Abstract
Context: Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a metabolic disorder due to homozygous loss-of-function mutations in the SLC34A3 gene encoding the renal type IIc sodium-phosphate cotransporter (NaPi-IIc). The typical presentation is severe rickets and hypophosphatemia, and hypercalciuria is often discovered later or overlooked.
Objective: We sought to determine the genetic basis for severe hypercalciuria and nephrolithiasis/nephrocalcinosis in an adolescent male with elevated serum levels of calcitriol but normal serum levels of calcium and phosphorus.
Design and Setting: We used PCR to analyze the SLC34A3 gene in the proband and members of his family.
Results: The proband was a compound heterozygote for two SLC34A3 missense mutations, a novel c.544C→T in exon 6 that results in replacement of arginine at position 182 by tryptophan (R182W) and c.575C→T in exon 7 that results in replacement of serine at position 192 by leucine (S192L). The R182W and S192L alleles were inherited from the mother and father, respectively, both of whom had hypercalciuria. A clinically unaffected brother was heterozygous for S192L.
Conclusion: We report a novel mutation in the SLC34A3 gene in a patient with an unusual presentation of HHRH. This report emphasizes that moderate and severe hypercalciuria can be manifestations of heterozygous or homozygous loss-of-function mutations in the SLC34A3 gene, respectively, providing further evidence for a gene dosage effect in determining the phenotype. HHRH may be an underdiagnosed condition that can masquerade as idiopathic hypercalciuria or osteopenia.
Hypophosphatemic rickets can present as hypercalciuria without typical clinical features of rickets.
Although nutritional vitamin D deficiency is the most common cause of rickets and osteomalacia, these conditions can also be due to genetic defects that impair activation of vitamin D, reduce target tissue responsiveness to 1,25-dihydroxyvitamin D [1,25(OH)2D] or calcitriol, or impair phosphorus reabsorption in the kidney. Regardless of the etiology, the classic signs of rickets, skeletal deformity and growth plate defects, are typically observed on physical examination and/or radiological examination. We report a patient in whom hypercalciuria and renal stone disease were the principal manifestations of hereditary hypophosphatemic rickets with hypercalciuria (HHRH) and in whom proper diagnosis required molecular genetic analysis.
Case reports
A 12-yr-old male was referred for evaluation of severe hypercalciuria of uncertain cause. His medical history disclosed nephrolithiasis beginning in infancy, medullary nephrocalcinosis, and two fractures of the right forearm at age 4 yr. Physical examination revealed height at the 4.6% tile (Z-score −1.73 and adjusted Z-score for height −1.95) and weight at the 5% tile. There were no skeletal deformities. The parents and an older brother were of normal stature and had no history of bone or mineral disorders. In addition, there was no history of consanguinity. Analysis of the extended family did not disclose any other members with a history of a bone disorder, short stature, or recurrent nephrolithiasis. Laboratory evaluation disclosed normal age-adjusted serum levels of calcium (2.47 mmol/liter; 2.13–2.63 mmol/liter) and phosphate (1.19 mmol/liter; 1.16–1.87 mmol/liter). At presentation, the serum concentration of intact PTH was low at 0.7 pmol/liter (normal 1.1–6.4 pmol/liter), serum 25-hydroxyvitamin D [25(OH)D] was 50 nmol/liter, and serum 1,25(OH)2D was markedly elevated at 980 pmol/liter (60–158 pmol/liter). Total alkaline phosphatase was elevated at 581 U/liter (60–485 U/liter) and bone-specific alkaline phosphatase was 419 U/liter (60–250 U/liter). The molar ratio of urinary Ca/Cr was elevated at 1.15 (mmol/mmol) (normal ≤ 0.50). The fasting tubular reabsorption of phosphate (TRP) was normal at 83% (>80%), but the maximal renal phosphate reabsorption per glomerular filtration rate (TmP/GFR) was slightly reduced at 0.79 μmol/ml (0.81–1.10 μmol/ml). Bone density of the L1–L4 spine, as determined by dual-energy x-ray absorptiometry, was reduced (Z = −2.2).
