Abstract
Context: Increased oxidant stress and inflammation (OS/infl) are linked to both aging-related diseases and advanced glycation end products (AGEs). Whereas AGE receptor-1 (AGER1) reduces OS/infl in animals, this has not been assessed in normal humans.
Objective: The objectives of the study were to determine whether AGER1 correlates with AGEs and OS/infl and a reduction of dietary AGEs (dAGEs) lowers OS/infl in healthy adults and chronic kidney disease (CKD-3) patients.
Design: This study was cross-sectional with 2-yr follow-up studies of healthy adults and CKD-3 patients, a subset of which received a reduced AGE or regular diet.
Setting: The study was conducted at general community and renal clinics.
Participants: Participants included 325 healthy adults (18–45 and >60 yr old) and 66 CKD-3 patients.
Intervention: An isocaloric low-AGE (30–50% reduction) or regular diet was given to 40 healthy subjects for 4 months and to nine CKD-3 patients for 4 wk.
Main Outcome: Relationships between age, dAGEs, serum AGEs, peripheral mononuclear cell AGE-receptors, and OS/Infl before and after reduction of dAGE intake were measured.
Results: AGEs, oxidant stress, receptor for AGE, and TNFα were reduced in normal and CKD-3 patients after the low-AGE diet, independently of age. AGER1 levels in CKD-3 patients on the low-AGE diet resembled 18- to 45-yr-old normal subjects. Dietary, serum, and urine AGEs correlated positively with peripheral mononuclear cell AGER1 levels in healthy participants. AGER1 was suppressed in CKD-3 subjects, whereas receptor for AGE and TNFα were increased.
Conclusions: Reduction of AGEs in normal diets may lower oxidant stress/inflammation and restore levels of AGER1, an antioxidant, in healthy and aging subjects and CKD-3 patients. AGE intake has implications for health outcomes and costs and warrants further testing.
Reduction of advanced glycation endproducts (AGE) in normal diets lowers oxidant stress/inflammation, and restores levels of AGE receptor-1 in healthy, aging, and chronic kidney disease-3 patients.
It is widely thought that oxidative stress (OS) inevitably increases with aging and plays a causal role in cardiovascular (CVD) or kidney (CKD) disease (1,2,3,4). However, it has been recently questioned whether unopposed OS is an obligatory process of normal aging in humans, whether it is causally related to chronic diseases of late adulthood, and whether it can be modified (5,6,7). Understanding whether OS is a normal component of aging, when it begins and whether it can be reduced in healthy adults or in patients with chronic diseases, such as CKD, may lead to new treatment options.
Prooxidants such as advanced glycation end products (AGEs) play a significant role in the pathogenesis of chronic diseases, such as CVD, CKD, and diabetes (8,9,10). Until recently, studies of AGEs concentrated on complications in diabetics (8,9). AGEs are byproducts of spontaneous addition reactions between reducing sugars and free amines on polypeptides or lipids and may be found as preformed products in heat-processed foods (11,12,13,14,15). AGEs in the diet are pathogenic, as demonstrated by the fact that the addition of specific AGEs to animal food increases OS, and increased dietary AGEs are associated with CKD and CVD (16). This also applies to humans, suggesting that dietary AGEs are directly linked to increased OS and the risk of developing CVD, CKD, and diabetes (17). Consistent with this hypothesis, the steady-state serum levels of common AGE substances, such as εN-carboxy-methyl-lysine (CML) or methyl-glyoxal derivatives (MG) are influenced by the intake of dietary AGEs in animals (18,19,20). In particular, AGEs promote diabetes and vascular and renal disease in mice, whereas restriction of the intake of AGEs prevents these disorders. In addition, there is a strong correlation between circulating AGEs and markers of OS or inflammation in healthy adults (21).
In vitro studies reveal that AGEs activate multiple cellular pathways, such as MAPK and Ras and promote nuclear factor-κB activity and cytokine production, in part via generation of reactive oxygen species (ROS). These effects are attributed to the stimulation of specific receptors, such as receptor for AGEs (RAGE) (22) or more recently to epithelial growth factor receptor, via the ERK and Shc pathways (23,24). One of the three isoforms of the Shc adaptor proteins, p66shc, is key in the regulation of ROS production, apoptosis, and life span in mice (25,26). Although normally suppressed (27), p66shc is elevated in diabetes, vascular disease, and renal disease (28,29), three conditions often associated with elevated AGEs and OS (12,30,31).
The detoxification of AGEs and counterregulation of their prooxidant effects is in part attributed to AGE receptor-1 (AGER1), a 50-kDa integral plasma membrane protein, which blocks ROS generation, as well as signaling pathways linked to RAGE, epithelial growth factor receptor, and ERK (23,24,32). Interestingly, AGER1 also suppresses p66shc expression and its tyr- and ser-36 phosphorylation as well its oxidant effects in vitro (22,23) and in vivo (16,33). This suggests that there is a mechanistic link between the levels of AGER1 and the regulation of prooxidant genes such as p66shc. Concordant with this hypothesis AGER1 expression/function is reduced in conditions generally associated with high OS, such as diabetes and aging, whereas restriction of dietary AGEs and restriction of calories in mice increases AGER1 levels (16,33,34,35). These data point to the hypothesis that the levels of AGER1 expression play a role in the accumulation of prooxidant molecules, tissue injury, and the subsequent development of chronic diseases.
