Abstract
Objectives
To compare the proportion of antimicrobial-resistant strains among bacterial isolates from younger and older hospital patients and to quantify changes in the proportion of antimicrobial-resistant strains in both groups over time.
Patients and methods
A retrospective analysis of microbiology data from two centres in Maryland and Chicago was performed. Adult hospital inpatients with positive clinical cultures for specific antimicrobial-resistant bacterial pathogens between 1999 and 2005 (55 427 isolates) were included. The proportions of isolates not susceptible to specific antimicrobial agents were compared between patients ≥65 and <65 years. Additional analyses examined temporal trends in the frequency of resistance and the frequency of resistance among the oldest patients (≥80 years), in bacteria isolated from blood cultures and in bacteria obtained from intensive care unit patients.
Results
Heterogeneity was observed in the frequency of resistance among different bacteria between older and younger patients, between the two centres and over the study period. Staphylococcus aureus isolates were more likely to be resistant to methicillin when obtained from older patients at Chicago (50.9% versus 40.9%; P < 0.001). In contrast, younger patients yielded a greater proportion of enterococci resistant to vancomycin at Maryland (19.4% versus 16.5%; P = 0.009). Results were variable when resistance to fluoroquinolones, cephalosporins and imipenem were compared for Pseudomonas aeruginosa, Escherichia coli and Klebsiella spp.
Conclusions
Overall, advanced patient age was not uniformly associated with a greater likelihood of antimicrobial resistance among all bacterial pathogens. Moreover, the frequency of resistance in older and younger patients varied considerably at the two sites over the study period. Variability in the frequency of resistance precludes simplistic conclusions regarding the relationship between age and resistance.
Keywords: drug resistance, bacterial, age
Introduction
Older hospital patients appear to disproportionately suffer the burden of infections that complicate medical care, probably as a result of the age-related decline in the protective immune response, the higher prevalence of co-morbid conditions and the complicated and often invasive therapy that they sometimes require.1 In addition to enduring more frequent episodes of healthcare-associated infections, older patients, when compared with younger hospital patients, often experience increased morbidity and mortality resulting from these infections.2
While the frequency and severity of healthcare-associated infections are often higher in older hospital patients, less is known about the incidence of infections with antimicrobial-resistant pathogens in this group.3–5 Many clinicians assume that antimicrobial resistance will necessarily be more common among all pathogens infecting older patients, due to the higher prevalence of other established risks for antimicrobial resistance in this group, including prior antimicrobial exposure, a greater number of co-morbid conditions and recent hospitalization or institutionalization.6,7 A higher frequency of antimicrobial resistance among older patients could increase the likelihood of delay in the initiation of antimicrobial agents to which the causative pathogen is susceptible, further increasing the risk of subsequent morbidity and mortality for these patients.8,9
Much of the evidence suggesting an increased risk of infection with antimicrobial-resistant pathogens among older patients is based on studies of methicillin-resistant Staphylococcus aureus (MRSA).6,7 However, the presumed association between age and other common antimicrobial-resistant pathogens has not been established.10 There are no published studies that systematically compare the frequency of antimicrobial resistance among young and older hospital patients for a number of common pathogens. As a result, clinicians who presume that the frequency of resistance is higher among older patients on the basis of generalizations about this population as a whole could make faulty decisions regarding the selection of antimicrobial therapy. The characterization and quantification of antimicrobial resistance in bacteria from older versus younger hospital patients has important clinical implications. Specifically, assessment of the frequency of resistance could inform decisions regarding the appropriate empirical coverage for older patients with healthcare-associated infections.
The primary aim of the present study was to quantify and compare the proportion of antimicrobial-resistant strains of common bacterial isolates from both younger and older hospital patients at two healthcare facilities in distinct geographical areas. We further aimed to quantify changes in the frequency of antimicrobial resistance in both groups over time and to examine how the frequency of antimicrobial resistance varies between important clinical subgroups, including bloodstream isolates, intensive care unit (ICU) patients and from the oldest old (age ≥80 years). Based on the results of these analyses, the potential usefulness of age-stratified institutional antibiotic susceptibility reports (antibiograms) is also discussed.