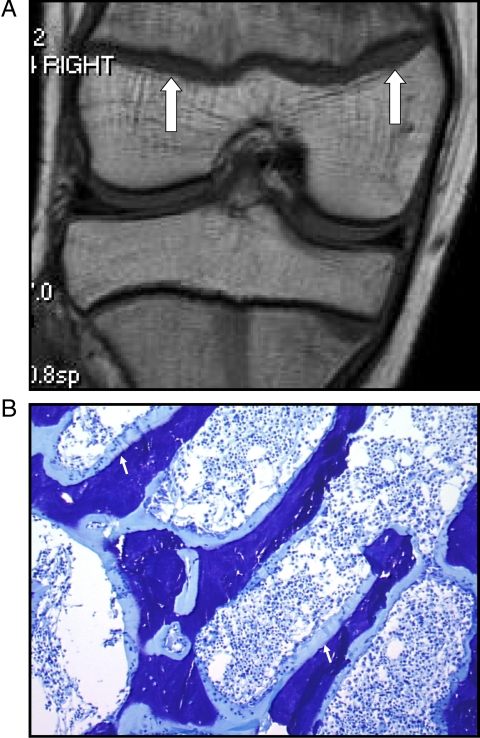
Radiological examination of the skull, spine, ribs, and long bones failed to demonstrate a skeletal deformity or a growth-plate abnormality. By contrast, magnetic resonance imaging (MRI) of the right knee (Fig. 1) was consistent with rickets, with bilateral widening of the distal femoral physis ranging from 2–6 mm, which was also present to a lesser extent in the tibia. The combination of an elevated serum level of 1,25(OH)2D, hypercalciuria, and rickets suggested a diagnosis of HHRH (MIM 241530) due to loss-of-function mutations in the SLC34A3 gene encoding the renal type IIc sodium-phosphate cotransporter (NaPi-IIc) (1,2,3).
Figure 1.
Skeletal studies in proband. A, MRI of right distal femoral and proximal tibial physes shows irregularity and widening of the physes (arrows), suggesting rickets. B, Metabolic bone biopsy, stained with toluidine blue. Image shows markedly widened osteoid seam (arrows) and increased cellularity. Fluorescent microscopy demonstrated no consistent uptake of tetracycline label (not shown).
Materials and Methods
The protocol was approved by the appropriate institutional review boards, and informed consent and/or assent was obtained from all subjects. All blood tests were obtained in the morning after an overnight fast and represent the means of multiple determinations.
Metabolic bone biopsy
A transilial bone biopsy was obtained after double tetracycline labeling with a 14-d interlabel interval. Specimens were embedded in plastic and sectioned undecalcified (3 or 5 μm thick) and were stained with a modified Goldner-Masson stain for general marrow histology, demonstration of bone cells, and distinction between osteoid and mineralized bone. Unstained 10-μm sections were used for fluorescent microscopy of the tetracycline label. Static histomorphometric measurements were performed using a computerized digitizing system. Histomorphometric analysis was performed using parameters as defined by Parfitt et al. (4).
Mutational analysis
Using peripheral blood leukocyte genomic DNA as templates, all 13 exons (one noncoding and 12 coding) as well as adjacent intronic sequences of SLC34A3 were amplified in eight amplicons and directly sequenced as previously described (2). The prevalence of the novel c.544C→T transition in exon 6 in healthy individuals was determined by PCR-restriction fragment length polymorphism analysis. Exons 6 and 7 were amplified by PCR and subjected to restriction endonuclease analysis using AvaII (New England Biolabs, Beverly, MA). The digested PCR products were electrophoresed in a 2% agarose gel and visualized under UV light.
Fibroblast growth factor 23 (FGF23) assay
Serum FGF23 concentration was determined using FGF23 ELISA kit (Kainos Laboratories Inc., Tokyo, Japan), which recognizes only intact FGF23.
Results
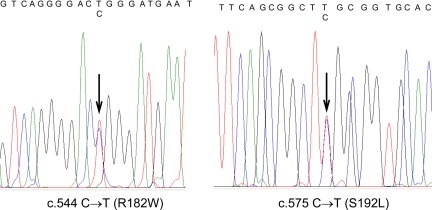
The patient was a compound heterozygote for two missense mutations in SLC34A3 (Fig. 2): c.544C→T in exon 6 that results in replacement of arginine at position 182 by tryptophan (R182W) and c.575C→T in exon 7 that results in replacement of serine at position 192 by leucine (S192L). The R182W and S192L alleles were inherited from the mother and father, respectively. A clinically unaffected brother was heterozygous for S192L. The NaPi-IIc protein has an intracytoplasmic NH2 tail and eight transmembrane segments (5), and both R182 and S192 are in the predicted first intracellular loop, which appears to be important for transport function (6). The c.575C→T mutation has been previously described in other patients with HHRH (1,3), but the c.544C→T transition is a novel finding. Two lines of evidence indicate that c.544C→T is a mutation: 1) the C→T replacement was not found in 100 healthy Caucasian controls, and 2) the R182 residue occurs within a highly conserved region of NaPi-IIc that is identical in NaPi-IIa, -b, and -c proteins from a variety of species, suggesting that an amino acid substitution would be poorly tolerated.