In the present studies, we asked whether levels of AGEs and OS in healthy persons 18–45 yr old or older than 60 yr are: 1) correlated with dietary AGEs, 2) modifiable by restricting the dietary intake of AGEs, and c) correlated with changes in AGER1 levels. We found that the intake of dietary AGEs strongly affect the levels of circulating AGEs, OS, and proinflammatory markers. Furthermore, the levels of AGER1 in PMNC correlate with serum and urine AGEs and oxidant stress markers positively in healthy participants, and negatively in CKD-3 patients, suggesting that abnormal AGER1 function may contribute to high OS. Reducing dietary AGE intake significantly decreases OS in both healthy participants and CKD-3 patients. This safe and efficient intervention may improve outcomes in age-related diseases.
Patients and Methods
Observational study
Healthy adult volunteers (NL) (18–45 and >60 yr old) were recruited from the community and the Bronx, NY, Veterans Affairs Hospital clinics (n = 325) (Table 1). Exclusion criteria were major medical conditions including diabetes, cardiovascular or kidney disease, and cancer. Information was collected on medical history and dietary intake. Of the initial 325 participants, 49 healthy participants also participated in a 2-yr follow-up, at which time all measures collected at baseline were repeated.
Table 1.
Baseline comparisons of demographic and biochemical parameters in healthy participants 18–45 yr old and older than 60 yr (n = 325)
| 18–45 yr old | Older than 60 yr | Pa | Older than 60 yr (GFR >90) | Older than 60 yr (GFR 30–60) | Pb | Pc | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 30 ± .7 | 80 ± .7 | 0.000 | 74 ± 1 | 85 ± 1 | 0.000 | 0.000 |
| BMI (kg/m2) | 24 ± 0.5 | 28 ± 0.7 | 0.000 | 27 ± 1 | 29 ± 2 | 0.505 | 0.002 |
| Systolic BP (mm Hg) | 114 ± 2 | 128 ± 2 | 0.000 | 126 ± 3 | 123 ± 9 | 0.739 | 0.108 |
| Diastolic BP (mm Hg) | 66 ± 1 | 69 ± 2 | 0.108 | 70 ± 2 | 67 ± 4 | 0.518 | 0.716 |
| Triglycerides (mg/dl) | 65 ± 5 | 111 ± 4 | 0.000 | 105 ± 10 | 116 ± 7 | 0.348 | 0.000 |
| HDL cholesterol (mg/dl) | 66 ± 2 | 53 ± 1 | 0.000 | 60 ± 3 | 46 ± 2 | 0.000 | 0.000 |
| LDL cholesterol (mg/dl) | 93 ± 4 | 113 ± 2 | 0.000 | 106 ± 3 | 110 ± 4 | 0.441 | 0.004 |
| Glucose (mg/dl) | 73 ± 1 | 84 ± 2 | 0.000 | 84 ± 3 | 82 ± 2 | 0.556 | 0.012 |
| Creatinine clearance (ml/min) | 119 ± 4 | 86 ± 3 | 0.000 | 109 ± 2 | 47 ± 3 | 0.000 | 0.000 |
| Urinary protein (mg/d) | 100 ± 8 | 98 ± 9 | 0.924 | 116 ± 14 | 60 ± 8 | 0.001 | 0.052 |
BMI, Body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Statistically significant difference between healthy participants 18–45 yr old and those older than 60 yr.
Statistically significant difference between healthy participants older than 60 yr with GFR greater than 90 ml/min and those with GFR between 30 and 60 ml/min.
Statistically significant difference between healthy participants 18–45 yr old and those older than 60 yr with GFR 30–60 ml/min.
Also included in the cross-sectional study were 66 CKD patients (most of whom were National Kidney Foundation-Dialysis Outcomes Quality Initiatives stage 3) (36). These patients were participants in concurrent studies conducted at the Mount Sinai School of Medicine. All participants consented to participate in the study, and the study protocol was approved by the Mount Sinai School of Medicine Institutional Review Board.
Interventional study
Thirty healthy participants whose usual diet was rich in AGEs (dietary AGE intake >13 AGE Eq/d) (15), equally divided among 18- to 45-yr-olds and those older than 60 yr, were randomized to either a low-AGE diet or their usual diet for a period of 4 months. A fasting blood sample and previously validated 3-d food records were obtained at the beginning and end of the intervention (37). For this study, healthy participants prepared their own food at home after being individually instructed on how to prepare their meal to reduce dietary AGE intake by modifying the cooking time and temperature without changing the quality or quantity of food (13,37). Participants were instructed to avoid frying, baking, or grilling by boiling, poaching, stewing, or steaming their food. It has been previously demonstrated that switching to these suggested methods of cooking limits new AGE formation, particularly in animal food products (37). Participants were followed regularly by telephone calls (one to two times weekly) and monthly clinic visits to promote dietary compliance. Only two subjects did not finish the total period of study (4 months). These two subjects came for three of the five visits and were included as part of the analyses.
A subgroup of CKD-3 patients (n = 9), which had a similar dAGE intake at baseline as the healthy intervention group, were also enrolled in the study. They were randomly placed on either a low-AGE diet or their usual diet. Rather than preparing their meals at home, their meals were prepared in the clinical research center and given to them twice a week (13). The CKD patients were overseen by a dietitian in the same manner as the healthy participants. The study diets in the healthy and CKD-3 participants were designed to maintain the participants’ dietary requirements of calories, protein, carbohydrate, and fat.
Methods
Daily dietary AGE content from the 3-d food records, which emphasized cooking methods, was estimated from a database of about 200 foods that listed AGE values expressed as AGE equivalents (equivalents per day) (one AGE equivalent equals 1000 kilo units). Nutrient calculations were estimated from the same food records using a nutrient software program (Food Processor version 10.1; ESHA Research, Salem, OR).