Patients and methods
Study sites
The study was conducted using clinical microbiology data collected at two different acute care hospitals. The University of Chicago Medical Center is a 480 bed facility that serves both a diverse primary care population and a large number of patients referred for subspecialty care. The University of Maryland Medical Center in Baltimore, MD, is a 669 bed tertiary-care centre. Both institutions each have more than 60 adult ICU beds. The study was approved by the institutional review board at each of the two participating centres prior to study commencement.
Antibiotic susceptibility testing
At each institution, routine antimicrobial susceptibility data are determined for bacterial isolates recovered from clinical specimens, irrespective of the specimen's source. Additionally, at both hospitals, local policies dictate the practice of compiling hospital-wide antimicrobial susceptibility reports used by clinicians in the management of patients. The present study includes data collected by the clinical microbiology laboratories at each of the institutions during the 7 year period from 1 January 1999 to 31 December 2005. For the purposes of this study and consistent with CLSI guidelines for antimicrobial susceptibility reports, only the first organism-specific clinical culture per patient, per year, was included.11
The method for determining susceptibility to specific antimicrobial agents varied slightly between pathogens and between the two institutions, but always conformed to established national standards for laboratory practice. Laboratory methods did not vary at either Chicago or Maryland during the period in which data were collected for the study. In some cases, the range of pathogens or antimicrobial agents that was tested was limited for practical reasons unrelated to the conduct of the study. For example, at both institutions, only Enterococcus faecium and Enterococcus faecalis were included from among all available enterococcal isolates. At Maryland, strains of Pseudomonas aeruginosa, Escherichia coli and Klebsiella spp. were tested for susceptibility to gatifloxacin, levofloxacin and ciprofloxacin. Isolates susceptible to any of the three drugs were considered susceptible to fluoroquinolones. At Chicago, ciprofloxacin was the only drug of this class against which these isolates were routinely tested. To determine the susceptibility of Klebsiella spp. and E. coli isolates to higher-generation cephalosporins, isolates were tested against cefepime at Maryland and ceftriaxone at Chicago. Bacterial isolates for which antimicrobial susceptibility results were not available for a particular agent were excluded only from the analysis of that specific agent, but remained eligible for inclusion in the analysis of other agents for which susceptibility data were available.
Data collection
All eligible clinical isolates from adult patients (≥18 years) at each institution were included for the analysis of the overall prevalence of antimicrobial resistance and temporal trends in resistance at both Chicago and Maryland. Data were abstracted electronically from clinical microbiology databases at each institution.
Statistical analysis
The χ2 test was used to compare the annual and cumulative prevalence of antimicrobial resistance among bacterial isolates from older (≥65 years) versus younger patients. To examine the trend in the prevalence of antimicrobial resistance for each of the organisms over time, the results were compared using the χ2 test for trend. For all analyses, the threshold for establishing statistical significance was set at P < 0.05. A Bonferroni correction for multiple comparisons was not used to set a different significance level for sub-analyses. Statistical analysis was completed using the SAS statistical package (Version 9.1; SAS Institute, Cary, NC, USA).
To better understand the epidemiology of resistance in older and younger hospital patients a number of sub-analyses were also performed. Because the proportion of antimicrobial-resistant isolates tends to be higher among critically ill patients, the prevalence of resistance among isolates collected from patients in the ICU at each institution was examined separately. To ensure that the results were not skewed by the inclusion of a high proportion of colonizing strains from otherwise non-sterile sites, an additional analysis included bloodstream isolates exclusively. Lastly, to more closely examine the relationship between patient age and the prevalence of antimicrobial resistance, a final analysis compared patients aged <80 years with those ≥80 years of age, who may be at particular risk for colonization and infection with resistant organisms.