Figure 2.
Chromatograms of direct sequence analysis of the SLC34A3 gene from the proband. The left panel demonstrates heterozygosity for a novel R182W mutation inherited from the proband’s mother. The right panel demonstrates heterozygosity for a previously reported S192L mutation, inherited from the proband’s father.
Histomorphometric analysis of a transilial bone biopsy (Fig. 1B) showed decreased trabecular thickness and marked hyperosteoidosis (Table 1). Fifty-five percent of the trabecular surface was covered with osteoid (normal is <12%), and the osteoid thickness (20.1 vs. 10.34 ± 2.05 μm) and osteoid volume per bone volume (26.1 vs. 1.5 ± 0.9%) were markedly increased. There was no consistent uptake of the tetracycline, likely reflecting the extreme defect in mineralization, and therefore dynamic bone parameters could not be assessed. Osteoblast surface was increased (13.5%), but osteoclast surface was normal (0.68%), and there did not appear to be high resorptive activity. There was no evidence of hyperparathyroidism. These features are consistent with the histomorphometric parameters that have been described previously in HHRH patients (1,7). Similarly, the reduced bone density observed by both bone histology and dual-energy x-ray absorptiometry in the patient that we describe has also been found in other patients with HHRH as well as their heterozygous relatives (1).
Table 1.
Bone histomorphometry
| Value | Normal values (mean ± sd) | |
|---|---|---|
| Static indices | ||
| Bone volume (%) | 37.6 | 23.2 ± 4.4 |
| Osteoid volume/trabecular volume (%) | 9.8 | 0.32 ± 0.19 |
| Osteoid volume/bone volume (%) | 26.1 | 1.48 ± 0.93 |
| Total osteoid surface (%) | 55.6 | 12.10 ± 4.64 |
| Osteoblast surface (%) | 13.5 | 3.90 ± 1.94 |
| Eroded surface (%) | 3.2 | 4.09 ± 2.33 |
| Osteoclast surface (%) | 0.68 | 0.69 ± 0.61 |
| Labeled surface (%) | ND | 9.69 ± 4.95 |
| Trabecular thickness (%) | 96.1 | 133.0 ± 22.0 |
| Structural indices | ||
| Trabecular separation (μm) | 159.8 | 570.6 ± 98.9 |
| Trabecular number (mm−1) | 3.9 | 1.75 ± 0.23 |
ND, Not done.
The concentration of intact FGF23 was undetectable (<3 pg/ml, normal 29.7 ± 20.7 pg/ml) in the patient’s serum (8). Serum concentrations of 1,25(OH)2D and phosphorus were normal in other family members, who were all carriers of a single mutant SLC34A3 allele, but the brother had a slightly depressed TRP. Twenty-four-hour urinary excretion of calcium was elevated in both mother (6.23 mmol) and father (8.83 mmol), but normal (2.15 mmol) in the brother (Table 2).
Table 2.
Biochemical features of family members
| Genotype | Serum phosphorus (mg/dl) | Serum calcium (mg/dl) | Alkaline phosphatase (U/liter) | 24-h urine calcium (mg) | Urine Ca/Cr (mg/mg) | TRPa (%) | TmP/GFRa (μmol/ml) | 25(OH)D (ng/ml) | 1,25(OH)2D (pg/ml) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Proband | R182W/S192L | 3.7 | 9.9 | 581–765 | 235 | 0.46 | 83 | 0.79 | 20 | 377 |
| Mother | R182W | 3.6 | 9.6 | 108 | 249 | 0.23 | 83 | 41 | 61 | |
| Father | S192L | 3.2 | 9.7 | 71 | 353 | 0.20 | 84 | 35 | 59 | |
| Brother | S192L | 3.2 | 10.0 | 113 | 86 | 0.08 | 75 | 47 | 55 | |
| Normal for adult | 2.4–4.7 | 8.6–10.4 | 42–128 | Male, <250; female, <200 | <0.18 | >80 | 0.81–1.10 | 20–100 | 20–71 |
To convert to SI units, multiple phosphorus by 0.323 (mmol/liter), calcium by 0.25 (mmol/liter), urine calcium by 0.025 (mmol), 25(OH)D by 2.5 (nmol/liter), and 1,25(OH)2D by 2.6 (pmol/liter).
Urine sample obtained in fasting state.