Participants
All participants received an evaluation of medical history and a physical examination at baseline and at each follow-up. In addition, participants donated a blood sample and a 24-h urine to estimate creatinine clearance and excretion of proteins and AGE. Routine blood and urine tests were performed in the hospital clinical laboratory by standard methods.
Serum measures related to AGEs and OS
Serum and urine samples were tested for CML (4G9 mab; Alteon, Inc., Northvale, NJ) and MG derivatives (MG3D11) by non-cross-reactive monoclonal antibodies, as previously described (12,13,21,38,39,40,41). CML-BSA and MG-BSA, characterized by HPLC and gas chromatography/mass spectrometry, were used as standards in respective ELISAs. Methylglyoxal hydroimidazolone derivative of arginine (purchased from NeoMPS, Inc., San Diego, CA) but not carboxy-ethyl-lysine (purchased from NeoMPS) was highly cross-reactive with MG3D11. High-sensitivity C-reactive protein (hsCRP) was measured by using a high-sensitivity kit and an IMMAGE protein analyzer (nephelometer; Beckman Coulter, Brea, CA). Plasma 8-isoprostane and vascular cell adhesion molecule (VCAM-1) were tested by ELISA kits (13,21).
Peripheral blood mononuclear cells (PMNCs)
PMNCs were separated from fasting, EDTA anticoagulated blood by Ficoll-Hypaque Plus gradient (Amersham Biosciences, Uppsala, Sweden), and used to isolate mRNA and protein (13). Total RNA was extracted by Trizol (Molecular Probes, Inc., Eugene, OR). The extracted RNA had an OD 280:260 ratio between 1.8 and 2.0. Total RNA was reverse transcribed using Superscript III RT (Invitrogen, Carlsbad, CA).
PCR assay
Quantitative SYBR Green real-time PCR was performed to analyze expression of mRNA for AGE-R1, RAGE, and p66Shc. Briefly, 5 μl of template cDNA were added to a final volume of 10 μl containing 1× SYBR Green PCR master mix and 10 μm of the primers. Amplification was performed with 40 cycles of denaturation at 95 C for 15 sec, annealing at 55 C for 20 sec, and elongation at 72 C for 30 sec. Sequences of the primers used for real-time PCR were: AGE-R1, forward primer, 5′-CTGGGGCTCTTCATCTTCAG-3′, reverse primer 5′-GTTGCATCTCCCACAGAGGT-3′; RAGE, forward primer, 5′-AGGAGCGTGCAGAACTGAAT-3′, reverse primer, 5′-TTGGCAAGGTGGGGTTATAC-3′; for p66shc, forward primer, 5′-AGGAAGGGCAGCTGATGAT-3′, reverse primer, 5′-GCGTGGGCTTATTGACAAAG-3′. During thermal cycling, emission from each sample was recorded, and the raw fluorescence data were processed using SDS software (Applied Biosystems, Foster City, CA) to produce threshold cycle values for each sample. β-Actin and glyceraldehyde-3-phosphate dehydrogenase housekeeping genes were used for internal normalization. The transcript copy number of target genes was determined based on their threshold cycle values (41).
Western analysis
PBMN proteins for AGE receptors (AGER1 and RAGE), p66shc, and TNFα were obtained by brief sonication of cells followed by centrifugation at 10000 × g for 5 min. Cell lysates were then subjected to Western blot analysis using the respective antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were reprobed by anti-β-actin antibody to assess protein loading (23,33).
Statistical analysis
Data are presented as mean ± sem in tables and figures. The Kolmogorov-Smirnov goodness-of-fit test was used to test for normal distribution. All variables not normally distributed were logarithmically converted for further analyses. Differences of means between groups were analyzed by Student t test or ANOVA (followed by Bonferroni correction for multiple comparisons), depending on the number of groups. Correlation analyses were also examined by Pearson’s or Spearman’s correlation coefficients. Stepwise multiple regression analysis was performed to assess variables, i.e. serum CML, serum MG, RAGE, AGER1, etc., that were independently associated with dietary AGEs. Significance of changes during the interventional study was assessed by comparing the percent change between baseline and end of the study between the low-AGE and the regular-AGE diet groups and analyzed by the Mann-Whitney test. Significant differences were defined as P < 0.05 and are based on two-sided tests. Data analysis used the SPSS statistical program (SPSS 15.0 for Windows, Chicago, IL).
Results
Cross-sectional study
Both serum (s) CML and sMG were higher on average in healthy participants older than 60 yr than 18- to 45-yr-old healthy participants (Tables 1 and 2) and as a group, independently correlated with dietary AGE (r = 0.452, P < 0.0001) (21). However, there was a large variability of values so that some of the 18- to 45-yr-old participants had sCML values in the range found in participants older than 60 yr (Fig. 1, A). Mean values of fasting blood glucose, hsCRP, fibrinogen, systolic blood pressure, plasma triglycerides, and low-density lipoprotein cholesterol were higher, but high-density lipoprotein cholesterol and estimated glomerular filtration rate (eGFR) for creatinine were lower in the age group older than 60 yr. Most markers of OS, including plasma 8-isoprostanes, VCAM-1, and PBMN-derived TNFα did not differ significantly between the two age groups, with the exception of hsCRP, p66shc, and fibrinogen, which were higher in participants older than 60 yr (Table 2).
Table 2.