Results
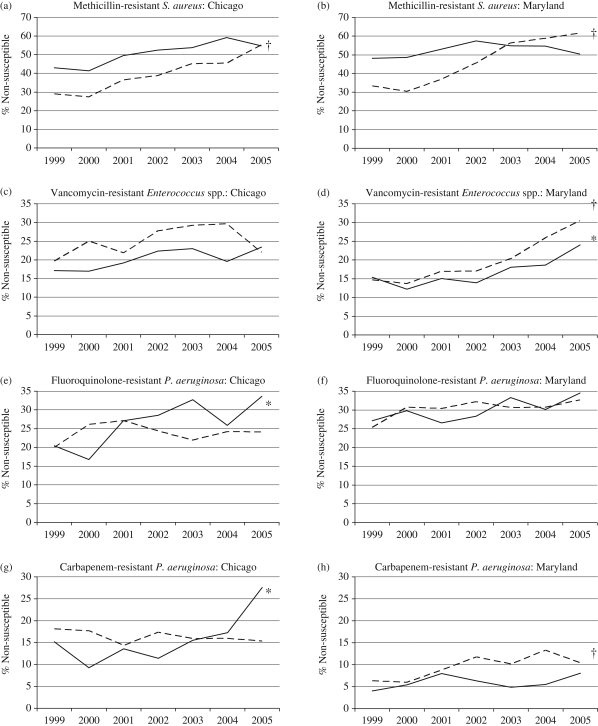
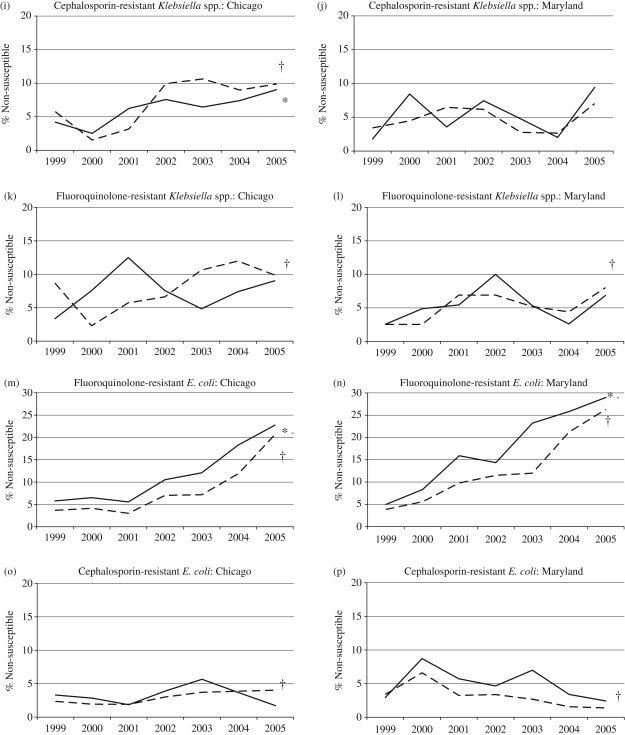
Susceptibility testing results were available for 55 427 isolate/antimicrobial pairings at the two institutions (32 543 for Maryland and 22 884 at Chicago) during the 7 year study period. When examined by individual calendar year, there was considerable variation in the proportion of resistant isolates for both older and younger patients at each of the two institutions, although overall resistance rates increased for most organisms over the study period (Figure 1). Detailed results for each pathogen at both institutions are provided in Table 1.
Figure 1.
Percentage of isolates susceptible to individual antimicrobial agents at the University of Chicago and the University of Maryland by year. Continuous line, patients ≥65 years (asterisk indicates P < 0.05 for trend over time); broken line, patients <65 years (dagger indicates P < 0.05 for trend over time).
Table 1.
Percentage of bacterial isolates non-susceptible to specified antimicrobial agents among older versus younger inpatients at Maryland and Chicago between 1999 and 2005
| Non-susceptible (%) |
||||||
|---|---|---|---|---|---|---|
| University of Maryland |
University of Chicago |
|||||
| ≥65 years | <65 years | P | ≥65 years | <65 years | P | |
| S. aureus (methicillin) (n = all isolates) | 1172 | 5051 | 1380 | 2786 | ||
| all isolates | 51.9 | 48.8 | 0.055 | 50.9 | 40.9 | <0.001* |
| isolates from ICU patients only | 48.7 | 37.0 | <0.001* | 49.3 | 40.9 | 0.002* |
| all isolates (>80 versus ≤80 years old) | 49.8 | 49.3 | 0.874 | 52.1 | 43.4 | 0.002* |
| isolates from bloodstream only | 50.2 | 50.6 | 0.238 | 48.3 | 38.9 | 0.004* |
| Enterococcus (vancomycin) (n) | 1775 | 3960 | 702 | 1137 | ||
| all isolates | 16.5 | 19.4 | 0.009* | 20.2 | 25.0 | 0.019* |
| isolates from ICU patients only | 18.4 | 18.9 | 0.779 | 26.1 | 23.4 | 0.409 |
| all isolates (>80 versus ≤80 years old) | 16.0 | 18.7 | 0.184 | 17.0 | 23.9 | 0.032* |
| isolates from bloodstream only | 27.0 | 28.7 | 0.598 | 24.0 | 30.5 | 0.047* |
| Pseudomonas (fluoroquinolones) (n) | 877 | 1966 | 805 | 1201 | ||
| all isolates | 30.1 | 30.3 | 0.931 | 26.2 | 24.0 | 0.257 |
