstract 14657, p. 639s [Google Scholar]
- de Groot JW, Zonnenberg BA, van Ufford-Mannesse PQ, de Vries MM, Links TP, Lips CJ, Voest EE 2007 A phase II trial of imatinib therapy for metastatic medullary thyroid carcinoma. J Clin Endocrinol Metab 92:3466–3469 [DOI] [PubMed] [Google Scholar]
- Wells Jr SA, Gosnell JE, Gagel RF, Moley JF, Pfister DG, Sosa JA, Skinner M, Krebs A, Hou J, Schlumberger M 2007 Vandetanib in metastatic hereditary medullary thyroid cancer: follow-up results of an open-label phase II trial. J Clin Oncol 25(Suppl): Abstract 6018, p. 303e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, Fidias PH, Temel JS, Gurubhagavatula S, Heist RS, Clark JR, Lynch TJ 2008 A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid 18:317–323 [DOI] [PubMed] [Google Scholar]
- Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, Grever M, Villalona-Calero M, Shah MH 2009 Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab 94:164–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.