Baseline comparisons of serum AGEs, AGE receptors, dietary AGE intake, OS, and inflammatory factors in healthy participants 18–45 yr old and those older than 60 yr (n = 325)
| 18–45 yr old | Older than 60 yr | Pa | Older than 60 yr (GFR >90) | Older than 60 yr (GFR 30–60) | Pb | Pc | |
|---|---|---|---|---|---|---|---|
| Serum CML (U/ml) | 8 ± .6 | 23 ± 1 | 0.000 | 16 ± 2 | 26 ± 3 | 0.001 | 0.000 |
| Serum MG (nmol/ml) | 0.74 ± 0.04 | 0.99 ± 0.05 | 0.000 | 1.0 ± .1 | 1.0 ± .1 | 0.938 | 0.003 |
| hsCRP (mg/liter) | 2.0 ± .4 | 3.4 ± .4 | 0.008 | 2.4 ± .3 | 5.2 ± .7 | 0.022 | 0.003 |
| Fibrinogen (mg/dl) | 316 ± 9 | 375 ± 9 | 0.000 | 349 ± 11 | 408 ± 24 | 0.041 | 0.000 |
| VCAM-1 (ng/ml) | 820 ± 28 | 908 ± 49 | 0.124 | 877 ± 67 | 1034 ± 127 | 0.290 | 0.008 |
| 8-Isoprostane (ng/ml) | 216 ± 20 | 192 ± 18 | 0.371 | 177 ± 23 | 238 ± 62 | 0.274 | 0.686 |
| TNFα (ng/mg protein) | 9.6 ± .6 | 9.7 ± .6 | 0.853 | 9 ± 1 | 11 ± 1 | 0.195 | 0.270 |
| RAGE (gene copies) | 452 ± 74 | 552 ± 74 | 0.307 | 638 ± 134 | 484 ± 149 | 0.452 | 0.651 |
| AGER1 (gene copies) | 176 ± 34 | 295 ± 53 | 0.064 | 270 ± 60 | 304 ± 96 | 0.768 | 0.087 |
| p66 (gene copies) | 27 ± 3 | 175 ± 43 | 0.001 | 95 ± 52 | 243 ± 94 | 0.148 | 0.000 |
| Caloric intake (kcal/d) | 1979 ± 63 | 1879 ± 90 | 0.362 | 2118 ± 145 | 1458 ± 57 | 0.000 | 0.015 |
| AGE intake (AGE Eq/d) | 15 ± 1 | 13 ± 1 | 0.069 | 14 ± 1 | 12 ± 2 | 0.328 | 0.135 |
| Urinary AGE (U/d) | 52.0 ± 4 | 36.8 ± 3 | 0.014 | 50.3 ± 6.7 | 26.1 ± 3.4 | 0.002 | 0.046 |
Statistical significant difference between healthy participants 18–45 yr old and those older than 60 yr.
Statistical significant difference between healthy participants older than 60 yr with GFR greater than 90 ml/min and those with GFR between 30 and 60 ml/min.
Statistical significant difference between healthy participants 18–45 yr old and those older than 60 yr with a GFR 30–60 ml/min.
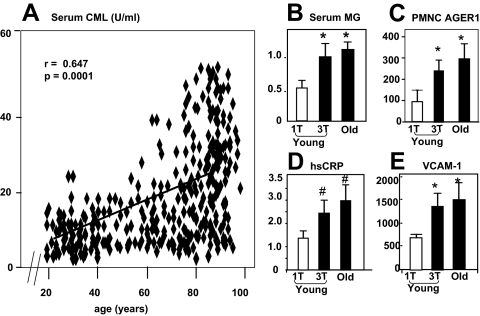
Figure 1.
A, Associations between serum CML, shown in AGE (units per milliliter), and age (n = 194). Extreme tertiles for dietary AGEs in young (<45 yr old) are compared with all healthy subjects older than 60 yr for sCML (B), sMG (C), PMNC AGER1 mRNA (D), and serum hsCRP (E) (n = 20/subgroup). Data are shown as means ± sem. *, P < 0.05 between lowest and highest dietary AGE tertile in healthy participants younger than 45 yr; **, P < 0.05 between lowest dietary tertile in the subjects 18–45 yr old and all subjects older than 60 yr.
Serum CML was negatively correlated with estimated glomerular filtration rate eGFR (r = −0.354, P < 0.0001) and positively with age (r = 0.518, P < 0.0001). Serum CML levels were higher and urine AGEs were lower in participants older than 60 yr with reduced eGFR than in those with a normal eGFR (Table 2). Among participants older than 60 yr, those with eGFR 30–60 ml/min had higher levels of sCML but not sMG, compared with those with a normal eGFR (Table 2). When compared with 18–45 yo subjects, those >60 yo had higher markers of inflammation and OS (hsCRP, fibrinogen, p66shc) (Table 2). This difference was largest in those older than 60 yr who had an eGFR less than 60 ml/min. However, the hsCRP values in this group were below those found in CKD-3 patients, even though the eGFR levels were similar (supplemental Table S3). Participants older than 60 yr, with a reduced eGFR (30–60 ml/min) had lower urinary AGE levels compared with healthy participants whose eGFR was greater than 90 ml/min (Table 2). Interestingly, their urine AGE values were not different from in CKD-3 patients (eGFR 30–60 ml/min) (supplemental Table S3).
Furthermore, stepwise multiple regression analysis in a model including age, eGFR, caloric intake, and dietary AGE intake showed that dietary AGE intake was an independent predictor of 8-isoprostane, VCAM-1, RAGE, p66shc (supplemental Table S1), whereas caloric intake and eGFR were not.