| isolates from ICU patients only | 24.7 | 29.2 | 0.118 | 27.2 | 22.7 | 0.131 |
| all isolates (>80 versus ≤80 years old) | 28.6 | 30.3 | 0.610 | 25.8 | 24.8 | 0.761 |
| isolates from bloodstream only | 30.7 | 24.0 | 0.253 | 26.8 | 24.8 | 0.764 |
| Pseudomonas (imipenem) (n) | 866 | 1977 | 800 | 1199 | ||
| all isolates | 5.9 | 9.5 | 0.002* | 15.8 | 16.3 | 0.759 |
| isolates from ICU patients only | 7.8 | 11.8 | 0.046* | 21.6 | 12.7 | <0.001* |
| all isolates (>80 versus ≤80 years old) | 5.8 | 8.6 | 0.171 | 12.4 | 16.4 | 0.149 |
| isolates from bloodstream only | 2.7 | 11.6 | 0.024* | 18.3 | 18.6 | 0.963 |
| Klebsiella (third-generation cephalosporin)a (n) | 765 | 1577 | 883 | 1048 | ||
| all isolates | 5.2 | 4.8 | 0.619 | 6.3 | 7.3 | 0.430 |
| isolates from ICU patients only | 2.3 | 6.1 | 0.019* | 8.3 | 5.1 | 0.073 |
| all isolates (>80 versus ≤80 years old) | 2.2 | 5.1 | 0.080 | 5.9 | 7.0 | 0.528 |
| isolates from bloodstream only | 6.3 | 7.9 | 0.571 | 6.9 | 8.6 | 0.589 |
| Klebsiella (fluoroquinolone)b (n) | 882 | 1778 | 883 | 1047 | ||
| all isolates | 5.1 | 5.3 | 0.840 | 7.5 | 8.1 | 0.600 |
| isolates from ICU patients only | 3.5 | 6.8 | 0.044* | 9.6 | 6.3 | 0.089 |
| all isolates (>80 versus ≤80 years old) | 3.2 | 5.4 | 0.167 | 5.9 | 8.1 | 0.222 |
| isolates from bloodstream only | 7.9 | 7.3 | 0.816 | 12.5 | 10.8 | 0.622 |
| E. coli (fluoroquinolone)b (n) | 1573 | 3588 | 1788 | 2718 | ||
| all isolates | 17.6 | 13.5 | 0.000* | 12.1 | 8.3 | <0.0001* |
| isolates from ICU patients only | 14.9 | 12.1 | 0.150 | 12.2 | 7.4 | 0.007* |
| all isolates (>80 versus ≤80 years old) | 16.7 | 14.6 | 0.234 | 11.7 | 9.5 | 0.065 |
| isolates from bloodstream only | 29.0 | 20.1 | 0.040* | 13.7 | 12.4 | 0.656 |
| E. coli (third-generation cephalosporin)a (n) | 1462 | 3274 | 1788 | 2719 | ||
| all isolates | 4.9 | 3.0 | 0.001* | 3.3 | 3.0 | 0.544 |
| isolates from ICU patients only | 4.6 | 3.4 | 0.328 | 6.5 | 2.2 | <0.0001* |
| all isolates (>80 versus ≤80 years old) | 4.3 | 3.5 | 0.397 | 3.0 | 3.1 | 0.860 |
| isolates from bloodstream only | 9.2 | 5.8 | 0.340 | 2.8 | 4.6 | 0.315 |
aCeftriaxone at Chicago and cefepime at Maryland.
bCiprofloxacin at Chicago and any fluoroquinolone at Maryland.
*P < 0.05, Fisher's exact test.