Discussion
We describe a patient with HHRH whose principal clinical manifestations of the disorder were hypercalciuria and recurrent nephrolithiasis. The diagnosis of rickets required MRI of growth plates, and a mineralization defect was documented by bone histomorphometry that showed markedly increased osteoid volume, a marked mineralization defect, but no features of hyperparathyroidism. These features are typical of HHRH but are unexpected in an affected patient with normal serum phosphate concentrations (7).
HHRH was confirmed by molecular genetic analysis that showed that the patient was a compound heterozygote for mutations in SLC34A3, including the novel missense mutation R182W, that are predicted to lead to inactivation of the renal NaPi-IIc sodium phosphate cotransporter. The patient had initially received a diagnosis of idiopathic hypercalciuria with coexisting osteopenia, and thus, this case emphasizes several points that are relevant both to hypercalciuria and rickets. First, although vitamin D deficiency is the principal cause of rickets and osteomalacia worldwide, these disorders can also be the result of defects in phosphate homeostasis. At least four inherited forms of hypophosphatemic rickets have now been described: X-linked hypophosphatemic rickets (MIM 307800) is caused by mutations in the PHEX gene (9), autosomal dominant hypophosphatemic rickets (MIM 193100) is caused by mutations in the FGF23 gene (10), autosomal recessive hypophosphatemic rickets (MIM 600980) is caused by mutations in the DMP1 gene (11,12), and autosomal recessive HHRH is caused by mutations in SLC34A3 encoding the NaPi-IIc renal sodium phosphate cotransporter (1,2,3). NaPi-IIc is coexpressed with NaPi-IIa in the renal brush border membrane of the proximal tubule, where most filtered phosphate is reabsorbed. NaPi-IIa and NaPi-IIc are both regulated by PTH, FGF23, and dietary phosphate; PTH and FGF23, in response to elevated levels of dietary and serum phosphorus, decrease the abundance of cotransporters at the cell membrane and thereby reduce the tubular reabsorption of phosphorus from the glomerular filtrate (1,13,14). Serum levels of FGF23 are appropriately suppressed in the presence of low serum phosphate levels in HHRH (3) (this study), and as a result, activity of the renal 25(OH)D 1α-hydroxylase (CYP27B1), the rate-limiting enzyme in the two-step activation process of vitamin D, is stimulated, leading to increased production of 1,25(OH)2D. This increased synthesis of 1,25(OH)2D acts to increase absorption of dietary calcium and phosphorus, with resultant hypercalciuria and continued phosphaturia. By contrast, circulating levels of FGF23 are inappropriately normal or elevated in other forms of hypophosphatemic rickets (15,16), calcium nephrocalcinosis, or hypercalciuria with hypophosphatemia (17). Thus, the unique biochemical pattern of elevated serum levels of 1,25(OH)2D, hypercalciuria phosphaturia, and suppressed serum levels of PTH and FGF23 distinguish HHRH from other hypophosphatemic and hypercalciuric disorders.
Second, the diagnosis of HHRH may not be straightforward. For example, vitamin D deficiency has been previously reported to mask some of the biochemical features of HHRH, particularly hypercalciuria (18). Although serum levels of alkaline phosphatase and 1,25(OH)2D were elevated in the patient that we report here, serum phosphate concentrations were consistently normal as was the TRP, and radiographs did not disclose typical features of rickets. Nevertheless, the presence of both rickets and suppressed serum concentrations of FGF23 implies that concentrations of phosphorus must be inadequate, if not in the serum then at least in the growth plate and mineralizing bone, and sensed as low by those bone cells that produce FGF23. The assessment of phosphate homeostasis can be challenging; serum phosphate concentrations are influenced by time of day, relationship to meals, and age of the subject, and none of the methods for determination of tubular reabsorption of phosphate is entirely satisfying (19). The TmP/GFR appears to be superior to the more commonly employed TRP, and in young children, TmP/GFR values obtained by calculation are more accurate than the values obtained by the nomogram of Walton and Bijvoet (19,20). Our comprehensive evaluation showed that the TmP/GFR was reduced, and MRI of the distal femoral epiphyses disclosed abnormalities that were consistent with rickets.
Third, the two parents in this family had mild hypercalciuria, whereas the heterozygous brother had normal urinary calcium excretion. Because HHRH is an autosomal recessive disease, biallelic mutations are required for complete manifestation of the phenotype. However, previous studies of HHRH kindreds have indicated that some subjects with a single mutation (i.e. carriers) can show clinical features of HHRH, including hypercalciuria and osteopenia (1,2,3). By contrast, hypercalciuria is a key hallmark of HHRH, and it is not surprising to find stone formation in these patients. However, stone formation in HHRH has been reported previously in only a few cases (1,2,18,21,22). The history of recurrent nephrolithiasis in our patient emphasizes that renal stone disease should be considered an important feature of HHRH.