Participants younger than 45 yr were further separated into extreme tertiles for dietary AGE intake. Subjects in the upper tertile were closely related to the group older than 60 yr with respect to sCML, sMG, AGER1, VCAM-1, and hsCRP, relative to those in the lowest tertile (Fig. 1, B–E). These data indicate that the levels of these markers in 18- to 45-yr-old subjects who consume a diet that has a high AGE content parallel the levels in persons older than 60 yr.
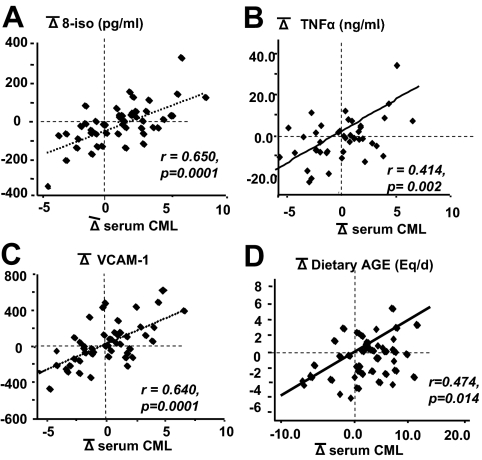
In the longitudinal analysis, serum CML levels underwent changes over the 2-yr follow-up period, and the direction and magnitude of the changes significantly correlated with changes in 8-isoprostanes, PMNC-derived TNFα and VCAM-1 (Fig. 2, A–C). Changes in dietary AGE intake during this same period strongly correlated with parallel changes in sCML (Fig. 2D) as well as sMG levels (data not shown).
Figure 2.
Temporal correlations of changes of circulating AGEs (CML) with changes of oxidant marker 8-isoprostanes (A), PMNC-derived TNF-α (B), VCAM-1 (C), and dietary AGE intake (D). Healthy participants (n = 49) were followed up for 2 yr while on their habitual diet. Statistical significance is indicated.
PMNC AGE receptors
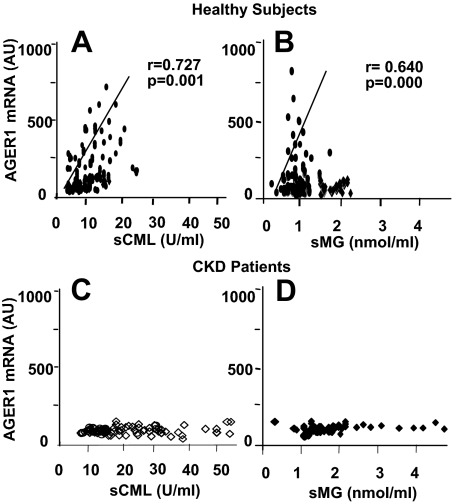
In healthy participants, AGER1 mRNA expression directly correlated with sCML and sMG levels (Fig. 3, A and B). AGER1 levels tended to be higher in healthy participants older than 60 yr than 18- to 45-yr-old healthy participants, although this difference did not reach statistical significance (P = 0.064) (Table 2). Age was not a significant independent predictor of RAGE or p66sch mRNA levels in healthy participants, and CKD patients had significantly higher levels of RAGE and p66sch mRNA (supplemental Tables S1 and S3). However, the levels of AGER1 mRNA in CKD patients were significantly lower than in healthy participants (Table 2 and supplemental Table S3) and did not vary as a function of sCML or sMG (Fig. 3, C and D).
Figure 3.
PMNC AGER1 mRNA expression in healthy subjects (n = 111) correlates with serum CML (A) and serum MG (B) but not in patients with CKD (n = 66) (C and D). Data are shown in human AGER1 gene copy numbers per cell by RT-PCR, CML (units per milliliter) and MG (nanomoles per milliliter) of fasting serum.
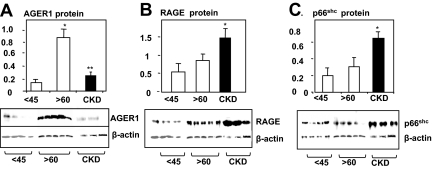
AGER1 protein levels were higher (>5-fold) in healthy participants older than 60 yr compared with 18- to 45-yr-old healthy participants, but they were lower in CKD-3 patients matched for eGFR (Fig. 4A). RAGE and p66Shc protein levels were not significantly different in healthy participants older than 60 yr and 18- to 45-yr-old healthy participants, but they were approximately greater than 3- to 4-fold higher in CKD-3 patients (Fig. 4, B and C).
Figure 4.
Relative PMNC protein expression levels of AGER1 (A), RAGE (B), and p66shc (C) in healthy 18- to 45-yr-old subjects, subjects older than 60 yr, and CKD patients. Freshly isolated PMNCs were used for Western analysis. Density data are shown as means ± sem. Western blots are representative of five subjects per group, each performed three times. *, P < 0.05; **, P < 0.01.
Because AGER1 is thought to mediate AGE removal, correlations between the levels of PMNC AGER1 with the levels of AGE excreted in the urine over 24 h were examined. Urine levels of both CML and MG derivatives significantly correlated with each other and the corresponding serum levels (supplemental Fig. 1, A and B). Furthermore, there was a highly significant linear relationship between PMNC AGER1 mRNA and urine levels of both CML and MG derivatives in healthy adults (supplemental Fig. 1, C and D).