Staphylococcus aureus
Taking the entire study period, the proportion of S. aureus isolates resistant to methicillin was greater among older than younger patients at both institutions. However, at both Maryland and Chicago, the proportion of S. aureus isolates resistant to methicillin increased in younger patients over time (P < 0.01), particularly near the end of the study period (Figure 1a and b). By 2003, the proportion of methicillin-resistant isolates from younger patients surpassed the proportion from older patients at Maryland. At Chicago, the proportion of methicillin-resistant isolates was higher among the older patients until 2005. The overall difference was found to be statistically significant at Chicago (50.9% versus 40.9%; P < 0.01) but not at Maryland (51.9% versus 48.8%; P = 0.06). The higher frequency of MRSA among older patients at Chicago was consistent whether examining specimens from patients in the ICU, from those older and younger than 80 years and when the analysis was limited to bloodstream isolates only (P < 0.01 for each). At Maryland, the proportion of resistant isolates was significantly higher only among ICU patients ≥65 years old (48.7% versus 37.0%; P < 0.01).
Vancomycin-resistant enterococci
The proportion of enterococcal isolates resistant to vancomycin was significantly greater in younger compared with older inpatients at both institutions (Maryland: 19.4% versus 16.5%; P = 0.01. Chicago: 25.0% versus 20.2%; P = 0.02). While this phenomenon was not observed among isolates from ICU patients at either centre, the difference in frequency of vancomycin resistance was even greater when the analysis was limited to bloodstream isolates at Chicago (30.5% versus 24.0%; P = 0.047). While the proportion of isolates resistant to vancomycin generally increased at both institutions and in both age groups during the study period, the increase was more pronounced and sustained at Maryland (Figure 1c and d).
Pseudomonas aeruginosa
Although the prevalence of fluoroquinolone resistance among P. aeruginosa isolates was observed to increase at both institutions during the study period (Figure 1e and f), there was no significant difference in the prevalence of resistant isolates in older versus younger patients at either site (Maryland: 30.1% versus 30.3%; P = 0.93. Chicago: 26.2% versus 24.0%; P = 0.26).
While the frequency of resistance to imipenem among P. aeruginosa isolates was generally higher at Chicago than at Maryland throughout the study period, there was a statistically significant higher frequency of imipenem resistance among P. aeruginosa isolates from younger patients at Maryland (9.5% versus 5.9%; P < 0.01), but not Chicago (16.3% versus 15.8%; P = 0.76). At Maryland, the difference was especially pronounced when the analysis was limited to bloodstream isolates only (11.6% versus 2.7%; P = 0.02). A sharp increase in the proportion of P. aeruginosa isolates resistant to carbapenems was detected over time among older patients at Chicago, but not at Maryland (Figure 1 g and h).
Klebsiella spp. and Escherichia coli
The proportion of Klebsiella species resistant to third-generation cephalosporins or ciprofloxacin did not differ between older and younger hospital patients at either Maryland or Chicago, neither cumulatively nor during any single year (Figure 1i–l).
The proportion of E. coli isolates resistant to fluoroquinolones was greater among older patients compared with younger patients at both Maryland and Chicago (Maryland: 17.6% versus 13.5%; P < 0.01. Chicago: 12.1% versus 8.3%; P < 0.01). Perhaps most striking was the sharp increase in the proportion of fluoroquinolone-resistant E. coli during the study period in all patient groups at both institutions (Figure 1 m and n). A small, but significantly greater proportion of E. coli isolates resistant to third-generation cephalosporins was observed among isolates from older patients at Maryland (4.9% versus 3.0%; P < 0.01) and from older ICU patients at Chicago (6.5% versus 2.2%; P < 0.01) (Figure 1o and p).
Discussion
The results of this study provide evidence that important but somewhat unpredictable differences exist in the frequency of antimicrobial resistance among common bacterial pathogens isolated from older versus younger adult hospital inpatients. Ultimately, this finding will not be surprising to clinicians and investigators familiar with the unique distribution of clinical risks and exposures that predict resistant infection in any population. Nonetheless, the observation that the frequency of resistance was not uniformly higher among bacteria isolated from older patients could influence the practice of clinicians who inappropriately infer that advanced age itself is a risk for resistance.