And lastly, this case illustrates the increasingly important roles that advanced imaging technology, newer immunoassays, and molecular genetic analysis play in the investigation of patients with unexplained or poorly characterized disorders of bone and mineral metabolism. This patient’s longstanding diagnosis was idiopathic hypercalciuria with elevated serum levels of 1,25(OH)2D, but the use of MRI to evaluate growth plates and molecular analysis to investigate the SLC34A3 gene yielded the correct diagnosis, HHRH.
Footnotes
This work was supported in part by the Cleveland Clinic Lerner Research Institute, the Friedman Family Fund, and National Institutes of Health Grants R01 AR42228 and R01 AR47866.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: FGF23, Fibroblast growth factor 23; HHRH, hereditary hypophosphatemic rickets with hypercalciuria; MRI, magnetic resonance imaging; NaPi-IIc, type IIc sodium-phosphate cotransporter; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D; TmP/GFR, maximal renal phosphate reabsorption per glomerular filtration rate; TRP, tubular reabsorption of phosphate.
References
- Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H 2006 SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78:179–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ 2006 Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab 91:4022–4027 [DOI] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum- Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM 2006 Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR 1987 Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ito M, Tatsumi S, Kuwahata M, Segawa H 2007 New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27:503–515 [DOI] [PubMed] [Google Scholar]
- Kohler K, Forster IC, Stange G, Biber J, Murer H 2002 Identification of functionally important sites in the first intracellular loop of the NaPi-IIa cotransporter. Am J Physiol Renal Physiol 282:F687–F696 [DOI] [PubMed] [Google Scholar]
- Gazit D, Tieder M, Liberman UA, Passi-Even L, Bab IA 1991 Osteomalacia in hereditary hypophosphatemic rickets with hypercalciuria: a correlative clinical-histomorphometric study. J Clin Endocrinol Metab 72:229–235 [DOI] [PubMed] [Google Scholar]
- Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ 2006 Sensitivity of fibroblast growth factor 23 measurements in tumor-induced osteomalacia. J Clin Endocrinol Metab 91:2055–2061 [DOI] [PubMed] [Google Scholar]
- 1995 A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet 11:130–136 [DOI] [PubMed] [Google Scholar]
- ADHR Consortium 2000 Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE 2006 Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM 2006 DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari SL, Bonjour JP, Rizzoli R 2005 Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 90:1519–1524 [DOI] [PubMed] [Google Scholar]
- Antoniucci DM, Yamashita T, Portale AA 2006 Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91:3144–3149 [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T 2007 FGF23 is a hormone-regulating phosphate metabolism: unique biological characteristics of FGF23. Bone 40:1190–1195 [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H 2003 Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348:1656–1663 [DOI] [PubMed] [Google Scholar]
- Rendina D, Mossetti G, De Filippo G, Cioffi M, Strazzullo P 2006 Fibroblast growth factor 23 is increased in calcium nephrolithiasis with hypophosphatemia and renal phosphate leak. J Clin Endocrinol Metab 91:959–963 [DOI] [PubMed] [Google Scholar]
- Kremke B, Bergwitz C, Ahrens W, Schutt S, Schumacher M, Wagner V, Holterhus PM, Juppner H, Hiort O 2009 Hypophosphatemic rickets with hypercalciuria due to mutation in SLC34A3/NaPi-IIc can be masked by vitamin D deficiency and can be associated with renal calcifications. Exp Clin Endocrinol Diabetes 117:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U 1993 Clinical assessment of plasma phosphate and renal tubular threshold for phosphate. In: Alon U, Chan JCM, eds. Phosphate in pediatric health and disease. Ann Arbor, MI: CRC Press; 103–114 [Google Scholar]
- Walton RJ, Bijvoet OL 1975 Nomogram for derivation of renal threshold phosphate concentration. Lancet 2:309–310 [DOI] [PubMed] [Google Scholar]
- Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA 1985 Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med:611–617 [DOI] [PubMed] [Google Scholar]
- Chen C, Carpenter T, Steg N, Baron R, Anast C 1989 Hypercalciuric hypophosphatemic rickets, mineral balance, bone histomorphometry, and therapeutic implications of hypercalciuria. Pediatrics 84:276–280 [PubMed] [Google Scholar]