Interventional study
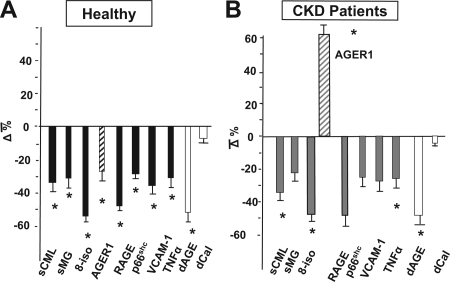
The low-AGE dietary intervention in healthy participants was associated with a significant reduction in the levels of sCML and sMG as well as AGER1, RAGE, and p66ShcmRNA (Fig. 5A and supplemental Table S2A). The low-AGE dietary intake was also followed by a significant reduction in 8-isoprostanes, VCAM-1, and PMNC TNFα, compared with the baseline values (Fig. 5A). There was no significant change in other parameters (supplemental Table S2A). Of note, the changes observed were similar in participants both 18–45 and older than 60 yr.
Figure 5.
A, Effects of a low-AGE dietary intervention on oxidant stress, inflammation, serum AGEs, and PMNC AGE in healthy subjects. Healthy subjects (n = 30) were randomly assigned to a regular diet or an isocaloric diet containing 50% lower AGEs for 4 months. *, P < 0.05. B, Effects of a low-AGE dietary intervention on OS, inflammation, serum AGEs, and PMNC AGE in CKD patients. CKD patients (n = 9) were randomly assigned to the low-AGE diet for 4 wk. *, P < 0.05.
The dietary intervention followed a similar pattern in CKD patients. Namely, there was a decline in the levels of sCML, sMG, 8-isoprostanes, VCAM-1 as well as TNFα (Fig. 5B and supplemental Table S2B). In addition, RAGE and p66Shc mRNA levels were significantly reduced in PMNC (by 50 and 25%, respectively). In contrast, in this group AGER1 increased by approximately 60% in PMNC (Fig. 5B), reaching levels approximating those found in the healthy 18- to 45-yr-old participants. These changes were statistically significant after only 4 wk on the low-AGE diet.
Discussion
The data of the current studies provide strong evidence that intake of a diet with a high AGE content is a major determinant of systemic OS in healthy adults. Three unexpected findings emerged from these studies. First, whereas OS may increase in the aging population, this increase may not be limited to that group. The data showing that elevated OS can be present at a young age, and is not an exclusive feature of aging, may have important health care implications. Second, OS and inflammation can be reduced by an isocaloric low-AGE diet, regardless of age or disease; thus, elevated OS may be remediable. Third, levels of AGER1, a receptor involved in AGE metabolism, correlate significantly with levels of circulating AGEs and markers of OS as well as with urine AGEs. This suggests that levels of AGER1 are important in the control of OS. Further data support the previously advanced hypothesis that a decrease in the level of AGER1 gene function may signal a compromise in innate defenses and a transition to disease, e.g. kidney disease. The effective removal of oxidant AGEs via AGER1 may also depend on effective AGE metabolism and excretion via the kidney. This potential relationship may provide one explanation for the increased OS encountered in the overall aging population as well as CKD patients.
The cross-sectional findings were confirmed by the longitudinal study. Variations in the daily consumption of AGEs correlated with parallel, positive or negative fluctuations in two serum AGEs, CML and MG. These in turn correlated with time-dependent changes in inflammatory markers. In particular, PMNC-derived TNFα, a key measure of the inflammatory state not affected by rapid plasma clearance, was sensitive to dietary AGE intake by healthy subjects, as shown for diabetic patients (13). Thus, the data identify AGEs in the average diet of healthy participants as agents that promote inflammation and OS (13,21,37).
MG derivatives are highly reactive and rapidly degraded by the glyoxalase system intracellularly (42,43). They are also rearranged to stable AGEs, such as CML, so that their levels in serum remain low, rising in the event of excessive ROS, e.g. hyperglycemia (44), CKD (45), or chronic intake of AGE-rich meals (13,16). This transience may explain the lower levels of sMG derivatives, relative to sCML, in healthy persons older than 60 yr. It may also partly explain the dissociation between the low sMG levels in healthy participants older than 60 yr with reduced eGFR compared with the high levels in CKD-3 patients. Because high ROS promotes de novo MG generation, elevated sMG may serve as a marker of active, uncompensated oxidative process in CKD-3 but not in uncomplicated aging as long as excess MG can be metabolized and excreted in the urine. This view, although in need of further investigation, is consistent with the linear relationship between AGER1 and urine MG levels in healthy persons. Because AGER1 expression levels in normal PMNC appear to be driven by both endogenous and exogenous AGEs across age, the findings suggest that the human AGER1 gene retains significant plasticity throughout life, actively suppressing OS. In this context, OS markers, 8-isoprostanes as well as RAGE and TNFα were significantly increased only in CKD patients, a group with suppressed AGER1 (32) and not in the participants older than 60 yr. PMNC AGER1 correlated with RAGE, which was also elevated in healthy participants consuming an AGE-rich diet. This finding suggests that a continuous excess of exogenous AGEs could, via excess ROS, lead to a perpetual induction of RAGE and inflammatory cytokines, such as TNFα, increasing the risk of disease.
An issue complicating the study of OS is related to the size of the oxidant pool, which is influenced by exogenous oxidants as well as disease (12,13,21). The role of dietary glycooxidants in healthy OS homeostasis is herein evaluated for the first time by the use of a probe that is neither drug nor calorie related. Namely, a moderate (30–50%) reduction of the intake of AGEs by healthy participants substantially reduced the normal baseline levels of serum AGEs, OS, and inflammation as well as AGER1, p66Shc, and RAGE mRNA. Because the participants were healthy and had average dietary habits, we believe this degree of OS reduction supports the view that the normal set point for OS may be elevated in that segment of the general population, which tends to consume diets with a higher content of AGEs.