Not surprisingly, an increased frequency of methicillin resistance among S. aureus isolates from older patients was observed. Similar findings have been reported in a number of previous studies.6,7 However, as is discussed below, this association was not observed for other antimicrobial-resistant pathogens. Moreover, even the rule associating older age and the risk for MRSA may soon be obsolete. Over the last 2–3 years of the study, a sharp increase in the frequency of methicillin resistance was observed especially among S. aureus isolates from younger patients at both Chicago and Maryland. This phenomenon may be attributable to the increase in community-associated MRSA that has been reported over the past several years.12
In contrast to the findings for MRSA, the observation that vancomycin resistance appears to be more common among enterococcal isolates from younger patients at both centres was unexpected. However, much like the risk for methicillin resistance among S. aureus isolates from older patients, it would be imprudent to assume that age itself independently predicts the risk for vancomycin resistance. Rather, other epidemiological exposures likely confound the relationship between age and vancomycin resistance. For example, it is possible that younger people sick enough to require hospital admission may actually be affected by chronic or severe medical conditions (e.g. malignancy or rheumatological disease) that render them more likely to have been previously colonized with antimicrobial-resistant bacteria than older patients.
There are practical implications to the findings reported in this study. We observed a higher frequency of resistance to imipenem among P. aeruginosa in younger patients at Maryland. Based on this finding, an older patient with suspected or confirmed pseudomonal infection may have no greater need for broader coverage than a similar but younger patient. According to several recent systematic reviews, the toxicity that can accompany the agents used to extend coverage (especially the aminoglycosides) may not only limit the benefit of this approach, but may actually prove detrimental. This may be especially true in older patients due to a greater physiological susceptibility to the adverse effects of these agents.13,14
The observed sharp increase in the proportion of E. coli isolates resistant to fluoroquinolones at both Chicago and Maryland is reminiscent of the findings of a number of other recent investigations. Lautenbach et al.15 linked a similar increase in resistance in isolates from both inpatients and outpatients to increased utilization of fluoroquinolones. Given the frequency with which fluoroquinolones are increasingly prescribed to treat infection or suspected infection among older patients, it is possible that a similar phenomenon may have contributed to the results reported in the present study.
The changes in frequency of antimicrobial resistance in both younger and older patients over time and between institutions further illustrates the potential problem of neglecting more nuanced epidemiological risks and making overly simplified assumptions concerning the risk for antimicrobial resistance among older patients. The 4- to 6-fold increase in fluoroquinolone resistance observed among E. coli isolates from all patients at both Chicago and Maryland over the 7 year period of this study illustrates why clinicians must have access to up-to-date information regarding the frequency of antimicrobial resistance in order to make the best decisions for patients, young and old. Similarly, the wide variation in the proportion of some resistant strains between the two institutions, such as the 5-fold higher frequency of resistance to imipenem among bloodstream isolates from older patients at Chicago mandates that such information must be determined and disseminated locally. Whether this phenomenon is attributable to differences in patient population, antibiotic prescribing pattern or infection control practice between the two institutions remains uncertain and beyond the scope of the present investigation. Based on the results of just this study, it is also unknown whether trends in antimicrobial resistance will be entirely unique at every acute care facility or whether common patterns will emerge when results from greater numbers of institutions are compared.
This study had several potential limitations. Although data from two distinct study sites were used, both were academic tertiary-care facilities and thus the external validity of these results may be limited. Furthermore, because the present study utilized aggregate data, we were unable to explore the more specific epidemiological and clinical risks that are ultimately responsible for the wide variability observed in the frequency of antimicrobial resistance. For example, we did not have data on antibiotic use, and differences in antibiotic use and corresponding selective pressure between the two facilities could explain some of the observed differences in the prevalence of resistance. Lastly, given some heterogeneity in the methods to assess antibiotic susceptibility between the two sites, some direct comparisons could not be made.
The observed differences in the proportion of antimicrobial-resistant strains among bacterial pathogens isolated from younger versus older hospital inpatients could impact selection of antimicrobial therapy. Clinicians and patients already benefit from the availability of institutional and, increasingly, unit-specific antimicrobial susceptibility reports16 and a similar tool highlighting the frequency of resistance stratified by both patient age and pathogen is likely to be of at least comparable benefit.
Finally, the heterogeneity in the frequency of antimicrobial resistance in younger versus older patients observed in this study serves as an important reminder that much remains to be understood regarding the interplay of age, more specific risk factors and colonization and infection with antimicrobial-resistant pathogens. Until these relationships are better elucidated, unfounded and potentially imprecise assumptions about antimicrobial resistance in older patients may persist.