It is particularly noteworthy that the suppressed AGER1 was restored in CKD patients after the low-AGE diet, implicating oxidants rather than genetic causes in the loss of AGER1 in high OS conditions. Although PMNC may not reflect other tissues with respect to AGER1, the data are in line with findings from aging mice fed a low-AGE or a low-calorie diet, linking increased tissue AGER1 to lower OS and extended life span (16,33).
These cross-sectional studies were limited in that associations with other significant variables, such as gender and race, could not be determined due to the small number of participants in these categories. Other limitations include the lack of direct measurements of renal function, the interventional study of CKD patients that was limited in sample size, and the interventions in both of the CKD and aging cohorts lacking unambiguous outcomes in terms of organ function. However, this new evidence suggests that reduction of exogenous oxidant burden can normalize OS, inflammatory markers, and previously suppressed innate defenses, such as AGER1. These are significant findings requiring further evaluation at the cellular, molecular, and clinical levels.
In summary, food-derived AGEs are a significant source of oxidants and a major modulator of innate anti-OS defenses during lifetime, gradually raising OS homeostatic set points to levels exceeding the native limits and increasing risk of disease. Abnormal OS may thus not be a natural accompaniment of human aging because it can be bypassed by physiological means. In the face of a rising incidence of chronic diseases such as diabetes, CVD, and CKD, reducing exposure to food AGEs may represent an important disease prevention strategy.
Supplementary Material
Acknowledgments
We thank Annabelle Rinaldo and Marisa Sherry, M.S., R.D., Bionutrition Research Manager, and the staff of the Mount Sinai School of Medicine General Clinical Research Center.
Footnotes
This work was supported by MERIT Grants AG-23188 and AG-09453 from the National Institute on Aging (to H.V.) and the National Institute of Research Resources Grant MO1-RR-00071 awarded to the General Clinical Research Center at Mount Sinai School of Medicine.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 9, 2009
Abbreviations: AGE, Advanced glycation end products; AGER1, AGE receptor-1; CKD, kidney disease; CML, εN-carboxy-methyl-lysine; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; hsCRP, high-sensitivity C-reactive protein; MG, methyl-glyoxal derivative; OS, oxidative stress; PMNC, peripheral blood mononuclear cell; RAGE, receptor for AGE; ROS, reactive oxygen species; s, serum; VCAM-1, vascular cell adhesion molecule.
References
- Bokov A, Chaudhuri A, Richardson A 2004 The role of oxidative damage and stress in aging. Mech Ageing Dev 125:811–826 [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ 2000 Oxidants, oxidative stress, and the biology of ageing. Nature 408:239–247 [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pederson M, Bruungsgaard H 2004 Inflammatory mediators in the elderly. Exp Gerontol 39:687–699 [DOI] [PubMed] [Google Scholar]
- Ershler WB, Ferrucci L, Finch C, Glaser R Inflammation, inflammatory mediators and aging. Proc National Institute of Aging Inflammation and Aging Workshop, National Institutes of Health, Bethesda, MD, 2004 [Google Scholar]
- Beharka AA, Meydoni M, Wu D, Leka LS, Meydani A, Meydani SN 2001 Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med 56:B81–B88 [DOI] [PubMed] [Google Scholar]
- Libby P 2002 Inflammation in atherosclerosis. Nature 420:868–874 [DOI] [PubMed] [Google Scholar]
- Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB, Shlipak MG 2007 Kidney function and markers of inflammation in elderly persons without chronic kidney disease: the health, aging, and body composition study. Kidney Int 71:239–244 [DOI] [PubMed] [Google Scholar]
- Brownlee M 2001 Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR 1999 Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 48:1–9 [DOI] [PubMed] [Google Scholar]
- Spiteller G 2008 Peroxyl radicals are essential reagents in the oxidation steps of the Maillard reaction leading to generation of advanced glycation end products. Ann NY Acad Sci 1126:128–133 [DOI] [PubMed] [Google Scholar]
- Finot PA 2005 Historical perspective of the Maillard reaction in food science. Ann NY Acad Sci 1043:1–8 [DOI] [PubMed] [Google Scholar]
- Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, Heitmann K, Vlassara H 1997 Orally absorbed reactive advanced glycation end products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci USA 94:6474–6479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ 2002 Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA 99:15596–15601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouillart P, Mauprivez H, Ait-Ameur L, Cayzeele A, Lecerf JM, Tessier FJ, Birlouez-Aragon I 2008 Strategy for the study of the health impact of dietary Maillard products in clinical studies. Ann NY Acad Sci 1126:173–176 [DOI] [PubMed] [Google Scholar]
- Goldberg T, Cai W, Peppa M, Dardaine V, Baliga BS, Uribarri J, Vlassara H 2004 Advanced glycooxidation end products in commonly consumed foods. J Am Diet Assoc 104:1287–1291 [DOI] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Chen X, Zheng F, Striker GE, Vlassara H 2008 Oral glycotoxins determine the effects of calorie restriction on oxidant stress, age-related diseases, and lifespan. Am J Pathol 173:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribarri J, Stirban A, Sander D, Cai W, Negrean M, Buenting CE, Koschinsky T, Vlassara H 2007 Single oral challenge by advanced glycation end products acutely impairs endothelial function in diabetic and non-diabetic subjects. Diabetes Care 30:2579–2582 [DOI] [PubMed] [Google Scholar]
- Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H 2005 Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes 54:2314–2319 [DOI] [PubMed] [Google Scholar]
- Lin RY, Reis ED, Dore AT, Lu M, Ghodsi N, Fallon JT, Fisher EA, Vlassara H 2002 Lowering of dietary advanced glycation endproducts (AGE) reduces neointiminal formation after arterial injury in genetically hypercholesterolemic mice. Atherosclerosis 163:303–311 [DOI] [PubMed] [Google Scholar]
- Zheng F, He C, Cai W, Hattori M, Steffes M, Vlassara H 2002 Prevention of diabetic nephropathy in mice by a diet low in glycooxidation products. Diab Metab Res Rev 18:224–237 [DOI] [PubMed] [Google Scholar]
- Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H 2007 Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci 62:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Naka Y, Schmidt AM 2003 Glycation, inflammation and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res 42:532–538 [DOI] [PubMed] [Google Scholar]
- Cai W, He C, Zhu L, Vlassara H 2006 Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. Proc Natl Acad Sci USA 103:13801–13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Chen X, Striker GE, Vlassara H 2008 AGE-receptor-1 counteracts cellular oxidant stress induced by AGEs via negative regulation of p66shc-dependent FKHRL1 phosphorylation. Am J Physiol 294:145–152 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Finkel T 2002 Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295:2450–2452 [DOI] [PubMed] [Google Scholar]
- Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, Condorelli M, Sica G, De Rosa G, Pelicci P 2003 Deletion of p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA 100:2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M, Ferney R, Wade J, Pawson T, Bowtell D 1993 The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature 363:83–85 [DOI] [PubMed] [Google Scholar]
- Pagnin E, Fadini G, de Toni R, Tiengo A, Calò L, Avogaro A 2005 Diabetes induces p66shc gene expression in human peripheral blood mononuclear cells: relationship to oxidative stress. J Clin Endocrinol Metab 90:1130–1136 [DOI] [PubMed] [Google Scholar]
- Menini S, Amadio L, Oddi G, Ricci C, Pesce C, Pugliese F, Giorgio M, Migliaccio E, Pelicci P, Iacobini C, Pugliese G 2006 Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress. Diabetes 55:1642–1650 [DOI] [PubMed] [Google Scholar]
- Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M 2005 High serum levels of advanced glycation end products predict increased coronary heart disease mortality in non-diabetic women but not in nondiabetic men. A population-based 18-year follow-up study. Arterioscler Thrombos Vasc Biol 25:815–820 [DOI] [PubMed] [Google Scholar]
- Linden E, Cai W, He JC, Chen X, Zhu L, Winston J, Vlassara H, Uribarri J 2008 Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin J Am Soc Nephrol 3:691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, He JC, Cai W, Liu H, Zhu L, Vlassara H 2004 Advanced glycation endproduct (AGE) receptor 1 is a negative regulator of the inflammatory response to AGE in mesangial cells. Proc Natl Acad Sci USA 101:11767–11772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, He JC, Zhu L, Chen X, Wallenstein S, Striker GE, Vlassara H 2007 Reduced oxidant stress and extended lifespan in mice exposed to low glycotoxin diet. Association with increased AGER1 expression. Am J Pathol 170:1893–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CJ, Koschinsky T, Buenting C, Vlassara H 2001 Presence of diabetic complications in type 1 diabetic patients correlates with low expression on mononuclear cell AGE-receptor-1 and elevated serum AGE. Mol Med 7:159–168 [PMC free article] [PubMed] [Google Scholar]
- He CJ, Zheng F, Stitt A, Striker L, Hattori M, Vlassara H 2000 Differential expression of renal AGE-receptor genes in NOD mice: possible role in nonobese diabetic renal disease. Kidney Int 58:1931–1940 [DOI] [PubMed] [Google Scholar]
- 2002 K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39(Suppl 1):S1–S266 [PubMed] [Google Scholar]
- Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, Vlassara H 2003 Restriction of dietary glycotoxin reduces excessive advanced glycation end products in renal failure patients. J Am Soc Nephrol 14:728–731 [DOI] [PubMed] [Google Scholar]
- Cai W, Gao QD, Zhu L, Peppa M, He C, Vlassara H 2002 Oxidative stress-inducing carbonyl compounds from common foods: novel mediators of cellular dysfunction. Mol Med 8:337–346 [PMC free article] [PubMed] [Google Scholar]
- Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H 1991 Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med 325:836–842 [DOI] [PubMed] [Google Scholar]
- Makita Z, Bucala R, Rayfield EJ, Friedman EA, Kaufman AM, Korbet SM, Barth RH, Winston JA, Fuh H, Manogue KR, Cerami A, Vlassara H 1994 Reactive glycosylation endproducts in diabetic uraemia and treatment of renal failure. Lancet 343:1519–1522 [DOI] [PubMed] [Google Scholar]
- Pierzchalska M, Soja J, Woś M, Szabó Z, Nizankowska- Mogielnicka E, Sanak M, Szczeklik A 2007 Deficiency of cyclooxygenases transcripts in cultured primary bronchial epithelial cells of aspirin-sensitive asthmatics. J Physiol Pharmacol 58:207–218 [PubMed] [Google Scholar]
- Abordo EA, Minhas HS, Thornalley PJ 1999 Accumulation of alpha-oxoaldehydes during oxidative stress: a role in cytotoxicity. Biochem Pharmacol 58:641–648 [DOI] [PubMed] [Google Scholar]
- Shangari N, O'Brien PJ 2004 The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol 68:1433–1442 [DOI] [PubMed] [Google Scholar]
- Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF 2003 Increased serum level of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 52:163–167 [DOI] [PubMed] [Google Scholar]
- Agalou AS, Ahmed N, Babaei-Jadidi RB, Dawnay A, Thornalley 2005 Profound mishandling of protein glycation degradation products in uremia and dialysis. J Am Soc Nephrol 16:1471–1485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.