Funding
Financial support was provided by a T. Franklin Williams Faculty Development Award from the Atlantic Philanthropies, the John A. Hartford Foundation, the Infectious Diseases Society of America and the Association of Subspecialty Professors (S. G. W.). Financial support was also provided by National Institute of Health Grant P60 AG12583 (R. R. M.) and grants 1K12RR023250-01 and 1K01AI071015-01 (J. P. F.). Centers for Disease Control and Prevention [grant 1 R01 CI000369-01 (A. D. H. and E. N. P.)] and Veterans Affairs Health Services Research and Development Service [grants IIR 04-123-2 and Level 2 Advanced Career Development Award (E. N. P.)] also provided funding.
Transparency declarations
S. G. W.: Merck, research support; and Pfizer, honorarium. R. R. M.: Merck, research support. D. P.: Abbot, BMS, Cubist and GSK, research support; Pfizer, speaker; and Gilead, advisor. Other authors: none to declare.
References
- 1.Saviteer SM, Samsa GP, Rutala WA. Nosocomial infections in the elderly. Increased risk per hospital day. Am J Med. 1988;84:661–6. doi: 10.1016/0002-9343(88)90101-5. [DOI] [PubMed] [Google Scholar]
- 2.McGarry SA, Engeman JJ, Schmader K, et al. Surgical-site infection due to Staphylococcus aureus among elderly patients: mortality, duration of hospitalization, and cost. Infect Control Hosp Epidemiol. 2004;25:461–7. doi: 10.1086/502422. [DOI] [PubMed] [Google Scholar]
- 3.Niclaes L, Buntinx F, Banuro E, et al. Consequences of MRSA carriage in nursing home residents. Epidemiol Infect. 1999;122:235–9. doi: 10.1017/s0950268898001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrison L, Stolarek I. Does MRSA affect patient outcomes in the elderly? A retrospective pilot study. J Hosp Infect. 2000;45:169–71. doi: 10.1053/jhin.2000.0727. [DOI] [PubMed] [Google Scholar]
- 5.Washio M, Kiyohara C, Hamada T, et al. The case fatality rate of methicillin-resistant Staphylococcus aureus (MRSA) infection among the elderly in a geriatric hospital and their risk factors. Tohoku J Exp Med. 1997;183:75–82. doi: 10.1620/tjem.183.75. [DOI] [PubMed] [Google Scholar]
- 6.Eveillard M, Ernst C, Cuviller S, et al. Prevalence of methicillin-resistant Staphylococcus aureus carriage at the time of admission in two acute geriatric wards. J Hosp Infect. 2002;50:122–6. doi: 10.1053/jhin.2001.1152. [DOI] [PubMed] [Google Scholar]
- 7.Hori S, Sunley R, Tami A, et al. The Nottingham Staphylococcus aureus population study: prevalence of MRSA among the elderly in a university hospital. J Hosp Infect. 2002;50:25–9. doi: 10.1053/jhin.2001.1130. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DJ, Engemann J, Harrell LJ, et al. Predictors of mortality in patients with bloodstream infection due to ceftazidime-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2006;50:1715–20. doi: 10.1128/AAC.50.5.1715-1720.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodise TP, McKinnon PS, Swiderski L, et al. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36:1418–23. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 10.Wiener J, Quinn JP, Bradford PA, et al. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–23. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data—Second Edition: Approved Guideline M39-A2. Wayne, PA, USA: CLSI; 2005. [Google Scholar]
- 12.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 13.Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in Gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519–27. doi: 10.1016/S1473-3099(04)01108-9. [DOI] [PubMed] [Google Scholar]
- 14.Chamot E, Boffi El Amari E, Rohner P, et al. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756–64. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lautenbach E, Strom B, Nachamkin I, et al. Longitudinal trends in fluoroquinolone resistance among Enterobacteriaceae isolates from inpatients and outpatients, 1989-2000: differences in the emergence and epidemiology of resistance across organisms. Clin Infect Dis. 2004;38:655–62. doi: 10.1086/381549. [DOI] [PubMed] [Google Scholar]
- 16.Binkley S, Fishman NO, LaRosa LA, et al. Comparison of unit-specific and hospital-wide antibiograms: potential implications for selection of empirical antimicrobial therapy. Infect Control Hosp Epidemiol. 2006;27:682–7. doi: 10.1086/505921. [DOI] [PubMed] [Google Scholar]